Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedure
2.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4. Computational Methods
2.4.1. Density Functional Theory Calculations
2.4.2. Molecular Dynamics Simulation
2.5. Preparation of Large Unilamellar Vesicles (LUVs)
2.6. Peroxidation of LUVs
2.7. Liposomal/Buffer Partitioning
2.8. Cell Treatment
2.9. Cell Viability (MTT Assay)
2.10. Statistical Analyses
3. Results and Discussion
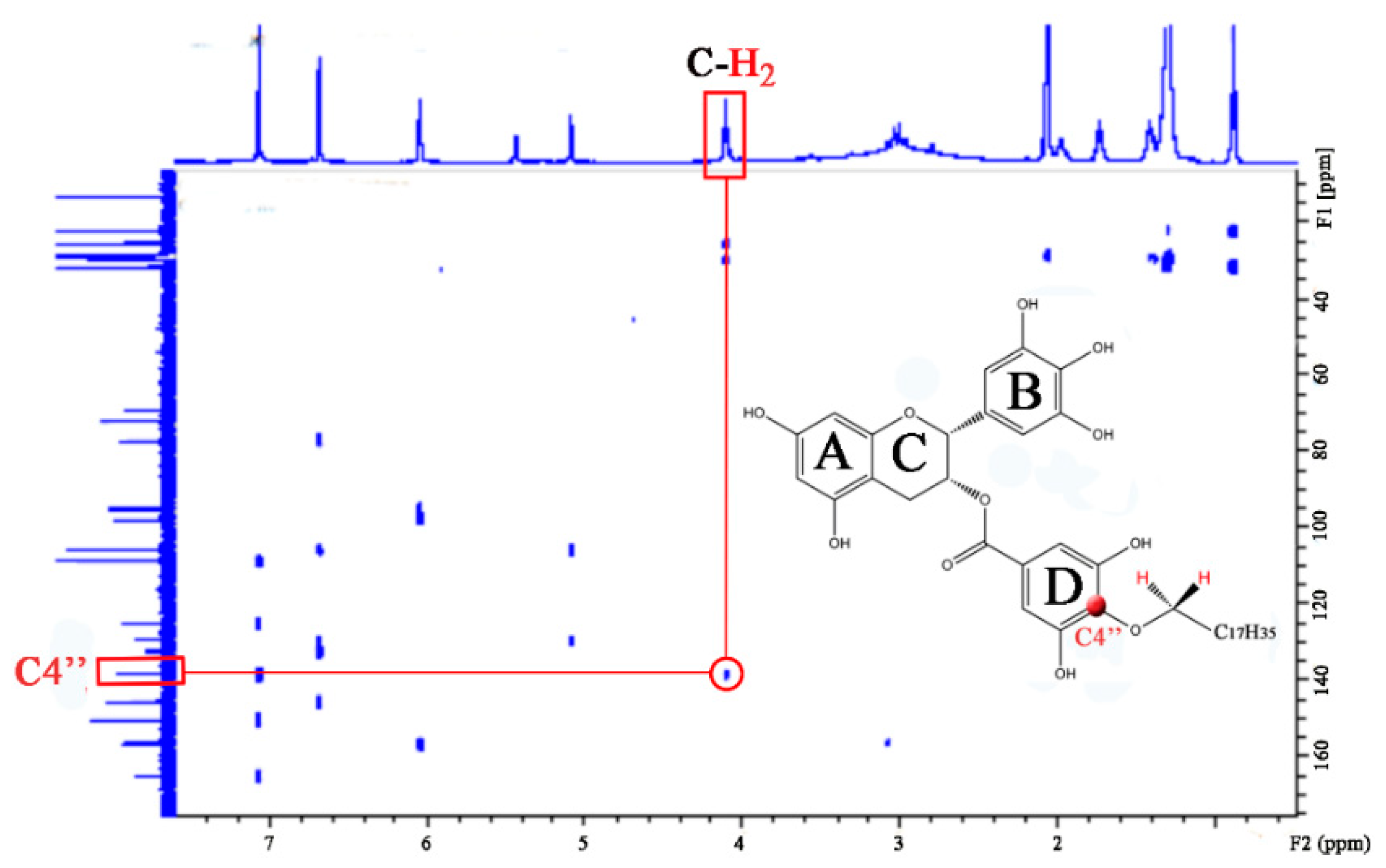
3.1. Synthesis and Structural Elucidation of C18-EGCG
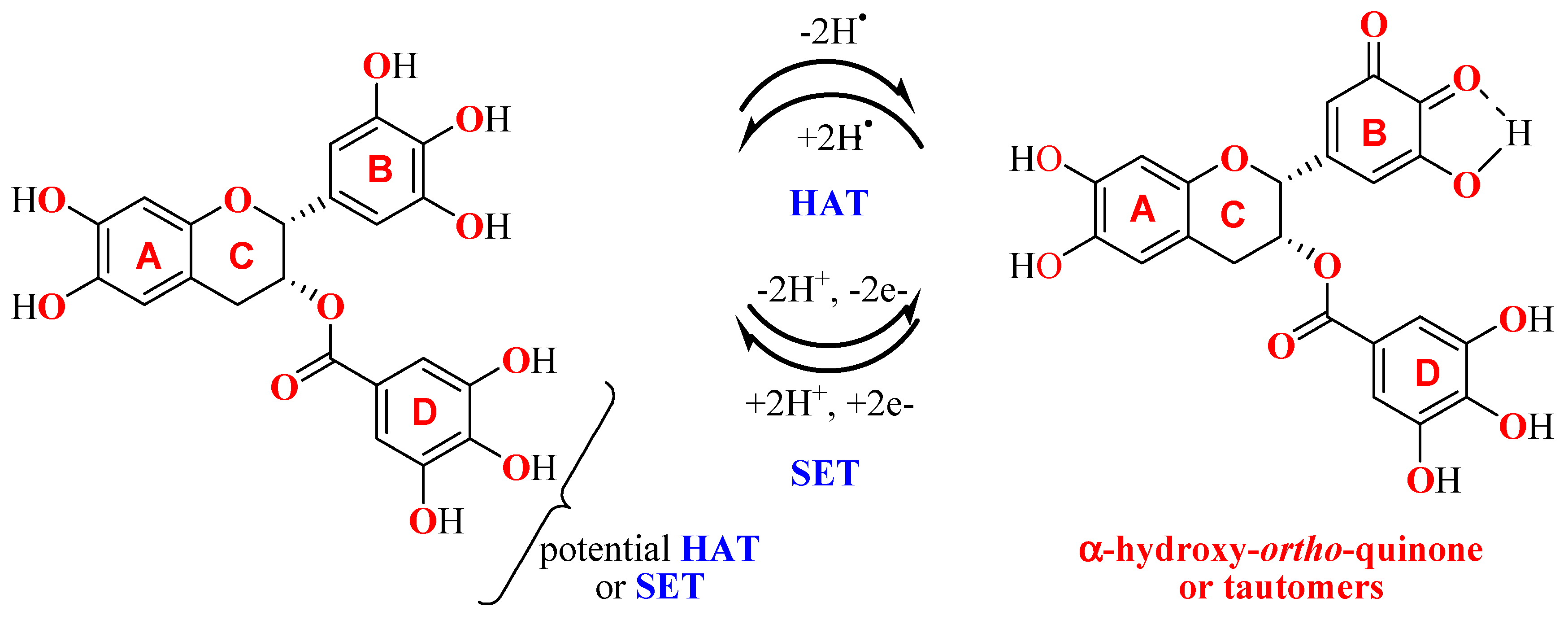
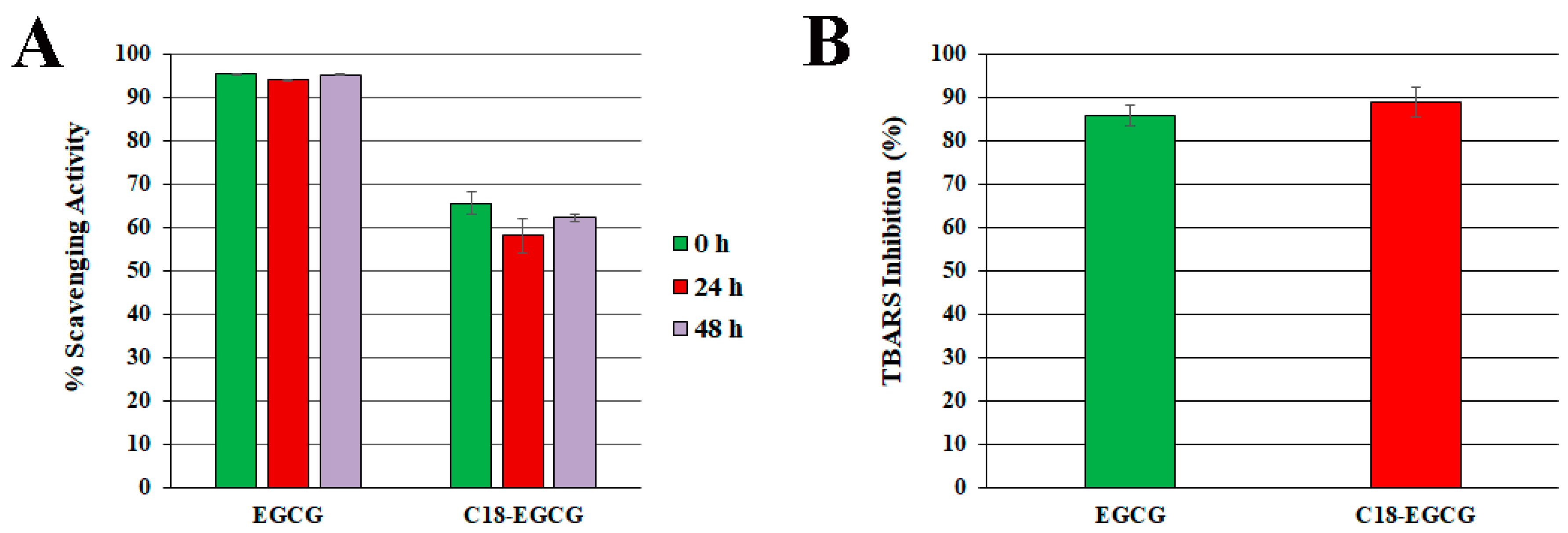
3.2. Reactions with DPPH Free Radical
3.3. Protection against Free Radical-Mediated Lipid Peroxidation
3.4. Studies on Interaction of EGCG and C18-EGCG with Liposome Cell Membrane Models
Affinity of EGCG and C18-EGCG for Lipid Bilayers
3.5. Structural Insight of C18-EGCG inside Lipid Bilayer: In Silico Studies
3.6. C18-EGCG Protective Efficacy against Oxidative Stress Induced Cell Death
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandel, S.A.; Amit, T.; Weinreb, O.; Youdim, M.B. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J. Alzheimers Dis. 2011, 25, 187–208. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Li, H.Y.; Lee, C.J.; Wen, Y.C.; Chen, S.J.; Huang, K.F. EGCG, a major polyphenol in green tea, protects human retinal pigment epithelium (ARPE-19) cells from viable blue light-induced disorders. Life Sci. J. 2014, 11, 424–429. [Google Scholar]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Mandel, S.; Amit, T.; Bar-Am, O.; Youdim, M.B. Iron dysregulation in Alzheimer’s disease: Multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog. Neurobiol. 2007, 82, 348–360. [Google Scholar] [CrossRef]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef]
- Hügel, H.M.; Jackson, N. Redox chemistry of green tea polyphenols: Therapeutic benefits in neurodegenerative diseases. Mini Rev. Med. Chem. 2012, 12, 380–387. [Google Scholar] [CrossRef]
- Damiani, E.; Astolfi, P.; Carloni, P.; Stipa, P.; Greci, L. Antioxidants: How they work. In Oxidants in Biology: A Question of Balance; Valacchi, G., Davis, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- Lambert, J.D.; Yang, C.S. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. 2003, 523–524, 201–208. [Google Scholar] [CrossRef]
- Dvorakova, K.; Dorr, R.T.; Valcic, S.; Timmermann, B.; Alberts, D.S. Pharmacokinetics of the green tea derivatives, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother. Pharmacol. 1999, 43, 331–335. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, L.; Yu, J.; Wang, Y.; Peng, B. Anti-osteoclastogenic effect of epigallocatechin gallate-functionalized gold nanoparticles in vitro and in vivo. Int. J. Nanomed. 2019, 14, 5017–5032. [Google Scholar] [CrossRef]
- Laudadio, E.; Minnelli, C.; Amici, A.; Massaccesi, L.; Mobbili, G.; Galeazzi, R. Liposomal formulations for an efficient encapsulation of epigallocatechin-3-gallate: An in-silico/experimental approach. Molecules 2018, 23, 441. [Google Scholar] [CrossRef]
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Rusciano, D.; Armeni, T.; Stipa, P.; Cantarini, M.; Mobbili, G. Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone. Antioxidants (Basel) 2019, 8, 258. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef]
- Astolfi, P.; Carloni, P.; Marini, M.G.; Mobbili, G.; Pisani, M.; Stipa, P. Benzoxazinic nitrones and nitroxides as possible antioxidants in biological systems. RSC Adv. 2013, 3, 22023–22030. [Google Scholar] [CrossRef]
- Laudadio, E.; Galeazzi, R.; Mobbili, G.; Minnelli, C.; Barbon, A.; Bortolus, M.; Stipa, P. Depth Distribution of Spin-Labeled Liponitroxides within Lipid Bilayers: A Combined EPR and Molecular Dynamics Approach. ACS Omega 2019, 4, 5029–5037. [Google Scholar] [CrossRef]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Galeazzi, R.; Pisani, M.; Crucianelli, E.; Bizzaro, D.; Armeni, T.; Mobbili, G. Selective induction of apoptosis in MCF7 cancer-cell by targeted liposomes functionalised with mannose-6-phosphate. J. Drug Target. 2018, 26, 242–251. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision A08; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, C.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Zavala-Oseguera, C.; Alvarez-Idaboy, J.R.; Merino, G.; Galano, A. Rate coefficient and mechanism of the gas phase OH hydrogen abstraction reaction from formic acid: A quantum mechanical approach. J. Phys. Chem. A 2009, 113, 13913–13920. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Macías-Ruvalcaba, N.A.; Campos, O.N.M.; Pedraza-Chaverri, J. Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: A combined theoretical and experimental study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef]
- Galano, A.; Francisco-Marquez, M.; Alvarez-Idaboy, J.R. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Phys. Chem. Chem. Phys. 2011, 13, 11199–11205. [Google Scholar] [CrossRef]
- Singleton, W.S.; Gray, M.S.; Brown, M.L.; White, J.L. Chromatographically homogeneous lecithin from egg phospholipids. J. Am. Oil Chem. Soc. 1965, 42, 53. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Dickson, C.J.; Madej, B.D.; Skjevik, A.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., 3rd; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; McGraw-Hill: New York, NY, USA; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Galeazzi, R.; Laudadio, E.; Massaccesi, L. Recent advances in Computational Simulations of Lipid Bilayer Based Molecular Systems. In Frontiers in Computational Chemistry; Ul-Haq, Z., Madura, J.D., Eds.; Bentham Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 326–388. [Google Scholar] [CrossRef]
- Eastwood, J.W.; Hockney, R.W.; Lawrence, D.N. P3M3DP The three-dimensional periodic particle-particle/ particle-mesh program. Comput. Phys. Commun. 1980, 19, 215–261. [Google Scholar] [CrossRef]
- Galeazzi, R.; Bruni, P.; Crucianelli, E.; Laudadio, E.; Marini, M.; Massaccesi, L.; Mobbili, G.; Pisani, M. Liposome-based gene delivery systems containing a steroid derivative: Computational and small angle X-ray diffraction study. RSC Adv. 2015, 5, 54070–54078. [Google Scholar] [CrossRef]
- Porasso, R.D.; Cascales, J.J.L. A criterion to identify the equilibration time in lipid bilayer simulations. Pap. Phys. 2012, 4, 040005. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cipollone, M.; Galli, M.C.; Pugnaloni, A.; Biagini, G.; Landi, L. Characterization of large unilamellar vesicles as models for studies of lipid peroxidation initiated by azocompounds. Free Radic. Res. 1994, 21, 329–339. [Google Scholar] [CrossRef]
- Liang, J.; Cao, L.; Zhang, L.; Wan, X.C. Preparation, characterization, and in vitro antitumor activity of folate conjugated chitosan coated EGCG nanoparticles. Food Sci. Biotechnol. 2014, 23, 569–575. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kida, K.; Suzuki, M.; Matsumoto, N.; Nanjo, F.; Hara, Y. Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J. Agric. Food Chem. 2000, 48, 4151–4155. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Ono, K.; Hashimoto, K. Affinity of antioxidative polyphenols for lipid bilayers evaluated with a liposome system. Biosci. Biotechnol. Biochem. 1998, 62, 1005–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys J. 2007, 92, 3178–3194. [Google Scholar] [CrossRef]
- Sun, Y.; Hung, W.C.; Chen, F.Y.; Lee, C.C.; Huang, H.W. Interaction of tea catechin (-)-epigallocatechin gallate with lipid bilayers. Biophys J. 2009, 96, 1026–1035. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Ito, H.; Chevyreva, V.; Makky, A.; Kaufmann, S.; Okano, K.; Kobayashi, N.; Suganuma, M.; Nakabayashi, S.; Yoshikawa, H.Y.; et al. Adsorption of galloyl catechin aggregates significantly modulates membrane mechanics in the absence of biochemical cues. Phys Chem Chem Phys. 2017, 19, 19937–19947. [Google Scholar] [CrossRef]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun. 1994, 204, 898–904. [Google Scholar] [CrossRef]
- Sang, S.; Yang, I.; Buckley, B.; Ho, C.T.; Yang, C.S. Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (−)-epigallocatechin-3-gallate: Studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radic. Biol. Med. 2007, 43, 362–371. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Fernández-Angulo, D.; Gil-Salido, A.A.; Lara-Espinoza, C.L.; Rubio-Pino, J.L. Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line. BMC Complement. Altern. Med. 2015, 15, 254. [Google Scholar] [CrossRef]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (−)-epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Simona Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]




| EGCG | C18-EGCG | |
|---|---|---|
| H-2 | 5.07 s | 5.07 s |
| H-3 | 5.56 m | 5.42 s |
| H-4a | 2.91 dd (2.2, 17.4) | 2.97 dd (2.4, 13.9) |
| H-4b | 3.04 dd (4.6, 17.4) | 3.03 dd (3.9, 13.9) |
| H-6 | 6.06 d (2.6) | 6.03 m |
| H-8 | 6.03 d (2.6) | 6.03 m |
| H-2′ | 6.64 s | 6.67 s |
| H-6′ | 6.64 s | 6.67 s |
| H-2″ | 7.03 s | 7.05 s |
| H-6″ | 7.03 s | 7.05 s |
| OCH2 | - | 4.09 t (5.5) |
| EGCG | C18-EGCG | |
|---|---|---|
| C-2 | 78.11 | 78.27 |
| C-3 | 69.45 | 70.23 |
| C-4 | 26.65 | 26.64 |
| C-5 | 157.54 | 157.53 |
| C-6 | 96.50 | 96.52 |
| C-7 | 157.80 | 157.91 |
| C-8 | 95.79 | 95.92 |
| C-9 | 157.08 | 157.13 |
| C-10 | 98.98 | 98.91 |
| C-1′ | 130.71 | 130.53 |
| C-2′ | 106.76 | 106.80 |
| C-3′ | 145.96 | 146.61 |
| C-4′ | 133.14 | 133.45 |
| C-5′ | 145.96 | 146.62 |
| C-6′ | 106.76 | 106.80 |
| C-1″ | 121.78 | 126.21 |
| C-2″ | 109.97 | 109.90 |
| C-3″ | 146.30 | 151.39 |
| C-4″ | 138.83 | 139.57 |
| C-5″ | 146.30 | 151.39 |
| C-6″ | 109.97 | 109.90 |
| CO | 166.17 | 166.31 |
| OCH2 | - | 73.33 |
| Ring | Oxyanion Position | ΔE (kJ/mol) |
|---|---|---|
| D | 4O | 0.00 |
| D | 3O | 2.70 |
| D | 5O | 12.06 |
| B | 4O | 4.64 |
| B | 3O | 10.45 |
| B | 5O | 5.56 |
| A | 5O | 6.48 |
| A | 7O | 9.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. https://doi.org/10.3390/antiox9030208
Minnelli C, Galeazzi R, Laudadio E, Amici A, Rusciano D, Armeni T, Cantarini M, Stipa P, Mobbili G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants. 2020; 9(3):208. https://doi.org/10.3390/antiox9030208
Chicago/Turabian StyleMinnelli, Cristina, Roberta Galeazzi, Emiliano Laudadio, Adolfo Amici, Dario Rusciano, Tatiana Armeni, Mattia Cantarini, Pierluigi Stipa, and Giovanna Mobbili. 2020. "Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation" Antioxidants 9, no. 3: 208. https://doi.org/10.3390/antiox9030208
APA StyleMinnelli, C., Galeazzi, R., Laudadio, E., Amici, A., Rusciano, D., Armeni, T., Cantarini, M., Stipa, P., & Mobbili, G. (2020). Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants, 9(3), 208. https://doi.org/10.3390/antiox9030208













