Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Eligibility Criteria
2.3. Inflammatory Markers of Interest
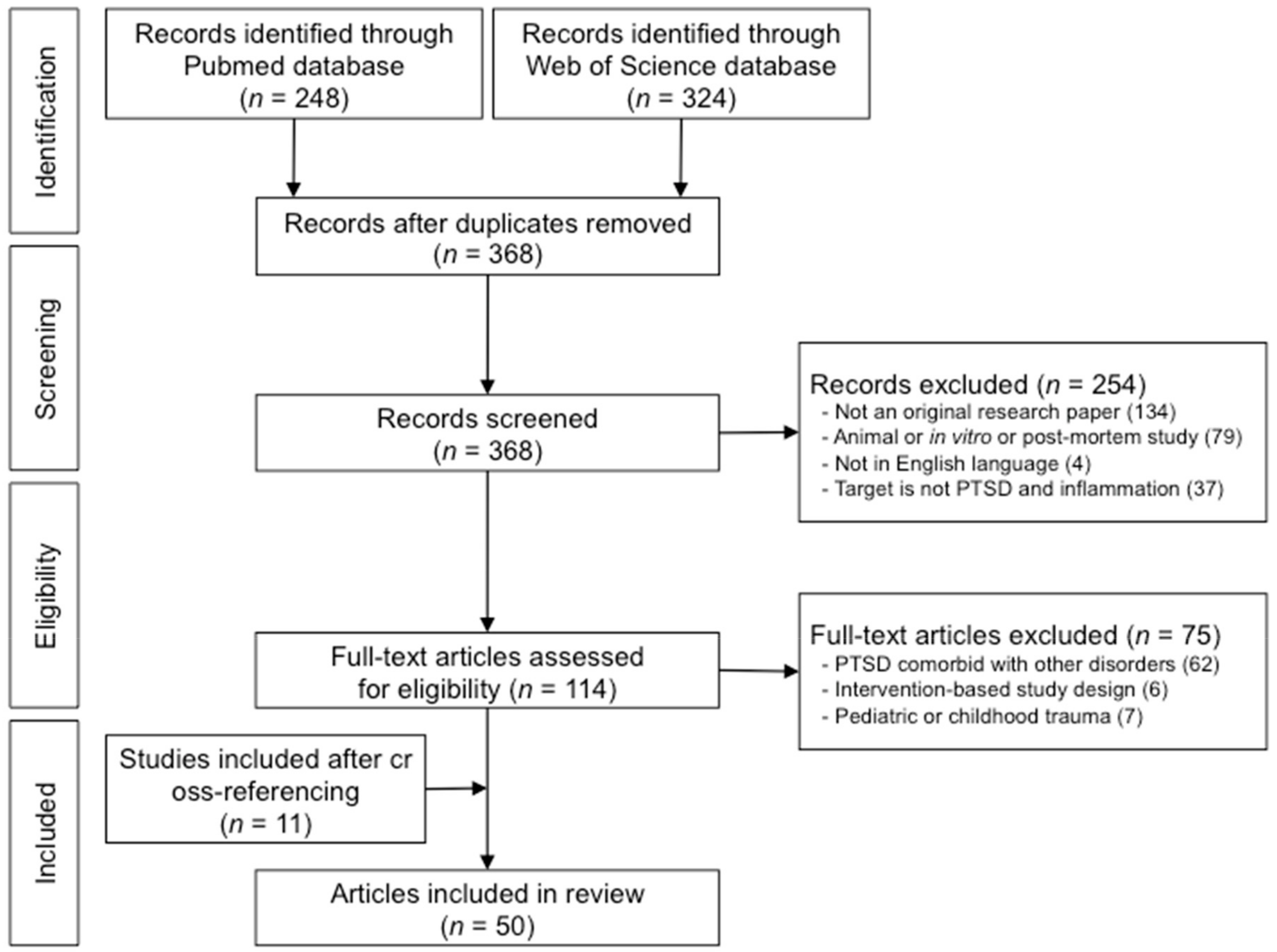
3. Results
3.1. Results from the Literature Search
3.2. Alterations of Serum Inflammatory Markers in Association with PTSD
3.2.1. Roles of Proinflammatory Cytokines in PTSD: IL-1β, IL-6, TNF-α, IFN-γ and CRP
3.2.2. Roles of Anti-Inflammatory Cytokines in PTSD: IL-4 and IL-10
3.3. Roles of PTSD- and Oxidative Stress-Related Genetic Markers in PTSD
3.4. Brain Alterations in Relation to Inflammation and Oxidative Stress in PTSD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kowalik, J.; Weller, J.; Venter, J.; Drachman, D. Cognitive behavioral therapy for the treatment of pediatric posttraumatic stress disorder: A review and meta-analysis. J. Behav. Ther. Exp. Psychiatry 2011, 42, 405–413. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; pp. 133–137. [Google Scholar]
- Sterling, M.; Hendrikz, J.; Kenardy, J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain 2011, 152, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Roley, M.E.; Claycomb, M.A.; Contractor, A.A.; Dranger, P.; Armour, C.; Elhai, J.D. The relationship between rumination, PTSD, and depression symptoms. J. Affect Disord. 2015, 180, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L.; Stappenbeck, C.A.; Varra, A.A.; Moore, S.A.; Kaysen, D. Symptoms of posttraumatic stress predict craving among alcohol treatment seekers: Results of a daily monitoring study. Psychol. Addict. Behav. 2012, 26, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Sautter, F.J.; Armelie, A.P.; Glynn, S.M.; Wielt, D.B. The development of a couple-based treatment for PTSD in returning veterans. Prof. Psychol. Res. Pr. 2011, 42, 63–69. [Google Scholar] [CrossRef]
- Bartoli, F.; Crocamo, C.; Alamia, A.; Amidani, F.; Paggi, E.; Pini, E.; Clerici, M.; Carrà, G. Posttraumatic stress disorder and risk of obesity: Systematic review and meta-analysis. J. Clin. Psychiatry 2015, 76, 1253–1261. [Google Scholar] [CrossRef]
- Vancampfort, D.; Rosenbaum, S.; Ward, P.B.; Steel, Z.; Lederman, O.; Lamwaka, A.V.; Richards, J.W.; Stubbs, B. Type 2 diabetes among people with posttraumatic stress disorder: Systematic review and meta-analysis. Psychosom. Med. 2016, 78, 465–473. [Google Scholar] [CrossRef]
- Edmondson, D.; Kronish, I.M.; Shaffer, J.A.; Falzon, L.; Burg, M.M. Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. Am. Heart J. 2013, 166, 806–814. [Google Scholar] [CrossRef]
- Kim, Y.K.; Amidfar, M.; Won, E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 103–112. [Google Scholar] [CrossRef]
- Palmer, C. A theory of risk and resilience factors in military families. Mil. Psychol. 2008, 20, 205–217. [Google Scholar] [CrossRef]
- Kim, J.E.; Dager, S.R.; Jeong, H.S.; Ma, J.; Park, S.; Kim, J.; Yera, C.; Suji, L.L.; Kang, I.; Ha, E.; et al. Firefighters, posttraumatic stress disorder, and barriers to treatment: Results from a nationwide total population survey. PLoS ONE 2018, 13, e0190630. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Beekman, A.T.F.; De Jonge, P.; Penninx, B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Wolkowitz, O.M.; Mellon, S.; Yehuda, R.; Flory, J.D.; Henn-Haase, C.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Neylan, T.C.; et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Mellon, S.H.; Dhabhar, F.S.; Yehuda, R.; Grenon, S.M.; Flory, J.D.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Makotkine, I.; et al. Increased circulating blood cell counts in combat-related PTSD: Associations with inflammation and PTSD severity. Psychiatry Res. 2017, 258, 330–336. [Google Scholar] [CrossRef]
- McFarlane, A.C.; Williamson, P.; Barton, C.A. The impact of traumatic stressors in civilian occupational settings. J. Public Health Policy 2009, 30, 311–327. [Google Scholar] [CrossRef]
- Weathers, F.W.; Bovin, M.J.; Lee, D.J.; Sloan, D.M.; Schnurr, P.P.; Kaloupek, D.G.; Keane, T.M.; Marx, B.P. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol. Assess 2018, 30, 383–395. [Google Scholar] [CrossRef]
- Ahmed-Leitao, F.; Spies, G.; van den Heuvel, L.; Seedat, S. Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Res. Neuroimaging 2016, 256, 33–43. [Google Scholar] [CrossRef]
- Karl, A.; Werner, A. The use of proton magnetic resonance spectroscopy in PTSD research—meta-analyses of findings and methodological review. Neurosci. Biobehav. Rev. 2010, 34, 7–22. [Google Scholar] [CrossRef]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef]
- Speer, K.; Upton, D.; Semple, S.; McKune, A. Systemic low-grade inflammation in post-traumatic stress disorder: A systematic review. J. Inflamm. Res. 2018, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhães, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Rohleder, N.; Aringer, M.; Boentert, M. Role of interleukin-6 in stress, sleep, and fatigue. Ann. N. Y. Acad. Sci. 2012, 1261, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Xu, H.; Cao, C.; Liu, P.; Luo, S.; Duan, Q.; Ellenbroek, B.; Zhang, X. Characteristics of pro-and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J. Affect Disord. 2019, 248, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.R.; Pfingst, K.; Carnevali, L.; Sgoifo, A.; Nalivaiko, E. In the search for integrative biomarker of resilience to psychological stress. Neurosci. Biobehav. Rev. 2017, 74, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, Y.; Katsuura, S.; Kuwano, Y.; Tanahashi, T.; Rokutan, K. Circulating cytokine signatures in healthy medical students exposed to academic examination stress. Psychophysiology 2012, 49, 991–997. [Google Scholar] [CrossRef]
- Jones, K.A.; Thomsen, C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013, 53, 52–62. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harvard Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef]
- Stein, D.J.; Benjet, C.; Gureje, O.; Lund, C.; Scott, K.M.; Poznyak, V.; van Ommeren, M. Integrating mental health with other non-communicable diseases. BMJ 2019, 364, l295. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Quagliato, L.A.; Nardi, A.E. Cytokine alterations in panic disorder: A systematic review. J. Affect Disord. 2018, 228, 91–96. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Kemp, D.E.; Soczynska, J.K.; McIntyre, R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry 2009, 70, 1078–1090. [Google Scholar] [CrossRef]
- Gray, S.M.; Bloch, M.H. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr. Psychiatry Rep. 2012, 14, 220–228. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro-and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef]
- Karavelioğlu, E.; Gönül, Y.; Kokulu, S.; Hazman, Ö.; Bozkurt, F.; Koçak, A.; Eser, O. Anti-inflammatory and antiapoptotic effect of interleukine-18 binding protein on the spinal cord ischemia-reperfusion injury. Inflammation 2014, 37, 917–923. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic immunology: Functions and disorders of the immune system; Elsevier Health Sciences: Philadelphia PA, USA, 2014; pp. 68–69. [Google Scholar]
- Raeburn, C.D.; Sheppard, F.; Barsness, K.A.; Arya, J.; Harken, A.H. Cytokines for surgeons. Am. J. Surg. 2002, 183, 268–273. [Google Scholar] [CrossRef]
- Newton, T.L.; Fernandez-Botran, R.; Miller, J.J.; Lorenz, D.J.; Burns, V.E.; Fleming, K.N. Markers of inflammation in midlife women with intimate partner violence histories. J. Womens Health (Larchmt) 2011, 20, 1871–1880. [Google Scholar] [CrossRef]
- Bersani, F.S.; Wolkowitz, O.M.; Lindqvist, D.; Yehuda, R.; Flory, J.; Bierer, L.M.; Makotine, I.; Abu-Amara, D.; Coy, M.; Reus, V.I.; et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav. Immun. 2016, 52, 153–160. [Google Scholar] [CrossRef]
- Blessing, E.M.; Reus, V.; Mellon, S.H.; Wolkowitz, O.M.; Flory, J.D.; Bierer, L.; Lindqvist, D.; Dhabhar, F.; Li, M.; Qian, M.; et al. Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology 2017, 82, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Devoto, C.; Arcurio, L.; Fetta, J.; Ley, M.; Rodney, T.; Kanefsky, R.; Gill, J. Inflammation relates to chronic behavioral and neurological symptoms in military personnel with traumatic brain injuries. Cell Transplant. 2017, 26, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Kanefsky, R.; Motamedi, V.; Mithani, S.; Mysliwiec, V.; Gill, J.M.; Pattinson, C.L. Mild traumatic brain injuries with loss of consciousness are associated with increased inflammation and pain in military personnel. Psychiatry Res. 2019, 279, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mandel, H.; Levingston, C.A.; Young, M.R.I. An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of Veterans with combat-related PTSD. Hum. Immunol. 2016, 77, 652–657. [Google Scholar] [CrossRef]
- Hammad, S.M.; Truman, J.P.; Al Gadban, M.M.; Smith, K.J.; Twal, W.O.; Hamner, M.B. Altered blood sphingolipidomics and elevated plasma inflammatory cytokines in combat veterans with post-traumatic stress disorder. Neurobiol. Lipids 2012, 10, 2. [Google Scholar]
- Dennis, P.A.; Weinberg, J.B.; Calhoun, P.S.; Watkins, L.L.; Sherwood, A.; Dennis, M.F.; Beckham, J.C. An investigation of vago-regulatory and health-behavior accounts for increased inflammation in posttraumatic stress disorder. J Psychosom Res. 2016, 83, 33–39. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef]
- Michopoulos, V.; Rothbaum, A.O.; Jovanovic, T.; Almli, L.M.; Bradley, B.; Rothbaum, B.O.; Gillespie, C.F.; Ressler, K.J. CRP genetic variation and CRP levels are associated with increased PTSD symptoms and physiological responses in a highly traumatized civilian population. JAMA Psychiatry 2015, 172, 353. [Google Scholar]
- Jiang, D.; Jiang, S.; Gong, F.; Yuan, F.; Zhao, P.; He, X.; Lv, G.; Chu, X. Correlation between depression, posttraumatic stress disorder, and inflammatory factors in patients with severe burn injury. Am. Surg. 2018, 84, 1350–1354. [Google Scholar]
- Gill, J.M.; Saligan, L.; Lee, H.; Rotolo, S.; Szanton, S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J. Psychosom Res. 2013, 74, 301–306. [Google Scholar] [CrossRef]
- Küffer, A.; Straus, L.D.; Prather, A.A.; Inslicht, S.S.; Richards, A.; Shigenaga, J.K.; Madden, E.; Metzler, T.J.; Neylan, T.C.; O’Donovan, A. Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology 2019, 102, 114–120. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.F.; Wiener, C.D.; Jansen, K.; Portela, L.V.; Lara, D.R.; de Mattos Souza, L.D.; da Silva, R.A.; Moreira, F.P.; Oses, J.P. Serum levels of interleukins IL-6 and IL-10 in individuals with posttraumatic stress disorder in a population-based sample. Psychiatry Res. 2018, 260, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.L.; Levy-Carrick, N.; Reibman, J.; Xu, N.; Shao, Y.; Liu, M.; Ferri, L.; Kazeros, A.; Caplan-Shaw, C.E.; Pradhan, D.R.; et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J. Psychiatr Res. 2017, 89, 14–21. [Google Scholar] [CrossRef]
- Teche, S.P.; Rovaris, D.L.; Aguiar, B.W.; Hauck, S.; Vitola, E.S.; Bau, C.H.; Freitas, L.H.; Grevet, E.H. Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Res. 2017, 250, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Kane, B.; Williams, S.N.; Duffy, A. Relationship of PTSD symptoms with combat exposure, stress, and inflammation in American soldiers. Biol. Res. Nurs. 2015, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann, M.; Lee, H.; Rak, H.; Livingston, W.; Barr, T.; Baxter, T.; Scattergood-Keepper, L.; Mysliwiec, V.; Gill, J. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Med. 2014, 15, 1565–1570. [Google Scholar] [CrossRef]
- Bruenig, D.; Mehta, D.; Morris, C.P.; Lawford, B.; Harvey, W.; Young, R.M.; Voisey, J. Correlation between interferon γ and interleukin 6 with PTSD and resilience. Psychiatry Res. 2018, 260, 193–198. [Google Scholar] [CrossRef]
- McCanlies, E.C.; Araia, S.K.; Joseph, P.N.; Mnatsakanova, A.; Andrew, M.E.; Burchfiel, C.M.; Violanti, J.M. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine 2011, 55, 74–78. [Google Scholar] [CrossRef]
- Bruenig, D.; Mehta, D.; Morris, C.P.; Harvey, W.; Lawford, B.; Young, R.M.; Voisey, J. Genetic and serum biomarker evidence for a relationship between TNFα and PTSD in Vietnam war combat veterans. Compr. Psychiatry 2017, 74, 125–133. [Google Scholar] [CrossRef]
- Van Exel, E.; Gussekloo, J.; de Craen, A.J.; Frölich, M.; Bootsma-van der Wiel, A.; Westendorp, R.G. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-Plus Study. Diabetes 2002, 51, 1088–1092. [Google Scholar] [CrossRef]
- Lio, D.; Candore, G.; Crivello, A.; Scola, L.; Colonna-Romano, G.; Cavallone, L.; Hoffman, E.; Caruso, M.; Licastro, F.; Caldarera, C.M.; et al. Opposite effects of interleukin 10 common gene polymorphisms in cardiovascular diseases and in successful ageing: Genetic background of male centenarians is protective against coronary heart disease. J. Med. Genet. 2004, 41, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Krukowska, A.; Gorska, M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care 2005, 28, 2036–2037. [Google Scholar] [CrossRef] [PubMed]
- Uceyler, N.; Valenza, R.; Stock, M.; Schedel, R.; Sprotte, G.; Sommer, C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006, 54, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Soni, S. An Overview on Inflammatory Biomarkers for Diabetes Mellitus. Madridge J. Diabetes 2019, 3, 64–66. [Google Scholar] [CrossRef]
- Zhang, J.M.; Jianxiong, A. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2009, 45, 27–37. [Google Scholar] [CrossRef]
- An, K.; Salyer, J.; Kao, H.F.S. Psychological strains, salivary biomarkers, and risks for coronary heart disease among hurricane survivors. Biol. Res. Nurs. 2015, 17, 311–320. [Google Scholar] [CrossRef]
- Dalgard, C.; Eidelman, O.; Jozwik, C.; Olsen, C.H.; Srivastava, M.; Biswas, R.; Eudy, Y.; Rothwell, S.W.; Mueller, G.P.; Yuan, P.; et al. The MCP-4/MCP-1 ratio in plasma is a candidate circadian biomarker for chronic post-traumatic stress disorder. Transl. Psychiatry 2017, 7, e1025. [Google Scholar] [CrossRef]
- Michopoulos, V.; Beurel, E.; Gould, F.; Dhabhar, F.S.; Schultebraucks, K.; Galatzer-Levy, I.; Rothbaum, B.O.; Ressler, K.J.; Nemeroff, C.B. Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am. J. Psychiatry 2020, 177, 58–65. [Google Scholar] [CrossRef]
- Gola, H.; Engler, H.; Sommershof, A.; Adenauer, H.; Kolassa, S.; Schedlowski, M.; Groettrup, M.; Elbert, T.; Kolassa, I.T. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 2013, 13, 40. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Guo, J.C.; Jiang, X.L.; Chen, F.; Gao, Y.S. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac. J. Trop Med. 2012, 5, 323–325. [Google Scholar] [CrossRef]
- Smith, A.K.; Conneely, K.N.; Kilaru, V.; Mercer, K.B.; Weiss, T.E.; Bradley, B.; Tang, Y.; Gillespie, C.F.; Cubells, J.F.; Ressler, K.J. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Wolf, E.J.; Sadeh, N.; Logue, M.; Spielberg, J.M.; Hayes, J.P.; Sperbeck, E.; Schichman, S.A.; Stone, A.; Carter, W.C.; et al. A novel locus in the oxidative stress-related gene ALOX12 moderates the association between PTSD and thickness of the prefrontal cortex. Psychoneuroendocrinology 2015, 62, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Benedek, D.M.; Fullerton, C.S.; Forsten, R.D.; Naifeh, J.A.; Li, X.X.; Hu, X.Z.; Li, H.; Jia, M.; Xing, G.Q.; et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol. Psychiatry 2014, 19, 8. [Google Scholar] [CrossRef]
- Bruenig, D.; Lurie, J.; Morris, C.P.; Harvey, W.; Lawford, B.; Young, R.M.; Voisey, J. A case-control study and meta-analysis reveal BDNF Val66Met is a possible risk factor for PTSD. Neural Plast. 2016, 6979435, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Logue, M.W.; Baldwin, C.; Guffanti, G.; Melista, E.; Wolf, E.J.; Reardon, A.F.; Wildman, D.; Galea, S.; Koenen, K.C.; Miller, M.W. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol. Psychiatry 2013, 18, 937. [Google Scholar] [CrossRef] [PubMed]
- Amstadter, A.B.; Sumner, J.A.; Acierno, R.; Ruggiero, K.J.; Koenen, K.C.; Kilpatrick, D.G.; Galea, S.; Gelernter, J. Support for association of RORA variant and post traumatic stress symptoms in a population-based study of hurricane exposed adults. Mol. Psychiatry 2013, 18, 1148. [Google Scholar] [CrossRef]
- Van der Kolk, B.; Greenberg, M.; Boyd, H.; Krystal, J. Inescapable shock, neurotransmitters, and addiction to trauma: Toward a psychobiology of post traumatic stress. Biol. Psychiatry 1985, 20, 314–325. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012, 37, 939–949. [Google Scholar] [CrossRef]
- Camps, J. Oxidative Stress and Inflammation in Non-Communicable Diseases-Molecular Mechanisms and Perspectives in Therapeutics; Springer International Publishing AG: Cham, Switzerland, 2014. [Google Scholar]
- Maes, M.; Lin, A.H.; Delmeire, L.; van Gastel, A.; Kenis, G.; De Jongh, R.; Bosmans, E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol. Psychiatry 1999, 45, 833–839. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yagi, S.; Yokokura, M.; Sakamoto, M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, S200–S204. [Google Scholar] [CrossRef]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pallast, S.; Arai, K.; Ziaoying, W.; Lo, E.H.; Leyen, K. 12/15-Lipoxygenasetargets neuronal mitochondria under oxidative stress. J. Neurochem. 2009, 111, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Porro, B.; Songia, P.; Squellerio, I.; Tremoli, E.; Cavalca, V. Analysis, physiological and clinical significance of 12-HETE: A neglected platelet-derived 12-lipoxygenase product. J. Chromatogr. B 2014, 964, 26–40. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Chao, L.L.; Paulson, J.; Samuelson, K.W.; Shigenaga, J.K.; Grunfeld, C.; Weiner, M.W.; Neylan, T.C. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 2015, 51, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Chmiel, J.; Berger, M. Chronic inflammation in the cystic fibrosis lung: Alterations in inter-and intracellular signaling. Clin. Rev. Allergy Immunol. 2008, 34, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Seixas, A.; Shetty, S.; Shenoy, S. Sleep duration and diabetes risk: Population trends and potential mechanisms. Curr. Diab. Rep. 2016, 16, 106. [Google Scholar] [CrossRef]
- Novak, P.; Cente, M.; Kosikova, N.; Augustin, T.; Kvetnansky, R.; Novak, M.; Filipcik, P. Stress-induced alterations of immune profile in animals suffering by Tau protein-driven neurodegeneration. Cell. Mol. Neurobiol. 2018, 38, 243–259. [Google Scholar] [CrossRef]
- Toczek, J.; Hillmer, A.T.; Han, J.; Liu, C.; Peters, D.; Emami, H.; Wu, J.; Esterlis, I.; Cosgrove, K.P.; Sadeghi, M.M. FDG PET imaging of vascular inflammation in post-traumatic stress disorder: A pilot case–control study. J. Nucl. Cardiol. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Sadeghi, M.M. 18F-FDG PET and vascular inflammation: Time to refine the paradigm? J. Nucl. Cardiol. 2015, 22, 319–324. [Google Scholar] [CrossRef]
- McColl, B.W.; Allan, S.M.; Rothwell, N.J. Systemic infection, inflammation and acute ischemic stroke. Neuroscience 2009, 158, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Villien, M.; Wey, H.Y.; Mandeville, J.B.; Catana, C.; Polimeni, J.R.; Sander, C.Y.; Zürcher, N.R.; Chonde, D.B.; Fowler, J.S.; Rosen, B.R.; et al. Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage 2014, 100, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.L. Anatomy of the lymphatic system and its disorders. In Lymphedema; Springer: London, UK, 2011; pp. 49–56. [Google Scholar]
- Khalili, S.; Liu, Y.; Kornete, M.; Roescher, N.; Kodama, S.; Peterson, A.; Piccirillo, C.A.; Tran, S.D. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjögren’s-like disease. PLoS ONE 2012, 7, e38615. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kondo, M.; Kelsoe, G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005, 201, 1771–1780. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Hu, H.H.; Shen, W.; Carmichael, O. Emerging technologies and their applications in lipid compartment measurement. Trends Endocrinol. Metab. 2015, 26, 688–698. [Google Scholar] [CrossRef]
- Shin, L.M.; Rauch, S.L.; Pitman, R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 67–79. [Google Scholar] [CrossRef]
- Henigsberg, N.; Kalember, P.; Petrović, Z.K.; Šečić, A. Neuroimaging research in posttraumatic stress disorder–focus on amygdala, hippocampus and prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 37–42. [Google Scholar] [CrossRef]
- Greenberg, M.S.; Tanev, K.; Marin, M.F.; Pitman, R.K. Stress, PTSD, and dementia. Alzheimers Dement. 2014, 10, S155–S165. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Buske-Kirschbaum, A.; Hellhammer, D.H.; Kirschbaum, C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology 2004, 29, 83–98. [Google Scholar] [CrossRef]
- Shalev, A.Y.; Videlock, E.J.; Peleg, T.; Segman, R.; Pitman, R.K.; Yehuda, R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: A longitudinal study. Part I: HPA axis responses. Int. J. Neuropsychopharmacol. 2008, 11, 365–372. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; Ebenezer, P.J.; McLaughlin, L.D.; Francis, J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE 2014, 9, e89104. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.; Doll, R.; Ma, R.; Cole, S.W. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatry 2008, 64, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.H.; Silverman, M.N.; Sternberg, E.M. Glucocorticoid dysregulations and their clinical correlates: From receptors to therapeutics. Ann. N. Y. Acad. Sci. 2009, 1179, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.; Heim, C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain Behav. Immun. 2011, 25, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; van Zuiden, M. Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri-and post-trauma glucocorticoid and inflammatory dysregulation. Curr. Opin. Psychol. 2017, 14, 132–137. [Google Scholar] [CrossRef]
- Morrison, F.G.; Miller, M.W.; Wolf, E.J.; Logue, M.W.; Maniates, H.; Kwasnik, D.; Cherry, J.D.; Svirsky, S.; Restaino, A.; Hilderbrandt, A.; et al. Reduced interleukin 1A gene expression in the dorsolateral prefrontal cortex of individuals with PTSD and depression. Neurosci. Lett. 2019, 692, 204–209. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Assessing the serum concentration levels of NT-4/5, GPX-1, TNF-α, and l-arginine as biomediators of depression severity in first depressive episode patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 1049–1058. [Google Scholar] [CrossRef]
| Trauma Types | Cytokine Markers | N (m/f) | Altered Direction | Main Findings | Ref. |
|---|---|---|---|---|---|
| Accident | IL-1β, IL-6, TNF-α | 52 (25/27) | Increase | - Individuals with PTSD had higher proinflammatory cytokine concentrations three months after the accident compared to healthy controls, even though clinical symptoms decreased. | [51] † |
| IL-1β, IL-6, TNF-α | 187 (71/116) | Increase | - Individuals with PTSD had higher IL-1β and TNF-α, and total proinflammatory cytokine scores than those without PTSD. | [25] | |
| - IL-1β levels in all subjects correlate with self-assessed PTSD symptom severity scores after controlling for trauma exposure severity. | |||||
| CRP | 641 (316/325) | Increase | - CRP was positively associated with PTSD severity, particularly with re-experiencing and avoidance symptoms. | [55] | |
| Assault | IL-6 | 77 (0/77) | Increase | - Individuals with PTSD had higher IL-6 concentrations than trauma-unexposed controls. | [52] † |
| - Individuals who recover from PTSD had similar IL-6 concentrations as trauma-unexposed controls. | |||||
| IL-6 | 68 (0/68) | Increase | - Physical assault history is significantly negatively correlated with phytohemagglutinin A-stimulated IL-6 production. | [41] | |
| IL-6 | 60 (10/50) | None | - Individuals with PTSD had statistically similar IL-6 cytokine levels as resilient trauma-exposed individuals. | [56] | |
| Combat | CRP, IL-6, TNF-α | 111 (111/0) | Increase | - Individuals with PTSD had lower nitric oxide synthetic capacity and higher CRP levels. | [42] |
| - Lower nitric oxide synthetic capacity is associated with higher IL-6, TNF-α, and PTSD symptom severity. | |||||
| IL-6, TNF-α | 166 (166/0) | Increase | - Individuals with PTSD had higher levels of IL-6, TNF-α, and other cardiovascular risk markers. | [43] | |
| IL-6, TNF-α | 299 (299/0) | Decrease, Increase | - PTSD symptom severity is correlated with significant decrease in IL-6, increase in TNF-α. | [61] | |
| - Resilience to PTSD is correlated with increased IL-6. | |||||
| TNF-α | 167 (87/80) | Increase | - PTSD symptom severity is positively associated with higher TNF-α levels, and is mediated by attenuated vagal activity, smoking, and alcohol dependence. | [48] | |
| IL-6, TNF-α | 83 (82/1) | Increase | - Individuals with PTSD and TBI had greater increased IL-6 and TNF-α concentrations than individuals with PTSD but without TBI. | [44] | |
| - IL-6 and TNF-α concentrations were greater in individuals with PTSD and TBI with high PTSD symptom severity than low PTSD symptom severity. | |||||
| IL-1β, IL-6, TNF-α | 52 (51/1) | None | - CRP and hair cortisol are correlated with symptoms of depression and PTSD. | [57] | |
| IL-6 | 66 (64/2) | None | - Individuals with PTSD and recovered insomnia since deployment had decreased CRP concentration than individuals with PTSD and consistent insomnia. | [58] | |
| IL-6 | 143 (138/5) | Increase | - Individuals with PTSD and TBI with loss of consciousness had elevated IL-6 compared to individuals with PTSD and TBI without loss of consciousness as well as individuals with PTSD without TBI. | [45] | |
| IL-1β, IL-6, TNF-α, IFN-γ | 104 (104/0) | Increase | - Individuals with PTSD had higher proinflammatory cytokine levels than those without PTSD. | [15] | |
| - Proinflammatory cytokine levels were not correlated with symptom severity of depression, PTSD diagnosis, or number of traumas. | |||||
| IL-6, TNF-α | 61 (61/0) | Increase | - Individuals with PTSD had higher total proinflammatory scores than those without PTSD after controlling for age, BMI, and smoking. | [16] | |
| - Total proinflammatory score is not correlated with PTSD symptom severity within the PTSD group. | |||||
| IL-6, TNF-α | 13 (12/1) | Increase | - Individuals with PTSD had higher IL-6, TNF-α than trauma-exposed individuals without PTSD. | [46] | |
| IFN-γ | 13 (13/0) | Increase | - Individuals with PTSD had higher levels of IL-6, IL-10, IFN-γ, and TNF-α than healthy controls. | [47] | |
| IFN-γ | 299 (299/0) | Decrease | - Individuals with PTSD showed small but significant decrease in levels of IL-6 and IFN-γ. | [59] | |
| IFN-γ | 30 (27/3) ‡ | Increase | - Individuals with PTSD had significantly higher IFN-γ levels compared to healthy controls. | [49] | |
| Police | CRP, IL-6 | 111 (68/43) | None | - There were no significant association between plasma inflammatory markers including levels of CRP and PTSD symptoms. | [60] |
| Other | IL-6, TNF-α | 44 (22/22) | Increase | - Individuals with PTSD had higher IL-6 and TNF-α levels at sleep onset but not at the end of the sleep cycle. | [53] † |
| - Men with PTSD show altered levels of TNF-α at the end of the sleep cycle than men without PTSD. | |||||
| IL-6 | 82 (16/66) | Increase | - Individuals with PTSD had higher IL-6 levels as compared to healthy controls. | [54] † | |
| - Significant sex-differences were present in IL-6 levels compared to healthy individuals, where men showed higher IL-6 levels than the control group, while women did not statistically differ according to PTSD. | |||||
| CRP | 2692 (800/1892) | Increase | - PTSD symptoms were positively associated with high CRP levels. | [50] |
| Trauma Types | N (m/f) | Altered Direction | Main Findings | Ref. |
|---|---|---|---|---|
| Accident | 19 (2/17) | None | - PTSD symptom severity is not significantly associated with IL-10 levels. | [68] |
| 187 (71/116) | None | - There are no differences in levels of IL-10 between individuals with PTSD and those without PTSD. | [25] | |
| Assault | 60 (10/50) | Decrease | - Individuals with PTSD presented lower IL-10 levels than the trauma-exposed individuals without PTSD. | [56] |
| 30 (10/20) | None | - There are no differences in levels of IL-10 between individuals with PTSD and those without PTSD. | [69] †,‡ | |
| Combat | 167 (87/80) | Increase | - PTSD symptom severity is positively associated with higher IL-10 levels, and is mediated by attenuated vagal activity, smoking, and alcohol dependence. | [48] |
| 83 (82/1) | None | - There were no anti-inflammatory cytokine level alterations between individuals with PTSD and TBI versus those with PTSD without TBI. | [44] | |
| 64 (60/4) | Increase | - PTSD individuals with mTBI had elevated IL-10 levels compared to individuals with PTSD but without mTBI. - IL-10 concentration is positively correlated with PTSD symptom severity. | [52] | |
| 299 (299/0) | Decrease | - PTSD symptom severity had a trend-line negative correlation with IL-10 levels. | [61] | |
| 52 (51/1) | None | - CRP and hair cortisol are correlated with symptoms of depression and PTSD. | [57] | |
| 143 (138/5) | None | - There are no between-group differences in IL-10 levels among individuals with PTSD and TBI with loss of consciousness versus individuals with PTSD and TBI without loss of consciousness, as well as individuals with PTSD but no TBI. - PTSD symptom severity is not significantly associated with IL-10 levels. | [45] | |
| 61 (61/0) | None | - There are no significant differences in IL-10 levels between individuals with PTSD and those without PTSD. | [16] | |
| 104 (104/0) | None | - Concentrations of IL-10 are not significantly altered in PTSD subjects. | [15] | |
| 13 (12/1) | Decrease | - Plasma and salivary levels of IL-10 are lower in veterans with PTSD compared to veterans without PTSD. | [46] | |
| Refugee | 60 (27/33) | None | - IL-10 levels are not significantly different between individuals with PTSD and healthy controls. | [71] † |
| Other | 273 (141/132) | None | - Anti-inflammatory cytokine levels are not different in the chronic PTSD group compared with those in the recovery and resilient group. | [70] |
| 104 (64/40) | Decrease | - Individuals with PTSD showed increased global DNA methylation and decreased IL-4 than to healthy controls. | [73] | |
| 100 (47/53) | Increase | - Individuals with PTSD showed increased cytokine levels compared to healthy controls in 6 cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, and TNF-α. | [72] | |
| 30 (27/3) § | None | - There are no significant difference in IL-4 levels between individuals with PTSD and healthy controls. | [49] | |
| 82 (16/66) | Increase | - Individuals with PTSD had a significant increase in the serum levels of IL-6 and IL-10 than the control group. | [54] † |
| Trauma Types | Genetic Marker | N (m/f) | Altered Direction | Main Findings | Ref. |
|---|---|---|---|---|---|
| Combat | BDNF | 461 (413/48) | Increase | - Individuals with PTSD had higher frequencies of Met/Met and 66Met allele than individuals without PTSD. | [75] |
| Val66Met | |||||
| (rs6265) | |||||
| BDNF | 257 (257/0) | None | - Individuals had similar frequencies of Met/Met and 66Met allele as resilience trauma-exposed individuals. | [76] | |
| Val66Met | |||||
| (rs6265) | |||||
| Other | RORA | 435 (260/175) | N/A | - A significant association was reported between a RORA SNP (rs8042149) and PTSD diagnosis. | [77] |
| SNPs | |||||
| RORA | 551 (193/358) | N/A | - A significant association was reported between a RORA SNP (rs8042149) and PTSD diagnosis. | [78] | |
| SNPs |
| Trauma Types | Inflammatory and Oxidative Stress Marker | Outcome Measure (Modality) | N (m/f) | Main Findings | Ref. |
|---|---|---|---|---|---|
| Combat | ALOX12 SNPs, ALOX15 SNPs | Cortical thickness(sMRI) | 218(194/24) | - The ALOX12 locus (rs1042357, rs10852889) moderated the association between PTSD and reduced cortical thickness in the middle frontal gyrus, superior frontal gyrus, rostral anterior cortex and medial orbitofrontal cortex. | [74] |
| - There were no associations between ALOX15 locus and cortical thickness in PTSD. | |||||
| IL-6, sTNF-RII | Hippocampal volume (sMRI) | 206 (170/36) | - Increased sTNF-RII is associated with reduced hippocampal volume. | [87] | |
| - There were no associations between levels of IL-6 and hippocampal volume. | |||||
| - Higher PTSD severity is associated with elevated sTNF-RII and reduced IL-6 levels. | |||||
| Unspecified | IL-1β, IL-6, TNF-α,FDG signal in spleen and bone marrow | FDG signal in amygdala (FDG-PET/CT) | 16(10/6) | - There were no associations between IL-1β, IL-6 and TNF-α levels and FDG signal in the amygdala, spleen and bone marrow. | [92] |
| - Positive correlations among FDG signals in the amygdala, spleen and bone marrow. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.D.; Lee, S.; Yoon, S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants 2020, 9, 107. https://doi.org/10.3390/antiox9020107
Kim TD, Lee S, Yoon S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants. 2020; 9(2):107. https://doi.org/10.3390/antiox9020107
Chicago/Turabian StyleKim, Tammy D., Suji Lee, and Sujung Yoon. 2020. "Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective" Antioxidants 9, no. 2: 107. https://doi.org/10.3390/antiox9020107
APA StyleKim, T. D., Lee, S., & Yoon, S. (2020). Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants, 9(2), 107. https://doi.org/10.3390/antiox9020107





