Transcriptomic Analysis Reveals Cu/Zn SODs Acting as Hub Genes of SODs in Hylocereus undatus Induced by Trypsin during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Materials
2.2. H. undatus Treatment
2.3. Library Construction and Illumina RNA-Sequencing
2.4. Difference of Gene Expression
2.5. GO and KEGG Enrichment Analyses
2.6. Gene Expression Analysis by Reverse Transcription-qPCR
2.7. Protein-Protein Interaction (PPI) Analyse
2.7.1. PPI Network Generation
2.7.2. Network Topological Parameters
2.7.3. Module
2.7.4. Analysis of Hub Nodes in the PPI Network
2.8. Weight Loss Rate
2.9. Quantification of Lipid Peroxides
2.10. Determination of ROS of H. undatus Peel
2.11. Statistical Analyses
3. Results
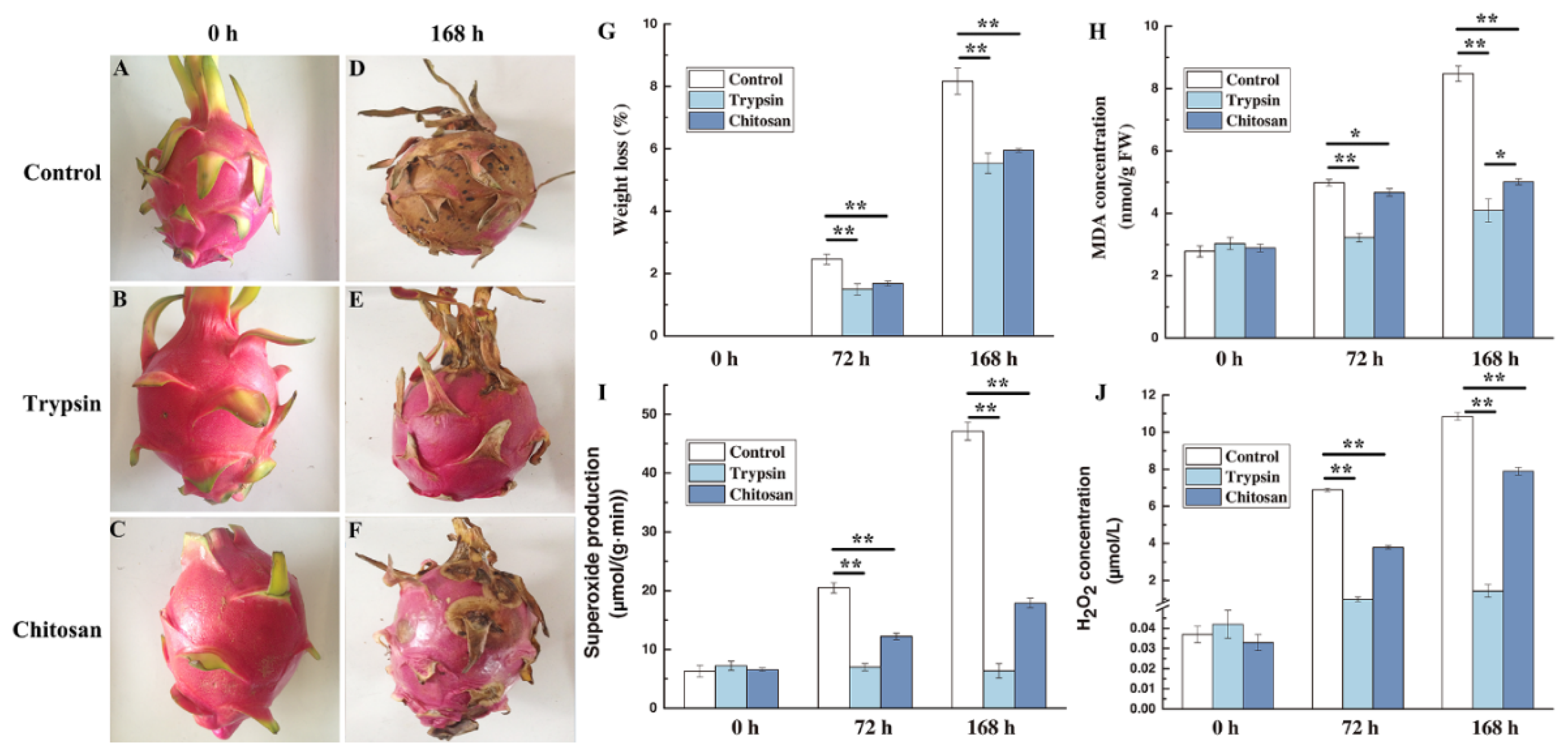
3.1. Effect on Fruit Quality of H. undatus
3.2. Impact on the Cell Injury
3.3. Impact on the ROS of H. undatus
3.4. Transcriptomic Analyses
3.4.1. Differentially Expressed Genes
3.4.2. Functional Annotation and Analyses
3.4.3. GO Enrichment Analyses
3.4.4. KEGG Enrichment Analyses
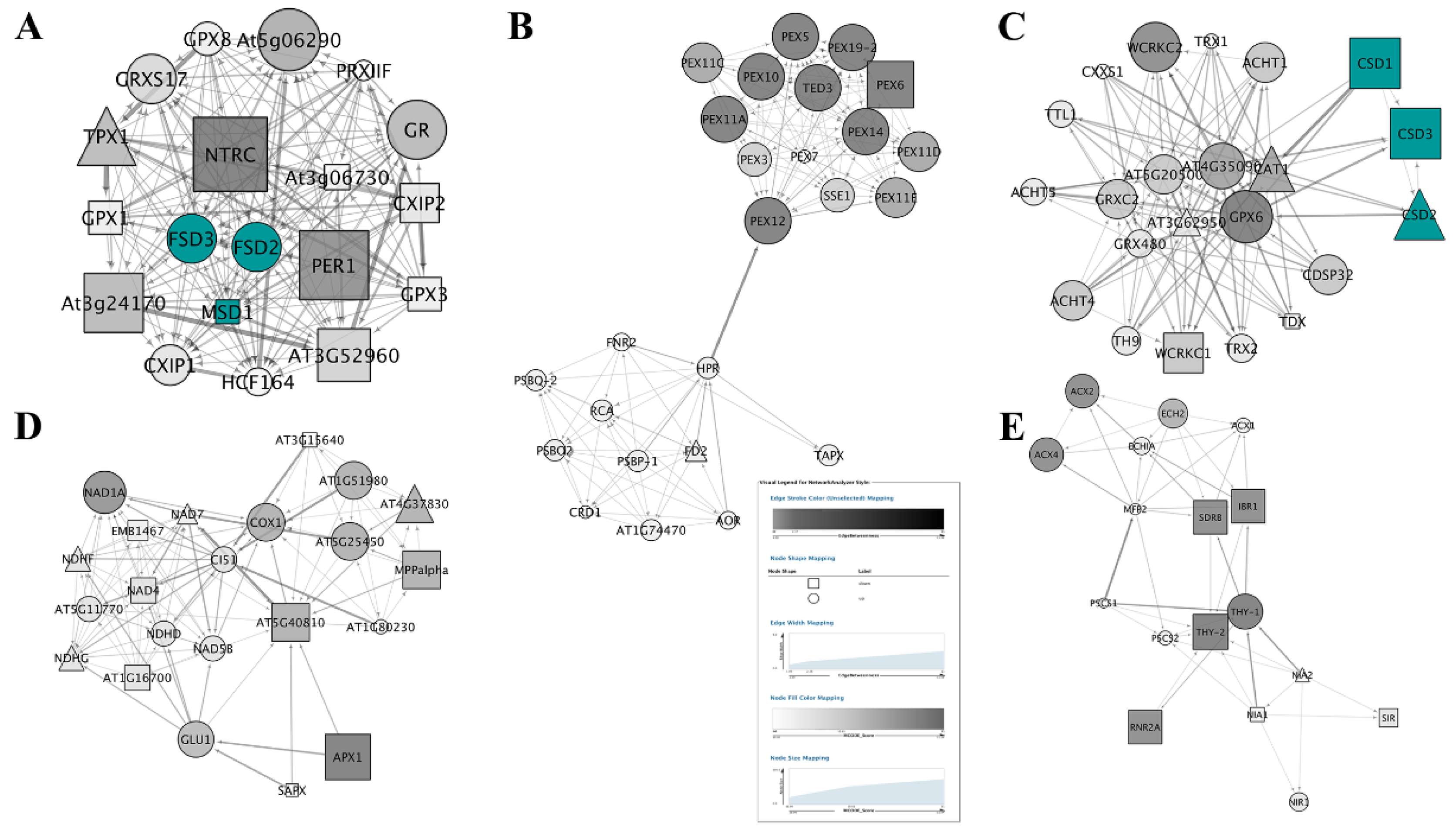
3.5. PPI Network Analysis
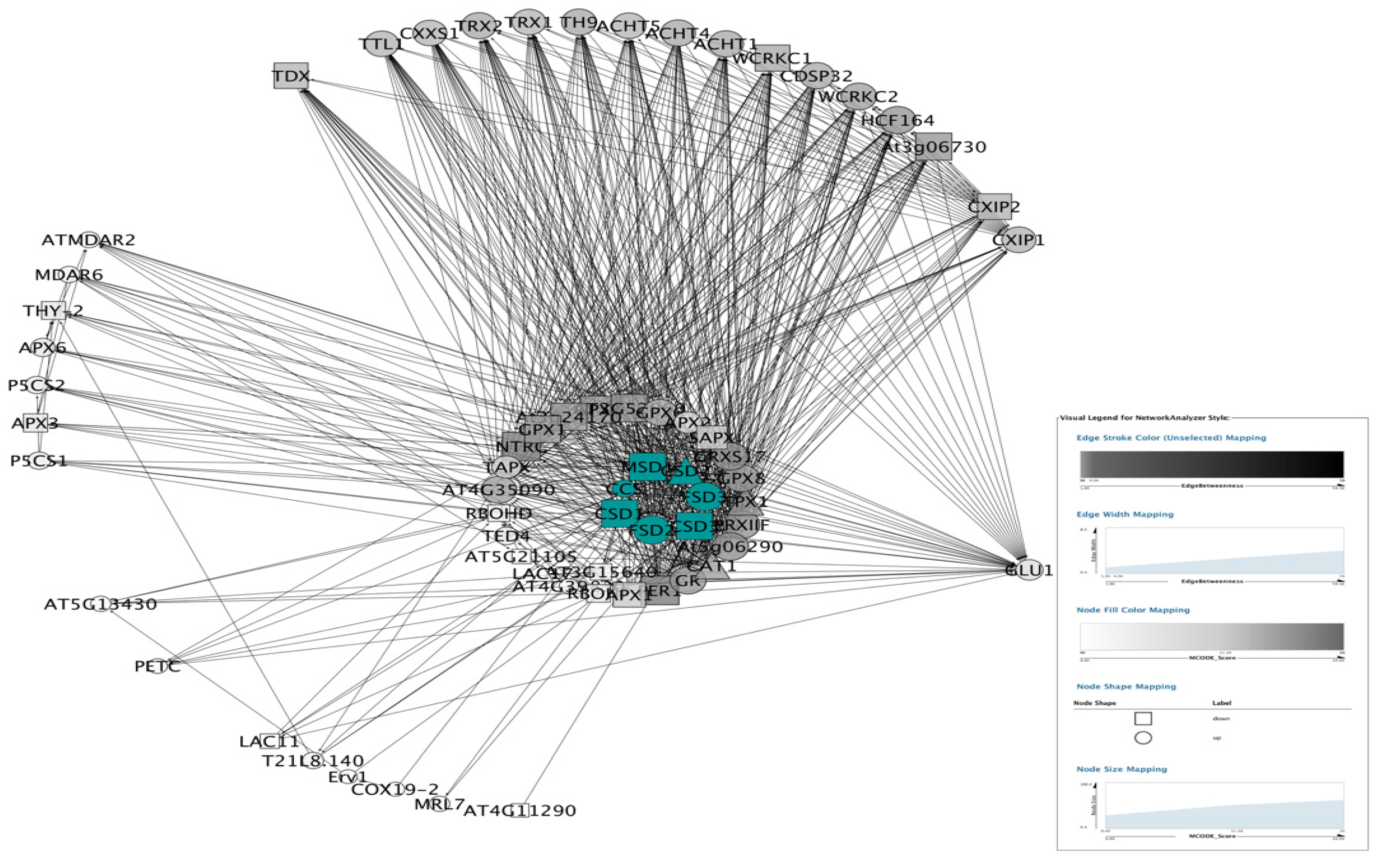
3.5.1. PPI Networks of SODs
3.5.2. Topological Properties of Networks.
3.6. Accuracy of the RNA-Seq Data Verification by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
References
- Fan, Q.J.; Yan, F.X.; Qiao, G.; Zhang, B.X.; Wen, X.P. Identification of differentially-expressed genes potentially implicated in drought response in pitaya (Hylocereus undatus) by suppression subtractive hybridization and cDNA microarray analysis. Gene 2014, 533, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.Z.; Chen, C.J.; Chen, Z.; Chen, P.K.; Ma, Y.W.; Wu, J.Y.; Zheng, J.; Hu, G.B.; Zhao, J.T.; Qin, Y.H. Transcriptomic analysis reveals key genes related to betalain biosynthesis in pulp coloration of Hylocereus polyrhizus. Front. Plant Sci. 2016, 6, 1179. [Google Scholar] [CrossRef]
- Freitas, S.T.D.; Mitcham, E.J. Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Sci. Agric. 2013, 70, 257–262. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhong, X.M.; Fan, Y.; Wang, H.C.; Li, J.G.; Huang, X.M. Burst of reactive oxygen species in pedicel-mediated fruit abscission after carbohydrate supply was cut off in longan (Dimocarpus longan). Front. Plant Sci. 2015, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sethu, R.; Imlay, J.A. Endogenous superoxide is a key effector of the oxygen sensitivity of a model obligate anaerobe. Proc. Natl. Acad. Sci. USA 2018, 115, E3266–E3275. [Google Scholar] [CrossRef]
- Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, Y.X.; Lin, H.T.; Zhang, S.; Chen, Y.H.; Shi, J. Inhibitory effects of propyl gallate on browning and its relationship to active oxygen metabolism in pericarp of harvested longan fruit. LWT Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Q.Y.; Yuan, E.D.; Yang, J.G.; Ning, Z.X. Interaction between four flavonoids and trypsin: Effect on the characteristics of trypsin and antioxidant activity of flavonoids. Int. J. Food Sci. Technol. 2014, 49, 1063–1069. [Google Scholar] [CrossRef]
- Li, X.; Tang, Z.C.; Zhao, C.Y.; Pang, X.Y.; Li, X.L.; Liu, Y.H. Trypsin slows the ageing of mice due to its novel superoxide scavenging activity. Appl. Biochem. Biotech. 2017, 181, 1–12. [Google Scholar] [CrossRef]
- Xia, C.J.; Li, S.F.; Hou, W.Y.; Fan, Z.F.; Xiao, H.; Lu, M.G.; Sano, T.; Zhang, Z.X. Global transcriptomic changes induced by infection of cucumber (Cucumis sativus L.) with mild and severe variants of hop stunt viroid. Front. Microbiol. 2017, 8, 2427. [Google Scholar] [CrossRef]
- Yu, X.J.; Chen, H.; Huang, C.Y.; Zhu, X.Y.; Wang, D.S.; Liu, X.Y.; Sun, J.; Zheng, J.Y.; Li, H.J.; Wang, Z.; et al. Transcriptomic mechanism of a phytohormone 6-benzylaminopurine (6-BAP) stimulating lipid and DHA synthesis in Aurantiochytrium sp. J. Agric. Food Chem. 2019, 67, 19. [Google Scholar] [CrossRef]
- Xu, M.; Liu, C.L.; Luo, J.; Qi, Z.; Yan, Z.; Fu, Y.; Wei, S.S.; Tang, H. Transcriptomic de novo analysis of pitaya (Hylocereus polyrhizus) canker disease caused by Neoscytalidium dimidiatum. BMC Genomics 2019, 20, 10. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Ding, B.; Fei, Z.J. Comprehensive transcriptome analyses reveal that potato spindle tuber viroid triggers genome-wide changes in alternative splicing, inducible trans-acting activity of phasiRNAs and immune responses. J. Virol. 2017, 91, e00247-17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.R.; Yin, Y.; Yu, H.C.; Zhang, M.; Jing, H.N.; Ma, Y.C.; Xiong, X.L.; Pang, X.Y. Transcriptomic analysis reveals key genes related to antioxidant mechanisms of Hylocereus undatus quality improving by trypsin during storage. Food Funct. 2019, 10, 8116–8128. [Google Scholar] [CrossRef] [PubMed]
- Azodi, M.Z.; Peyvandi, H.; Rostami-Nejad, M.; Safaei, A.; Rostami, K.; Vafaee, R.; Heidari, M.; Hosseini, M.; Zali, M.R. Protein-protein interaction network of celiac disease. Gastroenterol. Hepatol. Bed Bench 2016, 9, 268–277. [Google Scholar]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Zhang, H.Y.; He, P.; Kang, H.B.; Li, X.L. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT Food Sci. Technol. 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Hu, Y.S.; Pan, Z.H.; Hu, Y.; Zhang, L.; Wang, J. Network and Pathway-Based Analyses of Genes Associated with Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 4452–4465. [Google Scholar] [CrossRef]
- Liu, Y.H.; Sun, Y.; Yu, H.C.; Yin, Y.; Li, X.; Duan, X. Hot air drying of purple-fleshed sweet potato with contact ultrasound assistance. Dry. Technol. 2017, 35, 564–576. [Google Scholar] [CrossRef]
- Candar-Cakir, B.; Arican, E.; Zhang, B.H. Small RNA and degradome deep sequencing reveals drought-and tissue-specific micrornas and their important roles in drought-sensitive and drought-tolerant tomato genotypes. Plant Biotechnol. J. 2016, 14, 1727–1746. [Google Scholar] [CrossRef]
- Yang, A.M.; Yu, L.; Chen, Z.; Zhang, S.X.; Shi, J.; Zhao, X.Z.; Yang, Y.Y.; Hu, D.Y.; Song, B.A. Label-free quantitative proteomic analysis of chitosan oligosaccharide-treated rice infected with southern rice black-streaked dwarf virus. Viruses 2017, 9, 115. [Google Scholar] [CrossRef]
- Wu, B.L.; Xie, J.J.; Du, Z.P.; Wu, J.Y.; Zhang, P.X.; Xu, L.Y.; Li, E.M. PPI network analysis of mRNA expression profile of Ezrin knockdown in esophageal squamous cell carcinoma. BioMed Res. Int. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.L.; Li, C.Q.; Zhang, P.X.; Yao, Q.L.; Wu, J.Y.; Han, J.W.; Liao, L.D.; Xu, Y.J.; Lin, R.J.; Xiao, D.W.; et al. Dissection of miRNA-miRNA interaction in esophageal squamous cell carcinoma. PLoS ONE 2013, 8, e73191. [Google Scholar] [CrossRef]
- Vig, S.; Talwar, P.; Kaur, K.; Srivastava, R.; Srivastava, A.K.; Datta, M. Transcriptome profiling identifies p53 as a key player during calreticulin deficiency: Implications in lipid accumulation. Cell Cycle 2015, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, Z.H.; Zhu, B.A.; Xu, C.S. Identification of Key Pathways and Genes in the Dynamic Progression of HCC Based on WGCNA. Genes 2018, 9, 92. [Google Scholar] [CrossRef]
- Huang, H.J.; Luo, B.B.; Wang, B.Q.; Wu, Q.W.; Liang, Y.M.; He, Y. Identification of potential gene interactions in heart failure caused by idiopathic dilated cardiomyopathy. Med. Sci. Monit. 2018, 24, 7697–7709. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.C.; Wei, B.D.; Liu, X.Y.; Ji, S.J. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Schneider, K.; Schlegel, H.G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem. J. 1981, 193, 99–107. [Google Scholar] [CrossRef]
- Li, X.; Imlay, J.A. Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic. Biol. Med. 2018, 120, 217–222. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, Y.F.; Bi, Y. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Duan, X.W.; Liu, T.; Zhang, D.D.; Su, X.G.; Lin, H.T.; Jiang, Y.M. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Xi, H.H.; Liu, Y.H.; Guo, L.G.; Hu, R.R. Effect of ultrasonic power on drying process and quality properties of far-infrared radiation drying on potato slices. Food Sci. Biotechnol. 2020, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 2016, 253, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the response to temperature changes in ripe fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef]
- Verma, D.; Lakhanpal, N.; Singh, K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genomics 2019, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, Y.L.; Zhao, C.Y. Trypsin binding with copper ions scavenges superoxide: Molecular dynamics-based mechanism investigation. Int. J. Environ. Res. Public Health 2018, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Dong, J.F.; Qiao, H.L.; Wu, J.X. Molecular characterization, expression patterns and binding properties of two pheromone-binding proteins from the oriental fruit moth, Grapholita molesta (Busck). J. Integr. Agric. 2014, 13, 2709–2720. [Google Scholar] [CrossRef]
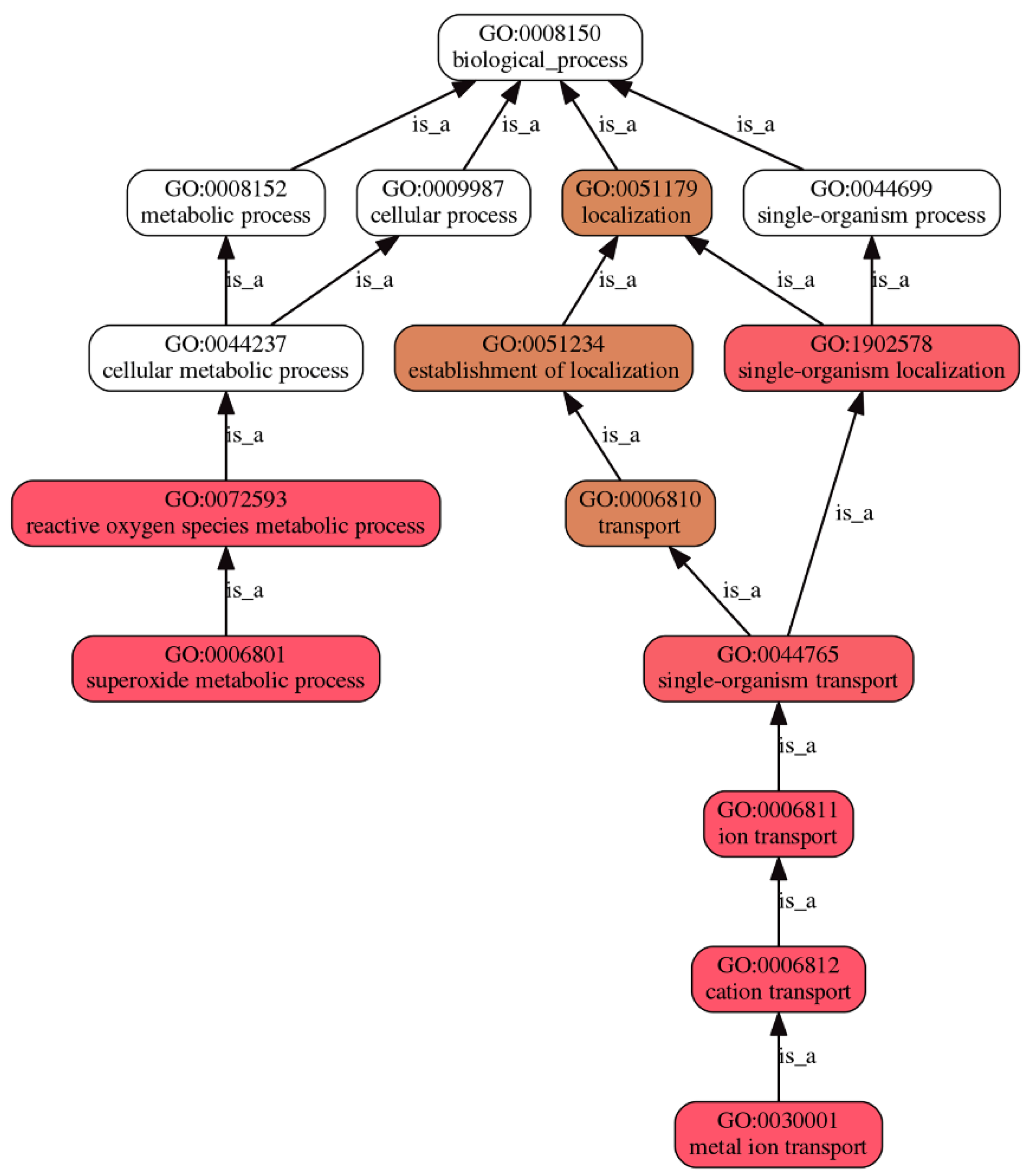



| Pattern | Num | GO ID | Term Type | Description | p-Value * | FDR a |
|---|---|---|---|---|---|---|
| Up-regulation | 1 | GO:0006801 | BP | superoxide metabolic process | 0.002383 | 1 |
| 1 | GO:0030001 | BP | metal ion transport | 0.009766 | 1 | |
| 1 | GO:0004784 | MF | superoxide dismutase activity | 0.001004 | 1 | |
| 1 | GO:0016721 | MF | oxidoreductase activity, acting on superoxide radicals as acceptor | 0.001004 | 1 | |
| Down-regulation | 3 | GO:0004784 | MF | superoxide dismutase activity | 3.3214 × 10−10 | 9.1654 × 10−7 |
| 3 | GO:0016721 | MF | oxidoreductase activity, acting on superoxide radicals as acceptor | 3.3214 × 10−10 | 9.1654 × 10−7 | |
| 3 | GO:0016209 | MF | antioxidant activity | 1.2739 × 10−6 | 0.002344 | |
| 4 | GO:0016491 | MF | oxidoreductase activity | 2.9523 × 10−5 | 0.04073 | |
| 1 | GO:0071493 | BP | cellular response to UV-B | 0.0002510 | 0.1732 | |
| 1 | GO:0071457 | BP | cellular response to ozone | 0.0002510 | 0.1732 |
| Pattern | Num | KO ID | Term | p-Value * | FDR a |
|---|---|---|---|---|---|
| Up-regulation | 2 | map04146 | Peroxisome | 0.00068 | 0.0020 |
| 1 | map03060 | Protein export | 0.023 | 0.035 | |
| 1 | map03440 | Homologous recombination | 0.039 | 0.039 | |
| Down-regulation | 3 | map04146 | Peroxisome | 4.84 × 10−6 | 1.45 × 10−5 |
| 1 | map03008 | Ribosome biogenesis in eukaryotes | 0.058 | 0.087 | |
| 1 | map03013 | RNA transport | 0.097 | 0.097 |
| PPI Subnetwork | y = βxα | R2 | Correlation | Clustering Coefficient | Network Centralization | Network Density | Total Num. of Nodes | Num. of Up-Regulated Nodes | Num. of Down-Regulated Nodes | Characteristic Path Length |
|---|---|---|---|---|---|---|---|---|---|---|
| Total ROS | Y = 109.39x−1.036 | 0.816 | 0.873 | 0.384 | 0.254 | 0.030 | 447 | 120 | 150 | 3.285 |
| First neighbors of SODs | Y = 1.727 x 0.004 | 0.000 | −0.006 | 0.598 | 0.414 | 0.333 | 65 | 38 | 23 | 1.758 |
| 7 SODs | Y = 0.161 x 1.708 | 0.936 | 0.924 | 0.914 | 0.200 | 0.857 | 7 | 3 | 3 | 1.143 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Li, X.; Liu, X.; Cai, L.; Li, B.; Li, X. Transcriptomic Analysis Reveals Cu/Zn SODs Acting as Hub Genes of SODs in Hylocereus undatus Induced by Trypsin during Storage. Antioxidants 2020, 9, 162. https://doi.org/10.3390/antiox9020162
Pang X, Li X, Liu X, Cai L, Li B, Li X. Transcriptomic Analysis Reveals Cu/Zn SODs Acting as Hub Genes of SODs in Hylocereus undatus Induced by Trypsin during Storage. Antioxidants. 2020; 9(2):162. https://doi.org/10.3390/antiox9020162
Chicago/Turabian StylePang, Xinyue, Xinling Li, Xueru Liu, Luning Cai, Bairu Li, and Xin Li. 2020. "Transcriptomic Analysis Reveals Cu/Zn SODs Acting as Hub Genes of SODs in Hylocereus undatus Induced by Trypsin during Storage" Antioxidants 9, no. 2: 162. https://doi.org/10.3390/antiox9020162
APA StylePang, X., Li, X., Liu, X., Cai, L., Li, B., & Li, X. (2020). Transcriptomic Analysis Reveals Cu/Zn SODs Acting as Hub Genes of SODs in Hylocereus undatus Induced by Trypsin during Storage. Antioxidants, 9(2), 162. https://doi.org/10.3390/antiox9020162