Cathelicidin Modulates Vascular Smooth Muscle Cell Phenotypic Switching through ROS/IL-6 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Primary VSMC Culture
2.3. Cell Viability Assay
2.4. Western Blot
2.5. Animal Experiments
2.6. Proliferation Assay
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.8. ROS Detection
2.9. Statistical Analysis
3. Results
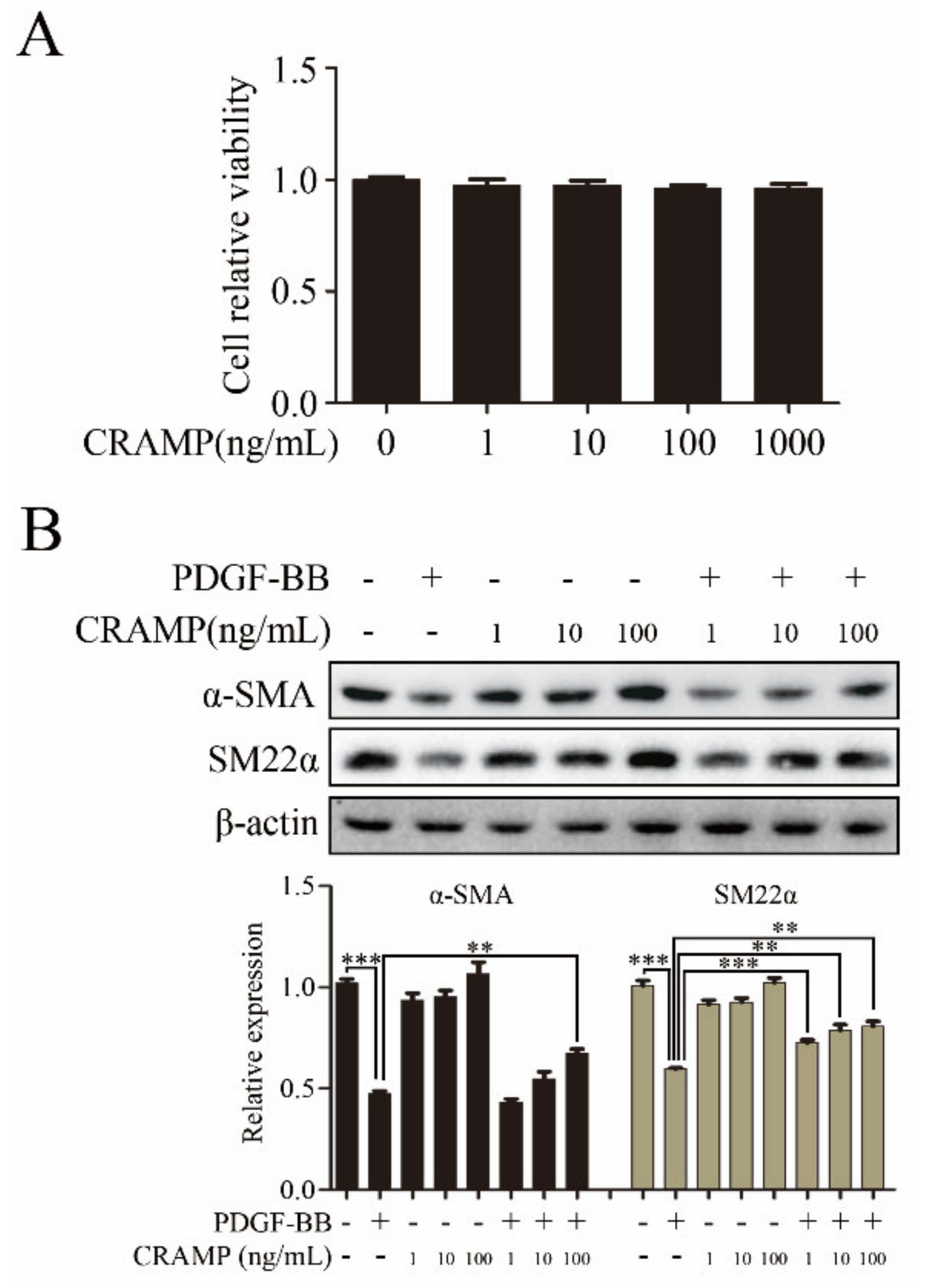
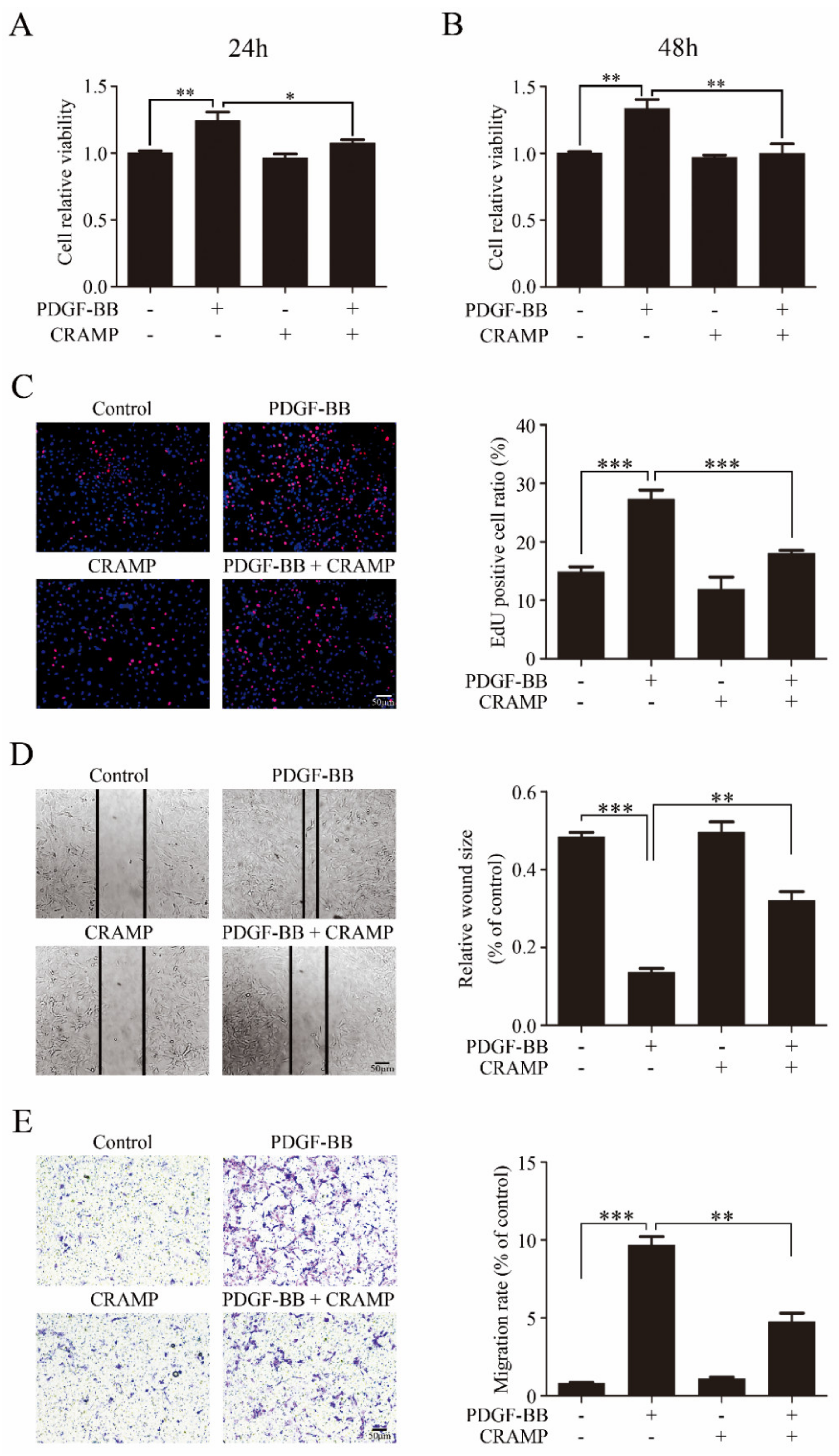
3.1. CRAMP Inhibits PDGF-BB-Induced VSMC Phenotypic Transformation, Proliferation and Migration
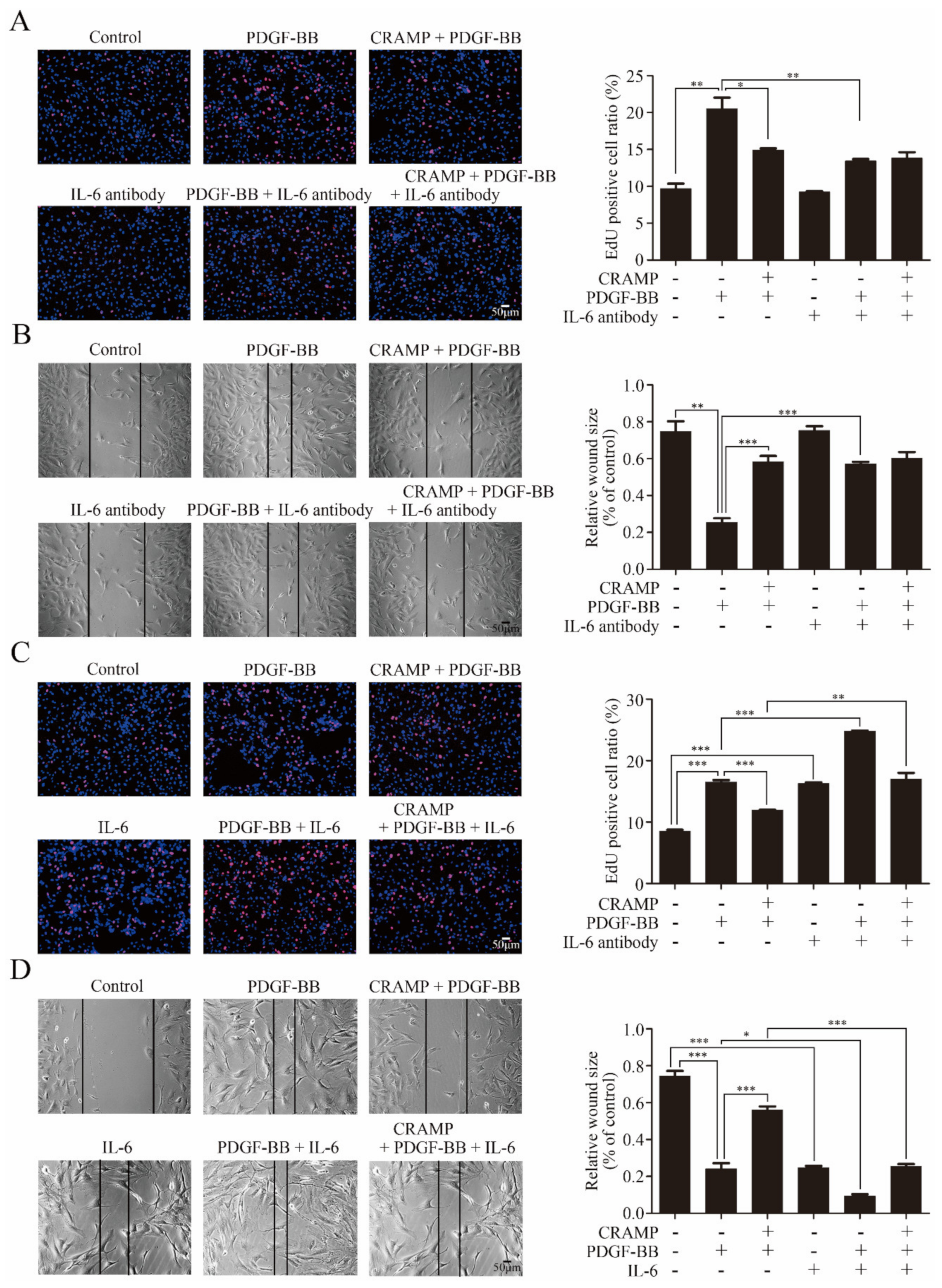
3.2. CRAMP Inhibited PDGF-Mediated IL-6/STAT3 Activation
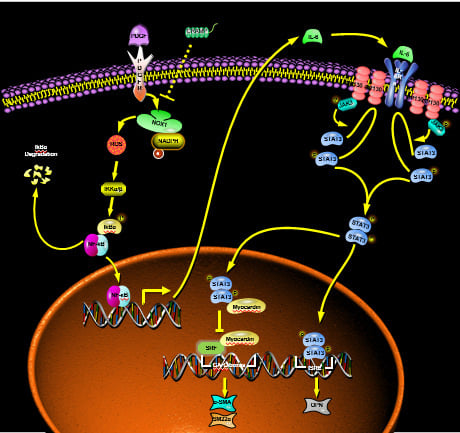
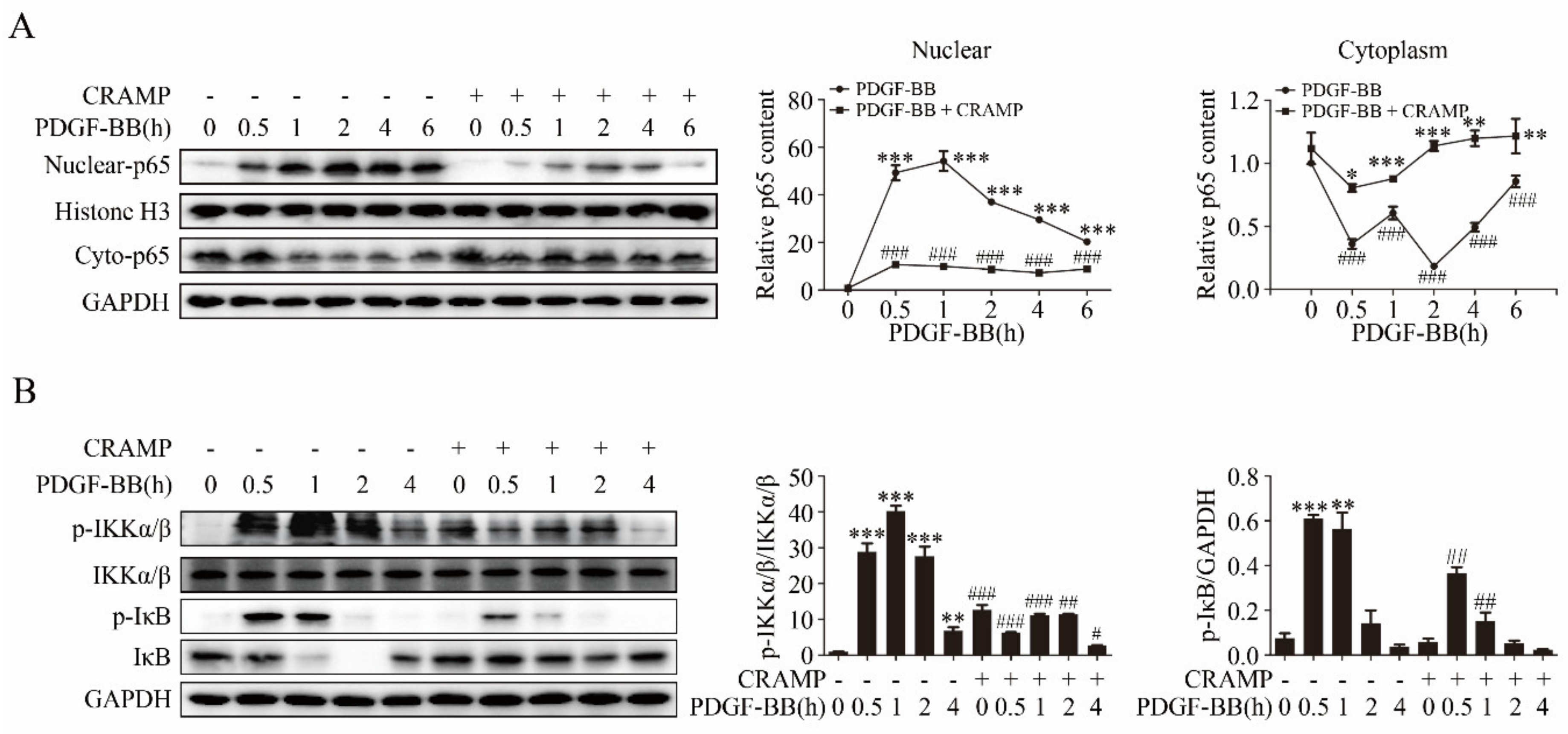
3.3. CRAMP Regulated IL-6 Autocrine via Targeting NF-κB Signaling
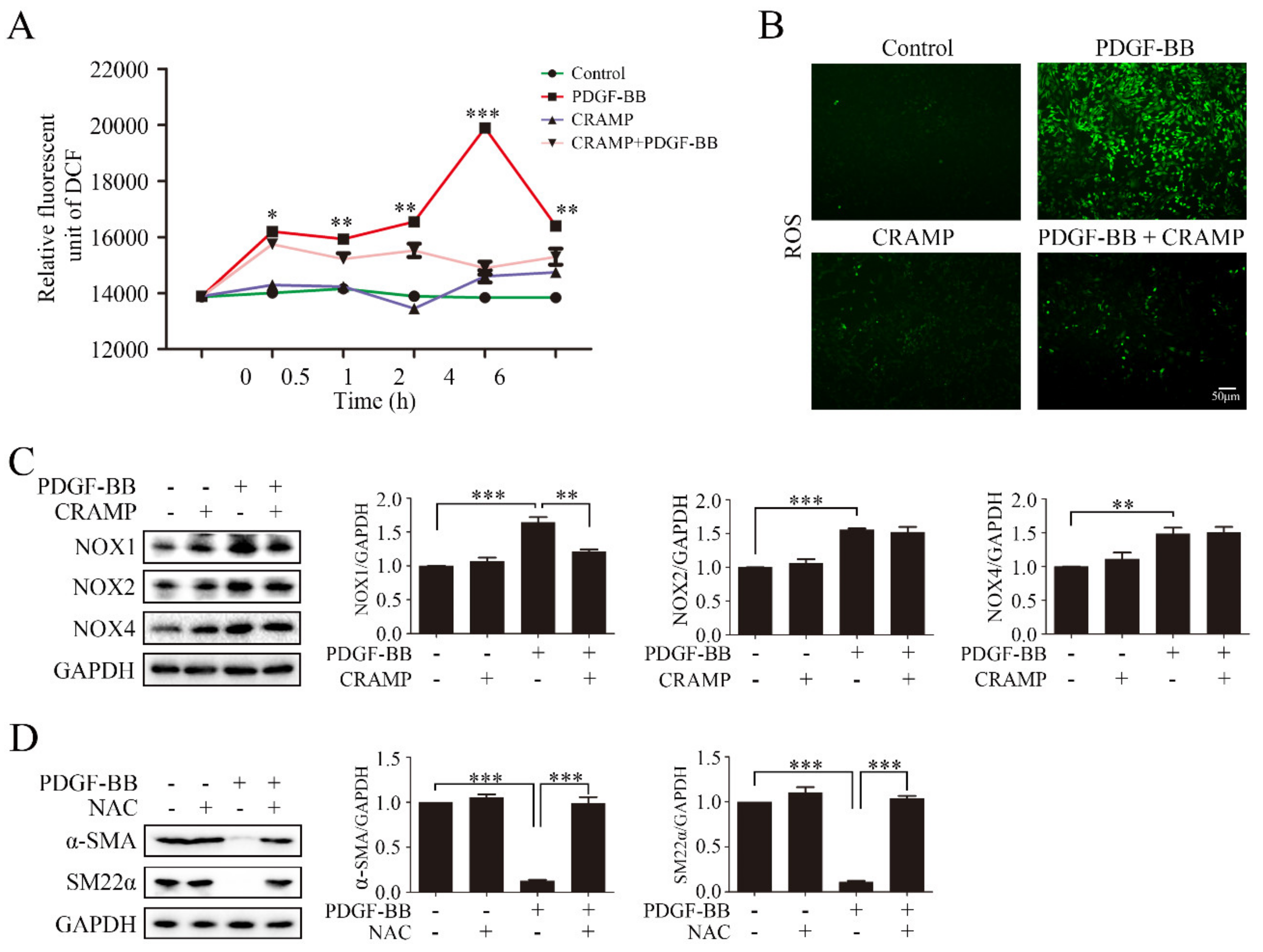
3.4. CRAMP Prevented PDGF-BB-Enhanced ROS by Targeting NOX1
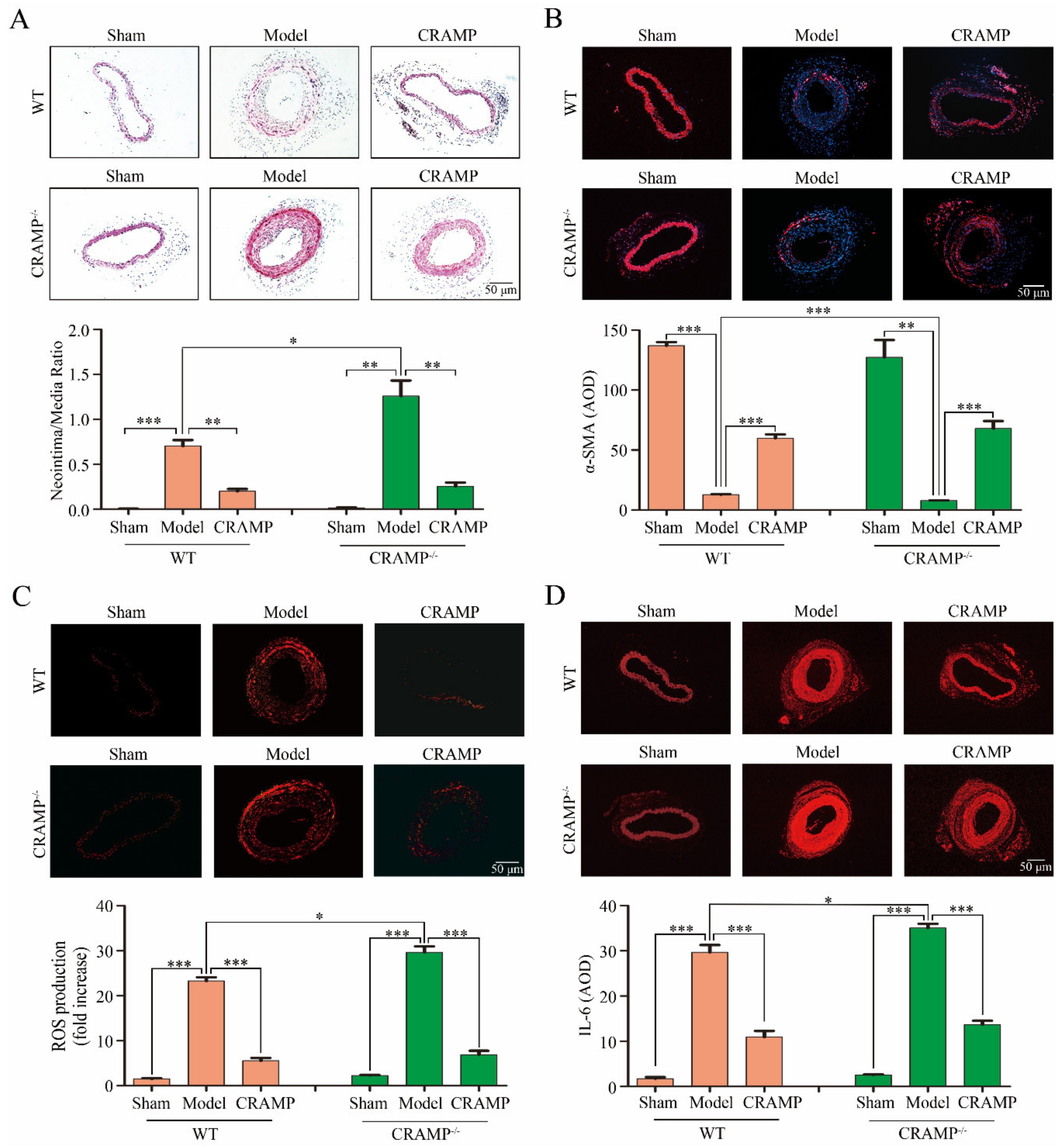
3.5. CRAMP Repressed Intimal Hyperplasia and Suppressed ROS/IL-6 Generation In Vivo
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: Https://www.who.int/health-topics/cardiovascular-diseases (accessed on 14 January 2020).
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. (Oxf) 2015, 214, 33–50. [Google Scholar] [CrossRef]
- Guo, X.; Shi, N.; Cui, X.B.; Wang, J.N.; Fukui, Y.; Chen, S.Y. Dedicator of cytokinesis 2, a novel regulator for smooth muscle phenotypic modulation and vascular remodeling. Circ. Res. 2015, 116, e71–e80. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. miR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation. Circulation 2018, 137, 1824–1841. [Google Scholar] [CrossRef]
- Zhan, J.K.; Wang, Y.J.; Wang, Y.; Tang, Z.Y.; Tan, P.; Huang, W.; Liu, Y.S. Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp. Cell Res. 2014, 323, 352–358. [Google Scholar] [CrossRef]
- Owens, G.K. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found. Symp. 2007, 283, 174–191, discussion 191–193, 238–241. [Google Scholar]
- Rzucidlo, E.M.; Martin, K.A.; Powell, R.J. Regulation of vascular smooth muscle cell differentiation. J Vasc. Surg. 2007, 45, A25–A32. [Google Scholar] [CrossRef]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Xu, F.; Ahmed, A.S.I.; Kang, X.; Hu, G.; Liu, F.; Zhang, W.; Zhou, J. MicroRNA-15b/16 Attenuates Vascular Neointima Formation by Promoting the Contractile Phenotype of Vascular Smooth Muscle through Targeting YAP. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2145–2152. [Google Scholar] [CrossRef]
- Kim, K.; Yang, D.K.; Kim, S.; Kang, H. miR-142-3p is a regulator of the TGFbeta-mediated vascular smooth muscle cell phenotype. J. Cell Biochem. 2015, 116, 2325–2333. [Google Scholar] [CrossRef]
- Althoff, T.F.; Offermanns, S. G-protein-mediated signaling in vascular smooth muscle cells-implications for vascular disease. J. Mol. Med. 2015, 93, 973–981. [Google Scholar] [CrossRef]
- Chaterji, S.; Lam, C.H.; Ho, D.S.; Proske, D.C.; Baker, A.B. Syndecan-1 regulates vascular smooth muscle cell phenotype. PLoS ONE 2014, 9, e89824. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Son, S.M. Reactive Oxygen and Nitrogen Species in Pathogenesis of Vascular Complications of Diabetes. Diabetes Metab. J. 2012, 3, 190–198. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Chang, K.C.; Chung, S.Y.; Chong, W.S.; Suh, J.S.; Kim, S.H.; Noh, H.K.; Seong, B.W.; Ko, H.J.; Chun, K.W. Possible superoxide radical-induced alteration of vascular reactivity in aortas from streptozotocin-treated rats. J. Pharmacol. Exp. Ther. 1993, 266, 992–1000. [Google Scholar]
- Pieper, G.M.; Langenstroer, P.; Siebeneich, W. Diabetic-induced endothelial dysfunction in rat aorta: Role of hydroxyl radicals. Cardiovasc. Res. 1997, 34, 145–156. [Google Scholar] [CrossRef]
- Yamagishi, S.i.; Yonekura, H.; Yamamoto, Y.; Katsuno, K.; Sato, F.; Mita, I.; Ooka, H.; Satozawa, N.; Kawakami, T.; Nomura, M.; et al. Advanced glycation end products-driven angiogenesis in vitro. Induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. J. Biol. Chern. 1997, 272, 8723–8730. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I.; et al. Diabetes associated sustained activation of the transcription factor nuclear factor kappa B. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Döring, Y.; Manthey, H.D.; Drechsler, M.; Lievens, D.; Megens, R.T.; Soehnlein, O.; Busch, M.; Manca, M.; Koenen, R.R.; Pelisek, J.; et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing self-DNA-peptide Complexes in Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.; Homey, B.; Cao, W.; Wang, Y.; Su, B.; Nestle, F.O.; et al. Plasmacytoid Dendritic Cells Sense self-DNA Coupled with Antimicrobial Peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Chen, S.; Zeqing, L.; Fengqin, W.; Yizhen, W. Cathelicidin-WA Polarizes, E. Coli K88-induced M1 Macrophage to M2-like Macrophage in RAW264.7 Cells. Int. Immunopharmacol. 2018, 54, 52–59. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Liu, Q.; Wang, X.; Shan, T.; Wang, Y. Cathelicidin-WA Attenuates LPS-induced Inflammation and Redox Imbalance through Activation of AMPK Signaling. Free Radic. Biol. Med. 2018, 129, 338–353. [Google Scholar] [CrossRef]
- Justyna, A.; Sylwia, R.; Magdalena, W.; Paulina, Ż.; Joanna, P.; Ewa, B. The RLR/NLR Expression and Pro-Inflammatory Activity of Tissue Mast Cells Are Regulated by Cathelicidin LL-37 and Defensin hBD-2. Sci. Rep. 2018, 8, 11750. [Google Scholar]
- Johnson, J.L. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc. Res. 2014, 103, 452–460. [Google Scholar] [CrossRef]
- Liao, X.H.; Xiang, Y.; Li, H.; Zheng, D.L.; Xu, Y.; Yu, C.X.; Li, J.P.; Zhang, X.Y.; Xing, W.B.; Cao, D.S.; et al. VEGF-A Stimulates STAT3 Activity via Nitrosylation of Myocardin to Regulate the Expression of Vascular Smooth Muscle Cell Differentiation Markers. Sci. Rep. 2017, 7, 2660. [Google Scholar] [CrossRef]
- Spin, J.M.; Maegdefessel, L.; Tsao, P.S. Vascular smooth muscle cell phenotypic plasticity: Focus on chromatin remodelling. Cardiovasc. Res. 2012, 95, 147–155. [Google Scholar] [CrossRef]
- Manabe, I.; Owens, G.K. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J. Clin. Investig. 2001, 107, 823–834. [Google Scholar] [CrossRef]
- Masamune, A.; Satoh, M.; Kikuta, K.; Suzuki, N.; Shimosegawa, T. Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J. Gastroenterol. 2005, 11, 3385–3391. [Google Scholar] [CrossRef]
- Zhan, Y.; Kim, S.; Izumi, Y.; Izumiya, Y.; Nakao, T.; Miyazaki, H.; Iwao, H. Role of JNK, p38, and ERK in Platelet-Derived Growth Factor–Induced Vascular Proliferation, Migration, and Gene Expression. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 795–801. [Google Scholar] [CrossRef]
- Yan, J.F.; Huang, W.J.; Zhao, J.F.; Fu, H.Y.; Zhang, G.Y.; Huang, X.J.; Lv, B.D. The platelet-derived growth factor receptor/STAT3 signaling pathway regulates the phenotypic transition of corpus cavernosum smooth muscle in rats. PLoS ONE 2017, 12, e0172191. [Google Scholar] [CrossRef]
- Mohrherr, J.; Uras, I.Z.; Moll, H.P.; Casanova, E. STAT3: Versatile Functions in Non-Small Cell Lung Cancer. Cancers (Basel) 2020, 29, 12. [Google Scholar] [CrossRef]
- Nonaka, K.; Kajiura, Y.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Lew, J.H.; Naruishi, K.; Ikuta, T.; Yoshida, K.; Kobayashi, T.; et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J. Periodontal. Res. 2018, 53, 334–344. [Google Scholar] [CrossRef]
- Yeh, C.C.; Wu, J.Y.; Lee, G.L.; Wen, H.T.; Lin, P.; Kuo, C.C. Vanadium Derivative Exposure Promotes Functional Alterations of VSMCs and Consequent Atherosclerosis via ROS/p38/NF-κB-Mediated IL-6 Production. Int. J. Mol. Sci. 2019, 20, E6115. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhao, M.X.; Ren, X.S.; Liu, T.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Wang, J.J.; Zhu, G.Q. Salusin-β Promotes Vascular Smooth Muscle Cell Migration and Intimal Hyperplasia after Vascular Injury via ROS/NFκB/MMP-9 Pathway. Antioxid. Redox Signal. 2016, 24, 1045–1057. [Google Scholar] [CrossRef]
- Xi, G.; Shen, X.; Wai, C.; Vilas, C.K. Clemmons DR. Hyperglycemia stimulates p62/PKCζ interaction, which mediates NF-κB activation, increased Nox4 expression, and inflammatory cytokine activation in vascular smooth muscle. FASEB J. 2015, 29, 4772–4782. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Meng, P.; Han, Y.; Shen, C.; Li, B.; Hakim, M.A.; Zhang, X.; Lu, Q.; Rong, M.; Lai, R. Mitochondrial DNA-LL-37 Complex Promotes Atherosclerosis by Escaping From Autophagic Recognition. Immunity 2015, 43, 1137–1147. [Google Scholar] [CrossRef]
- Seino, Y.; Ikeda, U.; Ikeda, M.; Yamamoto, K.; Misawa, Y.; Hasegawa, T.; Kano, S.; Shimada, K. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine 1994, 6, 87–91. [Google Scholar] [CrossRef]
- Lee, W.Y.; Allison, M.A.; Kim, D.J.; Song, C.H.; Barrett-Connor, E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study). Am. J. Cardiol. 2007, 99, 99–102. [Google Scholar] [CrossRef]
- Loppnow, H.; Libby, P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J. Clin. Investig. 1990, 85, 731–738. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.Z.; Hockemeyer, D.; McAnally, J.; Nordheim, A.; Olson, E.N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004, 428, 185–189. [Google Scholar] [CrossRef]
- Heiss, E.H.; Schachner, D.; Donati, M.; Grojer, C.S.; Dirsch, V.M. Increased aerobic glycolysis is important for the motility of activated VSMC and inhibited by indirubin-3′-monoxime. Vascul. Pharmacol. 2016, 83, 47–56. [Google Scholar] [CrossRef]
- Lassegue, B.; San Martin, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Lee, M.Y.; Martin, A.S.; Mehta, P.K.; Dikalova, A.E.; Garrido, A.M.; Datla, S.R.; Lyons, E.; Krause, K.H.; Banfi, B.; Lambeth, J.D.; et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 480–487. [Google Scholar] [CrossRef]
- Szocs, K.; Lassegue, B.; Sorescu, D.; Hilenski, L.L.; Valppu, L.; Couse, T.L.; Wilcox, J.N.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Upregulation of nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shriver, A.S.; Jagadeesha, D.K.; Chamseddine, A.H.; Szocs, K.; Weintraub, N.L.; Griendling, K.K.; Bhalla, R.C.; Jr, F.J.M. Increased expression of Nox1 in neointimal smooth muscle cells promotes activation of matrix metalloproteinase-9. J. Vasc. Res. 2012, 49, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH Oxidase Regulates Redox Signaling and Phenotype of Vascular Smooth Muscle Cell during Atherogenesis. Redox Biol. 2019, 21, 101063. [Google Scholar] [CrossRef]
- Prince, P.D.; Fischerman, L.; Toblli, J.E.; Fraga, C.G.; Galleano, M. LPS-induced Renal Inflammation Is Prevented by (-)-Epicatechin in Rats. Redox Biol. 2017, 11, 342–349. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Qi, J.; Liu, R.; Zhang, H.; He, L. Ferulic Acid Inhibits H2O2-induced Oxidative Stress and Inflammation in Rat Vascular Smooth Muscle Cells via Inhibition of the NADPH Oxidase and NF-κB Pathway. Int. Immunopharmacol. 2015, 28, 1018–1025. [Google Scholar] [CrossRef]
- Li, M.; Yao, W.; Li, S.; Xi, J. Norepinephrine Induces the Expression of interleukin-6 via β-adrenoreceptor-NAD(P)H Oxidase System-NF-κB Dependent Signal Pathway in U937 Macrophages. Biochem. Biophys. Res. Commun. 2015, 460, 1029–1034. [Google Scholar] [CrossRef]
- Alalwani, S.M.; Sierigk, J.; Herr, C.; Pinkenburg, O.; Gallo, R.; Vogelmeier, C.; Bals, R. The Antimicrobial Peptide Ll-37 Modulates the Inflammatory and Host Defense Response of Human Neutrophils. Eur. J. Immunol. 2010, 40, 1118–1126. [Google Scholar] [CrossRef]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H.; et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1523. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wu, D.; Zhang, Y.; Jia, L.; Pan, X.; Sun, J.; Pan, L.-L. Cathelicidin Modulates Vascular Smooth Muscle Cell Phenotypic Switching through ROS/IL-6 Pathway. Antioxidants 2020, 9, 491. https://doi.org/10.3390/antiox9060491
Dong X, Wu D, Zhang Y, Jia L, Pan X, Sun J, Pan L-L. Cathelicidin Modulates Vascular Smooth Muscle Cell Phenotypic Switching through ROS/IL-6 Pathway. Antioxidants. 2020; 9(6):491. https://doi.org/10.3390/antiox9060491
Chicago/Turabian StyleDong, Xiaoliang, Di Wu, Yihan Zhang, Lingling Jia, Xiaohua Pan, Jia Sun, and Li-Long Pan. 2020. "Cathelicidin Modulates Vascular Smooth Muscle Cell Phenotypic Switching through ROS/IL-6 Pathway" Antioxidants 9, no. 6: 491. https://doi.org/10.3390/antiox9060491
APA StyleDong, X., Wu, D., Zhang, Y., Jia, L., Pan, X., Sun, J., & Pan, L.-L. (2020). Cathelicidin Modulates Vascular Smooth Muscle Cell Phenotypic Switching through ROS/IL-6 Pathway. Antioxidants, 9(6), 491. https://doi.org/10.3390/antiox9060491