The Herbal Combination CPA4-1 Inhibits Changes in Retinal Capillaries and Reduction of Retinal Occludin in db/db Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the CPA4-1
2.2. HPLC Analysis
2.3. Determination of Preventive Effect of CPA4-1 on AGE Formation
2.4. Breaking Effect of CPA4-1 on Preformed AGE-Collagen Complexes
2.5. Cell Culture and Determination of Preventive Effect of CPA4-1 on AGE Formation in ARPE-19 Cells
2.6. Western Blot Analysis
2.7. Animals and Experimental Design
2.8. Measurement of BRB Permeability
2.9. Preparation of Trypsin-Digested Retinal Vessel
2.10. Immunohistochemical Staining
2.11. Statistical Analysis
3. Results
3.1. HPLC Analysis of CPA4-1
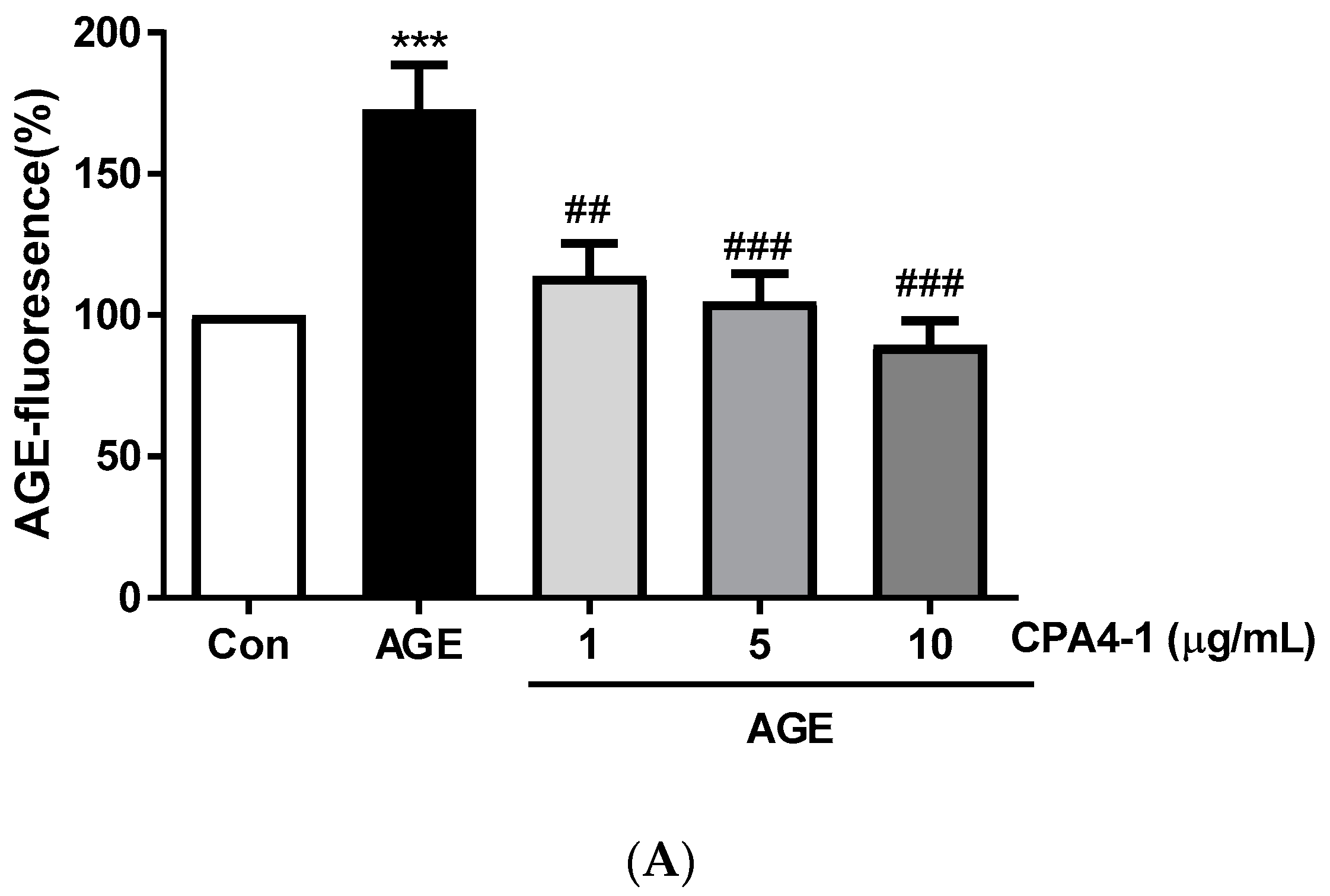
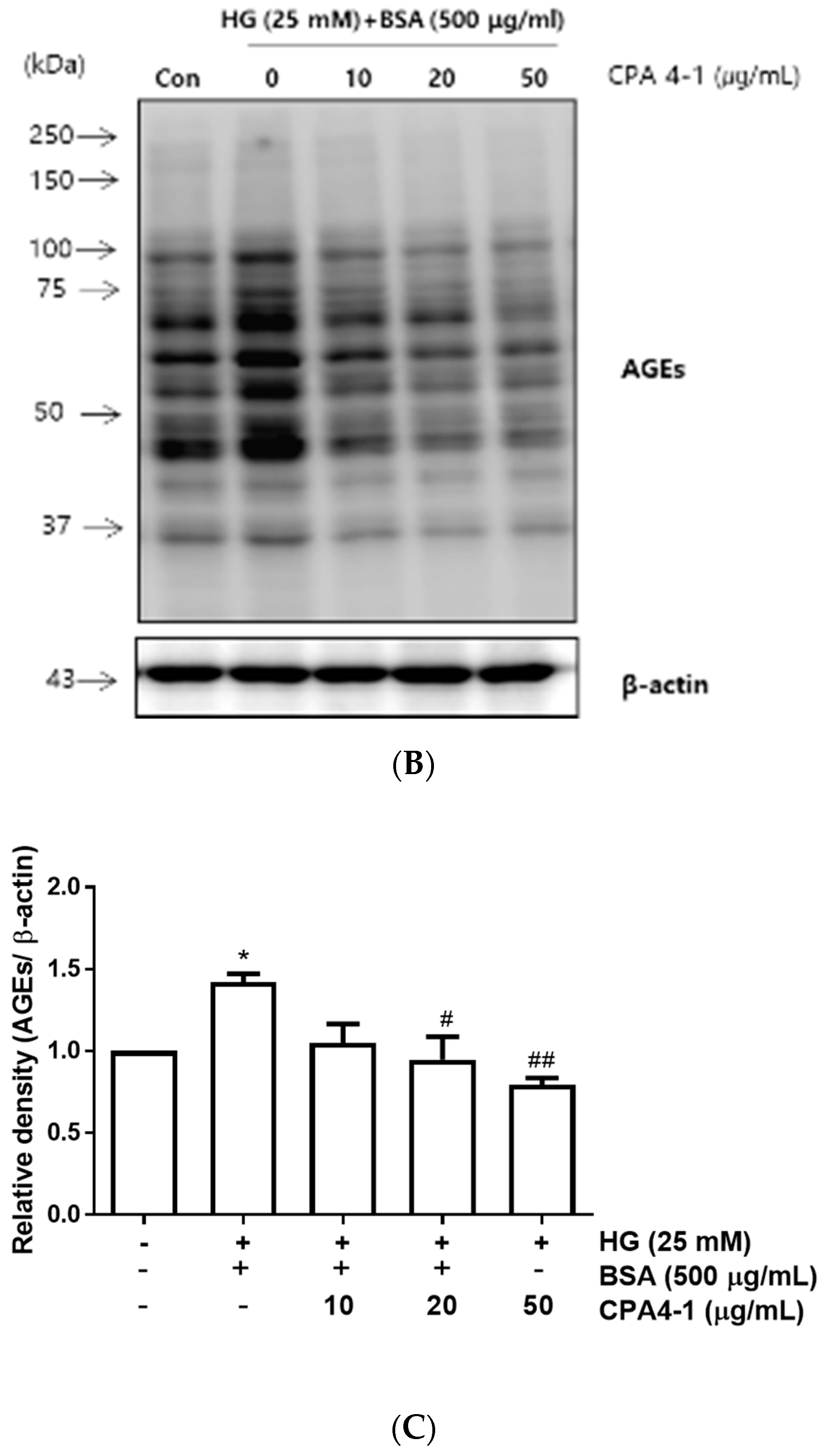
3.2. Inhibitory Effects of CPA4-1 on AGE Formation In Vitro and in ARPE-19 Cells
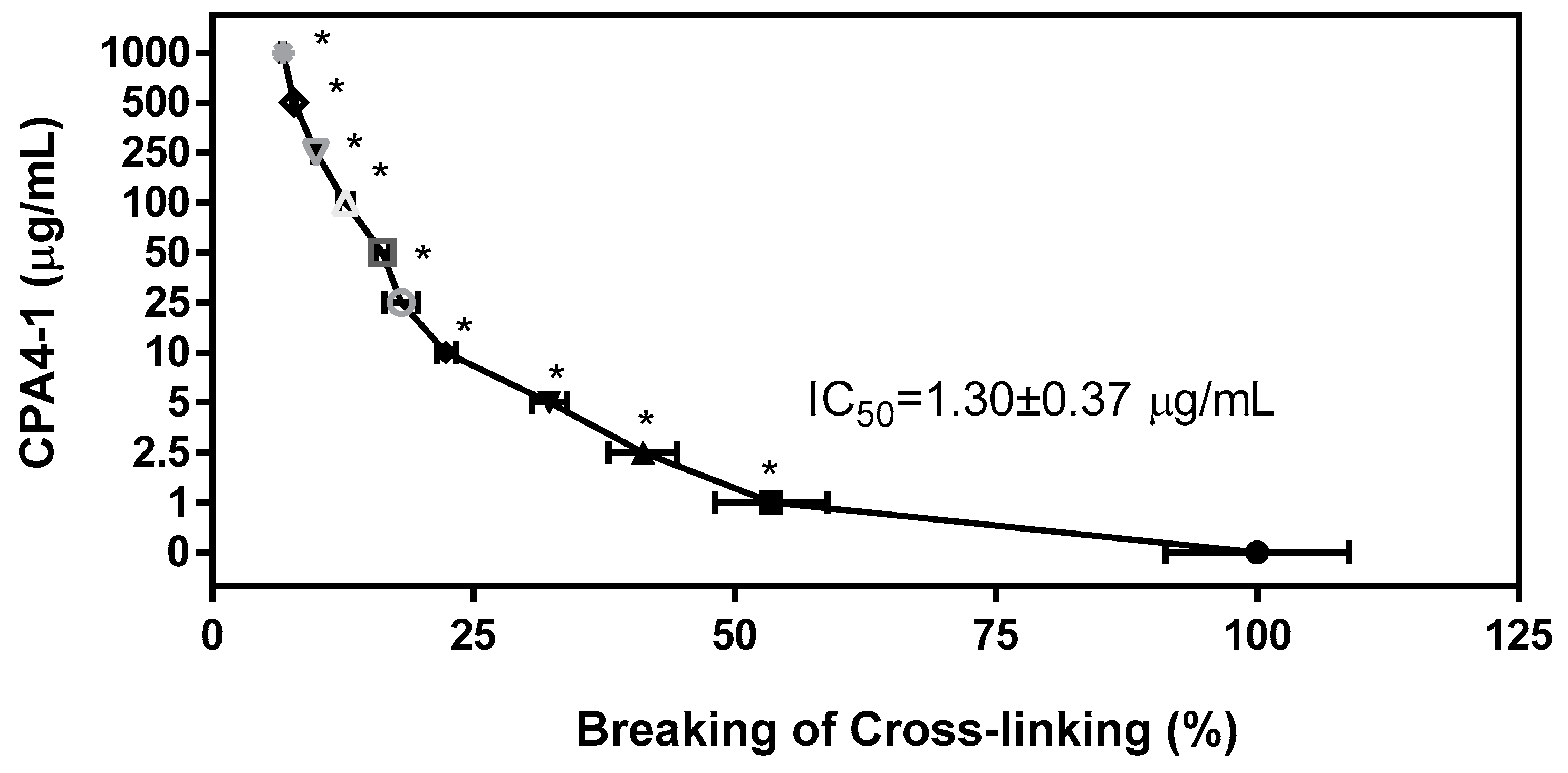
3.3. Breaking Effect of CPA4-1 on Preformed AGE-Collagen Complexes
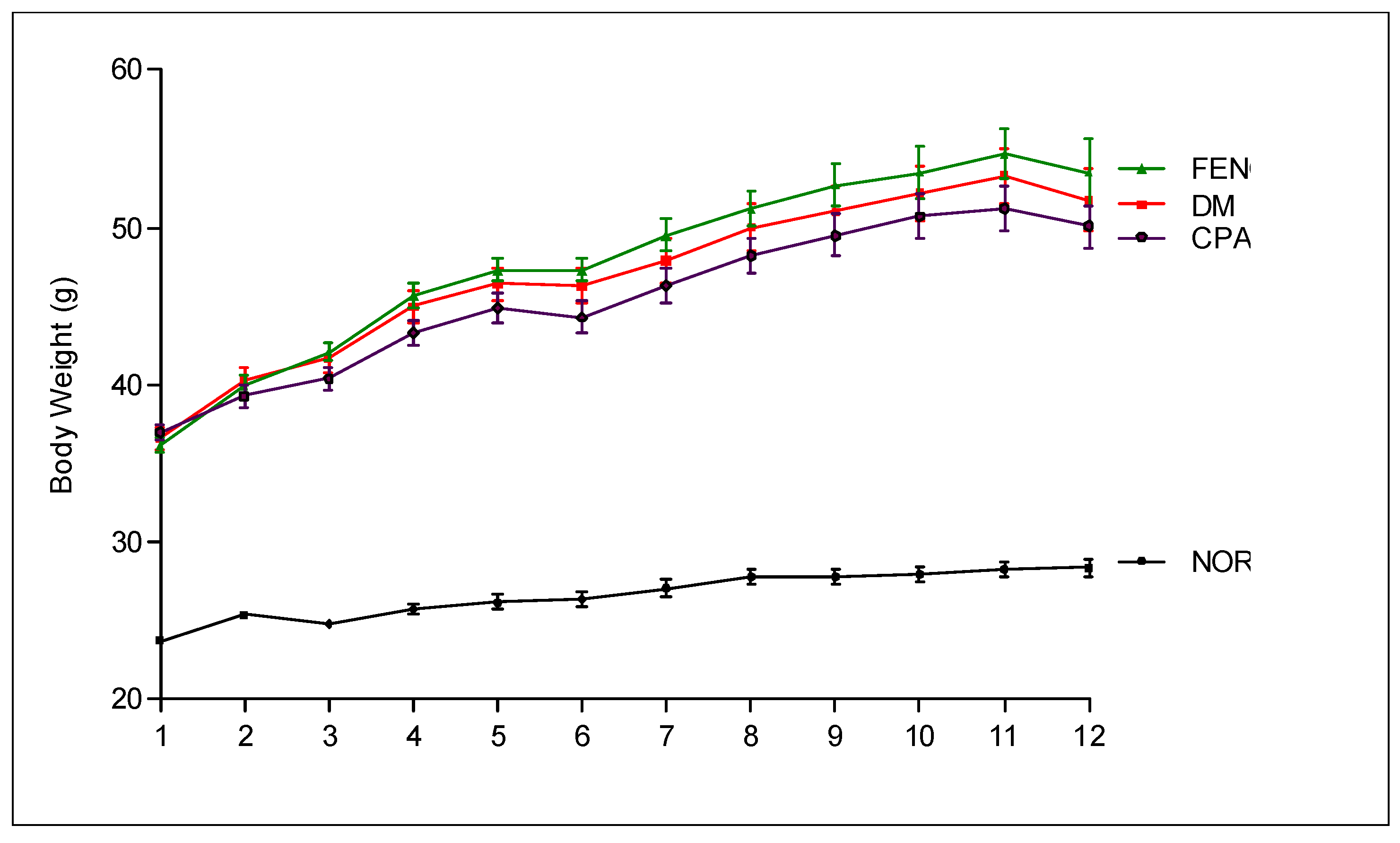
3.4. Metabolic and Physical Parameters
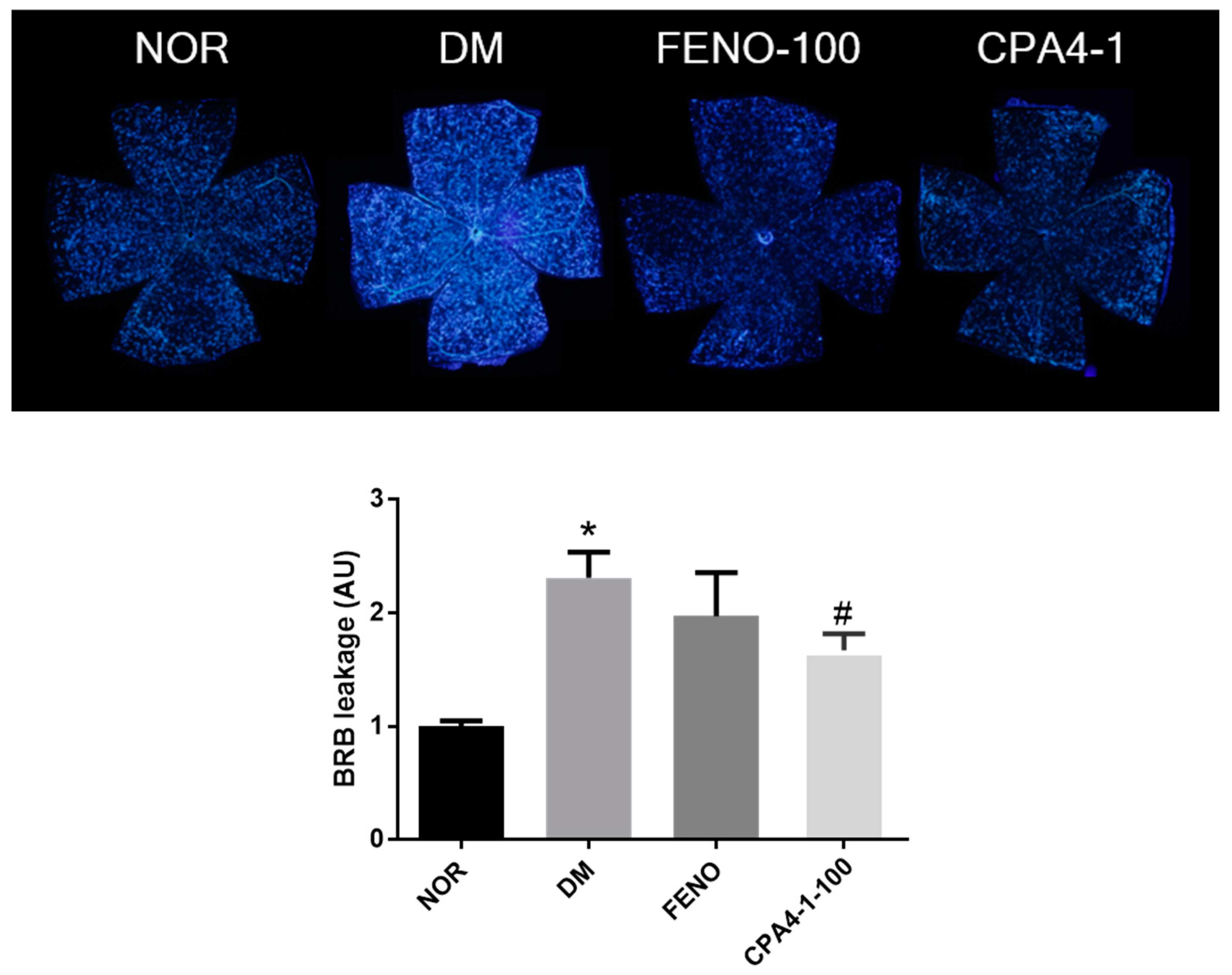
3.5. Preventive Effects of CPA4-1 on BRB Breakage in db/db Mice
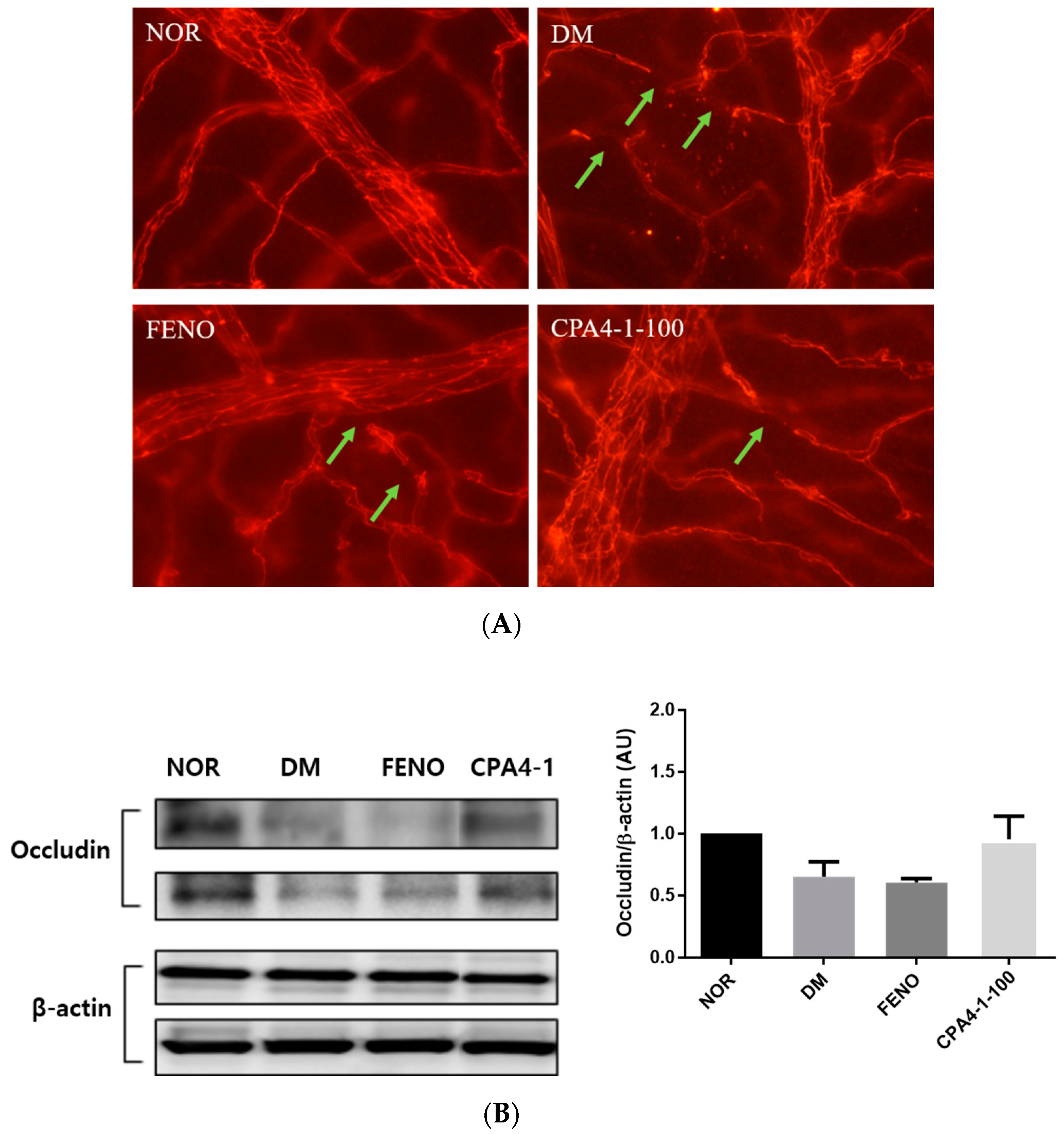
3.6. Preventive Effects of CPA4-1 on Tight Junction Protein Loss in db/db Mice
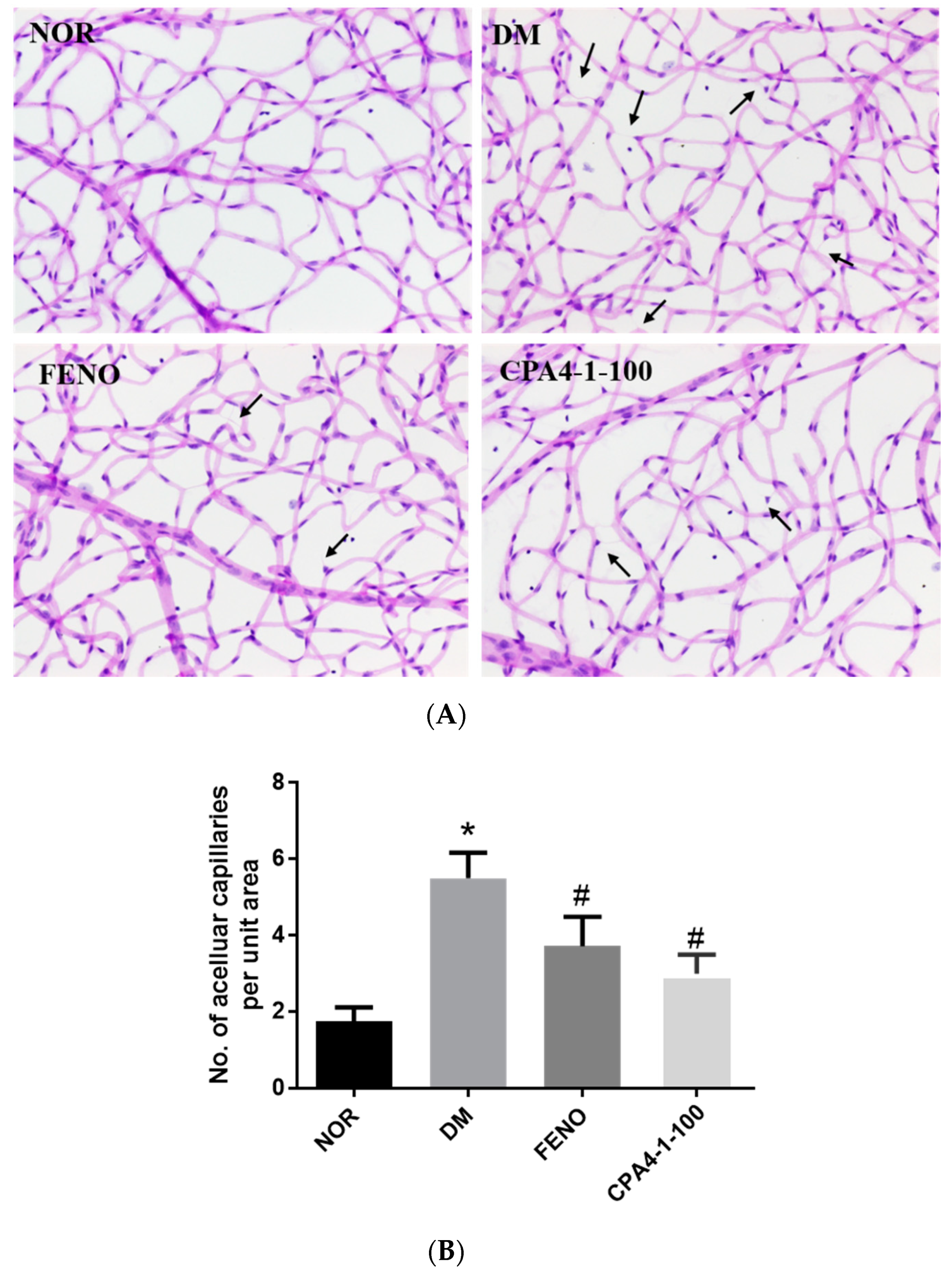
3.7. Effects of CPA4-1 on Acellular Capillary Formation in db/db Mice
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathological implications of protein glycation. Clin. Investig. Med. 1995, 18, 275–281. [Google Scholar]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 2016, 81 (Pt B), 403–418. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Sorenson, C.M.; Sheibani, N. Diabetes and retinal vascular dysfunction. J. Ophthalmic. Vis. Res. 2014, 9, 362–373. [Google Scholar]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Blood-retinal barrier in hypoxic ischaemic conditions: Basic concepts, clinical features and management. Prog. Retin. Eye Res. 2008, 27, 622–647. [Google Scholar] [CrossRef]
- Song, F.; Schmidt, A.M. Glycation and insulin resistance: Novel mechanisms and unique targets? Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1760–1765. [Google Scholar] [CrossRef]
- Sharma, N.; Ooi, J.L.; Ong, J.; Newman, D. The use of fenofibrate in the management of patients with diabetic retinopathy: An evidence-based review. Aust. Fam. Physician 2015, 44, 367–370. [Google Scholar]
- Unoki-Kubota, H.; Yamagishi, S.; Takeuchi, M.; Bujo, H.; Saito, Y. Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein Pept. Lett. 2010, 17, 1177–1181. [Google Scholar] [CrossRef]
- Lee, I.S.; Jung, S.H.; Kim, J.S. Polyphenols from Euphorbia pekinensis Inhibit AGEs Formation In Vitro and Vessel Dilation in Larval Zebrafish In Vivo. Planta Med. 2018, 84, 176–181. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, Y.J.; Jung, S.H.; Kim, J.H.; Kim, J.S. Flavonoids from Litsea japonica Inhibit AGEs Formation and Rat Lense Aldose Reductase In Vitro and Vessel Dilation in Zebrafish. Planta Med. 2017, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Kim, N.H.; Lee, Y.M.; Kim, C.S.; Kim, J.; Kim, J.H.; Kim, J.S. 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol. Pharm. Bull. 2010, 33, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, K.; Lee, I.S.; Kim, C.S.; Kim, J.S. The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction. Nutrients 2016, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Pyun, B.J.; Kim, Y.S.; Lee, I.S.; Jung, D.H.; Kim, J.H.; Kim, J.S. Osteomeles schwerinae Extract and Its Major Compounds Inhibit Methylglyoxal-Induced Apoptosis in Human Retinal Pigment Epithelial Cells. Molecules 2020, 25, 2605. [Google Scholar] [CrossRef]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Xu, H.; Bi, K.; Li, Q. Quality assessment of Cinnamomi Ramulus by the simultaneous analysis of multiple active components using high-performance thin-layer chromatography and high-performance liquid chromatography. J. Sep. Sci. 2014, 37, 2490–2498. [Google Scholar] [CrossRef]
- Chuang, W.C.; Lin, W.C.; Sheu, S.J.; Chiou, S.H.; Chang, H.C.; Chen, Y.P. A comparative study on commercial samples of the roots of Paeonia vitchii and, P. lactiflora. Planta Med. 1996, 62, 347–351. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumi, J.; Ramos, D.; Ruberte, J.; Simo, R.; Hernandez, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Jiang, Y.; Willard, L.; Ortiz, E.; Wark, L. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. (Maywood) 2011, 236, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Kim, Y.S.; Lee, I.S.; Kim, J.S. Jakyakgamcho-tang and Its Major Component, Paeonia Lactiflora, Exhibit Potent Anti-glycation Properties. J. Exerc. Nutr. Biochem. 2016, 20, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Kim, N.H.; Lee, Y.M.; Jang, D.S.; Song, G.Y.; Kim, J.S. KIOM-79 inhibits high glucose or AGEs-induced VEGF expression in human retinal pigment epithelial cells. J. Ethnopharmacol. 2007, 112, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Khin, S.; Lieth, E.; Tarbell, J.M.; Gardner, T.W. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef]
- Koh, E.H.; Kim, M.S.; Park, J.Y.; Kim, H.S.; Youn, J.Y.; Park, H.S.; Youn, J.H.; Lee, K.U. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: Comparison with PPAR-gamma activation. Diabetes 2003, 52, 2331–2337. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Lin, M.; Jenkins, A.J.; Keech, A.C.; Mott, R.; Lyons, T.J.; Ma, J.X. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 2013, 62, 261–272. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Chen, Z.; Yu, S.; Xu, H. Fenofibrate Ameliorates Oxidative Stress-Induced Retinal Microvascular Dysfunction in Diabetic Rats. Curr. Eye Res. 2018, 43, 1395–1403. [Google Scholar] [CrossRef]
- Bermudez, V.; Finol, F.; Parra, N.; Parra, M.; Perez, A.; Penaranda, L.; Vílchez, D.; Rojas, J.; Arráiz, N.; Velasco, M. PPAR-gamma agonists and their role in type 2 diabetes mellitus management. Am. J. Ther. 2010, 17, 274–283. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, Y.M.; Lee, G.Y.; Jang, D.S.; Bae, K.H.; Kim, J.S. Constituents of the roots of Pueraria lobata inhibit formation of advanced glycation end products (AGEs). Arch. Pharm. Res. 2006, 29, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients. 2017, 9, 163. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Bastos, D.H.; Schulze, J.; Souza, M.F. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Liu, B.Q.; Hao, M.J.; Fan, Y.X.; Qian, C.; Teng, P.; Zhou, X.W.; Hu, L.; Liu, W.T.; Yuan, Z.L.; et al. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-kappaB Pathway Through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation 2017, 40, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Cai, Z.; Cai, M.; Liu, K.; Liu, D.; Zhang, Q.; Tan, J.; Ma, Q. Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2016, 13, 2247–2252. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, Q.; Su, J.; Wu, Y.; Zhu, Y.; Wang, Y.; Fang, H.; Pang, M.; Li, B.; Chen, S.; et al. Effects of an Enriched Extract of Paeoniflorin, a Monoterpene Glycoside used in Chinese Herbal Medicine, on Cholesterol Metabolism in a Hyperlipidemic Rat Model. Med. Sci. Monit. 2017, 23, 3412–3427. [Google Scholar] [CrossRef]
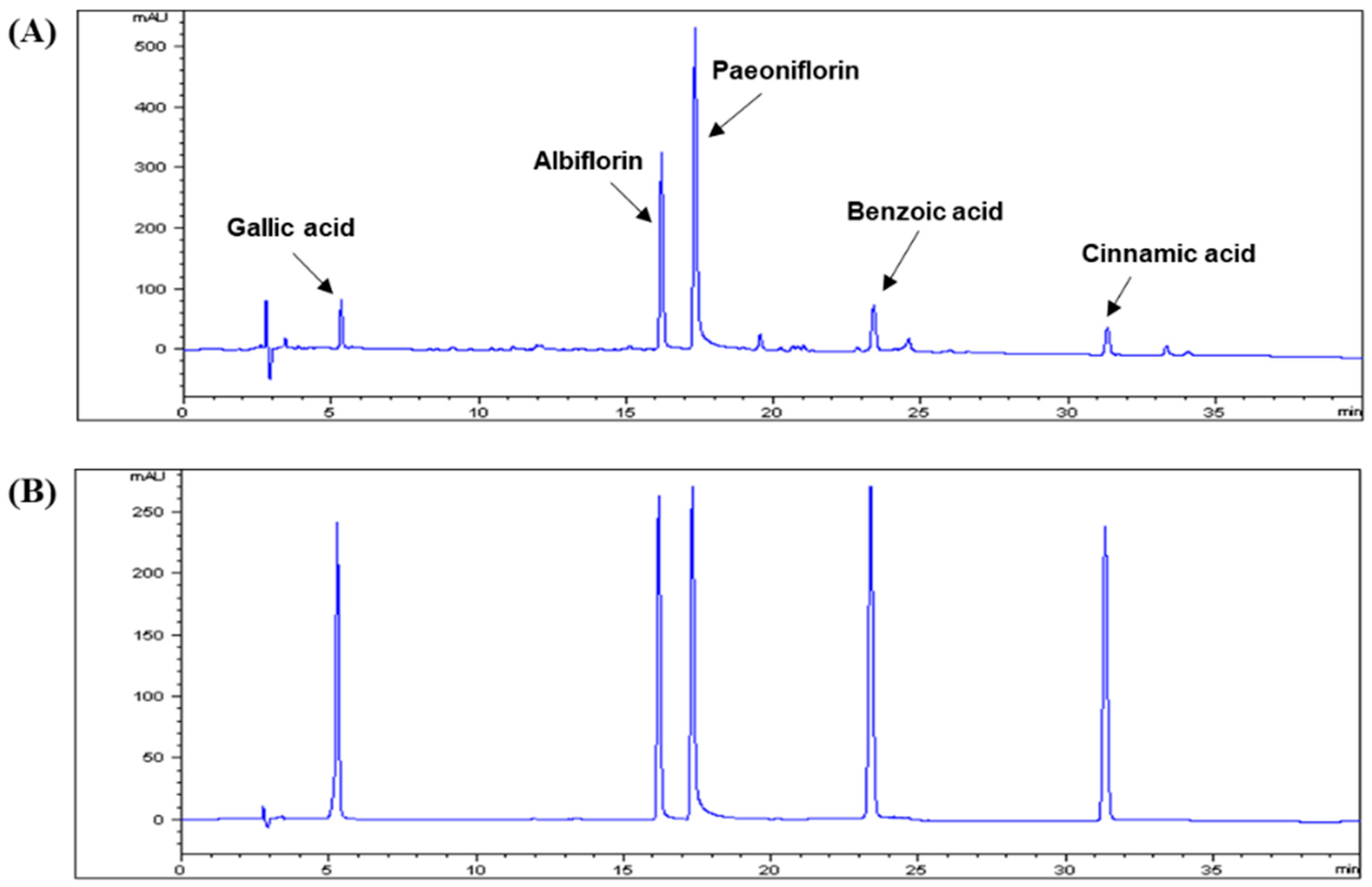
| Compound | Regression Equation a | Linear Range (μg/mL) | Linearity (R2) | LOD b (μg/mL) | LOQ c (μg/mL) |
|---|---|---|---|---|---|
| Gallic acid | y = 11.507x − 8.142 | 6.25–200 | 0.9998 | 0.06 | 0.20 |
| Albiflorin | y = 7.425x − 7.816 | 31.25–1000 | 0.9998 | 0.35 | 1.17 |
| Paeoniflorin | y = 6.751x − 46.907 | 31.25–1000 | 0.9998 | 0.38 | 1.27 |
| Benzoic acid | y = 29.191x − 0.899 | 3.15–100 | 0.9999 | 0.02 | 0.07 |
| Cinnamic acid | y = 22.359x − 2.634 | 1.0–50 | 0.9997 | 0.01 | 0.03 |
| Compounds | Source | Content in CPA4-1 (%) |
|---|---|---|
| Gallic acid | Paeoniae Radix | 1.1 |
| Albiflorin | Paeoniae Radix | 5.3 |
| Paeoniflorin | Paeoniae Radix | 8.5 |
| Benzoic acid | Paeoniae Radix | 0.4 |
| Cinnamic acid | Cinnamomi Ramulus | 0.1 |
| Sample | Conc., μg/mL | Inhibition, % | IC50, μg/mL |
|---|---|---|---|
| 2.5 | 16.64 ± 0.58 | ||
| CPA4-1 | 5 | 43.06 ± 0.71 | 6.84 ± 0.08 |
| 10 | 69.68 ± 1.67 | ||
| 55.55 (750 μM) | 44.53 ± 2.33 | ||
| AG | 74.06 (1000 μM) | 49.97 ± 0.59 | 78.28 ± 4.24 (1056.47 ± 57.25 μM) |
| 111.10 (1500 μM) | 56.37 ± 0.67 |
| NOR | DM | FENO | CPA4-1 | |
|---|---|---|---|---|
| Blood glucose (mg/dl) | 137.6 ± 16.2 | 447.2 ± 113.7 * | 423.5 ± 266.4 # | 459.6 ± 146.9 |
| HbA1c (%) | 2.66 ± 1.74 | 11.29 ± 3.81 * | 6.32 ± 5.14 # | 10.63 ± 1.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.S.; Kim, J.; Kim, C.-S.; Lee, I.S.; Jo, K.; Jung, D.H.; Lee, Y.M.; Kim, J.S. The Herbal Combination CPA4-1 Inhibits Changes in Retinal Capillaries and Reduction of Retinal Occludin in db/db Mice. Antioxidants 2020, 9, 627. https://doi.org/10.3390/antiox9070627
Kim YS, Kim J, Kim C-S, Lee IS, Jo K, Jung DH, Lee YM, Kim JS. The Herbal Combination CPA4-1 Inhibits Changes in Retinal Capillaries and Reduction of Retinal Occludin in db/db Mice. Antioxidants. 2020; 9(7):627. https://doi.org/10.3390/antiox9070627
Chicago/Turabian StyleKim, Young Sook, Junghyun Kim, Chan-Sik Kim, Ik Soo Lee, Kyuhyung Jo, Dong Ho Jung, Yun Mi Lee, and Jin Sook Kim. 2020. "The Herbal Combination CPA4-1 Inhibits Changes in Retinal Capillaries and Reduction of Retinal Occludin in db/db Mice" Antioxidants 9, no. 7: 627. https://doi.org/10.3390/antiox9070627
APA StyleKim, Y. S., Kim, J., Kim, C.-S., Lee, I. S., Jo, K., Jung, D. H., Lee, Y. M., & Kim, J. S. (2020). The Herbal Combination CPA4-1 Inhibits Changes in Retinal Capillaries and Reduction of Retinal Occludin in db/db Mice. Antioxidants, 9(7), 627. https://doi.org/10.3390/antiox9070627






