Abstract
Myoglobin (Mb), an oxygen-binding heme protein highly expressed in heart and skeletal muscle, has been shown to undergo oxidative modifications on both an inter- and intramolecular level when exposed to hydrogen peroxide (H2O2) in vitro. Here, we show that exposure to H2O2 increases the peroxidase activity of Mb. Reaction of Mb with H2O2 causes covalent binding of heme to the Mb protein (Mb-X), corresponding to an increase in peroxidase activity when ascorbic acid is the reducing co-substrate. Treatment of H2O2-reacted Mb with ascorbic acid reverses the Mb-X crosslink. Reaction with H2O2 causes Mb to form dimers, trimers, and larger molecular weight Mb aggregates, and treatment with ascorbic acid regenerates Mb monomers. Reaction of Mb with H2O2 causes formation of dityrosine crosslinks, though the labile nature of the crosslinks broken by treatment with ascorbic acid suggests that the reversible aggregation of Mb is mediated by crosslinks other than dityrosine. Disappearance of a peptide containing a tryptophan residue when Mb is treated with H2O2 and the peptide’s reappearance after subsequent treatment with ascorbic acid suggest that tryptophan side chains might participate in the labile crosslinking. Taken together, these data suggest that while exposure to H2O2 causes Mb-X formation, increases Mb peroxidase activity, and causes Mb aggregation, these oxidative modifications are reversible by treatment with ascorbic acid. A caveat is that future studies should demonstrate that these and other in vitro findings regarding properties of Mb have relevance in the intracellular milieu, especially in regard to actual concentrations of metMb, H2O2, and ascorbate that would be found in vivo.
1. Introduction
One of the major mechanisms of toxicity from reactive oxygen species (ROS) is the direct oxidation of protein side chains [1]. Although some oxidations are reversible, such as oxidation of the cysteine thiol to sulfenic acid, the majority are considered to be irreversible and to promote the destabilization of tertiary structure as well as the eventual loss of protein function [2,3]. One group of proteins that are particularly susceptible to oxidative damage is heme proteins [4,5,6]. This is likely due to the redox activity of the porphyrin-centered iron. When present in the ferric state (III), heme proteins are prone to oxidation by endogenously produced H2O2, resulting in a highly unstable oxoferryl form, which can then oxidize protein side chains either internally or on another protein [7].
One oxidative modification known to occur in myoglobin (Mb), especially under acidic conditions [8], involves the covalent linkage between the heme group and protein side chains [9,10,11]. It has been suggested that Mb cross-linked to heme be referred to as Mb-X to delineate it from the abbreviation for abnormal hemoglobin associated with Thalassemia (Mb-H) and the proposed sites of cross-linking, Mb-H when linked at a histidine and Mb-Y when linked via a tyrosine [8]. This paper will use the Mb-X notation to refer to heme-to-protein covalent bonds. The Mb-X form of Mb has increased NADH oxidase activity [12] and oxidizes low density lipoprotein (LDL), phospholipids, and cholesterol esters more rapidly than native Mb [13]. Mb-X also promotes cell death when taken up by cultured fibroblasts [14]. Mb-X and F2-isoprostanes, peroxidation products of arachidonic acid known to be produced by Mb [15] were increased in the urine of rhabdomyolysis patients [16], suggesting a role of Mb-X in the pathology of rhabdomyolysis.
Another oxidative modification shown to be present in H2O2-treated Mb is dityrosine. Dityrosine cross-links can be formed both intra- and intermolecularly [17,18] by the o,o’ coupling of two tyrosinyl radicals. Dityrosine cross-links have been viewed as markers of oxidative stress in vivo [19,20,21,22], although there is evidence that they might play a causal role in age-related pathologies such as Alzheimer’s [23] and Parkinson’s disease [24].
Here, we show that reaction of Mb with H2O2 increases peroxidase activity when ascorbate is the reducing co-substrate, a change that is associated with Mb-X formation. Furthermore, treatment of H2O2-reacted Mb with ascorbic acid reverses the Mb-X crosslink. We also show that Mb aggregates formed upon reaction of Mb with H2O2 are broken by subsequent treatment with ascorbic acid. In addition, it appears that Mb dimer reversal is protein catalyzed, as heat and detergent denatured Mb dimers were unable to reverse their cross-links. In summary, we find that oxidative modifications of Mb including formation of Mb-X and Mb aggregates are reversible by treatment with ascorbic acid, suggesting that Mb might serve a novel role of reversing oxidative modifications in proteins.
2. Materials and Methods
2.1. Reagents
Horse heart myoglobin, 30% H2O2, 3,3′,5,5′-tetramethylbenzidine (TMB), caffeic acid, resveratrol, N-acetylimidazole, methanol, acetonitrile, ethylenediaminetetraacetic acid (EDTA), dihydrobenzoic acid, and NADH were purchased from Sigma Aldrich Corporation (St. Louis, MO, USA). Ascorbic acid was from ICN Biomedicals Inc. (Aurora, OH, USA). NADPH was from Enzo Life Sciences (Farmingdale, NY, USA). 2-butanone was from Acros Organics/Thermo Fisher Scientific (Waltham, MA, USA). Sequencing grade trypsin was from Roche (Indianapolis, IN, USA).
2.2. Metmyoglobin Peroxidase Activity Assays
Metmyoglobin (metMb) solutions were prepared in 50 mM sodium phosphate buffer, pH 7.4. MetMb (111 µM) was incubated with 50–200 µM H2O2, and aliquots of these solutions were added to a reaction mixture containing 250 µM ascorbic acid or 500 µM TMB. Peroxidase activity was monitored by disappearance of ascorbate (ε290 nm = 2900 M−1 cm−1) or reduction of TMB (ε653 nm = 39,000 M−1 cm−1) as we have previously described [25].
2.3. Nuclear Magnetic Resonance (NMR) Methods
NMR procedures are based on NMR methods described for analysis of hemoproteins [26,27]. NMR samples were prepared in H2O with 10% D2O for locking. pH, uncorrected for the isotope effect, was adjusted with dilute HCl or NaOH. Proton NMR spectra were obtained on a Bruker AVANCE III HD 700 spectrometer equipped with a QCI cryoprobe (Bruker Biospin, Rheinstetten, Germany) at 16.44 Tesla at room temperature (298K). One-dimension (1D) 1H spectra were acquired with a p3919gp pulse sequence with ~50 ppm spectrum width and 200 ms recycle delay.
2.4. Heme Stain
metMb samples were incubated in the presence or absence of H2O2 and then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Heme stains were done using an established protocol [28] for assessing heme peroxidase activity in polyacrylamide gels. Staining solutions were made by first dissolving 23 mg TMB in 15 mL methanol, which was then added to 35 mL of 250 mM sodium acetate buffer, pH 5.0. After incubating gels in this staining solution for ~1 h, 180 μL of 30% H2O2 was added, and reactions were stopped by washing with deionized H2O after blue bands corresponding to oxidized TMB appeared. Bands were photo-documented with a LAS-4000 ImageQuant (GE Healthcare Life Sciences, Marlborough, MA, USA) or an iBright FL1000 (Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Heme Extraction
Non-covalently bound heme was removed using the acid-butanone method established by Catalano et al. [10]. Mb solutions (treated or untreated) were first cooled on ice for 10 min. Next, concentrated HCl was added to a pH of 1–1.5. After incubating the acidified solutions on ice for another 10 min, a 2:1 volume ratio of chilled 2-butanone was added before mixing and incubating on ice for 5 min. The amount of heme in the organic phase was determined by measuring the absorbance at 398 nm on a Genysis 5 spectrophotometer (Spectronic Instruments Inc., Fitchburg, WI, USA). To obtain Mb covalently linked to heme (Mb-X), the aqueous phase was removed, and the sample was dialyzed against 50 mM sodium phosphate buffer (pH 7.4) overnight to remove contaminant butanone.
2.6. Mb-X Peroxidase Activity Assays
The peroxidase activity of Mb-X was followed spectrophotometrically. Reactions were done in 50 mM sodium phosphate buffer containing 200 μM hydrogen peroxide. Reaction progress over time was measured using the following extinction coefficients: ε 312 nm = 11,200 M−1 cm−1 for caffeic acid; ε 304 nm = 19,406 M−1 cm−1 for resveratrol; ε 340 nm = 6270 M−1 cm−1 for NADH and NADPH.
2.7. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
Samples for analysis using MALDI-TOF MS were prepared as described by Shevchenko et al. [29]. Briefly, samples were first run on a pre-cast SDS-PAGE gel. Next, bands were excised and sliced into small pieces using a clean spatula. Gel slices were then shrunken by adding 0.5 mL acetonitrile for 10 min. In-gel digestion was then performed by immersing the dehydrated gel slices in a buffer containing 10 mM NH4HCO3, 10% acetonitrile, and 13 ng/μL trypsin (modified, sequencing grade). Samples were kept for either 4 h at 37 °C or overnight at room temperature before placing them at −20 °C until MALDI-TOF analysis. Proteolytic digests were added to a plate containing an equal volume of 20 g/L dihydroxybenzoic acid and were allowed to dry. Spectra were then acquired using a Shimadzu Resonance(tm) MALDI-QIT-TOF mass spectrometer (Shimadzu Scientific Instruments, Columbia, MD, USA).
2.8. Detection of Dityrosine Using Fluorescence
To detect dityrosine fluorescence using the fluorescence spectra described by Malencik et al. [17], fluorescence was measured at excitation and emission wavelengths of 290 nm and 400 nm, respectively, with a Synergy H1 platereader (Biotek, Winooski, VT, USA).
2.9. Western Blot Using Anti-dityrosine Antibody
Mb (2 mg/mL) samples were first reacted with 300 μM H2O2 for 5, 10, 20, and 40 min. Some of these H2O2-reacted samples were then treated with 833 μM ascorbic acid for the same duration of time they had been reacted with H2O2. Samples were detected via Western blotting using an anti-dityrosine antibody (Japan Institute for the Control of Aging, Shizuoka, Japan) and an Alexa™ 488 secondary antibody (with excitation and emission maxima of 490 nm and 525 nm respectively, Invitrogen, Carlsbad, CA, USA). We needed to use the fluorescent secondary antibody, because chemiluminescent detection would be incompatible with the presence of heme; if we were to use enhanced chemiluminescence substrate, bands would appear wherever heme were present (i.e., in the same pattern shown by the in-gel heme peroxidase activity stain using TMB), not just in dityrosine-containing bands.
2.10. Tyrosine Acetylation Using N-Acetylimidazole
Mb was acetylated according to a procedure described by Basu et al. [30]. Briefly, Mb was incubated in 50 mM sodium phosphate buffer, pH 7.4, containing a 10:1 molar excess of N-acetylimidazole, and samples were mixed overnight at room temperature. Excess N-acetylimidazole was removed by washes with 50 mM sodium phosphate buffer, pH 7.4, followed by spinning the acetylated samples in G-Sephadex Millipore spin filter columns (molecular weight cutoff = 10 kDa, Millipore, St. Louis, MO, USA).
2.11. Statistical Methods
For experiments with time courses, data were analyzed by analysis of variance (ANOVA) with time as a factor. When time course data involved more than two groups, an ANOVA using data from all groups with time as a factor was performed. If this model was significant (p < 0.05), post-hoc comparisons were separately made between the control group and each other group using ANOVA with time as a factor. Experiments with end-point data were analyzed by ANOVA with least significant difference (LSD) post-hoc comparisons when appropriate (p < 0.05).
3. Results
3.1. Pre-treatment with H2O2 Increases Mb Peroxidase Activity
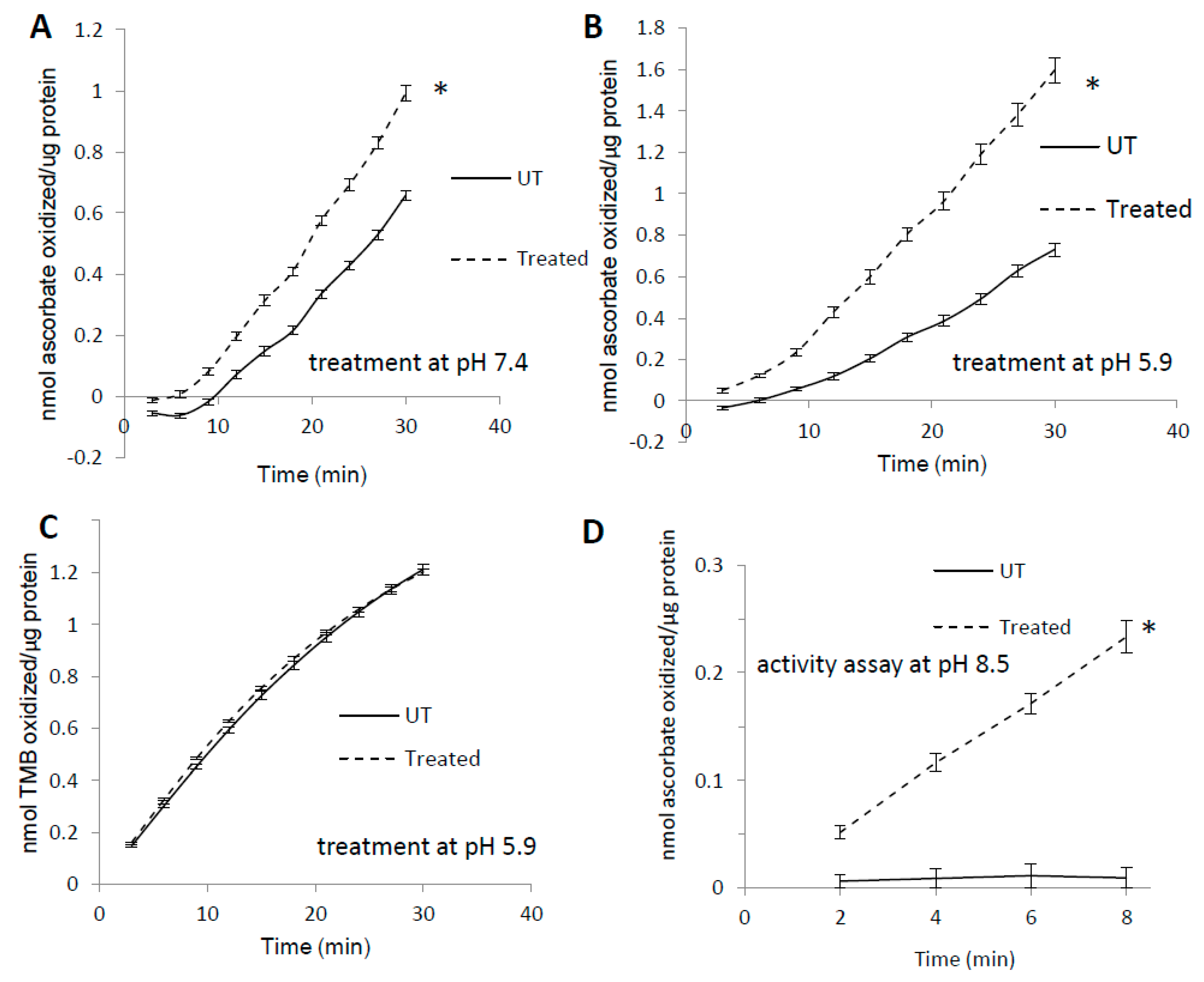
Like many other heme proteins [31], horse metMb has been shown to form heme-to-protein cross-links upon treatment with hydrogen peroxide [10,32]. To assess the effects of heme-protein cross-links on metMb peroxidase activity, we measured the activity of H2O2-reacted metMb with 3,3′,5,5′-tetramethylbenzidine (TMB) as well as ascorbic acid. Interestingly, pre-treatment of metMb with H2O2 at pH 7.4 significantly increased its peroxidase activity with ascorbic acid (Figure 1A). Further, this effect was more pronounced when the pre-treatment was performed at a pH of 5.9 (Figure 1B). When TMB was used as a substrate, however, pre-treatment had no effect on Mb peroxidase activity (Figure 1C). Given previous reports that heme-protein cross-links in H2O2-treated metMb are more readily formed at a lower pH [7], these data suggested to us that reaction of Mb with H2O2 might result in the formation of modified species with unique peroxidase activities. To further test this hypothesis, we sought to measure the peroxidase activity of H2O2-reacted metMb in an alkaline pH (pH 8.5) in which metMb has no peroxidase activity with ascorbate as substrate (Figure 1D). Interestingly, H2O2-reacted metMb retained peroxidase activity even under these alkaline conditions (Figure 1D), suggesting that H2O2-reacted metMb possesses distinct peroxidase activities relative to metMb.
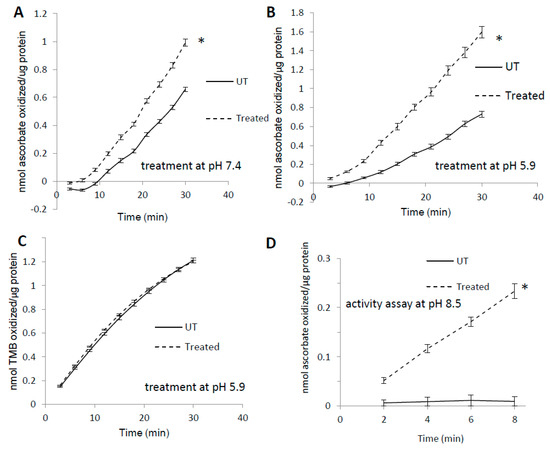
Figure 1.
Pre-treatment with H2O2 increases MetMb peroxidase activity in a substrate-dependent manner. MetMb (111 μM, pH 5.9) was untreated (UT) or was pre-reacted with 50 μM H2O2 for 15 min at (A) pH 7.4 (n = 12/group, * p < 0.05) and (B) pH 5.9 (n = 7/group, * p < 0.05) before 2 μL of this solution was added to a 200 μL reaction mixture on a 96-well plate containing 250 μM ascorbic acid and 200 μM H2O2. (C) metMb was reacted with H2O2 at pH 5.9 as described for (B), and peroxidase activity was measured using 500 μM TMB and 200 μM H2O2 (n = 10/group). (D) MetMb (222 μM) was pre-reacted with 100 μM H2O2 for 30 min before 1 μL of this solution was added to a 200 μL reaction mixture containing 250 μM ascorbic acid and 200 μM H2O2 at pH 8.5. * p ≤ 0.05, n = 12/group.
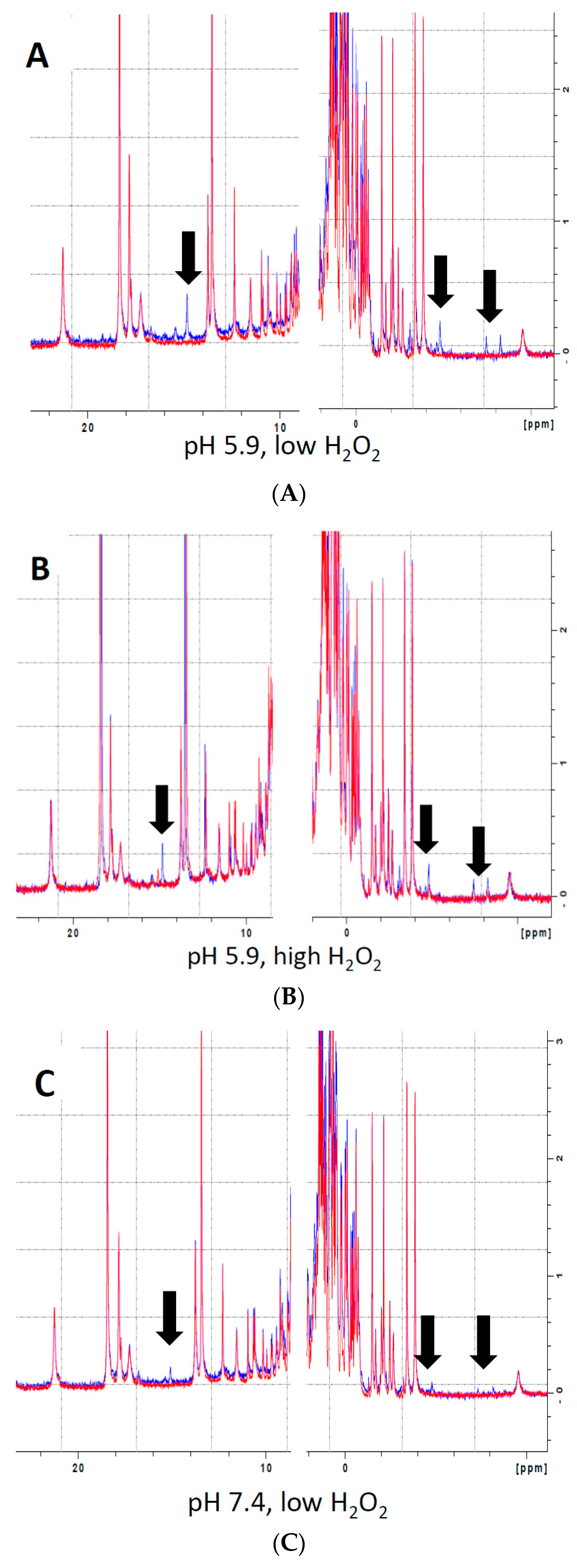
Analysis of the effects of H2O2 on heme electronic structure in Mb via 1H NMR spectroscopy revealed the presence of multiple novel peaks in the heme region (Figure 2, black arrows), indicating the presence of covalent modifications to the heme itself or to residues in its immediate vicinity. The presence of several novel peaks in the heme region might indicate either the presence of individual Mb-X molecules with multiple modifications, or, alternatively, that reaction of metMb with H2O2 results in various distinct Mb-X species. Since pre-treatment at lower pH further enhanced the ascorbate peroxidase activity of Mb (Figure 1B,C), we hypothesized that increased concentration of Mb-X species might be responsible for these effects. Consistent with our hypothesis, reaction of metMb with H2O2 at pH 5.9 produced ed the same novel heme resonances as found in metMb that was reacted with H2O2 at pH 7.4, though the intensity of these novel peaks was substantially increased at pH 5.9 (Figure 2A–C). We then sought to determine whether reaction at a higher H2O2 concentration would result in increased Mb-X formation. To our surprise, the higher H2O2 concentration had no effect on the amount of Mb-X present, as indicated by identical 1D 1H NMR spectra for low and high H2O2 concentrations (Figure 2A,B).
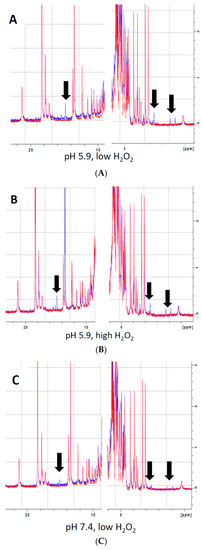
Figure 2.
Analysis of heme-protein crosslinks by 700 MHz 1H NMR spectra. (A–B) MetMb (555 µM, pH 5.9) was reacted with 110 µM H2O2 (A) or 1.1 mM H2O2 (B) for 10 min before adding 3 mM NaCN to generate low-spin Mb. Sample preparation and 1H NMR procedures are described in the methods section. (C) Same as (A) and (B), only metMb was reacted with 110 µM H2O2 at pH 7.4. Data for untreated metMb are in red, and data for metMb pretreated with H2O2 are in blue. Arrows indicate novel peaks in the heme region for H2O2-treated metMb.
3.2. Heme Activity Stains of H2O2-Reacted metMb
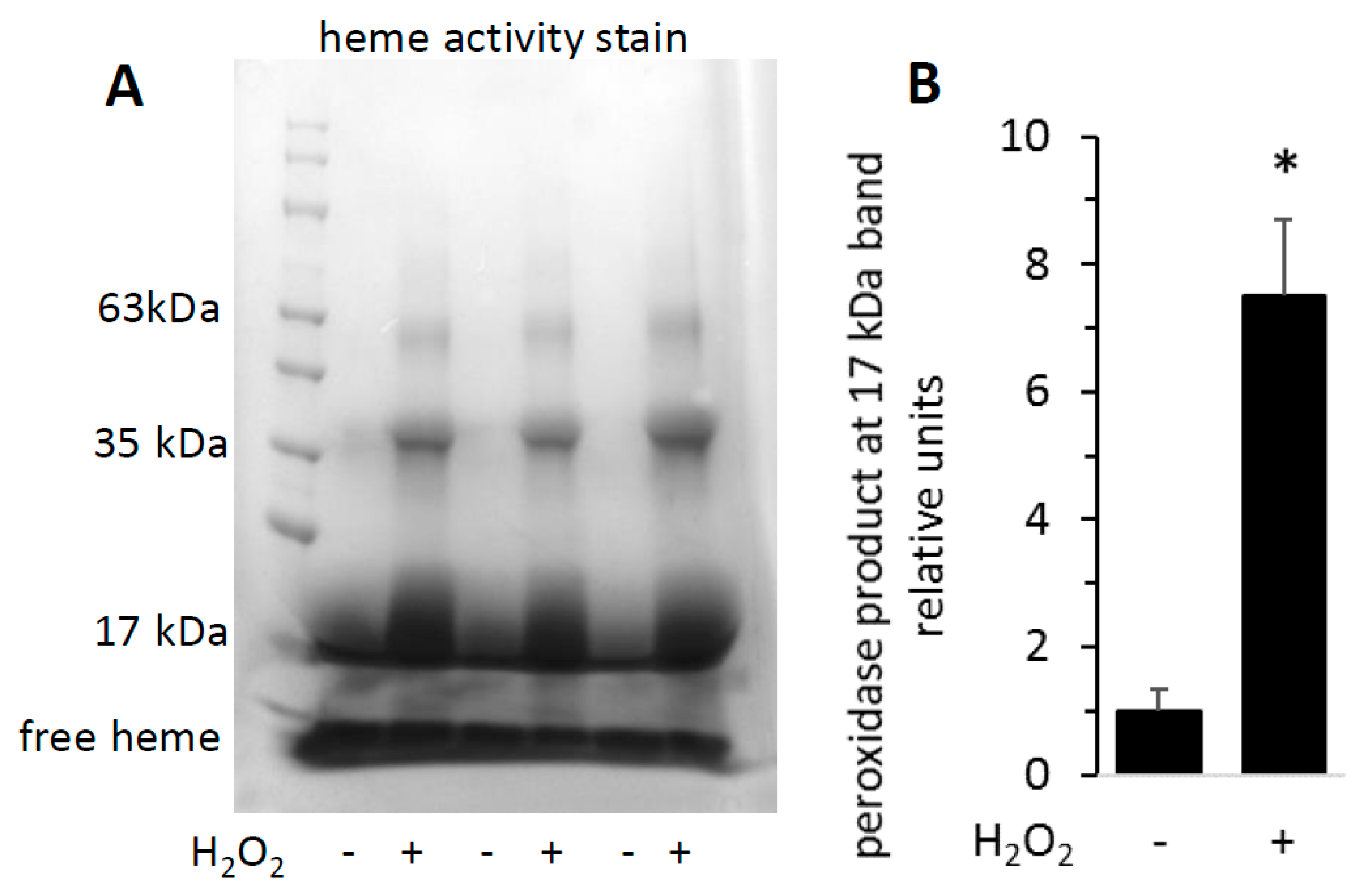
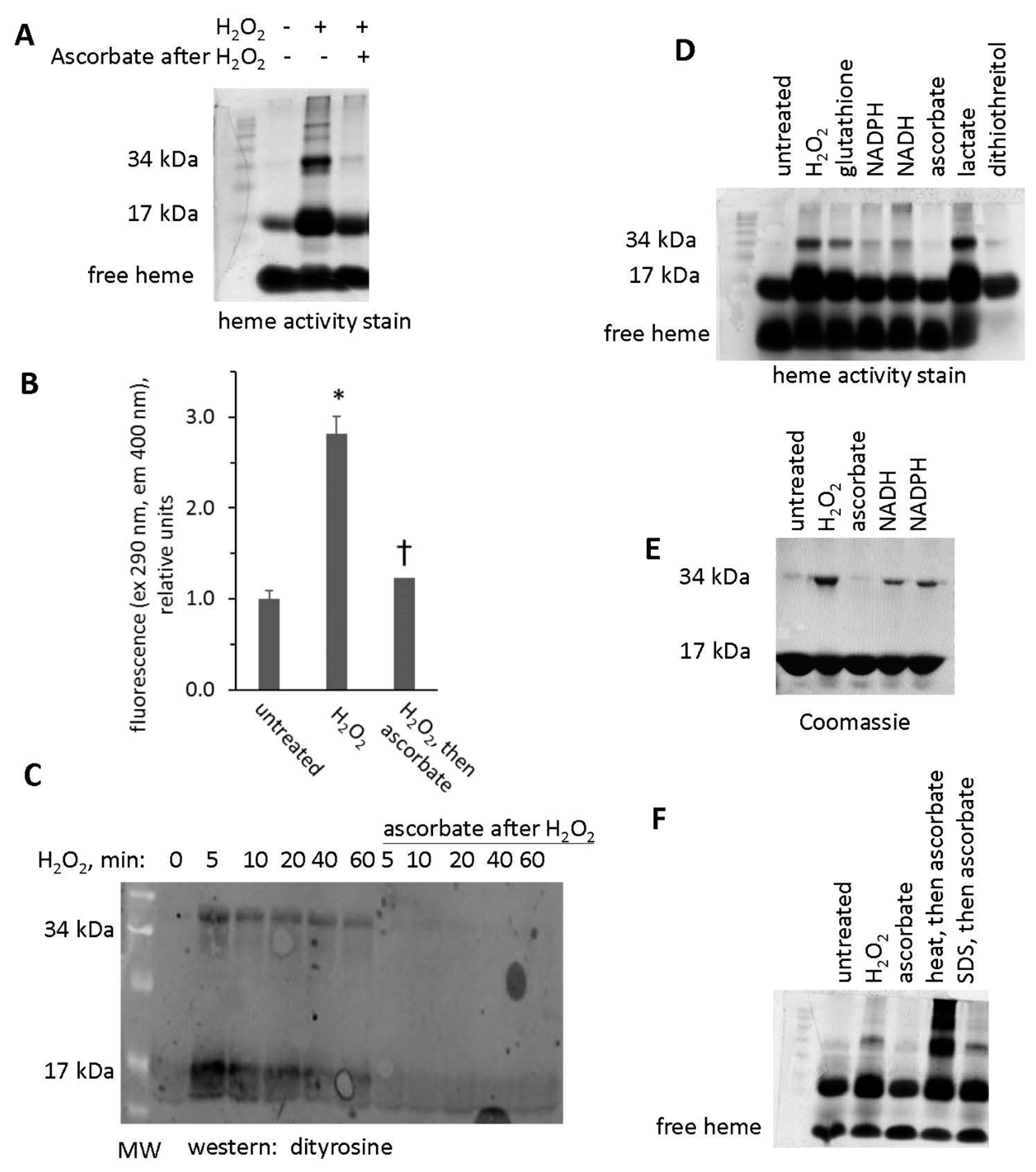
To visualize the increased activity of H2O2-reacted metMb on a polyacrylamide gel, we subjected both untreated and H2O2-treated metMb to SDS-PAGE and stained the gels for heme peroxidase activity. As expected, the heme-stain bands were much more intense for H2O2-reacted Mb than the untreated control (Figure 3). Interestingly, exposure of Mb to H2O2 caused appearance of bands corresponding to the molecular weights of Mb dimers and trimers, an effect that will be addressed later in this study.
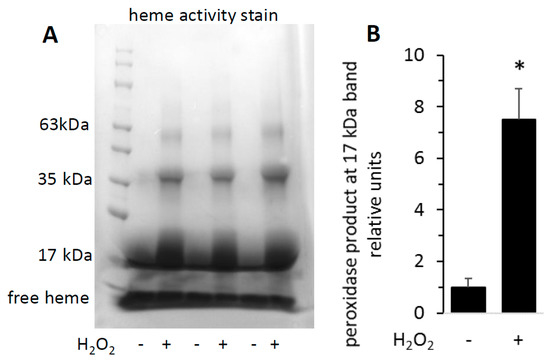
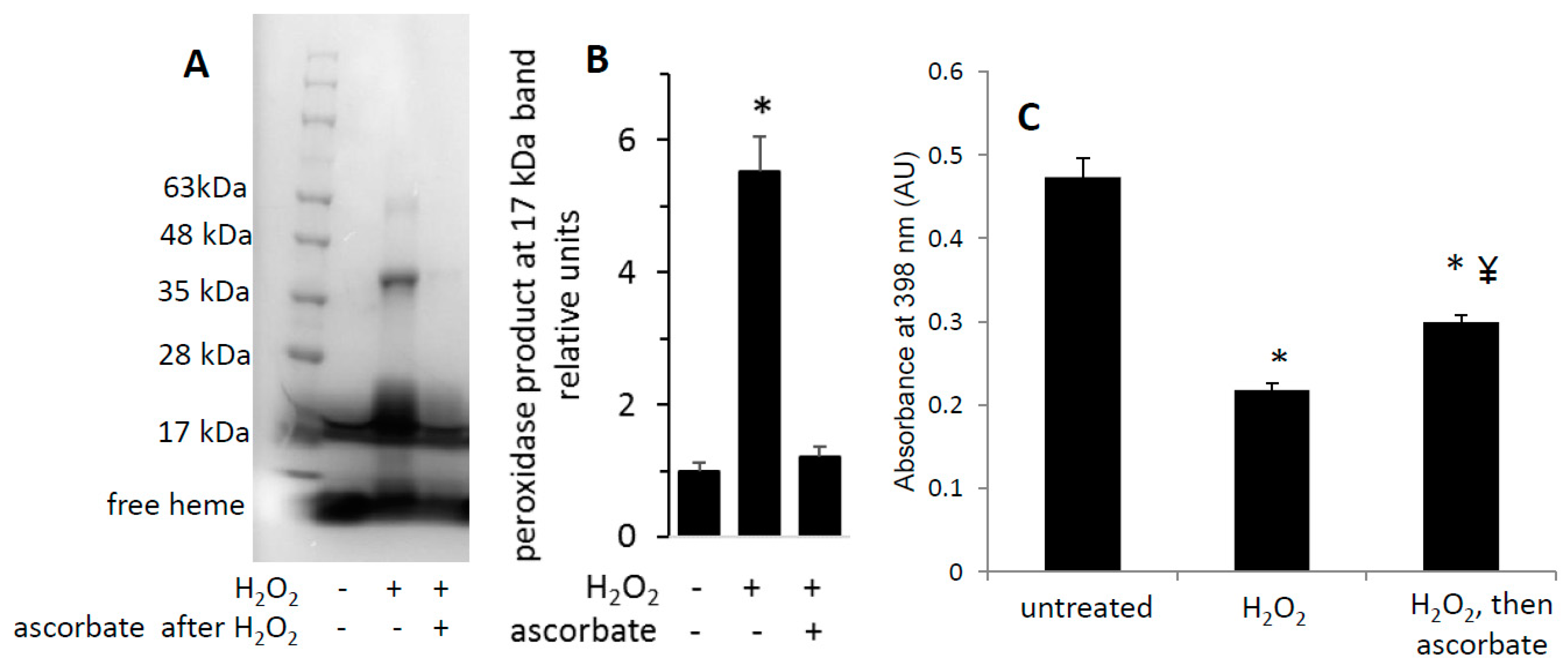
Figure 3.
Reaction of metMb with H2O2 increases peroxidase activity as visualized on polyacrylamide gels. metMb (555 μM, pH 5.9) was incubated in the absence or presence of 800 μM H2O2 for 15 min before performing SDS-PAGE and (A) heme-peroxidase activity stains (right) as described in methods. (B) Quantitation of the peroxidase product at the 17 kDa band, n = 3/group, * p < 0.01.
3.3. Unique Activity of Heme-Coupled Mb (Mb-X)
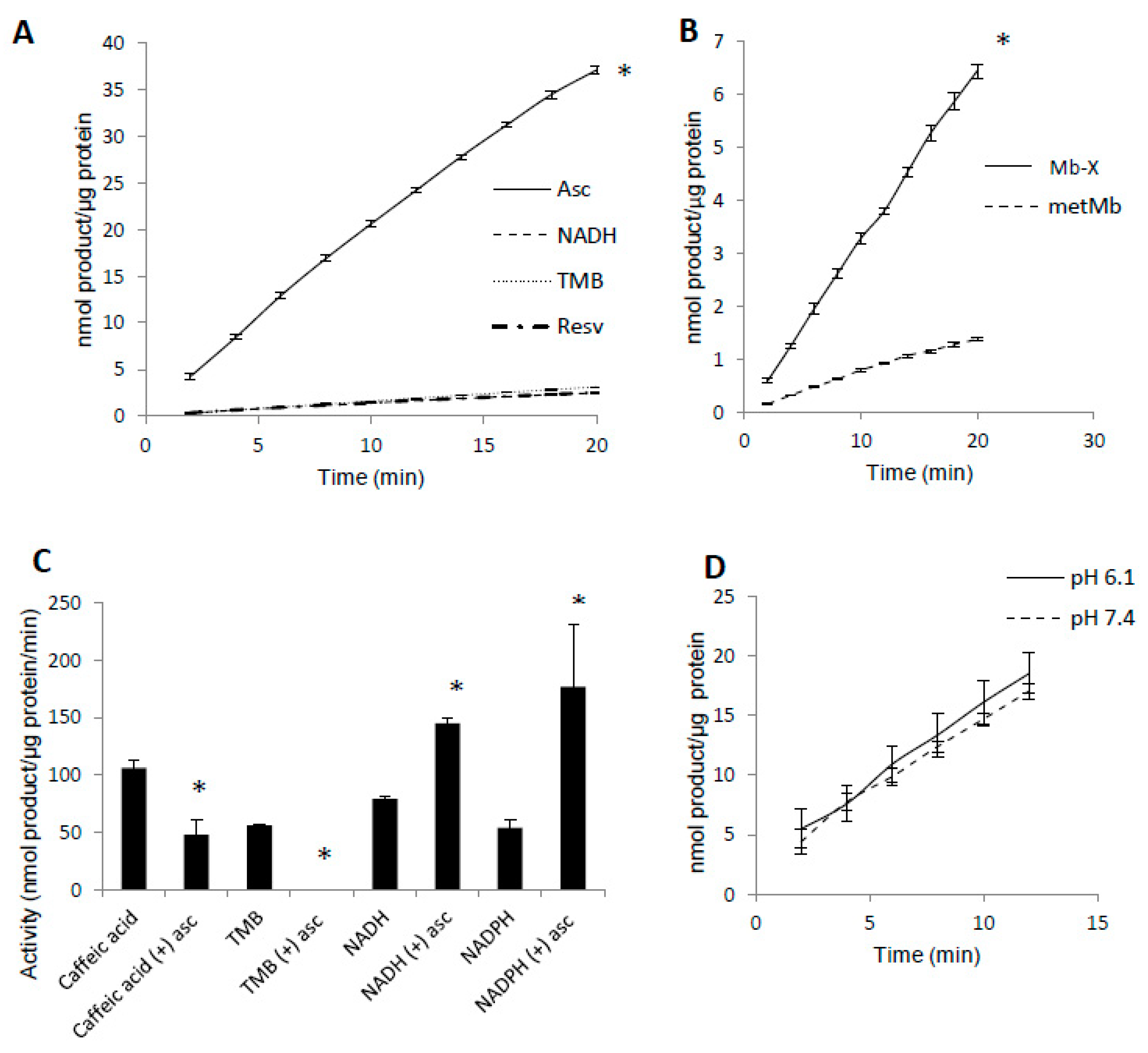
Since prior reaction with H2O2 increased metMb peroxidase activity, we hypothesized that most metMb activity is due to Mb-X species. To test this, we first treated metMb in conditions that favor Mb-X formation (as described in Section 2) and then removed non-covalently bound heme molecules with acid-butanone treatment. Next, we measured the activity of the Mb-X using several different substrates. Interestingly, Mb-X displayed unique peroxidase activities relative to metMb. Whereas metMb is a promiscuous peroxidase in terms of substrate selectivity [25], Mb-X displayed little to no activity with substrates other than ascorbic acid (Figure 4A). To quantitatively compare the activity of Mb-X to that of metMb, we estimated the concentration of heme-containing Mb molecules by measuring the amount of heme remaining in the organic phase after acid-butanone extraction. Using this estimated Mb-X concentration, we found Mb-X to possess four-fold greater activity with ascorbic acid compared to an equivalent concentration of metMb (Figure 4B).
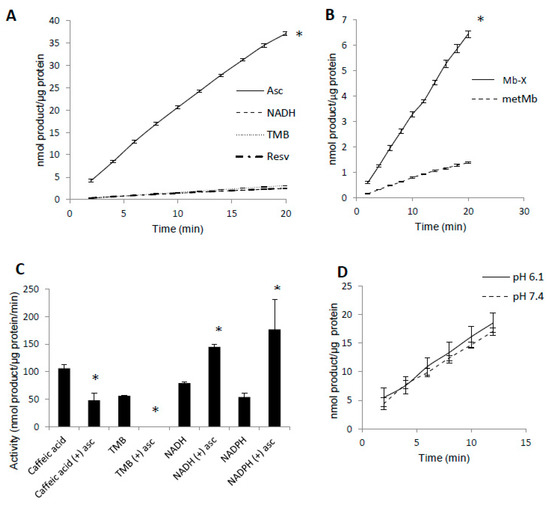
Figure 4.
Mb-X species possess unique peroxidase activities compared to metMb. (A) Mb-X peroxidase activity was measured using ascorbic acid (Asc, 250 μM), resveratrol (Resv, 125 μM), NADH (250 μM) and TMB (500 μM) and 200 μM H2O2 at pH 6.1. The activity plots for all substrates except ascorbic acid overlap, so they are not all visible. n = 6/group, * p <0.05 vs. all other groups. (B) Mb-X concentration was estimated as described in the methods section. Peroxidase activity with ascorbic acid was then measured with 250 μM ascorbic acid and 200 μM H2O2 at pH 6.1. * p ≤ 0.05 compared to metMb, n = 3/group. (C) Mb-X peroxidase activity using caffeic acid (125 μM), TMB (500 μM) and NAD(P)H (250 μM) and 200 μM H2O2, pH 6.1 both in the presence and absence of 50 μM ascorbic acid. * p ≤ 0.05 compared to control without ascorbic acid, n = 3/group. (D) Mb-X peroxidase activity was measured at pH 7.4 and 6.1 using 250 μM ascorbic acid and 200 μM H2O2 (n = 3/group).
In addition to switching Mb’s substrate preference for ascorbate, we found that pre-treating Mb-X with low concentrations (50 μM) of ascorbic acid actually enhanced its activity with NADH and NADPH, whereas its activity with other substrates (i.e., TMB and caffeic acid) was either strongly reduced or completely abolished by the same pre-treatment [25] (Figure 4C). We also found no difference in Mb-X peroxidase activity at pH 7.4 versus 6.1 (Figure 4D), although we previously showed that metMb peroxidase activity was increased nearly three-fold at pH 6.1 relative to 7.4 [25], suggesting that the pH dependence of metMb peroxidase activity might be explained by formation of Mb-X.
3.4. Reversibility of Mb-X Species
Interestingly, H2O2-treated metMb samples that were incubated with ascorbic acid prior to SDS-PAGE did not retain an increased heme activity stain (Figure 5A,B), suggesting that, in the presence of excess ascorbic acid, Mb could reverse its cross-linkage to heme. To test this hypothesis, we treated H2O2-reacted metMb with ascorbic acid and then measured the corresponding amount of heme that was lost after acid-butanone treatment. In support of our hypothesis, treating H2O2-reacted metMb with ascorbic acid prior to acid-butanone treatment significantly increased the corresponding amount of free heme (Figure 5C), indicating that ascorbic acid partially reversed the heme:protein cross-link under these conditions.
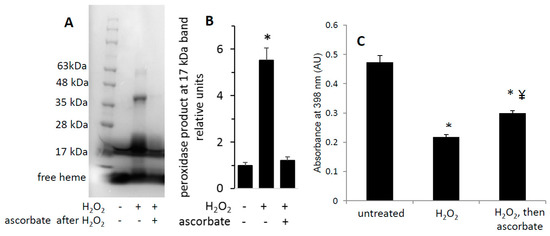
Figure 5.
Heme-dependent reaction of H2O2-oxidized metMb with ascorbic acid is sufficient to reverse heme-protein crosslinks. (A) MetMb (111 μM, pH 6.1) was incubated in the absence or presence of 300 μM H2O2 for 10 min prior to adding 833 μM ascorbic acid for an additional 10 min and performing SDS-PAGE followed by (A) a heme peroxidase stain. (B) Quantitation of the peroxidase product at the 17 kDa band, n = 5/group, * p < 0.001. (C) Acid-butanone heme extraction was performed on metMb that was either untreated, reacted with H2O2 alone for 10 min (H2O2) or treated with ascorbic acid after 10 min of H2O2 oxidation. Free heme was assessed by absorbance at 398 nm. * p ≤ 0.05 compared to untreated control; ¥ p ≤ 0.05 compared to H2O2-only; n = 3/group.
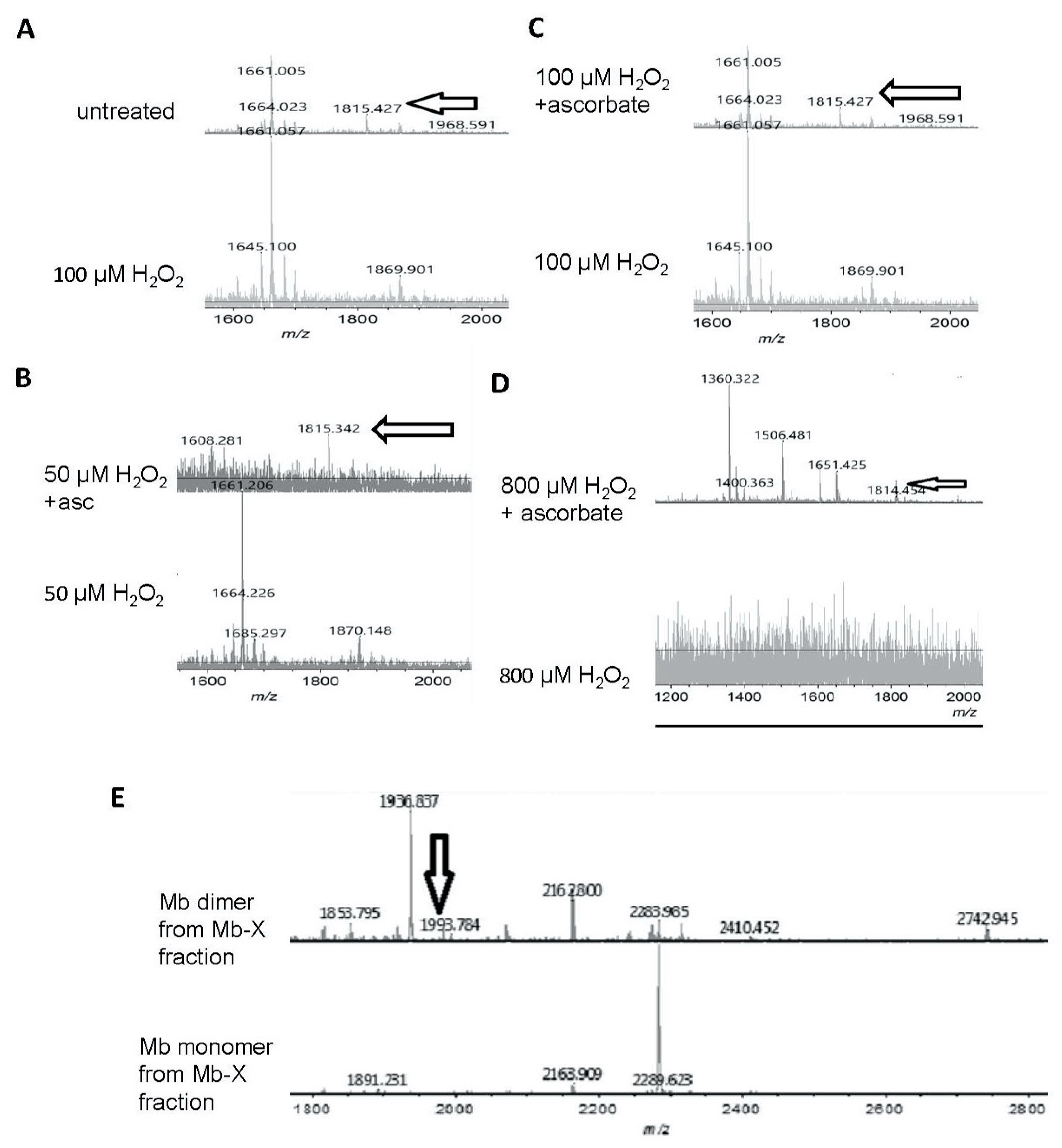
To investigate the reversibility of H2O2-induced modifications of Mb, we analyzed H2O2-treated metMb with and without ascorbic acid treatment using matrix assisted laser desorption mass spectrometry-time of flight (MALDI-TOF) mass spectrometry. Tryptic digests of H2O2-reacted metMb showed a missing peak at 1815 m/z (Figure 6A), suggesting that this peptide could participate in a cross-link. The peptide at 1815 m/z re-appeared in the ascorbic acid-treated tryptic digests of Mb that had been initially incubated with various H2O2 concentrations (50 μM, 100 μM, and 800 µM (Figure 6B–D)), indicating that ascorbic acid-treatment was sufficient to reverse this cross-linked species. The tryptic peptide corresponding to the 1815 m/z peak contains a tryptophan (position in peptide denoted by underlining, GLSDGEWQQVLNVWGK), which is known to harbor a radical upon reaction with H2O2 [33], making it a plausible candidate for oxidative modification or cross-linking.
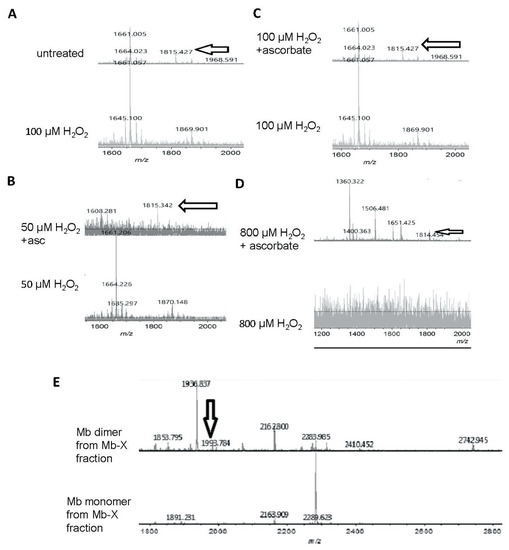
Figure 6.
Matrix assisted laser desorption mass spectrometry-time of flight (MALDI-TOF) mass spectrometric analysis of ascorbic acid-mediated reversal of heme-protein crosslinks in H2O2-reacted metMb. (A) (Bottom spectra) MetMb tryptic digests that were reacted with 100 μM H2O2 displayed loss of the N-terminal peptide (1815.9 m/z) indicated by arrows in the untreated MetMb (top spectra). (B–D) Tryptic digests of H2O2-reacted metMb that was then treated with ascorbic acid revealed the re-appearance of the N-terminal peptide. (E). MetMb monomer (bottom) and dimer (top) tryptic digests that were reacted with 300 µM H2O2 and analyzed with MALDI-TOF MS. The arrow indicates a peak at 1993 m/z, which suggests presence of a heme crosslinked to a peptide containing the distal histidine of Mb helix E.
Remarkably, after subjecting metMb to harsh oxidation conditions (800 μM H2O2 for 30 min at pH 5.9), no tryptic peptides were detected above the signal-to-noise threshold (Figure 6D), indicating that these stringent conditions had severely modified metMb. After these samples were treated with excess ascorbic acid, however, multiple peptides re-appeared (Figure 6D).
In addition to forming Mb-X when treated with H2O2, Mb also forms dimers as is visible in Figure 3A. We extracted Mb-X from H2O2-treated Mb, separated the products SDS-PAGE, gels, and performed MALDI-TOF on tryptic digests of the Mb monomer and dimer. We found a novel peak at 1993.8 m/z present in the H2O2-reacted Mb dimer (Figure 6E) that amounts to theoretical mass of a heme-crosslinked Mb peptide when accounting for the loss of two protons from the crosslinking reaction (1378.8 + 616.5 − 2.0 Da). Notably, this peptide contains the distal histidine (position in peptide denoted by underlining, HGTVVLTALGGILK), which has previously been implicated in heme-protein crosslinks in H2O2-treated Mb [8].
3.5. Complex Substrate Specificity of metMb Peroxidase Activity
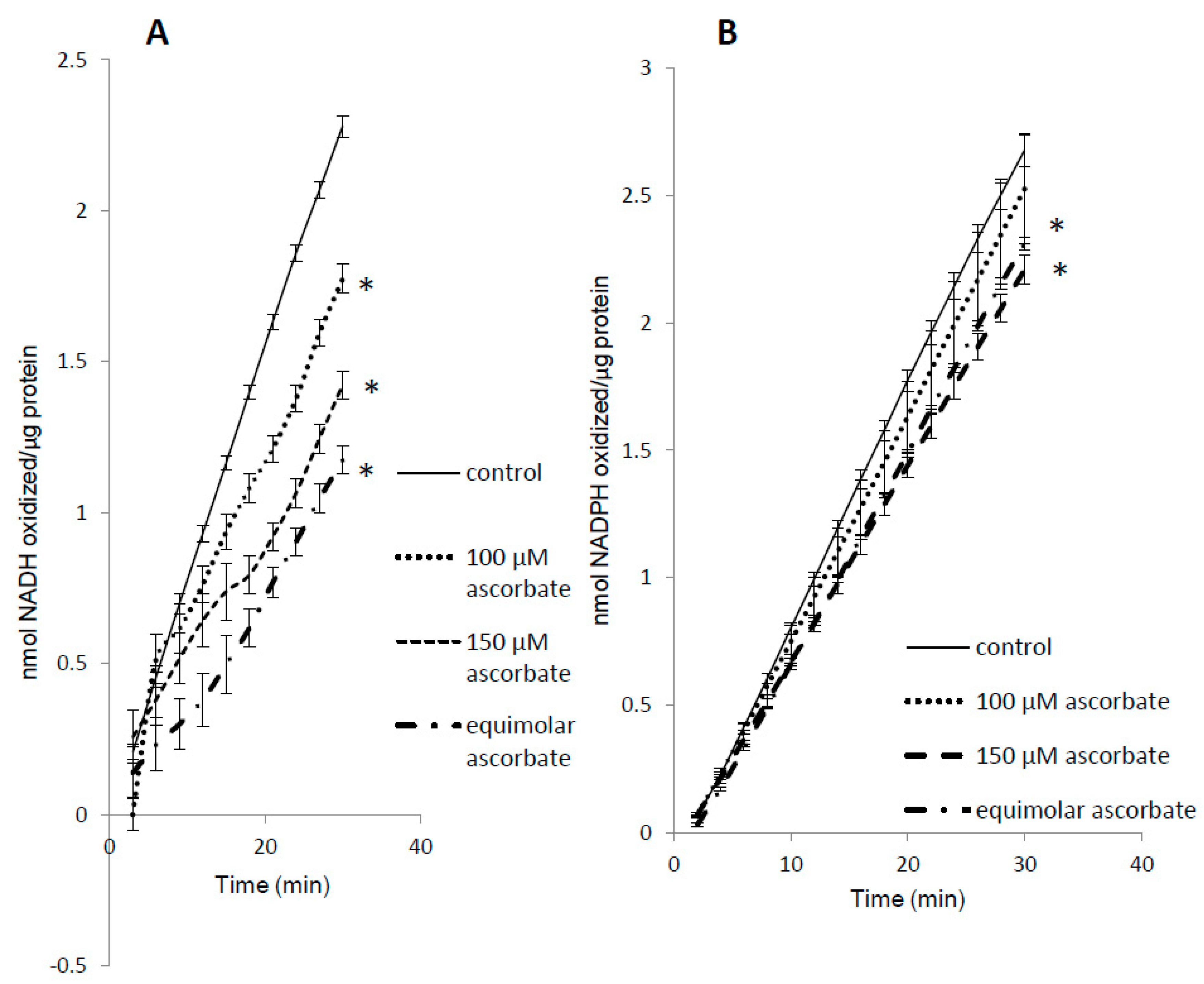
We found that ascorbic acid differentially competed with NADH and NADPH as substrates for metMb (Figure 7A,B). Even at equimolar concentrations of ascorbic acid, the rate of NADPH oxidation was minimally affected (Figure 7B), whereas the same ratio of ascorbic acid reduced metMb peroxidase activity with NADH by ~50% (Figure 7A). Notably, we have previously shown that metMb has similar peroxidase activity [25] with NADH and NADPH, which would imply that they both should be equally affected by the same competing substrate.
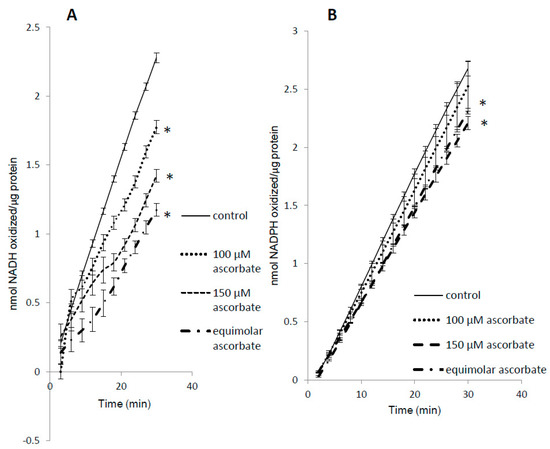
Figure 7.
Substrate competition for metMb peroxidase activity. MetMb peroxidase activity with NADH (A) or NADPH (B) was measured in the presence of varying concentrations of ascorbic acid. * p ≤ 0.05 compared to NAD(P)H without ascorbic acid present. n = 6/group in (A) and n = 11–12/group in (B).
3.6. H2O2-Dependent Dimerization of metMb
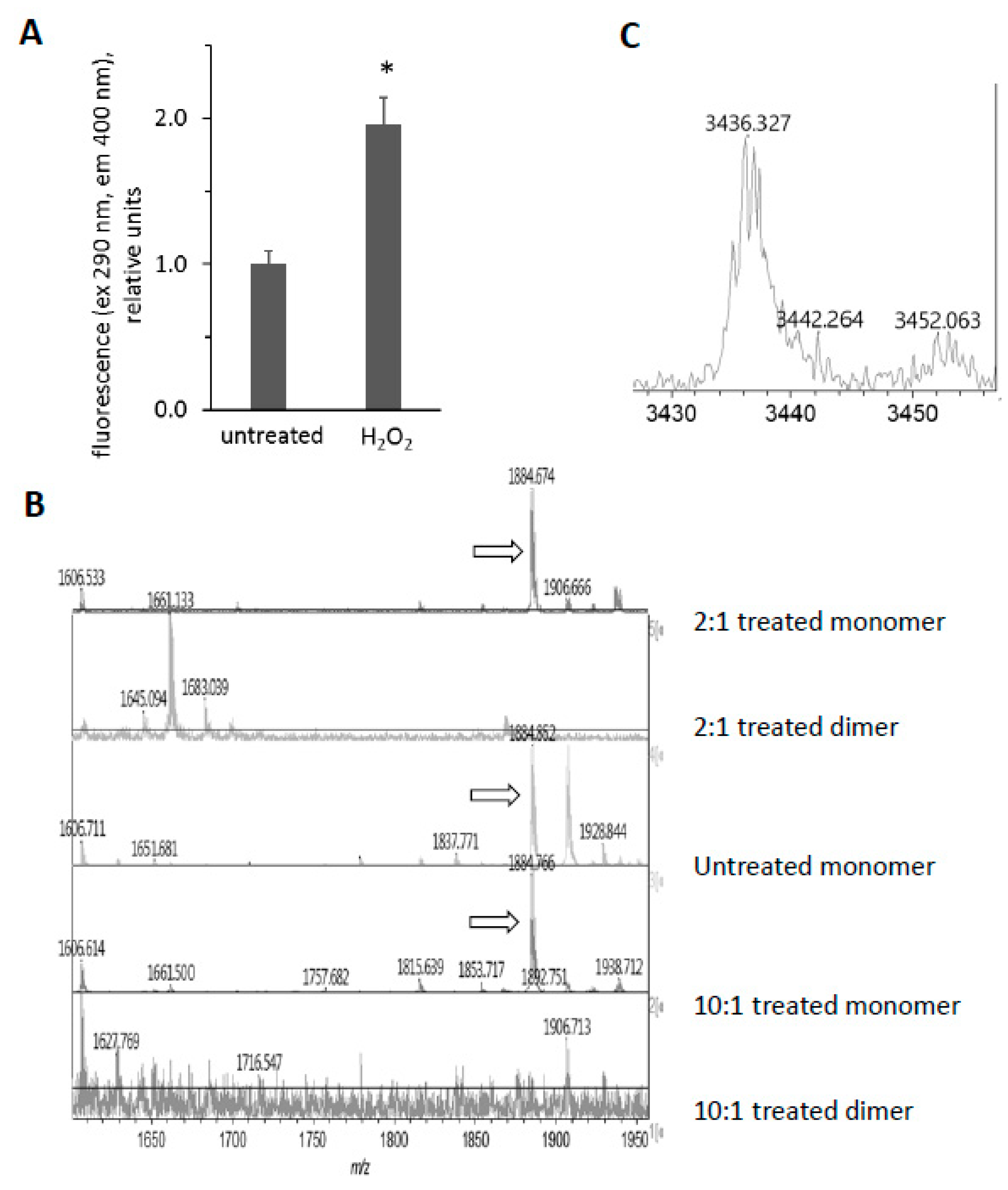
We were intrigued by the appearance of high molecular weight bands after treatment of Mb with H2O2 and the disappearance of these bands after subsequent exposure to ascorbic acid (Figure 3 and Figure 5). Since horse Mb does not contain cysteine residues and thus the crosslinks could not be disulfide bonds, we sought to determine the nature of the oxidative cross-link. Previous reports [34] have found dityrosine cross-links in the H2O2-treated Mb dimer. Since dityrosine has a unique fluorescence spectrum, we first measured the fluorescence of H2O2-treated metMb (Figure 8A). Consistent with the presence of dityrosine, H2O2-reacted Mb samples displayed significantly increased fluorescence corresponding to the excitation and emission wavelengths in the range of the dityrosine spectra [17]. Mass spectrometric analysis showed that the peptide containing tyrosine 103 is absent in the H2O2-treated dimer, while it is present in both the treated and untreated monomer (1885 m/z, Figure 8B). This corresponds to the tryptic peptide (K)YLEFISDAIIHVLHSK(H) containing Y103. In addition, a novel peptide at 3436.4 m/z appeared in the H2O2-reacted dimer (Figure 8C). Presumably, this is a species with the peptide containing Y103 cross-linked to the C-terminal tryptic missed cleavages peptide containing Y146, (R)NDIAAKYKELGFQG(-). The 3436.4 m/z peak corresponds to the theoretical mass of a Y103-Y146 cross-link (1885.0 m/z + 1553.8 m/z − 2 protons lost). Although these results do not rule out the possibility of other cross-links, they strongly indicate the presence of dityrosine cross-links in H2O2-reacted horse Mb.
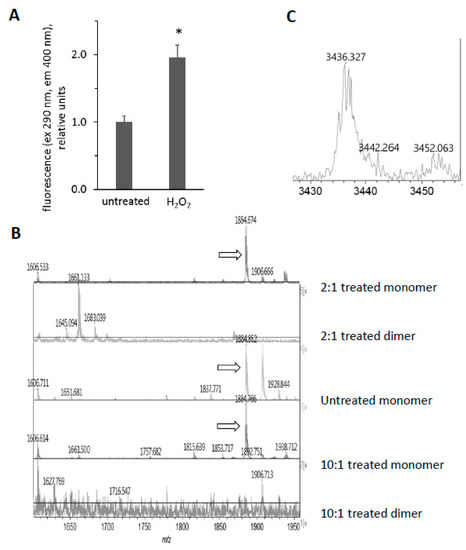
Figure 8.
Reaction of horse metMb with H2O2 results in dityrosine formation. (A) Fluorescence of metMb was measured before and after oxidation with H2O2 using excitation and emission wavelengths of 290 nm and 400 nm, respectively. * p ≤ 0.005 compared to untreated, n = 6/group. (B) MALDI-TOF analysis of metMb tryptic digests revealed that the tryptic peptide containing tyrosine 103 (1885 m/z) was absent in the H2O2-treated dimer. From bottom: dimer from 222 μM Mb reacted with 2.2 mM H2O2; monomer from 222 μM Mb reacted with 2.2 mM H2O2; untreated monomer; 222 μM Mb dimer reacted with 444 μM H2O2; 222 μM Mb monomer reacted with 444 μM H2O2. Arrows indicate peaks for 1885 m/z. (C) MALDI-TOF analysis of oxidized metMb dimers indicate the presence of a Y103-Y146 dityrosine cross-linked peptide with a m/z of 3436.3.
3.7. Reversal of Protein-to-Protein Cross-Links
Data discussed above suggests that H2O2 can induce protein-to-protein cross links in Mb and that subsequent treatment with ascorbic acid can break the crosslinks. This is shown for samples subjected to a heme peroxidase stain after exposure of Mb to H2O2 and then ascorbic acid (Figure 9A). For data shown in Figure 9A, we let the peroxidase stain proceed long enough to develop multiple bands at high molecular weights, corresponding to Mb dimers, trimers, and larger aggregates. We found that ascorbic acid treatment significantly reduced the fluorescence of H2O2-reacted metMb (Figure 9B), which is consistent with the idea that ascorbic acid facilitates the cleavage of Mb’s protein-to-protein crosslinks.
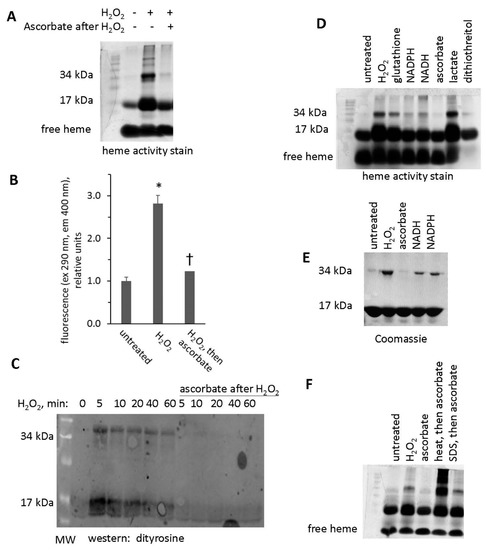
Figure 9.
Treatment with ascorbic acid reverses crosslinking of Mb dimers. (A) Heme peroxidase activity stain of metMb that was untreated, H2O2-treated, or H2O2-treated followed by exposure to ascorbic acid. (B) Fluorescence of metMb treated as for panel A was assessed at excitation 290 nm and emission 400 nm, which correspond to the fluorescence characteristics of dityrosine [17], n = 8/group, * p < 0.001 vs untreated and group treated with peroxide and then ascorbic acid, † p = 0.05 vs untreated. (C) Western blot of ascorbic acid-treated oxidized metMb using an anti-dityrosine antibody. (D) Heme stain of H2O2-reacted metMb that was then treated with various reducing substrates. (E) Coomassie stain of Mb exposed to H2O2 and then reducing agents. (F) Heme stain of oxidized metMb that was denatured by heating for 10 min at 90 °C or by adding SDS-PAGE sample loading buffer prior to the addition of ascorbic acid.
Western blot analysis using an anti-dityrosine antibody suggests that intra- and intermolecular dityrosine is present in H2O2-reacted metMb monomer and dimer, respectively (Figure 9C). Treatment of H2O2-reacted metMb with ascorbic acid eliminated reactivity with the antibody against dityrosine. In addition, treatment with other biological reducing substrates (glutathione, NADPH, NADH, and dithiothreitol) also reversed the dimer, although to differing extents (Figure 9D,E).
We next sought to determine the extent to which the Mb protein itself was responsible for cleavage of protein-to-protein bonds. To do this, we subjected oxidized metMb to both heat and detergent denaturation prior to ascorbic acid treatment. As shown in Figure 9F, heat and detergent-mediated denaturation inhibited the ability of ascorbic acid treatment to reverse metMb dimers, thus confirming that the native Mb protein plays a role in the mechanism of breaking protein-to-protein crosslinks.
3.8. Potential Role of Tyrosine Residues in Breaking Mb–Mb Cross-Links
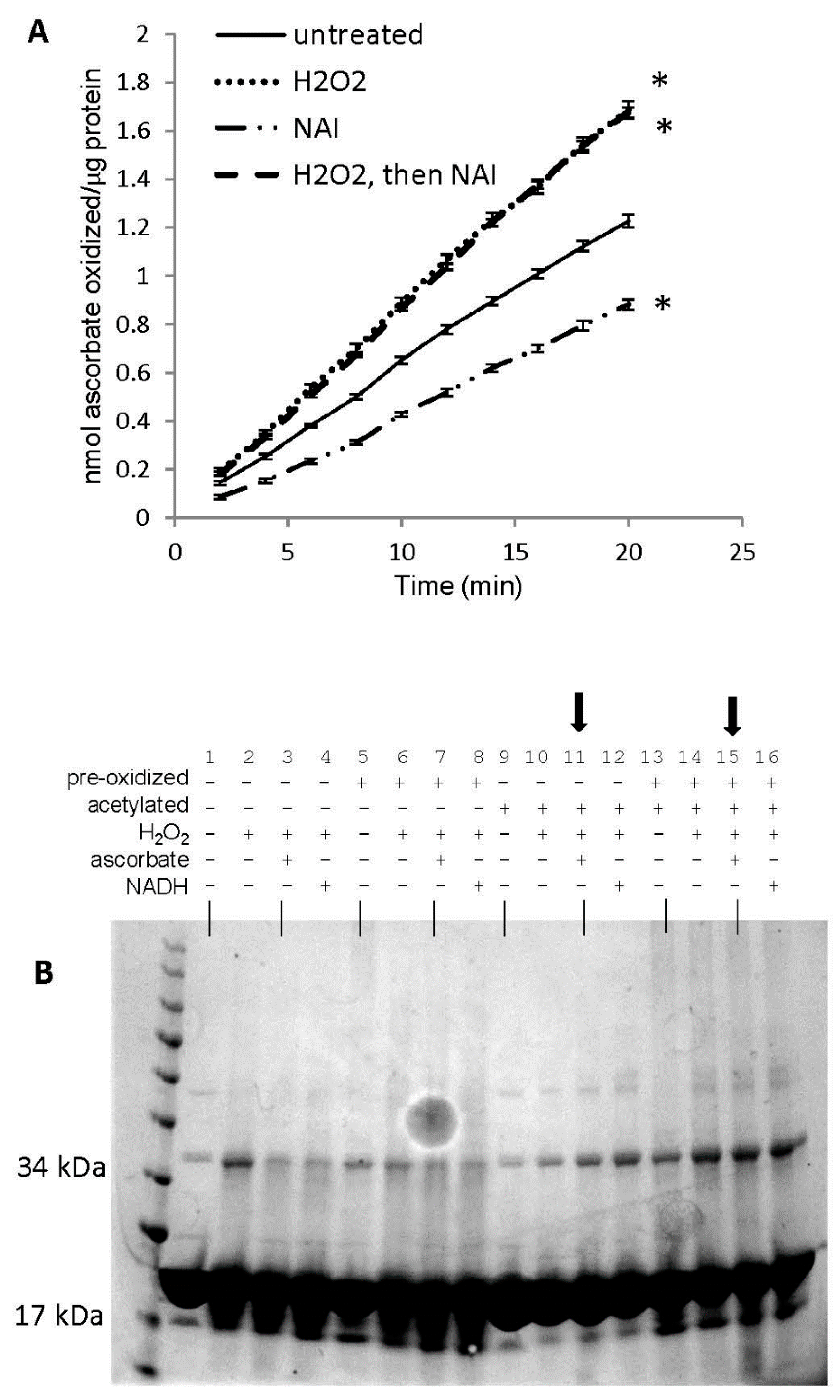
We have previously shown that acetylation of tyrosine residues in horse metMb differentially affects its peroxidase activity depending on the reducing co-substrate used [25]. We hypothesized that this discrepancy might be due to tyrosine-mediated formation of oxidatively-modified Mb species (i.e., metMb monomer, metMb dimer, Mb-X monomer, Mb-X dimer, etc.) with different peroxidase activities with different reducing co-substrates. As displayed in Figure 10A, tyrosine acetylation significantly decreased the peroxidase activity of metMb using ascorbic acid as a reducing co-substrate. However, the inhibitory effect of tyrosine acetylation was completely abrogated if metMb was first pre-treated with H2O2, suggesting that the role of the tyrosine residues is directing crosslinks as opposed to directly participating in redox cycling. In addition, the activity of metMb that was first treated with H2O2 and then acetylated was nearly identical to the activity of metMb that was only pre-treated with H2O2, indicating that the increased activity-conferring oxidative modifications had already occurred prior to acetylation.
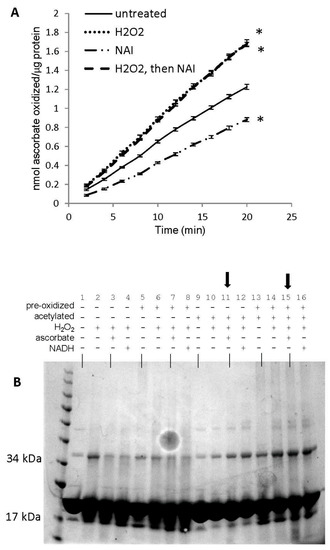
Figure 10.
Role of tyrosine residues in regulating oxidatively-modified metMb species. (A) Peroxidase activity assay of metMb that had been treated with H2O2 alone, N-acetylimidazole (NAI) alone or H2O2 and then NAI (as described in methods and materials) using 200 μM H2O2 and 250 μM ascorbic acid at pH 6.1. * p ≤ 0.05 compared to untreated control, n = 6/group. Activity plots for H2O2 and NAI groups overlap, so they are not both visible. (B) Coomassie stains of the samples prepared in (A). Lanes 1–4: untreated, control metMb; H2O2-treated, control metMb; H2O2-treated control metMb + ascorbic acid; H2O2-treated control metMb + NADH. Lanes 5–8: same as (1–4) only using met Mb that had been reacted with H2O2 (pre-oxidized). Lanes 9–12: same as (1–4) and (5–8) only using NAI-treated metMb. Lanes 13–16: same only using H2O2-treated then NAI-treated metMb. Lanes showing that tyrosine-acetylated Mb cannot reverse Mb dimerization in the presence of ascorbic acid are indicated by arrows.
SDS-PAGE of these samples revealed that both acetylated (with or without pre-H2O2 treatment) and unacetylated metMb could form dimers upon reaction with H2O2 (Figure 10B). However, neither acetylated sample could reverse its dimer(s) after treatment with ascorbic acid, indicating that tyrosine residues are not necessary to form dimers but that they are necessary for reversing them.
4. Discussion
The new information provided by this study includes the novel findings of reversible oxidative modifications of Mb upon treatment with ascorbic acid. For example, exposure to H2O2 increases Mb peroxidase activity and preference for ascorbate as the reducing co-substrate for Mb peroxidase activity. This increase of peroxidase activity was associated with Mb-X formed by reaction of Mb with H2O2, and both the increase in peroxidase activity and the Mb-X crosslink were reversed by treatment with ascorbic acid. While H2O2-reacted Mb forms intramolecular crosslinks to form dimers, trimers, and larger Mb aggregates, an important novel finding of the current study is that these interprotein bonds are broken by treatment with ascorbate. This action does not occur if the aggregates are first denatured by heat or incubation with SDS, suggesting that the native protein plays a role in reversal of interprotein crosslinks.
While the increase in peroxidase activity caused by exposure of Mb to H2O2 is a novel finding, there are reports of other Mb redox activities being enhanced by reaction of Mb with H2O2. For example, treatment of sperm whale or horse Mb with H2O2 reportedly increases NADH oxidase activity by up to 20-fold, and this activity when assessed for horse Mb was associated with Mb-X [12]. The stoichiometry of this reaction was 1 mol of NADH oxidized per 1 mol of O2 consumed, which is consistent with a two-electron transfer from NADH to O2, forming H2O2 [12]. Osawa and Korzekwa suggested that this NADH oxidase activity of Mb-X could contribute to toxicity by promoting further production of H2O2 [12]. Other deleterious reactions mediated by H2O2-reacted Mb or Mb-X include peroxidation of lipids, phospholipids, LDL, and cholesterol esters [13,35]. Holt et al. reported increased presence of Mb-X and the free-radical-induced peroxidation of arachidonic acid, F2-isoprostanes, in urine of patients with rhabomyolysis [16], suggesting a central role of Mb-X in rhabodomyolysis-related tissue damage. In contrast to the reactions described above, which are oxidative in nature (i.e., either promoting peroxidation or producing H2O2), an increase in peroxidase activity as shown in the current study would be a means to counteract an increase in reactive oxygen species.
Although myoglobin in the presence of H2O2 can produce hydroxyl radical, scavengers of hydroxyl radical had no effect on peroxidation of either uric acid or arachidonic acid peroxidation in the presence of Mb and H2O2 [35]. This suggests that the Mb itself—as opposed to Mb-produced hydroxyl radical—mediates uric acid or acachidonic peroxidation. In contrast, sulfhydryl reducing agents can prevent peroxidation of uric acid or arachidonic acid by myoglobin in the presence of H2O2 [35]. A suggested mechanism of the protective effects of the reducing agents was that they prevented formation of a reactive derivative of Mb [35], such as Mb-X. Mb-X, originally known as the green-pigmented species formed by reaction of Mb with H2O2, was found to be stable in solution at room temperature for months [11]. The new information provided by the current study is that reducing agents can reverse—as opposed to simply prevent—formation of Mb-X. Because it seems apparent that Mb-X plays a toxic role in conditions such as rhabdomyolytis [15,16], this novel demonstration of reversal of the Mb-X crosslink by ascorbic acid has important therapeutic implications.
Catalano et al. reported that treatment of horse Mb with H2O2 causes a ~50% decrease in tyrosine (Y) content [10]. Tryptic digests of H2O2-treated Mb contained a species not present in untreated Mb that had a molecular weight consistent with a heme group covalently bound to a peptide beginning at Y103 (YLEFISDAIIHVLHSK), though this peptide had virtually no Y content in H2O2-treated Mb compared to untreated Mb [10]. Taking these data together, the authors suggested that reaction with H2O2 produces Mb with the heme covalently bound to Y103 [10]. Reeder et al. [8] re-examined the hypothesis that Y103 mediates the heme-to-Mb cross link by using site-directed mutagenesis of sperm whale Mb. While the Y103F mutation did not affect Mb-X formation, the H64V mutation of the E7 helix distal histidine almost fully prevented generation of Mb-X [8]. Consistent with this, wild-type Aplysia limacina that lacks the E helix distal histidine does not form Mb-X, while introduction of a histidine residue into aplysia promotes Mb-X formation [8]. We have detected a tryptic fragment of H2O2-reacted Mb that is consistent with the analysis of Reeder et al. [8] that the cross-link of Mb-X can be mediated by histidine.
Reeder et al. reported that H2O2 reaction with Mb forms Mb-X at increasing rates when pH decreases [36]. The authors suggested that formation of Mb-X requires both the protonated oxoferryl heme and a protein radical. It also appears that a protein radical is required for protein-to-protein Mb cross linking [37]. Detweiler et al. used 3,4-dihydro-2,3-dimethyl-2H-pyrrole 1-oxide (DMPO) to trap radicals formed by reaction of sperm whale Mb with H2O2 [37]. DMPO prevented formation of sperm whale Mb dimers and trimers [37]. Use of DMPO followed by electrospray mass spectrometry showed that a peptide containing Y103 was the site of the radical [37]. This is consistent with the suggestion of Svistunenko et al. that given the proximity of Y103 to the heme, the radical originates on Y103 before passing to other sites in Mb [38]. Iodinization of horse Mb prevented subsequent formation of Mb dimers by reaction with H2O2, consistent of a role of a role of tyrosinyl side chains in generation of protein-to-protein cross links [37]. Further, Y151 of sperm whale Mb, a tyrosine lacking in horse Mb, was required for Mb dimer formation [37]. Our findings stand in contrast to those of Detweiler et al. [37] in that tyrosine acetylation did not affect Mb dimer formation in the current study, suggesting that tyrosine-independent crosslinks can contribute to the Mb aggregation in the current study.
Mb-X formation from metMb is modestly faster than Mb-X formation from oxygen-bound Mb (oxyMb) [36]. However, Mb-X formation from metMb is inhibited by presence of oxyMb [36]. This brings into question whether Mb-X formation could occur intracellularly under normoxic conditions in which oxyMb would be the predominant Mb form. On the other hand, it has been shown by magnetic resonance spectroscopy of human skeletal muscle that moderate aerobic exercise (about 50% or 60% of maximum oxygen consumption rate (O2max) causes about 50% of Mb to be in its deoxygenated form (deoxyMb) in the contracting skeletal muscle [39,40]. This deoxygenation of Mb sets in at moderate exercise intensity but does not further increase as exercise intensity increases up to O2max [39,40]. At the same time that exercise increases deoxyMb [39,40], muscle contractions also increase intracellular NADPH oxidase-generated superoxide [41], which rapidly dismutates to H2O2. Thus, it appears that aerobic exercise could create conditions under which Mb-X could potentially form (i.e., increases in both H2O2 and deoxyMb).
The dityrosine western blot showing reversal of dityrosine cross-links should be treated with caution, as dityrosine has such a high bond dissociation energy [42] that it would be unlikely to be broken. Consistent with a side chain other than tyrosine participating in protein crosslinking, acetylation of tyrosine residues did not prevent formation of Mb dimers. As a potential alternative mechanism of Mb–Mb cross-linking, it is possible that some other amino acid side chain, such as tryptophan, participates in protein-to-protein linkages. For example, radical-induced generation of ditryptophan (W–W) and tryptophan-tyrosine (W–Y) crosslinks has been shown to mediate protein and peptide dimerization [43,44,45]. W–W crosslinks have a fluorescence excitation and emission profile [46] that is similar to that of dityrosine [17] and so could potentially contribute to the fluorescence changes (e.g., Figure 8A and Figure 9B) in Mb exposed to H2O2. Mb contains a tryptophan residue that becomes a tryptophanyl radical after reaction with H2O2 [33]. Our MALDI-TOF data show that the peptide containing this W residue (and also the other W in horse Mb) disappears after treatment of Mb with H2O2 and reappears after subsequent treatment with ascorbic acid. Given the position of this W residue in Mb, it seems unlikely to be able to participate in binding to heme. However, the data are consistent with W being a candidate for forming reversible protein-to-protein crosslinks between Mb proteins, perhaps involving W–W bonds, W–Y bonds, or both. Our findings suggest the vital importance of denaturing H2O2-reacted Mb with heat and/or SDS before running blots under reducing conditions to ensure both detection of Mb dimers and larger aggregates and subsequent reversal, given the labile nature of the cross links when exposed to reducing agents in the presence of native (i.e., non-denatured) Mb.
The nature of ditryptophan bonds is not yet fully elucidated [47]. Available data suggest that C and N participate in ditryptophan crosslinking [47], giving the possibility of either C–C or C–N crosslinks. Notably, the ditryptophan dimer in superoxide dismutase or lysozyme can cleave under MS/MS conditions [47], suggesting that it is not as stable as a dityrosine link and thus is similar to the labile crosslinks we have detected in the current study. Paviani et al. have suggested that the susceptibility to cleavage of ditryptophan is more consistent with a C–N bond than a C–C bond [47]. Accordingly, the crosslink that is reversed by ascorbate in the current study is likely a C–N bond.
Our finding of a peptide corresponding to the mass of a peptide containing both Y103 and Y146 suggests that both tyrosines can participate in dityrosine crosslinks. We did not collect data on which tyrosine residues were acetylated by treatment of Mb with N-acetylimidazole. When horse heart Mb is incubated with a 100-fold excess of N-acetylimidazole, both tyrosines are acetylated, though the amount of tyrosine acetylated can be varied by titration with N-acetylimidazole and assessed by changes in tyrosine absorbance at 280 nm [48]. Our N-acetylimidazole-to-Mb molar ratio was 10-fold lower than that used by Giulivi et al. [49], so it is possible that Y103 was unaffected under these conditions. The finding that dimerization was not prevented by acetylation suggest that Y103 was not acetylated by N-acetylimidazole in our study. For example, Y103 is likely the site of radical initiation before the radical is passed to other residues [38], which would be necessary for radical-induced dimerization involving residues such as tyrosine or tryptophan. Interestingly, iodination of horse heart Mb prevents Mb dimerization [37]. This suggests that the dimerization reported by Detweiler et al. [37] is truly mediated by dityrosine, as opposed to the labile crosslink found in the current study.
Y103 is local to the heme, and it appears that formation of a radical occurs at Y103 before transferring to other residues [37,38]. We have detected a peptide containing both Y103 and Y146 in H2O2-treated Mb. This suggests that both the heme-localized tyrosine and the tyrosine in a helix closer to the protein surface can participate in dityrosine bonding. The distal histidine is in close proximity to the heme and has previously been demonstrated to form a cross link with heme [8]. W14, on the other hand, is distant from both the heme and Y103 but still reportedly forms a radical when Mb reacts with H2O2 [33]. For reference regarding positions of the heme and amino acids in Mb, a 3D structure of horse heart metMb (MMDB ID 57734) is available in the Molecular Modeling Database (MMDB) [50] housed by the National Center for Biotechnology Information.
Dityrosine occurs in various functional, structural elements such as silk proteins [51,52], elastin [53], and sea urchin eggs [54]. On the other hand, dityrosine can be a marker for both aging-associated oxidative damage [55] and acute bouts of oxidative stress, such as in myocardial infarction [56]. While the aforementioned studies consider dityrosine to be a biomarker for oxidative stress, others have proposed that it might play more of a harmful role in certain mammalian tissues. For example, dityrosine-mediated cross-linking of β-amyloid peptide [23,57] and α-synuclein protein [24] promotes the stabilization of their respective aggregates. Interestingly, overexpression of neuroglobin (Ngb), an oxygen-binding globin expressed mainly in neurons, has been shown to reduce Aβ fibril formation in vivo [58], and low Ngb levels correlate with Alzheimer’s disease [59]. The data from the current study suggest that ascorbic acid can break protein-to-protein crosslinks caused by reaction of Mb with H2O2. Thus, it seems possible that actions of globins might be a means through which deleterious protein aggregates could be broken in vivo.
Reaction schemes for reduction of ferrylMb (Mb with Fe4+ in an oxo complex) by ascorbate such as would occur in peroxidase activity [60], pH dependence of Mb redox reactions [61], generation of Mb-X [36], formation of dityrosine [17], and formation of ditryptophan [43,47] are presented in the literature. The increased peroxidase activity once Mb is treated with H2O2 is most likely due to formation of the heme-to-protein crosslink. The mechanism for the increased redox activity of Mb-X has not been elucidated, but it has been suggested to be attributable to a change in protein structure surrounding the heme [12]. Although we do not know the mechanism by which ascorbate becomes a preferred reducing substrate of Mb-X, we speculate that Mb-X retains the ability to be reduced by ascorbate at both sites for electron donation on Mb as described by Reeder et al. [60].
Translation of the in vitro data presented in this study to physiological conditions relies on the assumption that metMb would be present in vivo. While Mb protein is expressed in mammalian heart and skeletal muscle at about 400 µmol/kg [62,63], metMb concentration is relatively low in tissues in vivo if it is present at all. For example, Kreutzer et al. reported that metmyoglobin is undetectable by NMR in perfused rat heart [64]. On the other hand, it has been suggested that 1% of Mb in cardiac tissue is in the metMb form [65]. As measured spectroscopically in anoxic pig heart, metMb content was 32 µmol/dm3, which was about 6% of total Mb [66]. Ascorbate concentrations in human skeletal muscle are about 170 µmol/kg [67]. Unfortunately, H2O2 levels in skeletal muscle have been difficult to measure [68]. Palomero et al. [69] used extracellular H2O2 to calibrate intracellular H2O2 levels in isolated rat skeletal muscle fibers using the reactive oxygen species probe chloromethyl-2′,7′-dichlorofluorescin. They used this extracellular H2O2 standard to estimate that intracellular H2O2 reached about 1 µM during contractile activity [69]. Jackson later suggested that this H2O2 concentration would be closer to 0.1 µM due to a trans plasma membrane H2O2 gradient that would result from H2O2 being applied to the extracellular medium [68]. In summary, the in vitro concentrations of metMb and H2O2 used in the current study are much greater than would be found in the intracellular environment. Future study should be done to determine whether reversible modifications of Mb, such as Mb-X or labile Mb aggregates, can be found in skeletal muscle or heart under normal and pathological conditions. Of course, Mb can be also be found extracellularly, in conditions such as rhabdomyolysis. In the extracellular milieu, where serum H2O2 concentration and plasma ascorbate concentration are both about ~50 µM [67,70], the reversible modifications described in the in vitro studies of the current paper seem possible. Future work should investigate whether reversible modifications of Mb occur in vivo in both intracellular and extracellular spaces.
5. Conclusions
Collectively, our data indicate a redox role for Mb. We demonstrate that formation of heme-protein crosslinked species substantially increases Mb peroxidase activity as well as specificity with ascorbate as a reducing co-substrate. These findings point toward a potential regulatory role of ascorbate for Mb peroxidase activity in oxidatively-challenged muscle and heart. We also show that both heme-to-protein and protein-to-protein crosslinks in horse Mb can be broken by treatment with ascorbic acid, which indicates that Mb might play a role to reverse oxidative protein modifications.
Author Contributions
Conceptualization, M.H.M. and J.S.F.; methodology, M.H.M., R.S.P., B.E.J., D.C.W., and F.H.; investigation: M.H.M., R.S.P., B.E.J., A.M.E., D.C.W., F.H., and J.S.F.; writing—original draft preparation, M.H.M.; writing—review and editing, M.H.M., R.S.P., A.M.E., B.E.J., D.C.W., F.H., and J.S.F.; supervision, M.H.M., B.E.J., D.C.W., F.H., and J.S.F.; funding acquisition, J.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by USPHS award R15DK102122 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Jonathan Fisher. The manuscript content is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK or the National Institutes of Health. The funding source played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Acknowledgments
The authors would like to thank Andrei M. Gonzales, Megan M. Callahan, and Rodrigo A. Magnelli Perez for their technical support. We are grateful to the Saint Louis University High Resolution NMR Lab and the Protein Core Facility for technical assistance and access to instrumentation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Boronat, S.; Garcia-Santamarina, S.; Hidalgo, E. Gel-free proteomic methodologies to study reversible cysteine oxidation and irreversible protein carbonyl formation. Free Radic. Res. 2015, 49, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Reeg, S.; Grune, T. Protein oxidation in aging: Does it play a role in aging progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jeong, M.S.; Choi, S.Y.; Kang, J.H. Oxidative modification of cytochrome c by hydrogen peroxide. Mol. Cells 2006, 22, 220–227. [Google Scholar] [PubMed]
- Xiang, W.; Weisbach, V.; Sticht, H.; Seebahn, A.; Bussmann, J.; Zimmermann, R.; Becker, C.M. Oxidative stress-induced posttranslational modifications of human hemoglobin in erythrocytes. Arch. Biochem. Biophys. 2013, 529, 34–44. [Google Scholar] [CrossRef]
- Archakov, A.I.; Zgoda, V.G.; Karuzina, I.I. Oxidative modification of cytochrome P450 and other macromolecules during its turnover. Vopr. Med. Khim. 1998, 44, 3–27. [Google Scholar]
- Giulivi, C.; Cadenas, E. Ferrylmyoglobin: Formation and chemical reactivity toward electron-donating compounds. Methods Enzym. 1994, 233, 189–202. [Google Scholar]
- Reeder, B.J.; Cutruzzola, F.; Bigotti, M.G.; Watmough, N.J.; Wilson, M.T. Histidine and not tyrosine is required for the peroxide-induced formation of haem to protein cross-linked myoglobin. IUBMB Life 2007, 59, 477–489. [Google Scholar] [CrossRef]
- Svistunenko, D.A.; Reeder, B.J.; Wankasi, M.M.; Silaghi-Dumitrescu, R.L.; Cooper, C.E.; Rinaldo, S.; Cutruzzola, F.; Wilson, M.T. Reaction of Aplysia limacina metmyoglobin with hydrogen peroxide. Dalton Trans. 2007, 840–850. [Google Scholar] [CrossRef]
- Catalano, C.E.; Choe, Y.S.; Ortiz de Montellano, P.R. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J. Biol. Chem. 1989, 264, 10534–10541. [Google Scholar]
- Fox, J.B., Jr.; Nicholas, R.A.; Ackerman, S.A.; Swift, C.E. A multiple wavelength analysis of the reaction between hydrogen peroxide and metmyoglobin. Biochemistry 1974, 13, 5178–5186. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Korzekwa, K. Oxidative modification by low levels of HOOH can transform myoglobin to an oxidase. Proc. Natl. Acad. Sci. USA 1991, 88, 7081–7085. [Google Scholar] [CrossRef] [PubMed]
- Vuletich, J.L.; Osawa, Y.; Aviram, M. Enhanced lipid oxidation by oxidatively modified myoglobin: Role of protein-bound heme. Biochem. Biophys. Res. Commun. 2000, 269, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Williams, M.S. Covalent crosslinking of the heme prosthetic group to myoglobin by H2O2: Toxicological implications. Free Radic. Biol. Med. 1996, 21, 35–41. [Google Scholar] [CrossRef]
- Boutaud, O.; Moore, K.P.; Reeder, B.J.; Harry, D.; Howie, A.J.; Wang, S.; Carney, C.K.; Masterson, T.S.; Amin, T.; Wright, D.W.; et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc. Natl. Acad. Sci. USA 2010, 107, 2699–2704. [Google Scholar] [CrossRef]
- Holt, S.; Reeder, B.; Wilson, M.; Harvey, S.; Morrow, J.D.; Roberts, L.J., 2nd; Moore, K. Increased lipid peroxidation in patients with rhabdomyolysis. Lancet 1999, 353, 1241. [Google Scholar] [CrossRef]
- Malencik, D.A.; Anderson, S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 2003, 25, 233–247. [Google Scholar] [CrossRef]
- Cheng, G.; Li, H.; Cao, Z.; Qiu, X.; McCormick, S.; Thannickal, V.J.; Nauseef, W.M. Vascular peroxidase-1 is rapidly secreted, circulates in plasma, and supports dityrosine cross-linking reactions. Free Radic. Biol. Med. 2011, 51, 1445–1453. [Google Scholar] [CrossRef]
- DiMarco, T.; Giulivi, C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007, 26, 108–120. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Miura, Y.; Nabeno, Y.; Kato, Y.; Osawa, T.; Naito, M. Immunohistochemical detection of oxidative stress biomarkers, dityrosine and N(epsilon)-(hexanoyl)lysine, and C-reactive protein in rabbit atherosclerotic lesions. J. Atheroscler. Thromb. 2008, 15, 185–192. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Cucchiari, D.; Portinaro, N.M.; Giustarini, D.; Rossi, R.; Garavaglia, M.L.; Saino, N.; Milzani, A.; Badalamenti, S.; et al. Plasma protein-bound di-tyrosines as biomarkers of oxidative stress in end stage renal disease patients on maintenance haemodialysis. BBA Clin. 2017, 7, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilaly, Y.K.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.; Morris, K.L.; Sada, A.A.; Thorpe, J.R.; et al. A central role for dityrosine crosslinking of Amyloid-beta in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 83. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Biasetti, L.; Blakeman, B.J.; Pollack, S.J.; Zibaee, S.; Abdul-Sada, A.; Thorpe, J.R.; Xue, W.F.; Serpell, L.C. The involvement of dityrosine crosslinking in alpha-synuclein assembly and deposition in Lewy Bodies in Parkinson’s disease. Sci. Rep. 2016, 6, 39171. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.H.; Patel, R.S.; Eccardt, A.M.; Perez Magnelli, R.A.; Robinson, C.L.C.; Janowiak, B.E.; Warren, D.E.; Fisher, J.S. Myoglobin as a versatile peroxidase: Implications for a more important role for vertebrate striated muscle in antioxidant defense. Comp. Biochem. Physiol. Part B 2019, 234, 9–17. [Google Scholar] [CrossRef] [PubMed]
- La Mar, G.; Satterlee, J.; De Ropp, J. Nuclear magnetic resonance of hemoproteins. In The Porphrylin Handbook; Kadish, K., Smith, K., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; pp. 185–298. [Google Scholar]
- Yamamoto, Y. NMR study of active sites in paramagnetic haemoproteins. Annu. Rep. NMR Spectrosc. 1998, 36, 1–77. [Google Scholar] [CrossRef]
- Luthje, S.; Meisrimler, C.N.; Hopff, D.; Schutze, T.; Koppe, J.; Heino, K. Class III peroxidases. Methods Mol. Biol. 2014, 1072, 687–706. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Basu, S.; Kirley, T.L. Identification of a tyrosine residue responsible for N-acetylimidazole-induced increase of activity of ecto-nucleoside triphosphate diphosphohydrolase 3. Purinergic Signal. 2005, 1, 271–280. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.W. The broad diversity of heme-protein cross-links: An overview. Biochim. Biophys. Acta 2015, 1854, 844–859. [Google Scholar] [CrossRef]
- Silaghi-Dumitrescu, R.; Reeder, B.J.; Nicholls, P.; Cooper, C.E.; Wilson, M.T. Ferryl haem protonation gates peroxidatic reactivity in globins. Biochem. J. 2007, 403, 391–395. [Google Scholar] [CrossRef] [PubMed]
- DeGray, J.A.; Gunther, M.R.; Tschirret-Guth, R.; Ortiz de Montellano, P.R.; Mason, R.P. Peroxidation of a specific tryptophan of metmyoglobin by hydrogen peroxide. J. Biol. Chem. 1997, 272, 2359–2362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kroger-Ohlsen, M.V.; Ostdal, H.; Andersen, M.L. The effect of pH on the oxidation of bovine serum albumin by hypervalent myoglobin species. Arch. Biochem. Biophys. 2003, 416, 202–208. [Google Scholar] [CrossRef]
- Mitsos, S.E.; Kim, D.; Lucchesi, B.R.; Fantone, J.C. Modulation of myoglobin-H2O2-mediated peroxidation reactions by sulfhydryl compounds. Lab. Investig. 1988, 59, 824–830. [Google Scholar] [PubMed]
- Reeder, B.J.; Svistunenko, D.A.; Sharpe, M.A.; Wilson, M.T. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry 2002, 41, 367–375. [Google Scholar] [CrossRef]
- Detweiler, C.D.; Lardinois, O.M.; Deterding, L.J.; de Montellano, P.R.; Tomer, K.B.; Mason, R.P. Identification of the myoglobin tyrosyl radical by immuno-spin trapping and its dimerization. Free Radic. Biol. Med. 2005, 38, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Svistunenko, D.A.; Dunne, J.; Fryer, M.; Nicholls, P.; Reeder, B.J.; Wilson, M.T.; Bigotti, M.G.; Cutruzzola, F.; Cooper, C.E. Comparative study of tyrosine radicals in hemoglobin and myoglobins treated with hydrogen peroxide. Biophys. J. 2002, 83, 2845–2855. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Investig. 1995, 96, 1916–1926. [Google Scholar] [CrossRef]
- Richardson, R.S.; Newcomer, S.C.; Noyszewski, E.A. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: Response to graded exercise. J. Appl. Physiol. 2001, 91, 2679–2685. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- Rainey, W.T.J.; McDufie, H.F.; Hess, D.N.; Yeatts, L.B., Jr. Kinetics of the Thermal Decomposition of Biphenyl; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1964. [Google Scholar]
- Paviani, V.; Queiroz, R.F.; Marques, E.F.; Di Mascio, P.; Augusto, O. Production of lysozyme and lysozyme-superoxide dismutase dimers bound by a ditryptophan cross-link in carbonate radical-treated lysozyme. Free Radic. Biol. Med. 2015, 89, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Leinisch, F.; Mariotti, M.; Rykaer, M.; Lopez-Alarcon, C.; Hagglund, P.; Davies, M.J. Peroxyl radical- and photo-oxidation of glucose 6-phosphate dehydrogenase generates cross-links and functional changes via oxidation of tyrosine and tryptophan residues. Free Radic. Biol. Med. 2017, 112, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Leo, G.; Altucci, C.; Bourgoin-Voillard, S.; Gravagnuolo, A.M.; Esposito, R.; Marino, G.; Costello, C.E.; Velotta, R.; Birolo, L. Ultraviolet laser-induced cross-linking in peptides. Rapid Commun. Mass Spectrom. 2013, 27, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Osapay, K.; Tran, D.; Ladokhin, A.S.; White, S.H.; Henschen, A.H.; Selsted, M.E. Formation and characterization of a single Trp-Trp cross-link in indolicidin that confers protease stability without altering antimicrobial activity. J. Biol. Chem. 2000, 275, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Paviani, V.; Galdino, G.T.; dos Prazeres, J.N.; Queiroz, R.F.; Augusto, O. Ditryptophan cross-links as novel products of protein oxidation. J. Braz. Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Giulivi, C.; Romero, F.J.; Cadenas, E. The interaction of Trolox C, a water-soluble vitamin E analog, with ferrylmyoglobin: Reduction of the oxoferryl moiety. Arch. Biochem. Biophys. 1992, 299, 302–312. [Google Scholar] [CrossRef]
- Aguirre, E.; Rodriguez-Juarez, F.; Bellelli, A.; Gnaiger, E.; Cadenas, S. Kinetic model of the inhibition of respiration by endogenous nitric oxide in intact cells. Biochim. Biophys. Acta 2010, 1797, 557–565. [Google Scholar] [CrossRef]
- Madej, T.; Lanczycki, C.J.; Zhang, D.; Thiessen, P.A.; Geer, R.C.; Marchler-Bauer, A.; Bryant, S.H. MMDB and VAST+: Tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 2014, 42, D297–D303. [Google Scholar] [CrossRef]
- Wang, C.S.; Pan, H.; Weerasekare, G.M.; Stewart, R.J. Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Raven, D.J.; Earland, C.; Little, M. Occurrence of dityrosine in Tussah silk fibroin and keratin. Biochim. Biophys. Acta 1971, 251, 96–99. [Google Scholar] [CrossRef]
- LaBella, F.; Keeley, F.; Vivian, S.; Thornhill, D. Evidence for dityrosine in elastin. Biochem. Biophys. Res. Commun. 1967, 26, 748–753. [Google Scholar] [CrossRef]
- Foerder, C.A.; Shapiro, B.M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc. Natl. Acad. Sci. USA 1977, 74, 4214–4218. [Google Scholar] [CrossRef]
- Kato, Y.; Maruyama, W.; Naoi, M.; Hashizume, Y.; Osawa, T. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998, 439, 231–234. [Google Scholar] [CrossRef]
- Mayer, F.; Falk, M.; Huhn, R.; Behmenburg, F.; Ritz-Timme, S. Dityrosine as a marker of acute myocardial infarction? Experiments with the isolated Langendorff heart. Int. J. Leg. Med. 2016, 130, 1053–1060. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry 2004, 43, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mao, X.O.; Banwait, S.; Jin, K.; Greenberg, D.A. Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 19114–19119. [Google Scholar] [CrossRef]
- Szymanski, M.; Wang, R.; Fallin, M.D.; Bassett, S.S.; Avramopoulos, D. Neuroglobin and Alzheimer’s dementia: Genetic association and gene expression changes. Neurobiol. Aging 2010, 31, 1835–1842. [Google Scholar] [CrossRef]
- Reeder, B.J.; Cutruzzola, F.; Bigotti, M.G.; Hider, R.C.; Wilson, M.T. Tyrosine as a redox-active center in electron transfer to ferryl heme in globins. Free Radic. Biol. Med. 2008, 44, 274–283. [Google Scholar] [CrossRef]
- Reeder, B.J.; Wilson, M.T. The effects of pH on the mechanism of hydrogen peroxide and lipid hydroperoxide consumption by myoglobin: A role for the protonated ferryl species. Free Radic. Biol. Med. 2001, 30, 1311–1318. [Google Scholar] [CrossRef]
- Weller, P.A.; Price, M.; Isenberg, H.; Edwards, Y.H.; Jeffreys, A.J. Myoglobin expression: Early induction and subsequent modulation of myoglobin and myoglobin mRNA during myogenesis. Mol. Cell. Biol. 1986, 6, 4539–4547. [Google Scholar] [CrossRef]
- Moller, P.; Sylven, C. Myoglobin in human skeletal muscle. Scand. J. Clin. Lab. Investig. 1981, 41, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, U.; Jue, T. Role of myoglobin as a scavenger of cellular NO in myocardium. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H985–H991. [Google Scholar] [CrossRef] [PubMed]
- Gussakovsky, E.; Yang, Y.; Rendell, J.; Jilkina, O.; Kupriyanov, V. Mapping the myoglobin concentration, oxygenation, and optical pathlength in heart ex vivo using near-infrared imaging. Anal. Biochem. 2010, 407, 120–127. [Google Scholar] [CrossRef][Green Version]
- Swartling, J.; Pålsson, S.; Platonov, P.; Olsson, S.B.; Andersson-Engels, S. Changes in tissue optical properties due to radio-frequency ablation of myocardium. Med. Biol. Eng. Comput. 2003, 41, 403–409. [Google Scholar] [CrossRef]
- Mason, S.A.; Baptista, R.; Della Gatta, P.A.; Yousif, A.; Russell, A.P.; Wadley, G.D. High-dose vitamin C supplementation increases skeletal muscle vitamin C concentration and SVCT2 transporter expression but does not alter redox status in healthy males. Free Radic. Biol. Med. 2014, 77, 130–138. [Google Scholar] [CrossRef]
- Jackson, M.J. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic. Biol. Med. 2009, 47, 1267–1275. [Google Scholar] [CrossRef]
- Palomero, J.; Pye, D.; Kabayo, T.; Spiller, D.G.; Jackson, M.J. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid. Redox Signal. 2008, 10, 1463–1474. [Google Scholar] [CrossRef]
- Akl, M.G.; Fawzy, E.; Deif, M.; Farouk, A.; Elshorbagy, A.K. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: Association with insulin resistance. PLoS ONE 2017, 12, e0177268. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).