Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Scheme
2.2. Growth Parameters’ Determination
2.3. Examination of Leaf Anatomy and Ultrastructure
2.4. Evaluation of Photosynthetic Pigment Content
2.5. Determination of Lipid Peroxidation
2.6. Assessment of Antioxidative System Efficiency
2.6.1. Non-Enzymatic Antioxidants
Carotenoids
Proline
Phenolic Profile
2.6.2. Antioxidant Enzymes’ Assay
Superoxide Dismutase (SOD)
Catalase (CAT)
Peroxidase Family (POX)
Glutathione-S-Transferase (GST)
2.7. DPPH Radical Scavenging Assay
2.8. HMs Detection in Tissue using Scanning Electron Microscopy (SEM)
2.9. Statistics
3. Results
3.1. Culture Growth in the Presence of HMs
3.2. Anatomy of HM-Treated Leaves
3.3. Photosynthetic Pigments’ Accumulation and Chloroplast Ultrastructure
3.4. Lipid Peroxidation under Metallic Stress
3.5. Non-Enzymatic Antioxidants in Silene Shoots Cultured on HM-Containing Media
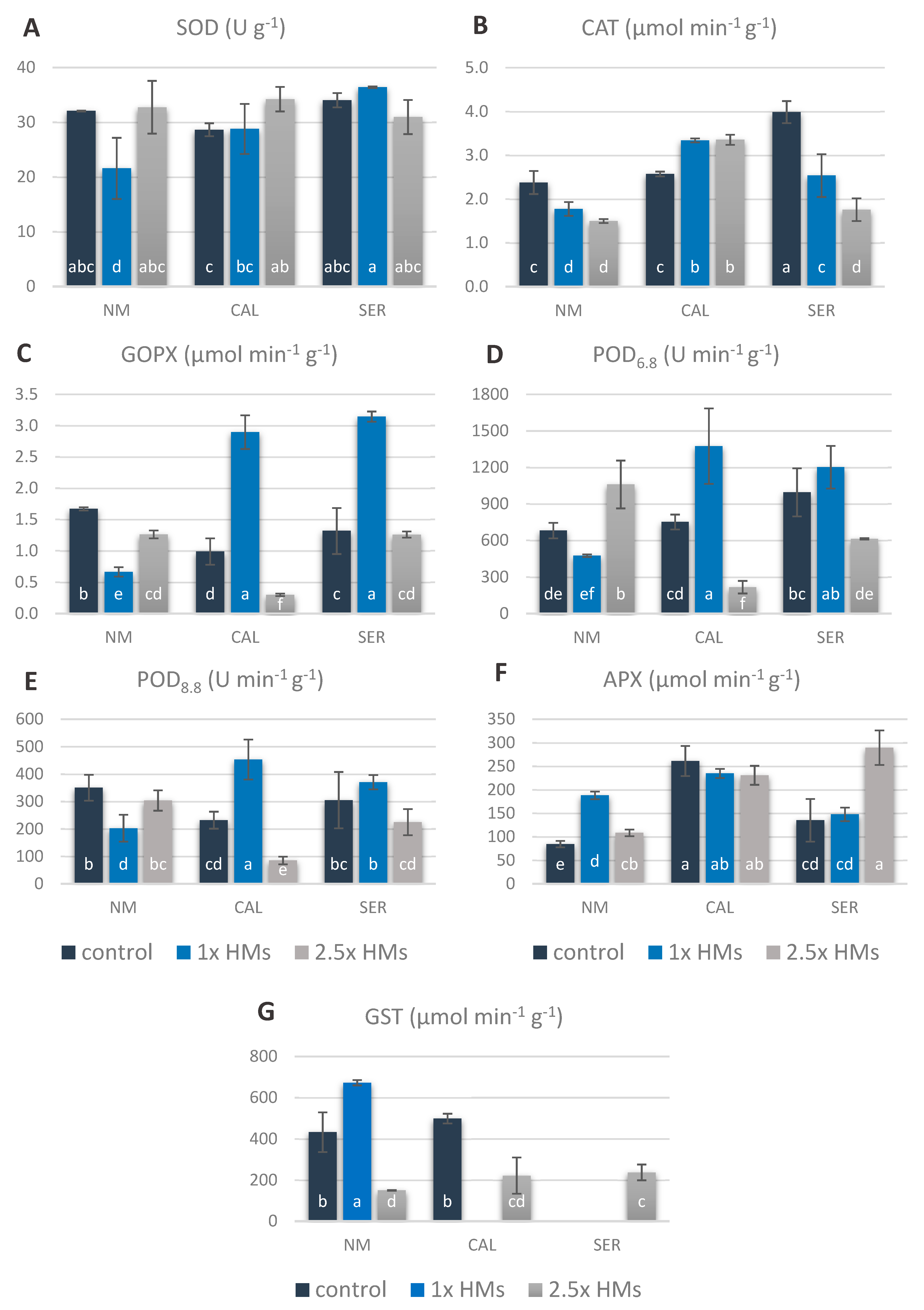
3.6. Antioxidant Enzyme Responses of Silene Shoots to HMs
3.7. Major Players in Antioxidant Machinery of Particular Ecotypes
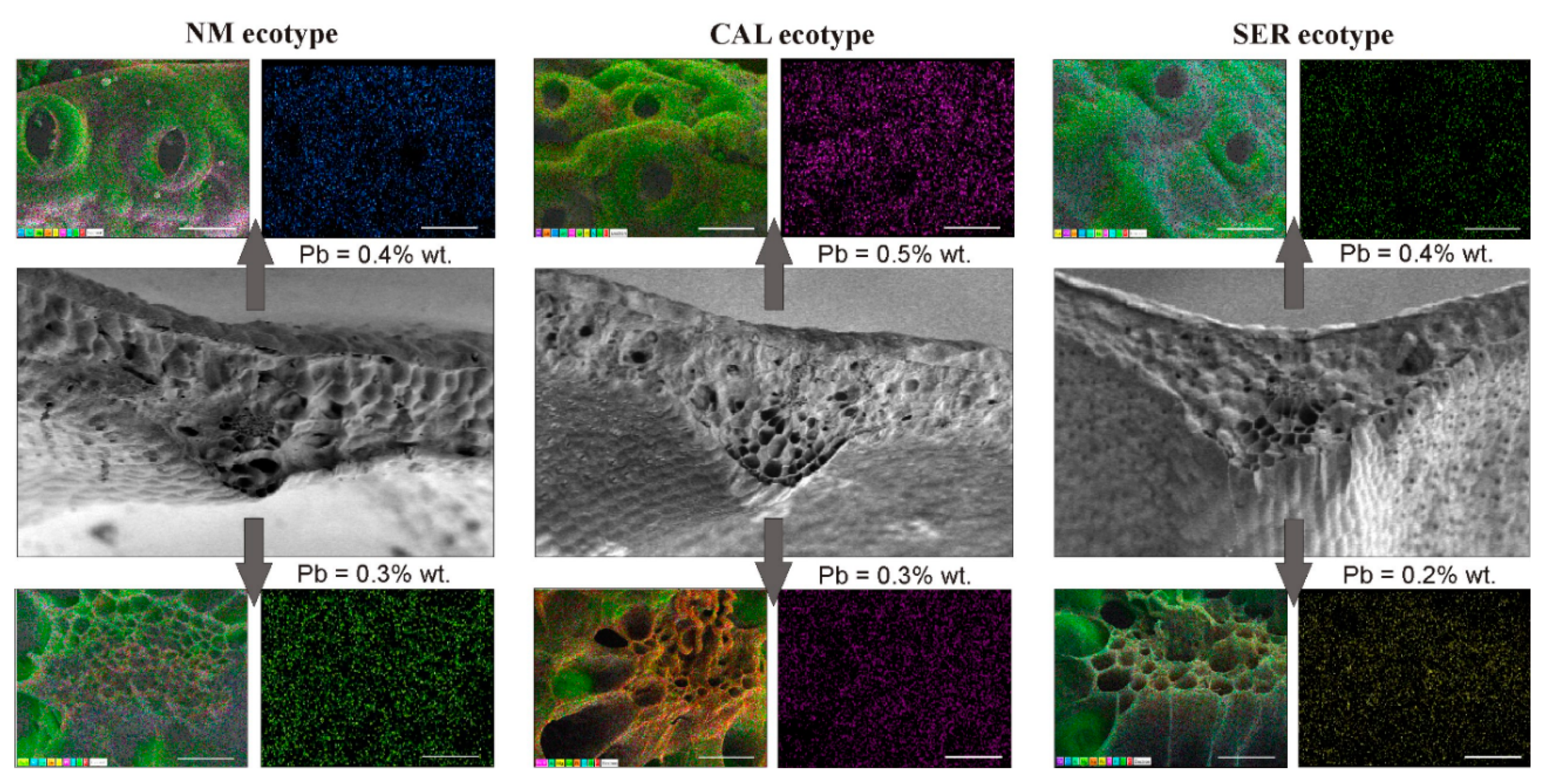
3.8. Cryo-SEM-EDX Analysis
4. Discussion
4.1. Morphogenetic Response of HM-Treated Cultures is Diversified
4.2. Interrelation of Structural and Physiological Features Responsible for Morphogenetic Reactions
4.3. Stromules as Unique Feature of CAL Chloroplasts
4.4. Changes in Antioxidant Accumulations are Ecotype-Specific
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Branco-Neves, S.; Soares, C.; de Sousa, A.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. An efficient antioxidant system and heavy metal exclusion from leaves make Solanum cheesmaniae more tolerant to Cu than its cultivated counterpart. Food Energy Secur. 2017, 6, 123–133. [Google Scholar] [CrossRef]
- Hasan, M.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress-a review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Jia, L.; Liu, Z.; Chen, W.; Ye, Y.; Yu, S.; He, X. Hormesis effects induced by cadmium on growth and photosynthetic performance in a hyperaccumulator, Lonicera japonica Thunb. J. Plant Growth Regul. 2015, 34, 13–21. [Google Scholar] [CrossRef]
- Woźniak, A.; Drzewiecka, K.; Kęsy, J.; Marczak, Ł.; Narożna, D.; Grobela, M.; Motała, R.; Bocianowski, J.; Morkunas, I. The influence of lead on generation of signalling molecules and accumulation of flavonoids in pea seedlings in response to pea aphid infestation. Molecules 2017, 22, 1404. [Google Scholar] [CrossRef]
- Durenne, B.; Druart, P.; Blondel, A.; Fauconnier, M.-L. How cadmium affects the fitness and the glucosinolate content of oilseed rape plantlets. Environ. Exp. Bot. 2018, 155, 185–194. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Ali, S.; Gill, R.A.; Mwamba, T.M.; Zhang, N.; Lv, M.T.; ul Hassan, Z.; Islam, F.; Ali, S.; Zhou, W.J. Differential cobalt-induced effects on plant growth, ultrastructural modifications, and antioxidative response among four Brassica napus (L.) cultivars. Int. J. Environ. Sci. Technol. 2018, 15, 2685–2700. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Kamińska, I.; Górecka, M.; Bederska-Błaszczyk, M. Evaluation of heavy metal-induced responses in Silene vulgaris ecotypes. Protoplasma 2019, 256, 1279–1297. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Labudda, M.; Różańska, E.; Czarnocka, W.; Sobczak, M.; Dzik, J.M. Systemic changes in photosynthesis and reactive oxygen species homeostasis in shoots of Arabidopsis thaliana infected with the beet cyst nematode Heterodera schachtii. Mol. Plant Pathol. 2018, 19, 1690–1704. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in proteolytic activity and protein carbonylation in shoots of Alyssum montanum ecotypes under multi-metal stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef]
- Ishtiyaq, S.; Kumar, H.; Varun, M.; Kumar, B.; Paul, M.S. Heavy metal toxicity and antioxidative response in plants: An overview. In Plants under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 77–106. ISBN 9789811322426. [Google Scholar]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Malesev, D.; Kuntic, V. Investigation of metal–flavonoid chelates and the determination of flavonoids via metal–flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Dai, L.-P.; Dong, X.-J.; Ma, H.-H. Molecular mechanism for cadmium-induced anthocyanin accumulation in Azolla imbricata. Chemosphere 2012, 87, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Pozgajova, M.; Navratilova, A.; Arvay, J.; Duranova, H.; Trakovicka, A. Impact of cadmium and nickel on ion homeostasis in the yeast Schizosaccharomyces pombe. J. Environ. Sci. Health B 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Ernst, W. Evolution of metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Lundholm, J.T.; Richardson, P.J. Habitat analogues for reconciliation ecology in urban and industrial environments: Habitat analogues. J. App. Ecol. 2010, 47, 966–975. [Google Scholar] [CrossRef]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Koszelnik-Leszek, A. Structural, physiological and genetic diversification of Silene vulgaris ecotypes from heavy metal-contaminated areas and their synchronous in vitro cultivation. Planta 2019, 249, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.-The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I. Superoxide-driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem. Int. 1989, 19, 1117–1124. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Lück, H. Peroxidase. In Methoden der Enzymatischen Analyse; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1962; pp. 895–897. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Pradas del Real, A.E.; Silvan, J.M.; de Pascual-Teresa, S.; Guerrero, A.; García-Gonzalo, P.; Lobo, M.C.; Pérez-Sanz, A. Role of the polycarboxylic compounds in the response of Silene vulgaris to chromium. Environ. Sci. Pollut. Res. 2017, 24, 5746–5756. [Google Scholar] [CrossRef]
- Koźminska, A.; Al Hassan, M.; Hanus-Fajerska, E.; Naranjo, M.A.; Boscaiu, M.; Vicente, O. Comparative analysis of water deficit and salt tolerance mechanisms in Silene. S. Afr. J. Bot. 2018, 117, 193–206. [Google Scholar] [CrossRef]
- Fernández, R.; Fernández-Fuego, D.; Bertrand, A.; González, A. Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: Role of the cell wall, non-protein thiols and organic acids. Plant Physiol. Biochem. 2014, 78, 63–70. [Google Scholar] [CrossRef]
- Malar, S.; Shivendra Vikram, S.; Favas, P.J.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2016, 55, 54. [Google Scholar] [CrossRef]
- Glińska, S.; Gapińska, M.; Michlewska, S.; Skiba, E.; Kubicki, J. Analysis of Triticum aestivum seedling response to the excess of zinc. Protoplasma 2016, 253, 367–377. [Google Scholar] [CrossRef]
- Antonovics, J.; Bradshaw, A.D.; Turner, R.G. Heavy metal tolerance in plants. In Advances in Ecological Research; Cragg, J.B., Ed.; Academic Press: Cambridge, MA, USA, 1971; Volume 7, pp. 1–85. [Google Scholar]
- Gomes, M.P.; Marques, T.C.L.L.D.; e Melo, S.; Nogueira, M.D.O.G.; Castro, E.M.D.; Soares, Â.M. Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ. Exp. Bot. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Rhizopoulou, S. Development and structure of drought-tolerant leaves of the mediterranean shrub Capparis spinosa L. Ann. Bot. 2003, 92, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Amin, N.; Ahmad, I.; Shah, S.; Hussain, K. Dust particles induce stress, reduce various photosynthetic pigments and their derivatives in Ficus benjamina: A landscape plant. Int. J. Agric. Biol. 2017, 19, 1469–1474. [Google Scholar]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Long-term exposure of rapeseed (Brassica napus L.) to ZnO nanoparticles: Anatomical and ultrastructural responses. Environ. Sci. Pollut. Res. 2015, 22, 10733–10743. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, Y.; Tian, R. Tolerance mechanism of Triarrhena sacchariflora (Maxim.) Nakai. seedlings to lead and cadmium: Translocation, subcellular distribution, chemical forms and variations in leaf ultrastructure. Ecotoxicol. Environ. Saf. 2018, 165, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Rottet, S.; Besagni, C.; Kessler, F. The role of plastoglobules in thylakoid lipid remodeling during plant development. Biochim. Biophys. Acta 2015, 1847, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, K.J.; Kessler, F. Plastoglobuli: Plastid microcompartments with integrated functions in metabolism, plastid developmental transitions, and environmental adaptation. Annu. Rev. Plant Biol. 2017, 68, 253–289. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Kwok, E.Y.; Hanson, M.R. Stromules and the dynamic nature of plastid morphology. J. Microsc. 2004, 214, 124–137. [Google Scholar] [CrossRef]
- Ho, J.; Theg, S.M. The formation of stromules in vitro from chloroplasts isolated from Nicotiana benthamiana. PLoS ONE 2016, 11, e0146489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanson, M.R.; Sattarzadeh, A. Trafficking of proteins through plastid stromules. Plant Cell 2013, 25, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast stromules function during innate immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef]
- Schattat, M.; Klösgen, R. Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Brunkard, J.O.; Runkel, A.M.; Zambryski, P.C. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA 2015, 112, 10044–10049. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; Hansen, M.R.; Shaw, D.J.; Graham, K.; Dale, R.; Smallman, P.; Natesan, S.K.A.; Newell, C.A. Plastid stromules are induced by stress treatments acting through abscisic acid: Stress induction of plastid stromules. Plant J. 2012, 69, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, G.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef]
- Degu, A.; Ayenew, B.; Cramer, G.R.; Fait, A. Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem. 2016, 212, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Kumar, R.; Seth, C.S.; Gupta, D.K. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 2006, 65, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C.; et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Harmens, H.; Koevoets, P.L.M.; Verkleij, J.A.C.; Ernst, W.H.O. The role of low molecular weight organic acids in the mechanism of increased zinc tolerance in Silene vulgaris (Moench) Garcke. New Phytol. 1994, 126, 615–621. [Google Scholar] [CrossRef]
- García-Gonzalo, P.; del Real, A.E.P.; Lobo, M.C.; Pérez-Sanz, A. Different genotypes of Silene vulgaris (Moench) Garcke grown on chromium-contaminated soils influence root organic acid composition and rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 25713–25724. [Google Scholar] [CrossRef]
- Štolfa, I.; Pfeiffer, T.Ž.; Špoljarić, D.; Teklić, T.; Lončarić, Z. Heavy metal-induced oxidative stress in plants: Response of the antioxidative system. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 127–163. ISBN 978-3-319-20421-5. [Google Scholar]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Van Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Burchett, M.D. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the Grey mangrove, Avicennia marina (Forsk.) Vierh. Mar. Pollut. Bull. 2001, 42, 233–240. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Parmar, N.G.; Vithalani, S.D.; Chanda, S.V. Alteration in growth and peroxidase activity by heavy metals in Phaseolus seedlings. Acta Physiol. Plant 2002, 24, 89–95. [Google Scholar] [CrossRef]
- Yan, H.; Li, Q.; Park, S.-C.; Wang, X.; Liu, Y.-J.; Zhang, Y.-G.; Tang, W.; Kou, M.; Ma, D.-F. Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol. Biochem. 2016, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
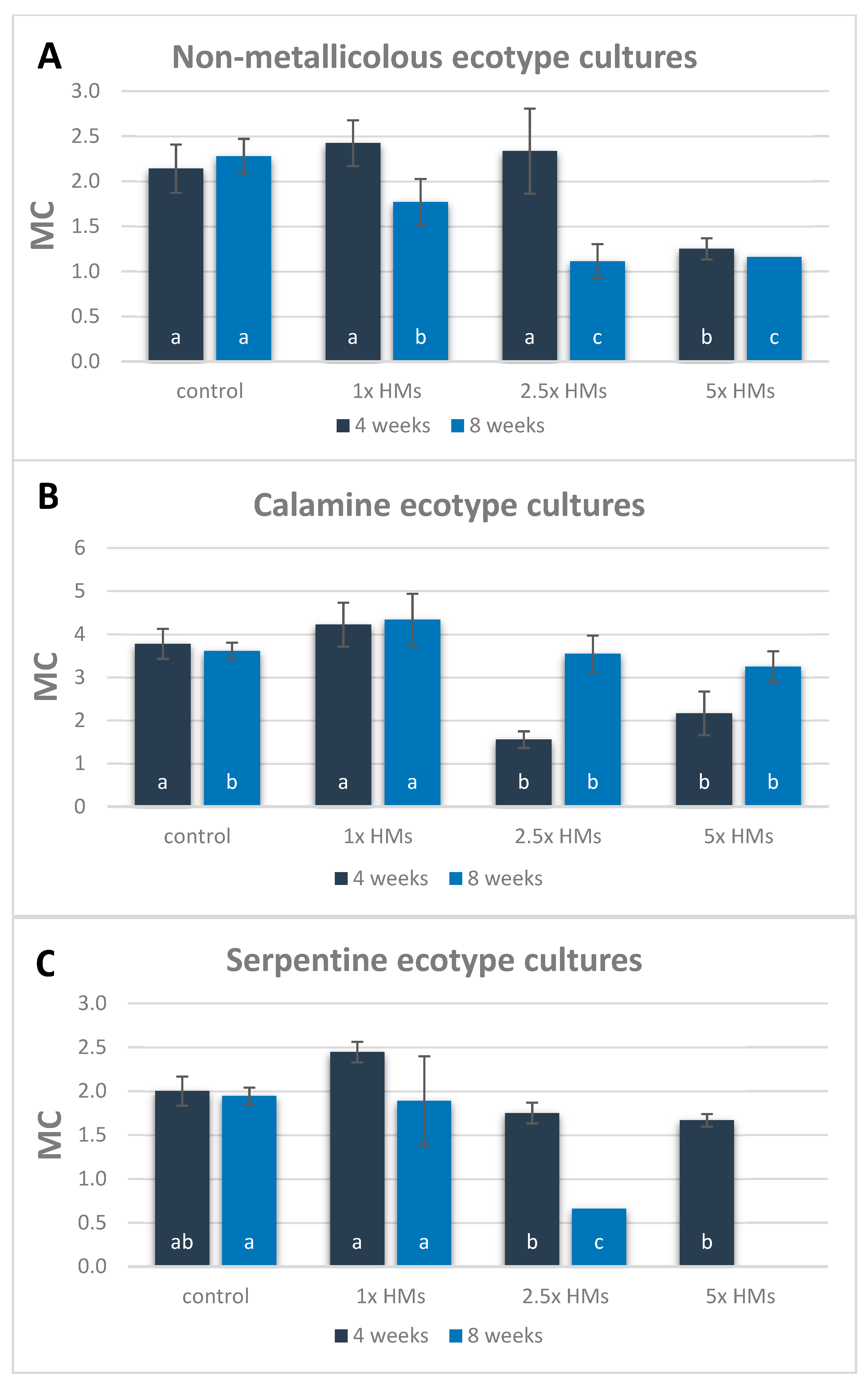
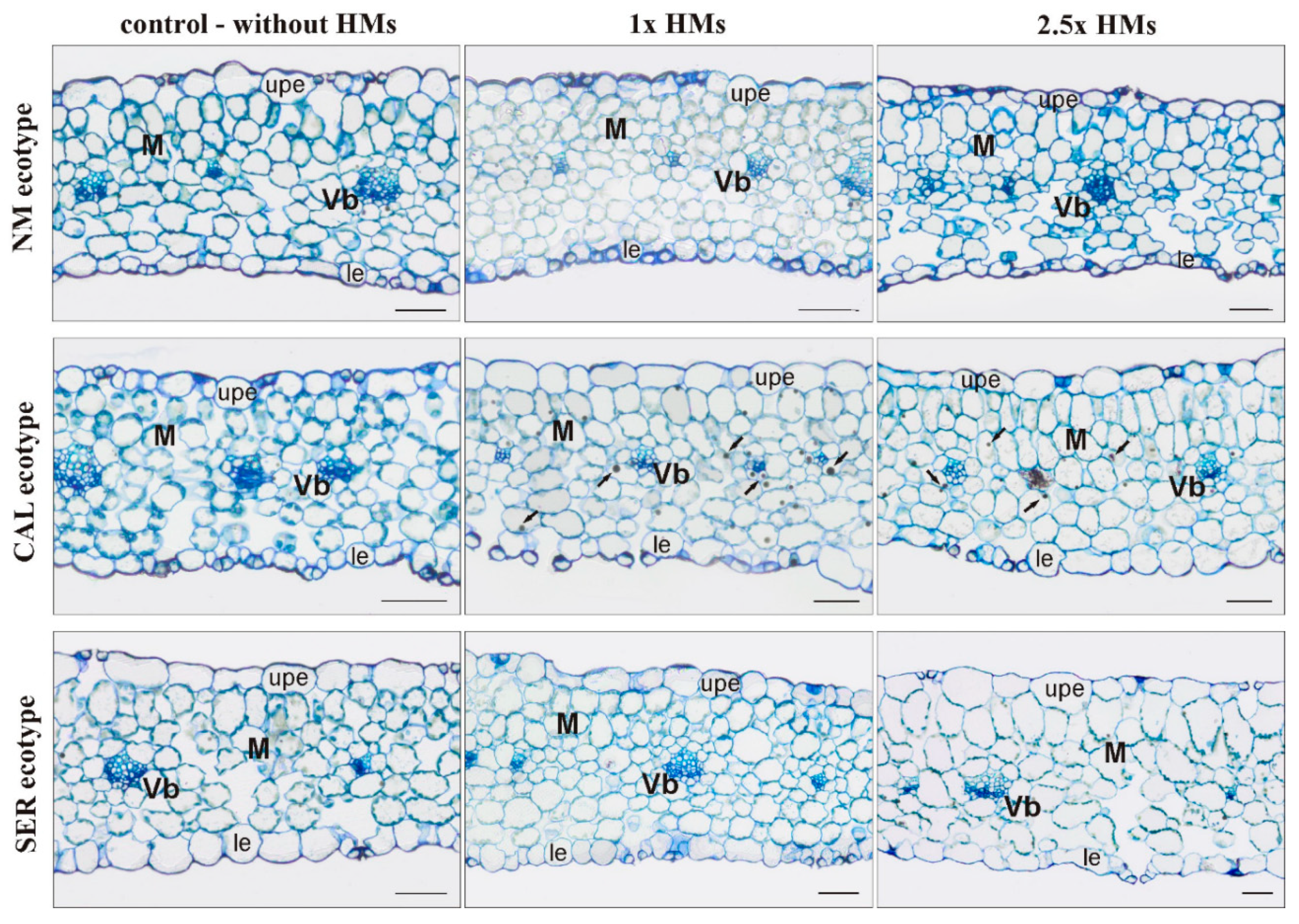
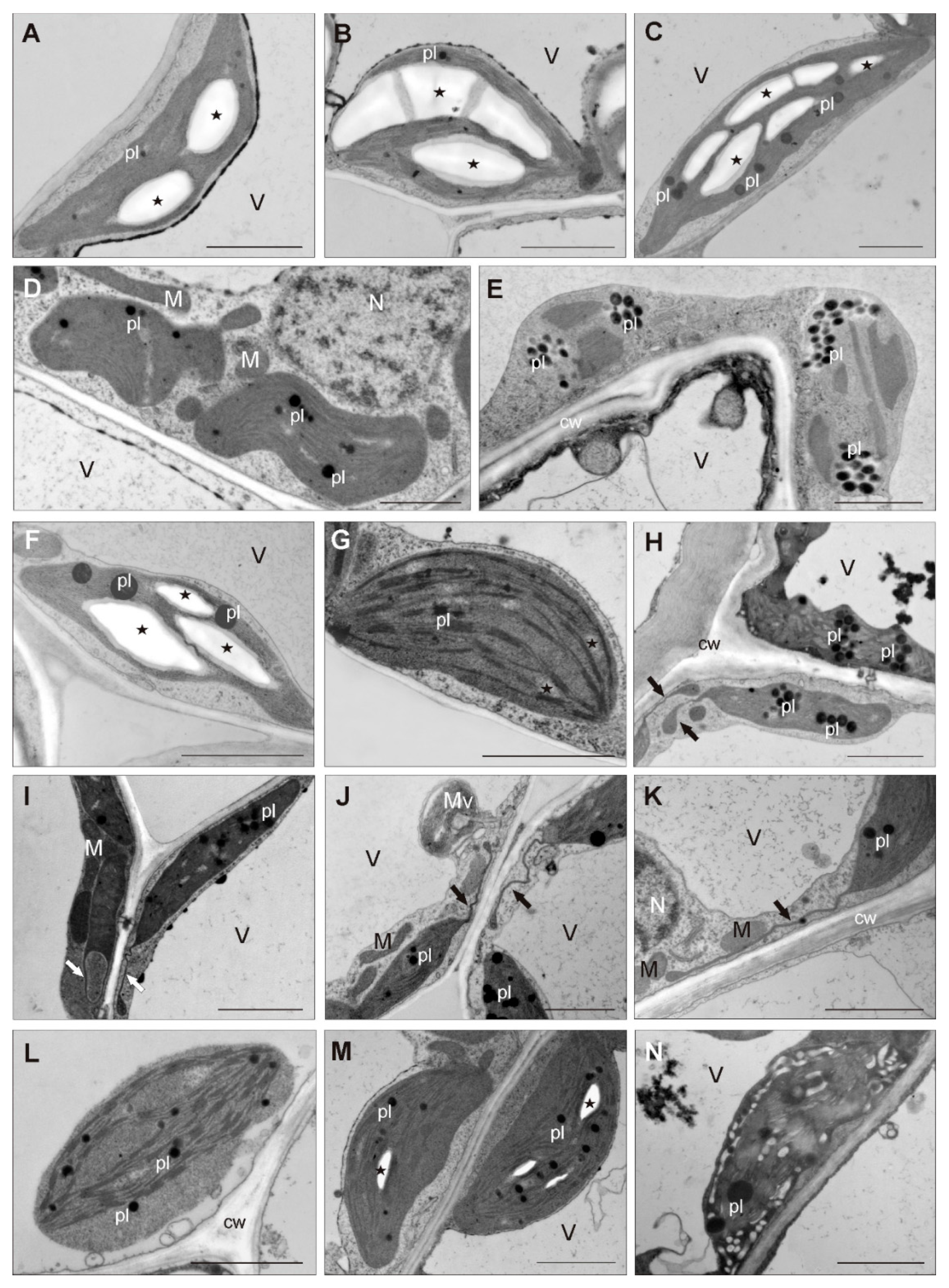
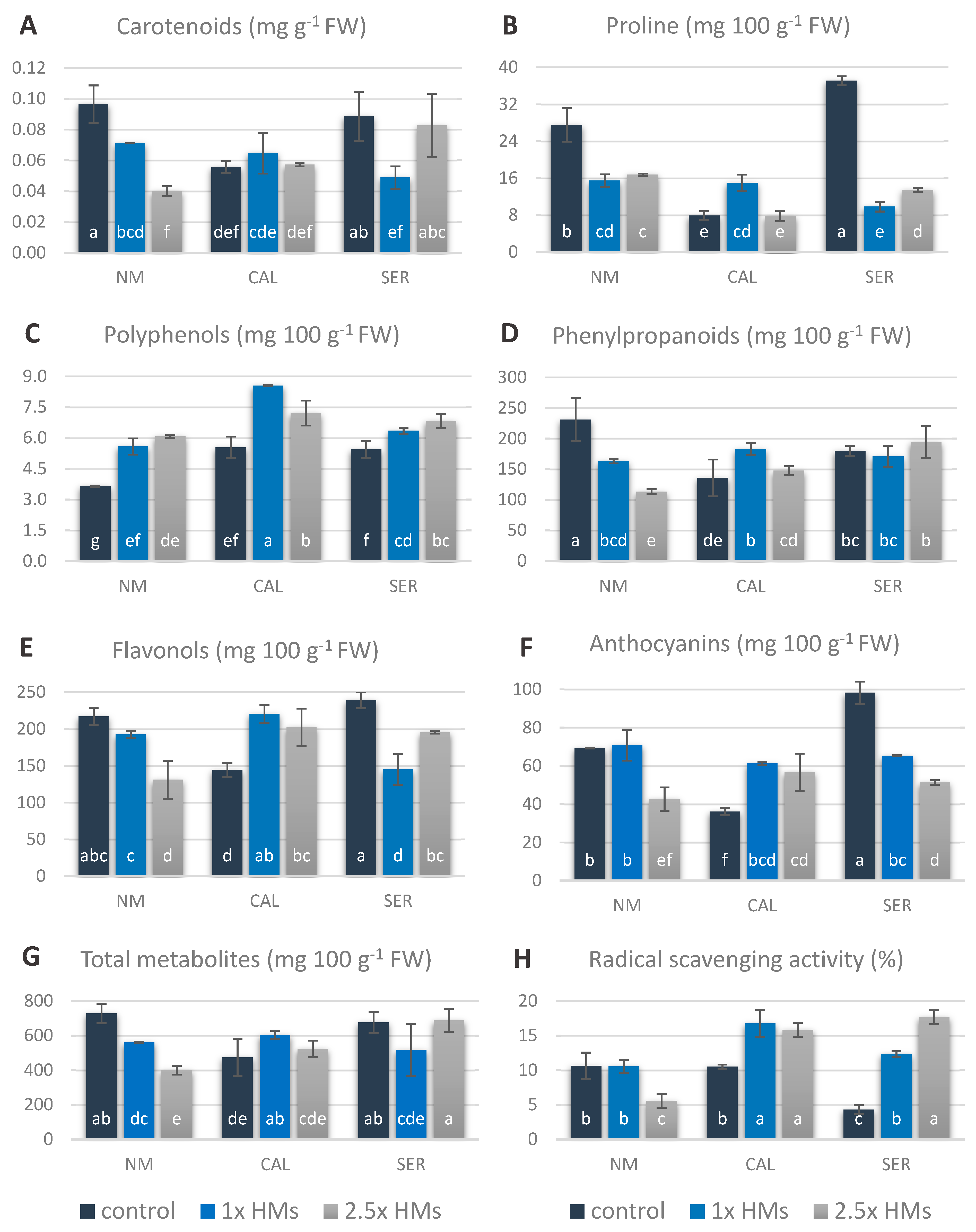


| Ecotype 1 | Treatment 2 | Minimum Length (mm) | Mean Length (mm) | Maximum Length (mm) | Median Value (mm) | Shoot FW (mg) | Shoot DW (mg) |
|---|---|---|---|---|---|---|---|
| NM | control | 7.78 | 21.76 ab* ± 9.94 | 47.47 | 18.73 | 1223.66 a* ± 81.74 | 119.06 a* ± 7.95 |
| 1× HMs | 8.14 | 27.67 a ± 15.33 | 69.75 | 24.03 | 896.65 b ± 78.62 | 104.55 b ± 9.16 | |
| 2.5× HMs | 9.02 | 16.98 bc ± 5.71 | 31.98 | 15.89 | 685.33 c ± 98.01 | 54.83 c ± 7.84 | |
| 5× HMs | 5.17 | 9.30 c ± 3.66 | 14.11 | 7.71 | 346.67 d ± 61.46 | 20.81 d ± 3.69 | |
| CAL | control | 7.70 | 32.78 b ± 21.12 | 106.01 | 25.43 | 947.00 b ± 23.51 | 94.87 b ± 2.36 |
| 1× HMs | 9.31 | 38.72 a ± 19.48 | 108.83 | 35.25 | 1252.67 a ± 57.55 | 132.91 a ± 6.11 | |
| 2.5× HMs | 7.17 | 16.71 c ± 0.04 | 45.70 | 14.00 | 658.67 c ± 44.77 | 64.65 c ± 3.84 | |
| 5× HMs | 4.58 | 9.88 c ± 6.05 | 26.90 | 7.11 | 505.13 d ± 14.11 | 57.33 c ± 4.51 | |
| SER | control | 11.95 | 39.23 a ± 22.50 | 105.73 | 33.59 | 742.67 a ± 58.86 | 90.01 a ± 7.13 |
| 1× HMs | 7.53 | 26.11 b ± 13.54 | 76.95 | 22.72 | 458.00 b ± 25.51 | 47.95 b ± 2.67 | |
| 2.5× HMs | 1.54 | 4.61 c ± 2.16 | 6.62 | 5.14 | 430.33 b ± 83.27 | 56.21 b ± 3.21 | |
| 5× HMs | n/a | n/a | n/a | n/a | n/a | n/a |
| Parameter | Ecotype 1 | Treatment 2 | ||
|---|---|---|---|---|
| Control | 1× HMs | 2.5× HMs | ||
| Chlorophyll a (mg g−1 FW) | NM | 0.436 a* ± 0.063 | 0.226 b ± 0.004 | 0.148 c ± 0.025 |
| CAL | 0.131 c ± 0.011 | 0.177 bc ± 0.014 | 0.132 c ± 0.006 | |
| SER | 0.389 a ± 0.060 | 0.163 c ± 0.016 | 0.236 b ±0.015 | |
| Chlorophyll b (mg g−1 FW) | NM | 0.121 a ± 0.024 | 0.082 bc ± 0.004 | 0.036 e ± 0.012 |
| CAL | 0.066 cd ± 0.006 | 0.047 de ± 0.006 | 0.040 e ± 0.002 | |
| SER | 0.089 b ± 0.016 | 0.046 de ± 0.006 | 0.065 cd ± 0.003 | |
| Chlorophyll a/b | NM | 3.557 cd ± 0.187 | 2.763 e ± 0.123 | 4.202 ab ± 0.636 |
| CAL | 1.993 f ± 0.026 | 4.080 abc ± 0.280 | 3.271 de ± 0.028 | |
| SER | 4.520 a ± 0.715 | 3.531 cd ± 0.167 | 3.644 bcd ± 0.075 | |
| Chlorophyll a+b/carotenoids | NM | 5.652 a ± 0.198 | 4.333 cd ± 0.094 | 4.594 bc ± 0.516 |
| CAL | 3.542 de ± 0.058 | 3.830 cde ± 0.772 | 3.006 de ± 0.194 | |
| SER | 5.539 ab ± 0.812 | 4.296 cd ± 0.208 | 3.765 cde ± 0.814 | |
| MDA (μmol g−1 FW) | NM | 37.301 e ± 0.510 | 56.925 c ± 1.078 | 73.548 a ± 2.183 |
| CAL | 59.258 c ± 1.860 | 62.925 cb ± 2.541 | 51.065 d ± 2.495 | |
| SER | 53.054 d ± 0.746 | 59.247 c ± 0.614 | 70.946 a ± 2.177 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muszyńska, E.; Labudda, M.; Kral, A. Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals. Antioxidants 2020, 9, 102. https://doi.org/10.3390/antiox9020102
Muszyńska E, Labudda M, Kral A. Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals. Antioxidants. 2020; 9(2):102. https://doi.org/10.3390/antiox9020102
Chicago/Turabian StyleMuszyńska, Ewa, Mateusz Labudda, and Adam Kral. 2020. "Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals" Antioxidants 9, no. 2: 102. https://doi.org/10.3390/antiox9020102
APA StyleMuszyńska, E., Labudda, M., & Kral, A. (2020). Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals. Antioxidants, 9(2), 102. https://doi.org/10.3390/antiox9020102







