1. Introduction
The role of selenium, the biological trace element and the related selenium-containing proteins (selenoproteins) has been described in numerous antioxidant processes [
1]. Selenocysteine, known as the twenty-first amino acid, is the only selenium-containing building block of proteins that contains selenium. The majority of selenoproteins are enzymes (such as glutathione peroxidase [
2], iodothyronine deiodinase [
3], thioredoxin reductase [
4]), most of which are involved in redox reactions. Their selenocysteine residue is the essential unit for their catalytic activity.
A new, specific way to quantify redox properties at the submolecular level has recently been introduced to characterize thiols of biological importance (cysteine, cystamine, homocysteine and glutathione [
5,
6]; ovothiol A and penicillamine [
5,
7]). A major conclusion of these studies is that there is a close correlation between the thiolate basicities and the thiolate-disulfide redox properties [
5,
6,
7,
8,
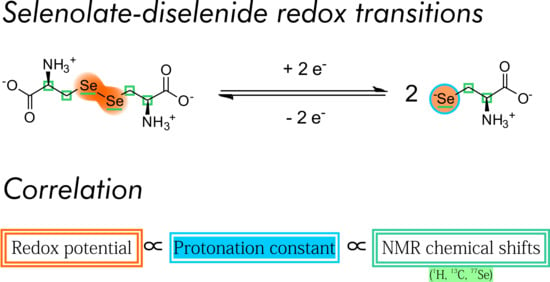
9]. The hypothesis of this work is that such a correlation between acid-base and redox characteristics exists for the selenium-analogues of the above compounds as well, namely for selenocysteine and selenocysteamine. We wish to demonstrate said correlation by determining the species-specific physico-chemical parameters of the acid-base and redox equilibria of the above compounds and extend the correlation to nuclear magnetic resonance (NMR) chemical shift values as well.
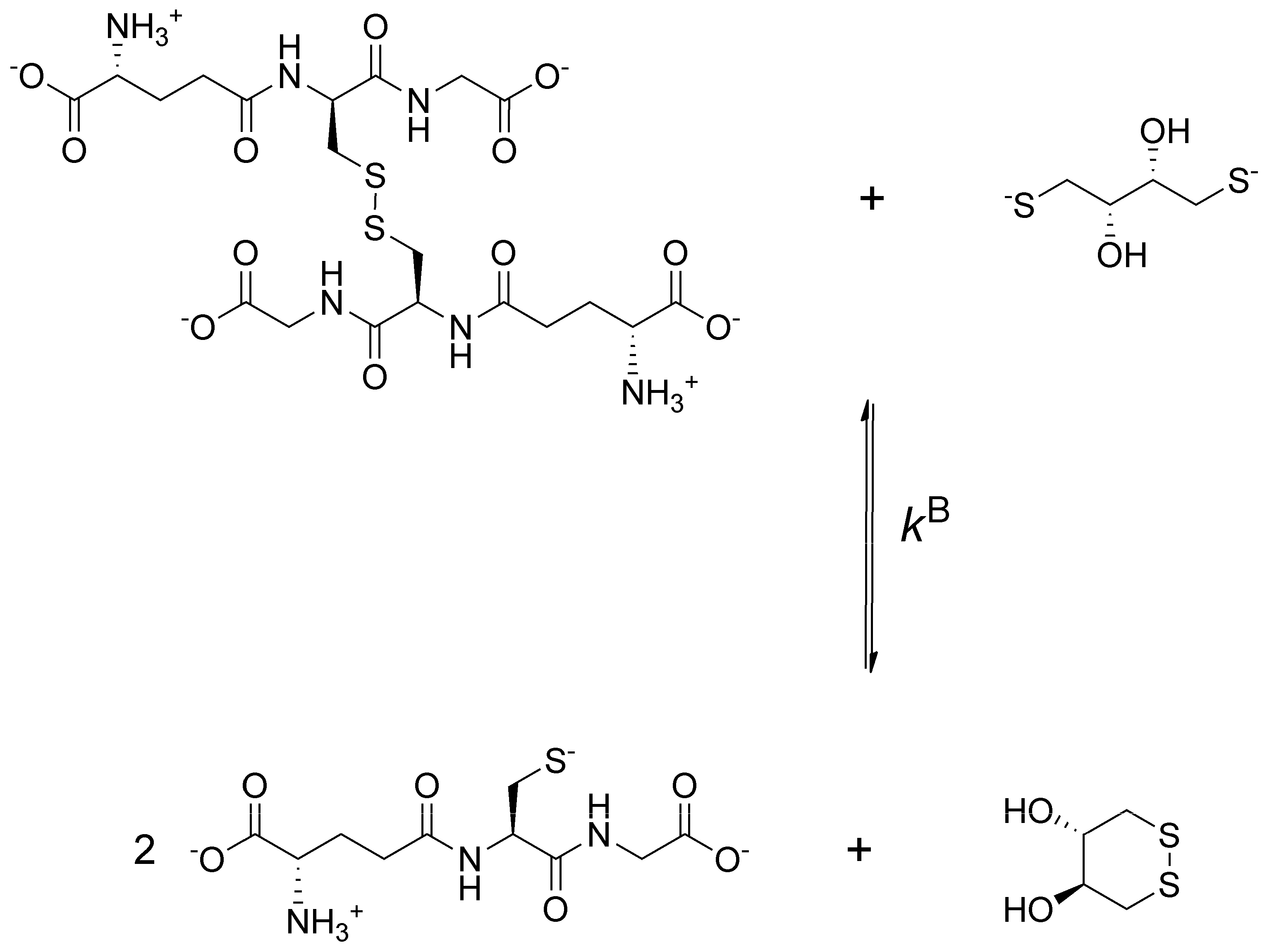
In this work, the species-specific redox equilibria of two biogenic selenols: selenocysteamine (CysASeH) and selenocysteine (CysSeH) with dithiothreitol (DTT) were studied. The oxidation of these compounds yields selenocystamine (CysASeSeCysA) and selenocystine (CysSeSeCys), respectively. The constitutional formulae of the studied selenium-containing compounds are depicted in
Figure 1.
Glutathione is known as a “gold standard” in thiol-disulfide biochemistry, hence, every thiol-containing antioxidant is compared to glutathione. Moreover, the species-specific physico-chemical acid-base [
9] and redox [
5,
6] parameters of glutathione are well-characterized. However, previous works examining selenoproteins or selenium-containing peptides found that the diselenide bridge cannot be reduced by glutathione [
10,
11]. We found that even a large excess of glutathione was ineffective in reducing the studied diselenide compounds, confirming the above observation. Thus, a more powerful reducing agent was needed to investigate selenolate-diselenide redox equilibria. Based on literature recommendations [
8,
12], dithiothreitol (DTT), a stronger reducing agent, was chosen. Dithiothreitol undergoes a one-step two-electron redox reaction, resulting in the formation of oxidized dithiothreitol (DTT
ox) with an intramolecular disulfide in a six-membered ring. During the redox processes between dithiothreitol and other agents, the presence of the intermediate compound, where only one of the thiolate groups of DTT
2− is oxidized is negligible [
13]. The main physico-chemical properties of DTT are already described (log
K1 = 10.1; log
K2 = 9.2 [
14];
E° = −0.323 V at pH = 7.0 [
15]).
To obtain data describing selenolate-diselenide redox equilibria comparable to those of thiolate-disulfide systems, first the glutathione-DTT
2− redox reaction was investigated (
Figure 2).
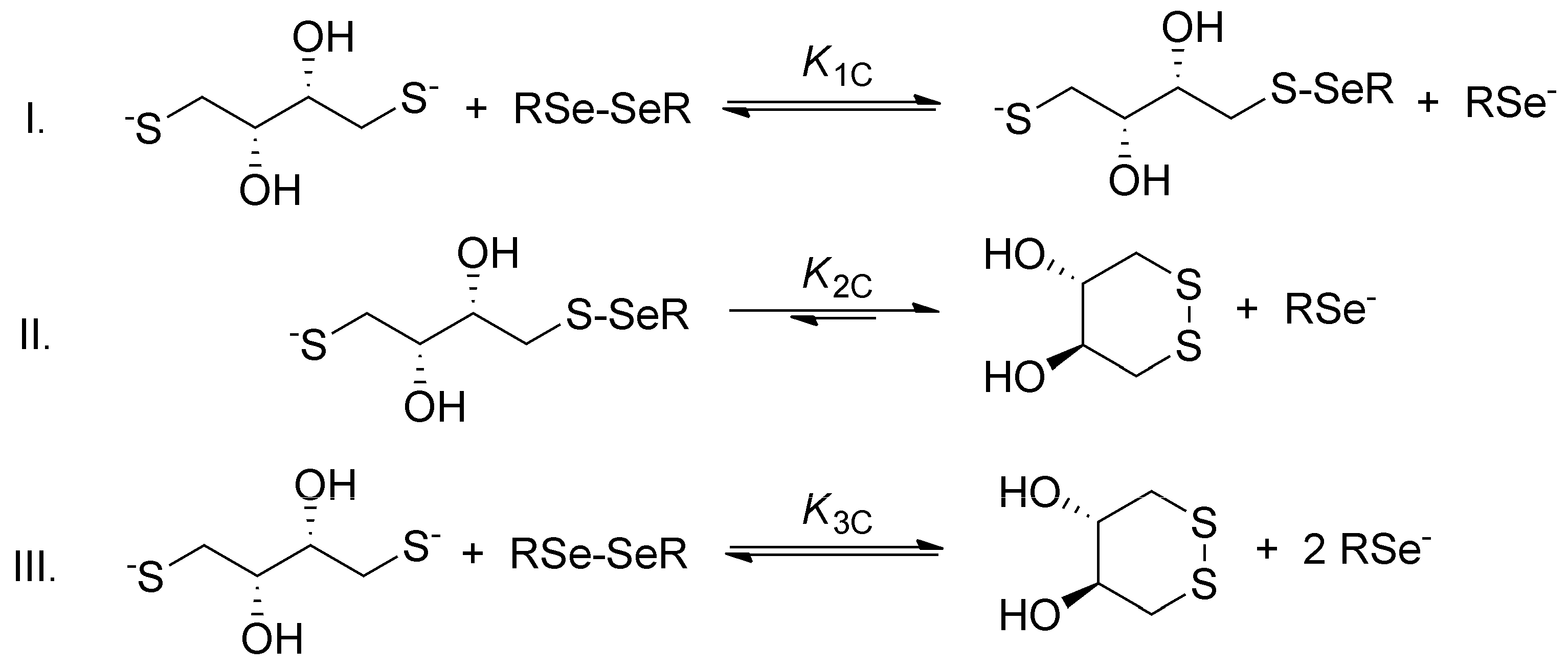
The general step-by-step scheme of selenolate-diselenide redox equilibria with DTT
2− is depicted in
Figure 3, where RSe
− and RSeSeR stand for the reduced and oxidized form of the selenium-containing compounds, respectively. The reaction between disulfides (e.g., oxidized glutathione) and DTT
2− is analogous (where RS
− and RSSR stand for the thiolate and disulfide, respectively).
Since DTT
2− is highly driven towards the ring-closing second oxidation step [
13], and diselenides are more stable than the selenylsulfides [
16], the reaction between diselenides and DTT
2− can be reasonably discussed in terms of the net reaction. The conditional equilibrium constant describing this net reaction is as follows:
Since redox and acid-base reactions coexist, the observed apparent redox equilibrium constants are pH-dependent. In order to get a clear insight into the redox equilibria, purified from the protonation effects, an improved evaluation method has been introduced [
5,
6]. This new method makes it possible to determine species-specific redox equilibrium constants (
kx) and the species-specific, i.e., the standard redox potential (
E°
x, where x is the general sign of the appropriate RSe
− microspecies in
Figure 4 and
Figure 5). This determination method requires all the species-specific protonation constants, including those of the minor microspecies; otherwise, redox processes could only be characterized at the level of phenomenon. The complete set of the microscopic protonation constants of the studied selenium-containing compounds has recently been established [
17]. The protonation constants of selenocysteine, selenocysteamine, and glutathione, relevant to this work, are collected in
Table 1 and used for further calculations.
Here, we report the comprehensive redox characterization of the selenolate-diselenide equilibria of selenocysteine and selenocysteamine in terms of pH-independent standard redox potentials (298 K, 95/5 v/v% H2O/D2O and 0.15 mol/L ionic strength). The correlation between the acid-base and redox parameters with NMR chemical shifts is also determined.
3. Results
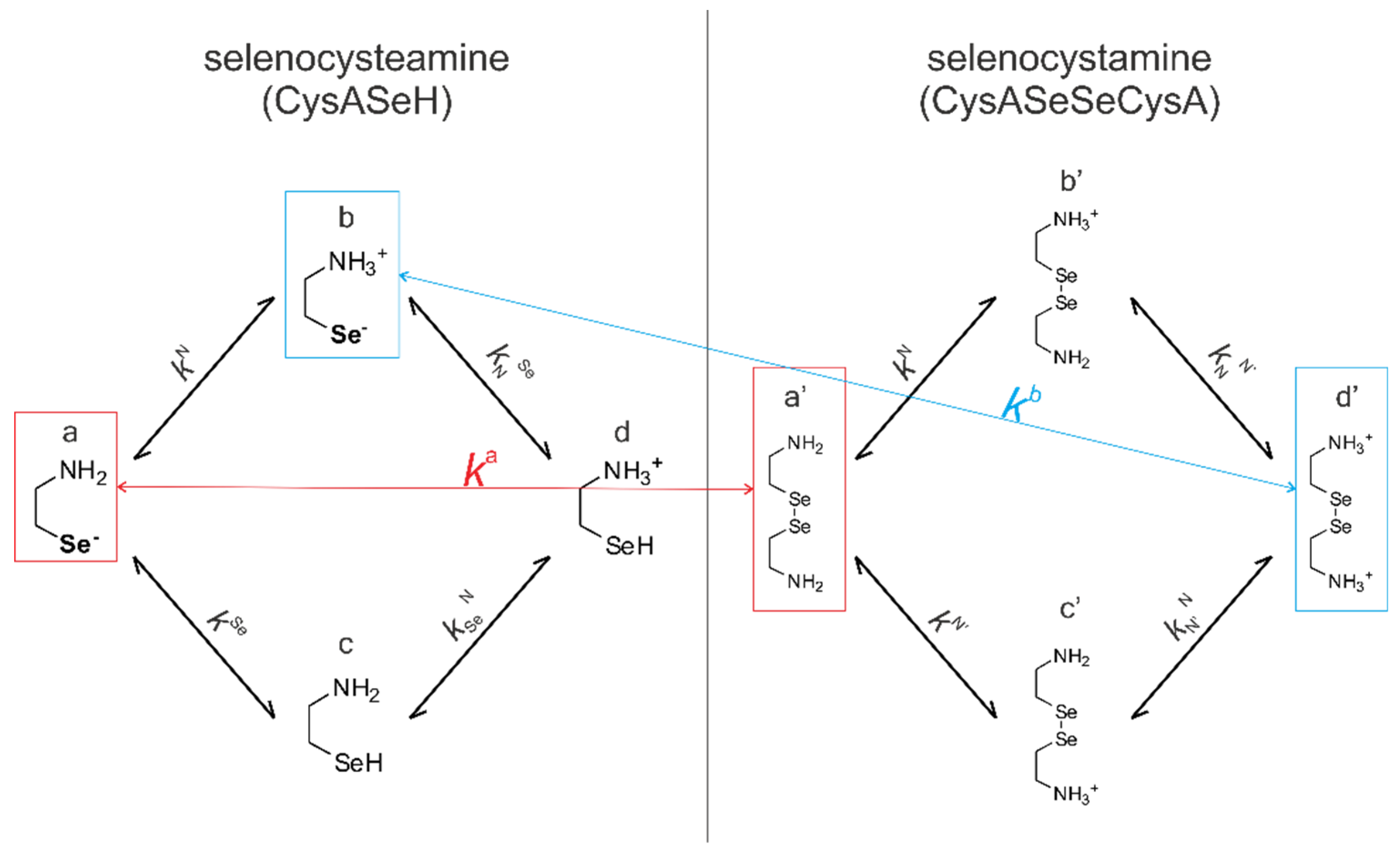
Figure 4 shows the species-specific protonation equilibria of selenocysteamine and selenocystamine, along with the selenolate → diselenide oxidation processes. Microspecies that take part in the redox processes are framed.
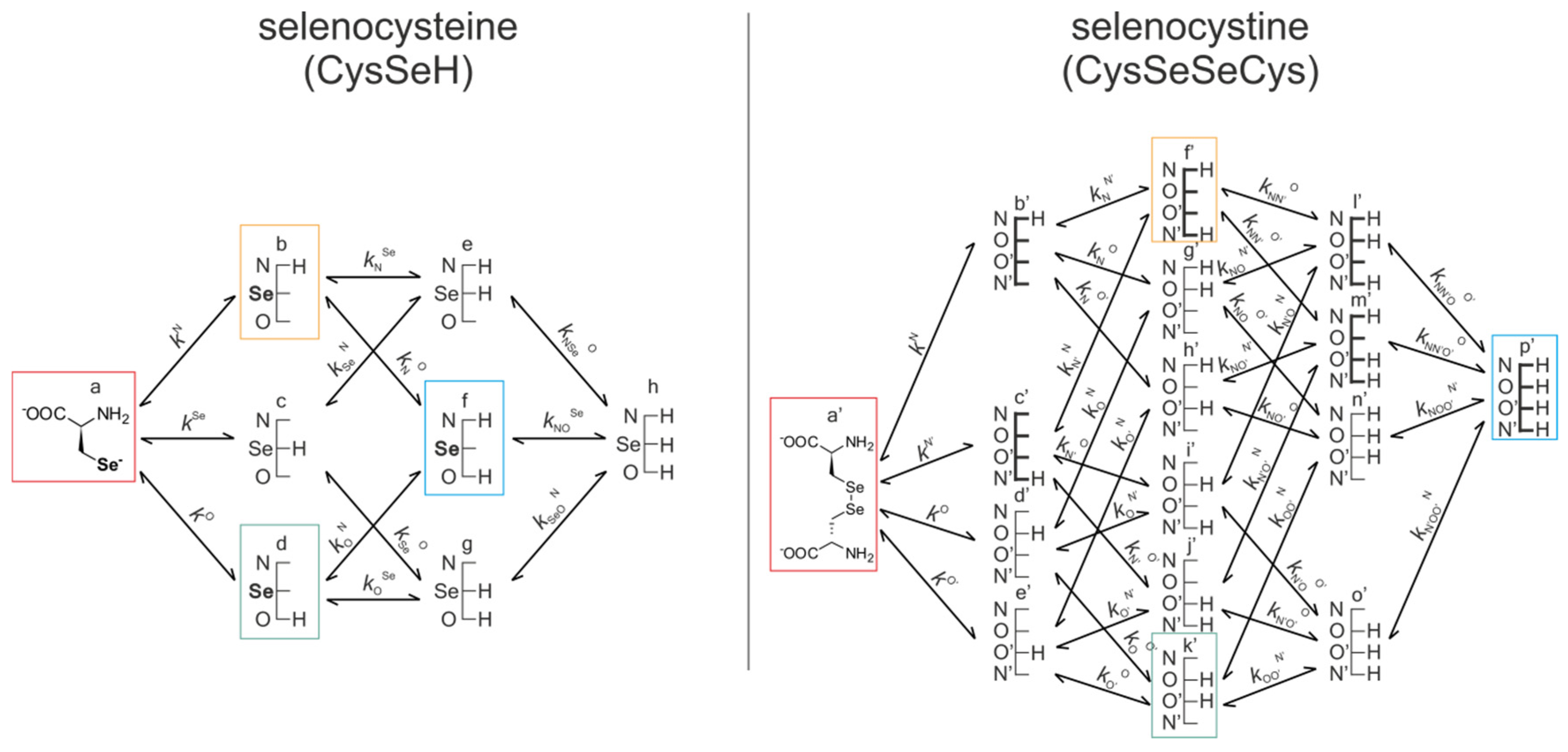
Figure 5 represents the species-specific acid-base equilibria of selenocysteine and selenocystine. The corresponding microspecies in the redox pairs have identical status of the amino and carboxylate sites.
Acid-base equilibria are often described with macroscopic protonation constants, which only reveal the stoichiometry of the successively protonated ligands. Microscopic protonation schemes used in this work, however, characterize the site of protonation as well. The protonation microspecies are discerned with their one-letter symbols (a, b, c, etc.) and the microscopic protonation constants are depicted using kN, kSe, kO, etc. The superscript of microscopic protonation constants k indicates the protonating group, while the subscript (if any) shows the site(s) already protonated. Se, N, O denote the selenolate, amino, and carboxylate sites, respectively.
The acid-base microequilibria are indispensable constituents in the evaluation of the species-specific, pH-independent redox equilibrium constants, as shown in Equations (7) and (8). Some protonation constant examples for selenocysteine are shown below:
where
K1,
K2,
K3 are successive macroconstants,
β3 is one of the cumulative macroconstants, and
kN is the microconstant representing the amino protonation in CysSeH, when its carboxylate and selenolate sites are unprotonated. The concentrations of the various macrospecies comprise the sum of the concentration of those microspecies that contain the same number of protons, for example, in CysSeSeCys:
For the selenolate-diselenide (and dithiolate-disulfide for dithiothreitol) redox equilibria, only the apparent (pH-dependent) or conditional equilibrium constants (
K3C) are directly available, by determining the equilibrium concentration of the compounds (RSeH, DTT, RSeSeR, and DTT
ox.) which correspond to the total concentration of variously protonated reactant species. The pH-dependent, apparent redox equilibrium constants can be decomposed into pH-independent, species-specific equilibrium constants, the number of which is large, but definite. For example, the selenolate moiety in CysSeH is adjacent to two other basic sites: the amino and the carboxylate. Therefore, the number of selenolate-bearing microspecies is four, corresponding to the number of protonation states of the amino and carboxylate moieties (amino-carboxylate, ammonium-carboxylate, amino-carboxyl. ammonium-carboxyl). Accordingly, the selenolate exists in four different electrostatic environments, having thus four different oxidizabilities. Considering the opposite direction of the redox equilibrium, dithiothreitol has only one microspecies, bearing both thiols in their deprotonated, oxidizable form. Therefore, the redox equilibria between selenocysteine and dithiothreitol can be characterized with four different microscopic, species-specific equilibrium constants. The method for determining the microscopic redox equilibrium constants is analogous for every case. The determination of one of the microscopic redox equilibrium constants,
kb will be demonstrated. The notation
kb refers to the reaction involving the ”b” microspecies of CysSeH and oxidized dithiothreitol on the products side, and the corresponding ”f′“ microspecies of CysSeSeCys and the deprotonated dithiothreitol on the reactants side. Note that ”b” defines that the diselenide microspecies is mandatorily ”f′”, since ”f′“ is the only diselenide microspecies with protonation states in the side-chain identical with ”b”. Superscript ”b” therefore unambiguously identifies all four microspecies in the microequilibrium in question. The example of this pH-independent, microscopic selenolate-diselenide equilibrium constant is in Equation (6):
For the next step of the calculation, the relative abundance of the protonation microspecies is needed. The mole fraction of microspecies ”b” relative to the total CysSeH concentration can be written with the following equation, as can be carried out for every microspecies provided the complete set of protonation microconstants is known.
By writing analogous equations as Equation (7) for the remaining microspecies in the formula of
kb, one can write the equation of the microscopic equilibrium constant expressed with the conditional equilibrium constant as follows:
Thus, if
K3C and the
χ values are known, the species-specific redox microconstants can be calculated. The conditional redox equilibrium constant values determined directly from the NMR spectra are listed in
Table 2 and
Supplementary Materials. These conditional redox equilibrium constants were determined in basic media only (above pH 9) due to the fact that the redox reaction did not commence at lower pH values. Only the negatively charged thiolate in DTT
2− participates in redox reactions, and below pH 9 the thiolate groups are overwhelmingly in protonated form. However, as the conditional redox equilibrium constants provide pH-independent redox microconstants, it is apparent from Equation (8) that the pH range of determination does not impede the calculation. It should be noted however, that the pH-independent redox microconstants pertaining to microspecies that are predominantly present at pH values outside the determined range (i.e.,
kf) will have a greater inherent uncertainty based on the present calculations.
The microscopic redox equilibrium constants were calculated and derived from the conditional equilibrium constants using analogous equations to Equation (8) based on at least three repeated measurements. The mean values of the calculated microscopic redox equilibrium constants and their standard deviation of determination are listed in
Table 3. Note that near physiological pH (i.e., around pH 7) the most abundant CysSe and CysASe selenolate microspecies are ”b” and ”b”, respectively. In fact, these microspecies have maximal relative abundance between pH 6.5 and 10, therefore the pH range of determination in our study is well-conditioned for the extraction of the physiologically relevant redox microconstants.
In order to obtain the standard redox potential values of the studied selenol compounds, the standard redox potential of dithiothreitol is needed. Although dithiothreitol has been extensively studied, only its apparent redox potential has been previously determined, therefore we determined the pH-independent, species-specific standard redox potential of DTT
ox./DTT
2− as well using glutathione as the reaction partner. In the state of a chemical equilibrium, the electrode potential of every existing redox system is equal. For example, for the reaction of dithiothreitol and glutathione one can write:
where the A’/A, E’/B symbols denote the microspecies of glutathione disulfide/glutathione redox pairs. We chose to perform further calculations with equations pertaining to glutathione/glutathione disulfide microspecies B/E’, since this redox pair has the highest relative abundance in the pH of interest, and therefore its calculation is least exposed to uncertainty. The above equation adapted for glutathione microspecies ”B”
Figure 6 and DTT
2− becomes:
where
E° is the standard redox potential of the redox system denoted in its subscript,
R is the universal gas constant,
T is the absolute temperature,
z is the number of electrons transferred in the redox half-reaction, and
F is the Faraday constant. Using the previously determined microscopic protonation constant [
9] and standard redox potential [
5] of glutathione microspecies ”B”, the species-specific standard redox potential was determined for the DTT
ox./DTT
2− redox system as −0.403 ± 0.007 V.
With the knowledge of the selenolate-diselenide species-specific redox equilibrium constants and the standard redox potential of the DTT
ox./DTT
2− redox couple, the species-specific standard redox potentials of the different selenolate-containing microspecies could be calculated. Equation (12) shows the continued example of CysSeH microspecies ”b”. The comprehensive set of determined species-specific standard redox potentials is listed in
Table 4.
By graphing these standard redox potentials against the concomitant selenolate-specific protonation constants compiled in
Table 1 we find a similar correlation to that of thiolates presented in
Figure 7.
4. Discussion
In this work, the highly interwoven acid-base and redox pathways of selenolate-diselenide systems were decomposed into species-specific, component equilibria. The standard redox potentials of biologically relevant selenolate-diselenide couples are determined for the first time; these values characterize the redox processes at the protonation microspecies level. The elucidation of these redox microequilibria reveals considerable differences between the various protonation species. The knowledge of species-specific standard redox potentials of selenocysteine and selenocystine, in particular, improves our knowledge on redox homeostasis and can lead to better interpretation of several biochemical phenomena. For example the cytoprotective potential of selenoproteins is thought to entail several mechanisms involving thio/seleno chemistry [
23]. One proposed mechanism of action is formation of stable diselenide bonds in thioredoxin reductase acting as a ”diselenide trap”, which can be further supported by exact standard redox potentials of the reaction partners. New selenoproteins are investigated for their function, likely related to catalyzing thiol/disulfide exchange in proteins, as the kinetics of selenolate-diselenide transitions is seven orders of magnitude greater than that of thiolate-disulfide transitions [
24]. In order to understand these catalytic effects purified from the protonation fraction of the thiolate and selenolate moieties at physiological pH, a comprehensive species-specific characterization is needed. The knowledge of detailed physico-chemical properties of selenocompounds presented in this work can also serve as the basis to develop artificial selenoenzymes [
25] of highly selective redox capacities.
The example of selenocystine microspecies ”a′” and ”f′” (
Table 4) shows that even side chain protonation changes can lead to significantly different redox characteristics, with nearly 160 mV difference in standard redox potential values. Therefore, small changes in pH can not only affect the redox processes of selenolate-diselenide transitions by changing the protonation fraction of the selenolate, but also by altering the protonation state of neighboring moieties.
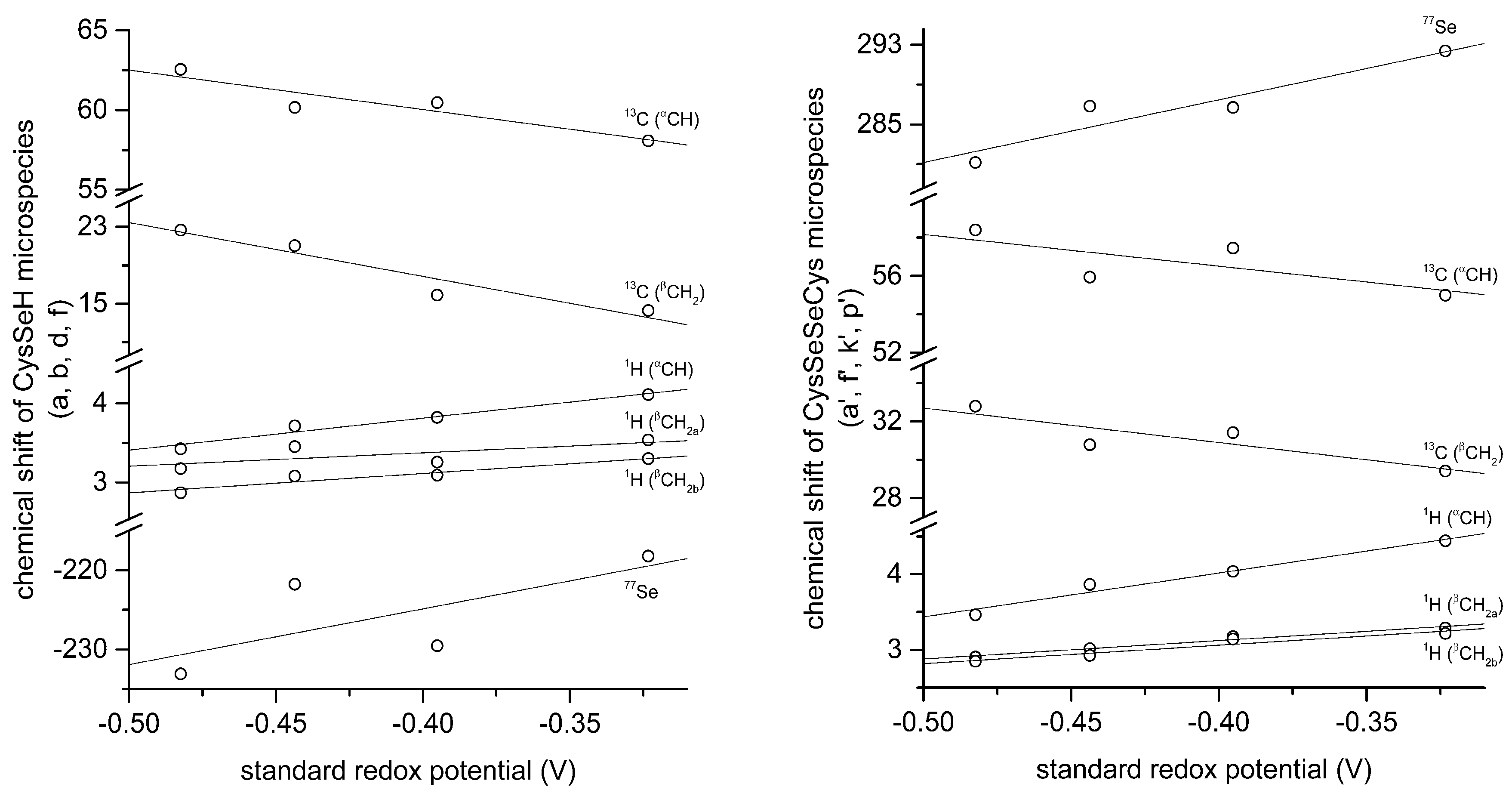
The correlation between selenolate basicity and standard redox potentials verifies the previous observations regarding thiolate basicity and its proportionality with thiolate oxidizability. It is interesting that the correlation line between selenolate basicity and the concomitant standard redox potential is parallel to the correlation line of thiolates, however shifted by ca. −246 mV units. This accentuates the fact that selenolates, apart from being less basic, are vastly stronger reducing agents than thiolates in general. It is noteworthy that the species-specific standard redox potentials of selenolate-containing microspecies also show linear correlation with species-specific NMR chemical shift. The chemical shift values were previously determined [
16], and the details of the correlation are shown in
Figure 8 and
Table 5. The use of chemical shifts in protein NMR (from
1H,
13C, and for selenoproteins the relatively undisrupted
77Se spectra) can now serve as sound means to predict selenolate oxidizability or diselenide reducibilty/stability in proteins: a key parameter to understand and influence oxidative stress.
The critical issue in designing preventive or therapeutic antioxidants is the narrow path of redox potentials, effective enough to reduce harmful oxidative agents, but keeps disulfides and other reducible units in useful biomolecules intact. Naturally, small reducing agents can hardly be as selective as substrate-specific enzymes of the biological antioxidant system; however, a finely tuned and designed selenolate-containing compound with an appropriate basicity, redox potential and concomitant selectivity can be confined to a narrower range. The correlation between the redox and NMR parameters serves now as a sound basis to better quantify the characteristics of diselenide moieties in selenoenzymes, allowing thus the development of potent, selective antioxidant compounds for serious ailments related to selenoenzyme deficiencies, such as autism [
26].