Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems
Abstract
1. Introduction
1.1. Molecular Hydrogen and Its Potential Use in Therapy
1.2. Molecular Hydrogen and The Cardiovascular System
1.3. Molecular Hydrogen and the Central Nervous System
2. Mechanisms and Cellular Systems Involved in the Actions of Molecular Hydrogen
2.1. Molecular Hydrogen as Regulator of Redox Signaling
2.2. Molecular Hydrogen and Mitochondria
2.3. Molecular Hydrogen and Modulation of Intracellular Protein Kinases Signal Transduction
2.3.1. Molecular Hydrogen and the PI3K/Akt/GSK-3β Signaling Pathway
2.3.2. Effects of Molecular Hydrogen on Wnt/β-catenin Signaling
2.4. Effects of Hydrogen on Gene Expression Regulation
2.5. Molecular Hydrogen and Autophagy
2.6. Molecular Hydrogen and Matrix Metalloproteinases
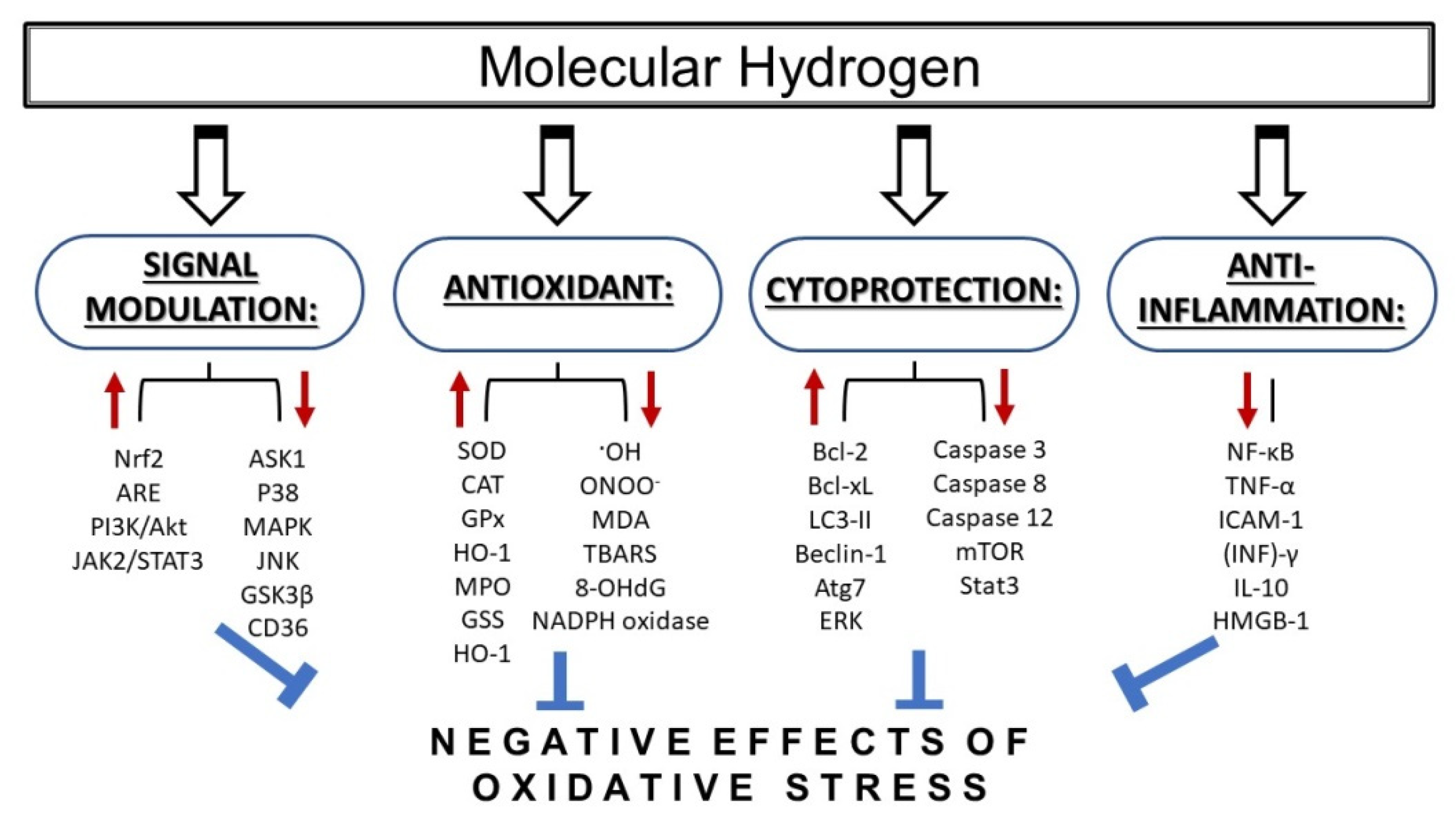
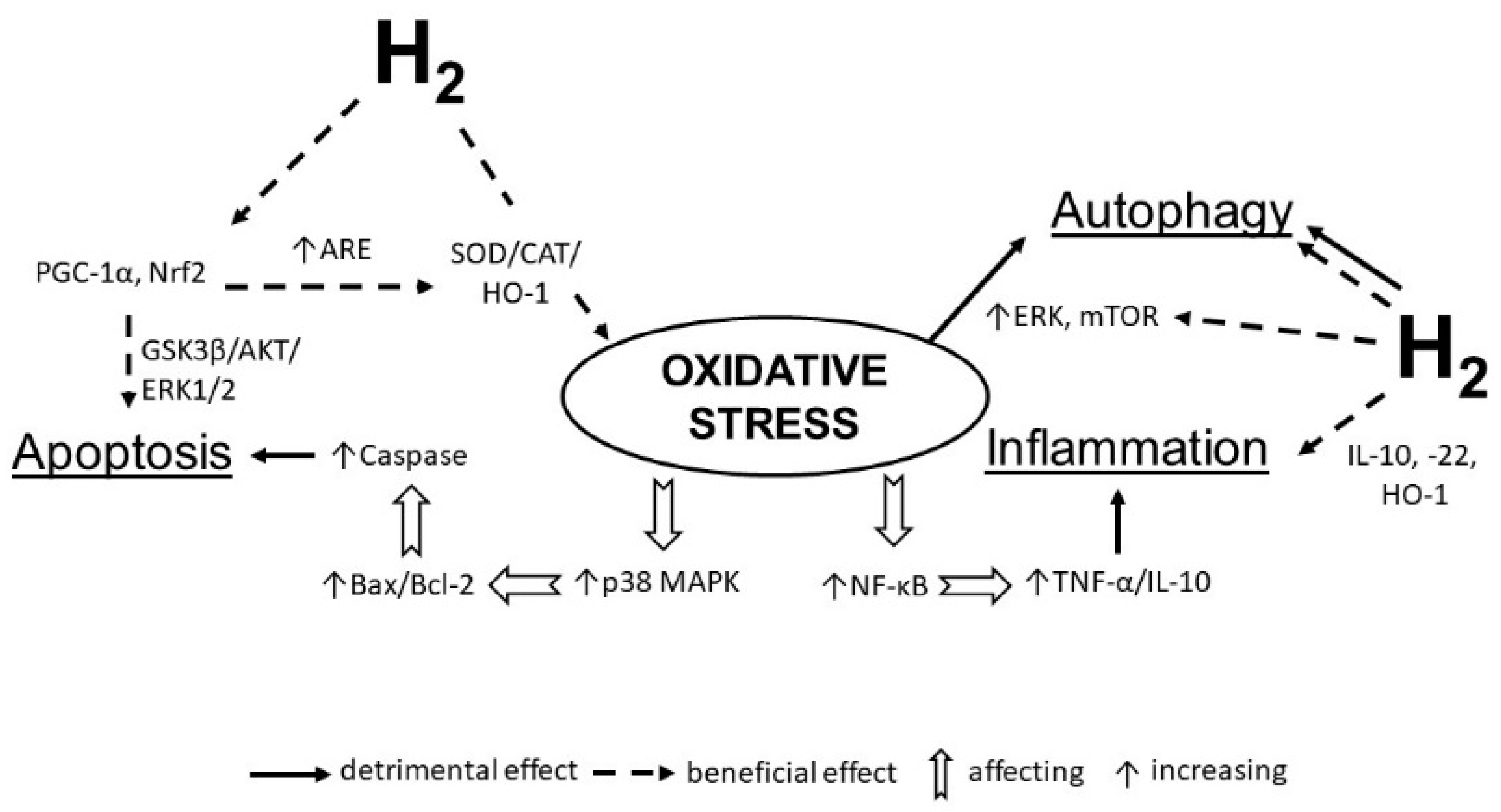
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Maekawa, Y.; Kawamura, A.; Abe, T.; Ohta, S.; Fukuda, K.; et al. H2 Gas Improves Functional Outcome After Cardiac Arrest to an Extent Comparable to Therapeutic Hypothermia in a Rat Model. J. Am. Heart Assoc. 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Zálešák, M.; Kura, B.; Graban, J.; Farkašová, V.; Slezák, J.; Ravingerová, T. Molecular hydrogen potentiates beneficial anti-infarct effect of hypoxic postconditioning in isolated rat hearts: A novel cardioprotective intervention. Can. J. Physiol. Pharmacol. 2017, 95, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, S.; Yuan, L.; Xie, Y.; Li, T.; Wang, L.; Wang, X.; Zhang, T.; Qin, S.; Song, G.; et al. Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 2016, 1646, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, Y.; Fan, X.; Chen, Y.; Yang, B.; Liu, K.X.; Zhou, J. Amelioration of Coagulation Disorders and Inflammation by Hydrogen-Rich Solution Reduces Intestinal Ischemia/Reperfusion Injury in Rats through NF- κ B/NLRP3 Pathway. Mediat. Inflamm. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ito, M.; Fukami, M.; Hashimoto, M.; Hirayama, M.; Ohno, K. Molecular hydrogen alleviates motor deficits and muscle degeneration in mdx mice. Redox Rep. 2017, 22, 26–34. [Google Scholar] [CrossRef]
- LeBaron, T.; Singh, R.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- Ming, Y.; Ma, Q.; Han, X.; Li, H. Molecular hydrogen improves type 2 diabetes through inhibiting oxidative stress. Exp. Ther. Med. 2020, 20, 359–366. [Google Scholar] [CrossRef]
- Qiu, X.; Ye, Q.; Sun, M.; Wang, L.; Tan, Y.; Wu, G. Saturated hydrogen improves lipid metabolism disorders and dysbacteriosis induced by a high-fat diet. Exp. Biol. Med. 2020, 245, 512–521. [Google Scholar] [CrossRef]
- Guan, P.; Sun, Z.-M.; Luo, L.-F.; Zhou, J.; Yang, S.; Zhao, Y.-S.; Yu, F.-Y.; An, J.-R.; Wang, N.; Ji, E.-S. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019, 225, 46–54. [Google Scholar] [CrossRef]
- Li, J.; Hong, Z.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front. Pharmacol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Meng, J.; Liu, L.; Wang, D.; Yan, Z.; Chen, G. Hydrogen gas represses the progression of lung cancer via down-regulating CD47. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia–reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M.; Takahashi, H.; Ohta, S.; Ohsawa, I. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, Y.; Sano, F.; Abe, T.; Tamura, T.; Fujisawa, T.; Shiraishi, Y.; Kohsaka, S.; Ueda, I.; Homma, K.; Suzuki, M.; et al. The Effects of Hydrogen Gas Inhalation on Adverse Left Ventricular Remodeling After Percutaneous Coronary Intervention for ST-Elevated Myocardial Infarction—First Pilot Study in Humans—. Circ. J. 2017, 81, 940–947. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef]
- Sakai, T.; Sato, B.; Hara, K.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T.; Ishibashi, T. Consumption of water containing over 3.5 mg of dissolved hydrogen could improve vascular endothelial function. Vasc. Health Risk Manag. 2014, 10, 591–597. [Google Scholar]
- Barancik, M.; Gresova, L.; Bartekova, M.; Dovinova, I. Nrf2 as a Key Player of Redox Regulation in Cardiovascular Diseases. Physiol. Res. 2016, 65, S1–S10. [Google Scholar] [CrossRef]
- Montezano, A.C.; Tsiropoulou, S.; Dulak-Lis, M.; Harvey, A.; Camargo, L.D.L.; Touyz, R.M. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 425–433. [Google Scholar] [CrossRef]
- Li, J.-M.; Gall, N.P.; Grieve, D.J.; Chen, M.; Shah, A.M. Activation of NADPH Oxidase During Progression of Cardiac Hypertrophy to Failure. Hypertension 2002, 40, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-X.; Ohmori, K.; Nagai, Y.; Fujisawa, Y.; Nishiyama, A.; Abe, Y.; Kimura, S. Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. J. Mol. Cell. Cardiol. 2007, 42, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef] [PubMed]
- van Zwieten, P. The influence of antihypertensive drug treatment on the prevention and regression of left ventricular hypertrophy. Cardiovasc. Res. 2000, 45, 82–91. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Montecucco, F.; Ashri, M.; Pelli, G.; Galan, K.; Frias, M.; Burger, F.; Quinderé, A.L.G.; Montessuit, C.; Krause, K.-H.; et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013, 64, 99–107. [Google Scholar] [CrossRef]
- Han, J.; Su, G.; Wang, Y.; Lu, Y.; Zhao, H.; Shuai, X. 18β-Glycyrrhetinic Acid Improves Cardiac Diastolic Function by Attenuating Intracellular Calcium Overload. Curr. Med. Sci. 2020, 40, 654–661. [Google Scholar] [CrossRef]
- Shanmugam, K.; Ravindran, S.; Kurian, G.A.; Rajesh, M. Fisetin Confers Cardioprotection against Myocardial Ischemia Reperfusion Injury by Suppressing Mitochondrial Oxidative Stress and Mitochondrial Dysfunction and Inhibiting Glycogen Synthase Kinase 3 β Activity. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Soltys, C.-L.M.; Rogan, K.J.; Chan, A.Y.M.; Nagendran, J.; Wang, S.; Dyck, J.R.B. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J. Mol. Med. 2015, 93, 413–425. [Google Scholar] [CrossRef]
- de Britto, R.M.; da Silva-Neto, J.A.; Mesquita, T.R.R.; de Vasconcelos, C.M.L.; de Almeida, G.K.M.; de Jesus, I.C.G.; dos Santos, P.H.; Souza, D.S.; Miguel-dos-Santos, R.; de Sá, L.A.; et al. Myrtenol protects against myocardial ischemia-reperfusion injury through antioxidant and anti-apoptotic dependent mechanisms. Food Chem. Toxicol. 2018, 111, 557–566. [Google Scholar] [CrossRef]
- Jin, L.; Sun, S.; Ryu, Y.; Piao, Z.H.; Liu, B.; Choi, S.Y.; Kim, G.R.; Kim, H.-S.; Kee, H.J.; Jeong, M.H. Gallic acid improves cardiac dysfunction and fibrosis in pressure overload-induced heart failure. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.P.; Cossu, M.; Rassu, G.; Giunchedi, P.; Cerri, G.; Pourová, J.; Najmanová, I.; Migkos, T.; Pilařová, V.; Nováková, L.; et al. Aqueous injection of quercetin: An approach for confirmation of its direct in vivo cardiovascular effects. Int. J. Pharm. 2018, 541, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Zheng, H. Chronic hydrogen-rich saline treatment reduces oxidative stress and attenuates left ventricular hypertrophy in spontaneous hypertensive rats. Mol. Cell. Biochem. 2012, 365, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Nomura, A.; Sakamoto, A.; Yasuda, Y.; Amatani, K.; Nagai, S.; Sen, Y.; Ijiri, Y.; Okada, Y.; Yamaguchi, T.; et al. Hydrogen gas attenuates embryonic gene expression and prevents left ventricular remodeling induced by intermittent hypoxia in cardiomyopathic hamsters. Am. J. Physiol. Circ. Physiol. 2014, 307, H1626–H1633. [Google Scholar] [CrossRef]
- Yao, L.; Chen, H.; Wu, Q.; Xie, K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int. J. Mol. Med. 2019, 44, 1048–1062. [Google Scholar] [CrossRef]
- Yoshida, A.; Asanuma, H.; Sasaki, H.; Sanada, S.; Yamazaki, S.; Asano, Y.; Shinozaki, Y.; Mori, H.; Shimouchi, A.; Sano, M.; et al. H2 Mediates Cardioprotection Via Involvements of KATP Channels and Permeability Transition Pores of Mitochondria in Dogs. Cardiovasc. Drugs Ther. 2012, 26, 217–226. [Google Scholar] [CrossRef]
- Colareda, G.A.; Ragone, M.I.; Bonazzola, P.; Consolini, A.E. The mKATP Channels and protein-kinase C Are Involved in the Cardioprotective Effects of Genistein on Estrogen-Deficient Rat Hearts Exposed to Ischemia/Reperfusion. J. Cardiovasc. Pharmacol. 2020, 75, 460–474. [Google Scholar] [CrossRef]
- Simoncíková, P.; Ravingerová, T.; Andelová, E.; Tribulová, N.; Barancík, M. Changes in rat myocardium associated with modulation of ischemic tolerance by diazoxide. Gen. Physiol. Biophys. 2007, 26, 75–85. [Google Scholar]
- Gao, Y.; Yang, H.; Chi, J.; Xu, Q.; Zhao, L.; Yang, W.; Liu, W.; Yang, W. Hydrogen Gas Attenuates Myocardial Ischemia Reperfusion Injury Independent of Postconditioning in Rats by Attenuating Endoplasmic Reticulum Stress-Induced Autophagy. Cell. Physiol. Biochem. 2017, 43, 1503–1514. [Google Scholar] [CrossRef]
- Song, D.; Liu, X.; Diao, Y.; Sun, Y.; Gao, G.; Zhang, T.; Chen, K.; Pei, L. Hydrogen-rich solution against myocardial injury and aquaporin expression via the PI3K/Akt signaling pathway during cardiopulmonary bypass in rats. Mol. Med. Rep. 2018, 18, 1925–1938. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Y.; Diao, Y.; Zhang, T.; Dong, W. Hydrogen-rich solution attenuates myocardial injury caused by cardiopulmonary bypass in rats via the Janus-activated kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 2018, 16, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood?brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood–Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Chiba, Y.; Matsumoto, K.; Murakami, R.; Fujihara, R.; Kawauchi, M.; Miyanaka, H.; Nakagawa, T. Blood-brain barrier damage in vascular dementia. Neuropathology 2016, 36, 115–124. [Google Scholar] [CrossRef]
- Wang, M.; Norman, J.E.; Srinivasan, V.J.; Rutledge, J.C. Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am. J. Neurodegener. Dis. 2016, 5, 171–177. [Google Scholar]
- Rochfort, K.D.; Cummins, P.M. The blood–brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Arima, Y.; Harada, M.; Kamimura, D.; Park, J.-H.; Kawano, F.; Yull, F.E.; Kawamoto, T.; Iwakura, Y.; Betz, U.A.K.; Márquez, G.; et al. Regional Neural Activation Defines a Gateway for Autoreactive T Cells to Cross the Blood-Brain Barrier. Cell 2012, 148, 447–457. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6–regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef]
- Jazwa, A.; Cuadrado, A. Targeting Heme Oxygenase-1 for Neuroprotection and Neuroinflammation in Neurodegenerative Diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef]
- Papa, S.; Zazzeroni, F.; Pham, C.; Bubici, C.; Franzoso, G. Linking JNK signaling to NF- B: A key to survival. J. Cell Sci. 2004, 117, 5197–5208. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Ma, Q.; Zhang, J.H.; Tang, J. Hydrogen Inhalation Ameliorated Mast Cell–Mediated Brain Injury After Intracerebral Hemorrhage in Mice. Crit. Care Med. 2013, 41, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med. Gas Res. 2011, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Lin, Y.; Ohkawara, B.; Ito, M.; Misawa, N.; Miyamoto, K.; Takegami, Y.; Masuda, A.; Toyokuni, S.; Ohno, K. Molecular hydrogen suppresses activated Wnt/β-catenin signaling. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Ito, M.; Iwamoto, T.; Qiao, S.; Ohkuwa, T.; Ichihara, M. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell. Biochem. 2015, 403, 231–241. [Google Scholar] [CrossRef]
- Hanaoka, T.; Kamimura, N.; Yokota, T.; Takai, S.; Ohta, S. Molecular hydrogen protects chondrocytes from oxidative stress and indirectly alters gene expressions through reducing peroxynitrite derived from nitric oxide. Med. Gas Res. 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Kiyoi, T.; Liu, S.; Takemasa, E.; Nakaoka, H.; Hato, N.; Mogi, M. Constitutive hydrogen inhalation prevents vascular remodeling via reduction of oxidative stress. PLoS ONE 2020, 15, e0227582. [Google Scholar] [CrossRef]
- Liu, Y.; Croft, K.D.; Hodgson, J.M.; Mori, T.; Ward, N.C. Mechanisms of the protective effects of nitrate and nitrite in cardiovascular and metabolic diseases. Nitric Oxide 2020, 96, 35–43. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, M.; Chen, Z.; Wu, G.; Fujino, M.; Zhang, C.; Zhou, W.; Zhao, M.; Hirano, S.; Li, X.-K.; et al. Hydrogen Gas Attenuates Hypoxic-Ischemic Brain Injury via Regulation of the MAPK/HO-1/PGC-1a Pathway in Neonatal Rats. Oxid. Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-S.; Zhang, Q.-Z.; Li, H.; Bai, G.; Jiao, Z.-H.; Wang, H.-B. Hydrogen-rich saline protects against hepatic injury induced by ischemia-reperfusion and laparoscopic hepatectomy in swine. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Wang, M.-H.; Chen, Y.; Fan, X.; Yang, B.; Du, J.; Wang, X.-B.; Liu, K.-X.; Zhou, J. Hydrogen-Rich Saline Attenuates Acute Lung Injury Induced by Limb Ischemia/Reperfusion via Down-Regulating Chemerin and NLRP3 in Rats. Shock 2019, 52, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, W.; Xie, K.; Liu, W.; Qu, Y.; Chao, X.; Chen, T.; Zhou, J.; Fei, Z. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2010, 1354, 196–205. [Google Scholar] [CrossRef]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; Lebaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharmacol. 2019, 97, 287–292. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.-S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, 646–656. [Google Scholar] [CrossRef]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Jain, A.K.; Jaiswal, A.K. GSK-3β acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007, 282, 16502–16510. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huo, R.; Cai, B.; Lu, Y.; Ye, B.; Li, X.; Li, F.; Xu, H. Activation of Wnt/β-catenin signaling by hydrogen peroxide transcriptionally inhibits NaV1.5 expression. Free Radic. Biol. Med. 2016, 96, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, F.; Guo, R.; Zhang, Y.; Qu, X.; Wu, X.; Yao, R. Hydrogen-Rich Saline Ameliorates Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice Via the Nrf2-ARE Signaling Pathway. Inflammation 2019, 42, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Bellance, N.; Jose, C.; Melser, S.; Nouette-Gaulain, K.; Rossignol, R. Multi-site control and regulation of mitochondrial energy production. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Morciano, G.; Giorgi, C.; Bonora, M.; Punzetti, S.; Pavasini, R.; Wieckowski, M.R.; Campo, G.; Pinton, P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 142–153. [Google Scholar] [CrossRef]
- Shen, J.; Xu, S.; Zhou, H.; Liu, H.; Jiang, W.; Hao, J.; Hu, Z. IL-1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef]
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Jovaisaite, V.; Mouchiroud, L.; Auwerx, J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 2014, 217, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sobue, S.; Inoue, C.; Hori, F.; Qiao, S.; Murate, T.; Ichihara, M. Molecular hydrogen modulates gene expression via histone modification and induces the mitochondrial unfolded protein response. Biochem. Biophys. Res. Commun. 2017, 493, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Nishimaki, K.; Kamimura, N.; Ohta, S. Molecular hydrogen suppresses free-radical-induced cell death by mitigating fatty acid peroxidation and mitochondrial dysfunction. Can. J. Physiol. Pharmacol. 2019, 97, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yu, Y.; Li, B.; Gu, X.; Xie, K.; Wang, G.; Yu, Y. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuropathy via activation of the mitochondrial ATP-sensitive potassium channel channels in rats. Mol. Med. Rep. 2020, 21, 282–290. [Google Scholar] [CrossRef]
- Szewczyk, A. The ATP-regulated K+ channel in mitochondria: Five years after its discovery. Acta Biochim. Pol. 1996, 43, 713–720. [Google Scholar] [CrossRef]
- Nozawa, Y.; Miura, T.; Miki, T.; Ohnuma, Y.; Yano, T.; Shimamoto, K. Mitochondrial K ATP channel-dependent and -independent phases of ischemic preconditioning against myocardial infarction in the rat. Basic Res. Cardiol. 2003, 98, 50–58. [Google Scholar] [CrossRef]
- Bajgar, R.; Seetharaman, S.; Kowaltowski, A.J.; Garlid, K.D.; Paucek, P. Identification and Properties of a Novel Intracellular (Mitochondrial) ATP-sensitive Potassium Channel in Brain. J. Biol. Chem. 2001, 276, 33369–33374. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, Y.; Wang, Y.; Meng, X.; Wang, Y.; Yu, Y.; Chen, H. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm. Res. 2020, 69, 697–710. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Kura, B.; Vančová, O.; Rausová, Z.; Sumbalová, Z.; Uličná, O.; Slezák, J. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Can. J. Physiol. Pharmacol. 2020, 98, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, G.; Kim, Y.-J.; Tran, Q.H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Hydrogen-rich medium protects mouse embryonic fibroblasts from oxidative stress by activating LKB1-AMPK-FoxO1 signal pathway. Biochem. Biophys. Res. Commun. 2017, 491, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Zhang, Z.; Liu, L.; Liu, T.; Li, S.; Liu, S.; Zhou, Y.; Liu, F. Effects of Hydrogen-rich Water on the PI3K/AKT Signaling Pathway in Rats with Myocardial Ischemia-reperfusion Injury. Curr. Mol. Med. 2020, 20, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Long, Z.; Wang, C.; Wang, L.; Sun, P.; Li, P.; Wang, T. Hydrogen (H2) inhibits isoproterenol-induced cardiac hypertrophy via antioxidative pathways. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Cuadrado, A.; Kügler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Teng, L.; Meng, Q.; Lu, J.; Xie, J.; Wang, Z.; Liu, Y.; Wang, D. Liquiritin modulates ERK- and AKT/GSK-3β-dependent pathways to protect against glutamate-induced cell damage in differentiated PC12 cells. Mol. Med. Rep. 2014, 10, 818–824. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Zhao, W.; Guo, L.; Li, X.; Li, Y.; Zhang, X.; Sun, Y. Gypenoside Protects against Myocardial Ischemia-Reperfusion Injury by Inhibiting Cardiomyocytes Apoptosis via Inhibition of CHOP Pathway and Activation of PI3K/Akt Pathway in Vivo and in Vitro. Cell. Physiol. Biochem. 2016, 39, 123–136. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Liu, H. The role of Wnt/β-catenin pathway in the protection process by dexmedetomidine against cerebral ischemia/reperfusion injury in rats. Life Sci. 2019, 236. [Google Scholar] [CrossRef]
- Liu, T.; Fang, Y.; Liu, S.; Yu, X.; Zhang, H.; Liang, M.; Ding, X. Limb ischemic preconditioning protects against contrast-induced acute kidney injury in rats via phosphorylation of GSK-3β. Free Radic. Biol. Med. 2015, 81, 170–182. [Google Scholar] [CrossRef]
- Sathiya Priya, C.; Vidhya, R.; Kalpana, K.; Anuradha, C.V. Indirubin-3′-monoxime prevents aberrant activation of GSK-3β/NF-κB and alleviates high fat-high fructose induced Aβ-aggregation, gliosis and apoptosis in mice brain. Int. Immunopharmacol. 2019, 70, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, G.; Zhou, X.; Wang, C.; Qiao, Y.; Liao, D.; Shi, D. Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3β/β-catenin pathway. Chemosphere 2019, 214, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Z.; Wang, F.; Han, Z.; Wang, Y.; Huang, S.; Hu, T.; Guo, M.; Lei, P. Hydrogen exerts neuroprotection by activation of the miR-21/PI3K/AKT/GSK-3β pathway in an in vitro model of traumatic brain injury. J. Cell. Mol. Med. 2020, 24, 4061–4071. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, N.; Diao, Y.; Dong, W.; Sun, Y.; Liu, L.; Wu, X. Hydrogen-Rich Saline Attenuates Brain Injury Induced by Cardiopulmonary Bypass and Inhibits Microvascular Endothelial Cell Apoptosis Via the PI3K/Akt/GSK3β Signaling Pathway in Rats. Cell. Physiol. Biochem. 2017, 43, 1634–1647. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Miao, H.; Chen, X.; Sun, X.; Li, Y. Hydrogen-rich saline attenuates vascular smooth muscle cell proliferation and neointimal hyperplasia by inhibiting reactive oxygen species production and inactivating the Ras-ERK1/2-MEK1/2 and Akt pathways. Int. J. Mol. Med. 2013, 31, 597–606. [Google Scholar] [CrossRef]
- Hong, Y.; Shao, A.W.; Wang, J.; Chen, S.; Wu, H.J.; McBride, D.W.; Wu, Q.; Sun, X.J.; Zhang, J.M. Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: Possible role of the Akt/GSK3β signaling pathway. PLoS ONE 2014, 9, e96212. [Google Scholar] [CrossRef]
- Chen, O.; Cao, Z.; Li, H.; Ye, Z.; Zhang, R.; Zhang, N.; Huang, J.; Zhang, T.; Wang, L.; Han, L.; et al. High-concentration hydrogen protects mouse heart against ischemia/reperfusion injury through activation of thePI3K/Akt1 pathway. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Ke, H.; Liu, D.; Li, T.; Chu, X.; Xin, D.; Han, M.; Wang, S.; Wang, Z. Hydrogen-Rich Saline Regulates Microglial Phagocytosis and Restores Behavioral Deficits Following Hypoxia-Ischemia Injury in Neonatal Mice via the Akt Pathway. Drug Des. Devel. Ther. 2020, 14, 3827–3839. [Google Scholar] [CrossRef]
- Voloshanenko, O.; Schwartz, U.; Kranz, D.; Rauscher, B.; Linnebacher, M.; Augustin, I.; Boutros, M. β-catenin-independent regulation of Wnt target genes by RoR2 and ATF2/ATF4 in colon cancer cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Liu, B.; Pan, W.; Farr, G.H.; Flynn, C.; Yuan, H.; Takada, S.; Kimelman, D.; Li, L.; et al. Low-Density Lipoprotein Receptor-Related Protein-5 Binds to Axin and Regulates the Canonical Wnt Signaling Pathway. Mol. Cell 2001, 7, 801–809. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, J.-J.; Shi, K.-H.; Li, J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism 2016, 65, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, V.; Lee, S.H.; Inge, L.; Guo, L.; Goldbeck, C.; Garrett, E.; Wiesmann, M.; Garcia, P.D.; Fuller, J.H.; Chan, V.; et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003, 63, 3145–3153. [Google Scholar]
- Lévy, L.; Neuveut, C.; Renard, C.-A.; Charneau, P.; Branchereau, S.; Gauthier, F.; Van Nhieu, J.T.; Cherqui, D.; Petit-Bertron, A.-F.; Mathieu, D.; et al. Transcriptional Activation of Interleukin-8 by β-Catenin-Tcf4. J. Biol. Chem. 2002, 277, 42386–42393. [Google Scholar] [CrossRef]
- Patapoutian, A.; Backus, C.; Kispert, A.; Reichardt, L.F. Regulation of Neurotrophin-3 Expression by Epithelial-Mesenchymal Interactions: The Role of Wnt Factors. Science 1999, 283, 1180–1183. [Google Scholar] [CrossRef][Green Version]
- Yi, H.; Hu, J.; Qian, J.; Hackam, A.S. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport 2012, 23, 189–194. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Edeling, M.; Ragi, G.; Huang, S.; Pavenstädt, H.; Susztak, K. Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016, 12, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Chen, W.; Zhang, Y.; Yang, P.; Liu, L. Tripterygium wilfordii mitigates hyperglycemia-induced upregulated Wnt/β-catenin expression and kidney injury in diabetic rats. Exp. Ther. Med. 2018, 15, 3874–3882. [Google Scholar]
- Luo, J.; Chen, J.; Deng, Z.-L.; Luo, X.; Song, W.-X.; Sharff, K.A.; Tang, N.; Haydon, R.C.; Luu, H.H.; He, T.-C. Wnt signaling and human diseases: What are the therapeutic implications? Lab. Investig. 2007, 87, 97–103. [Google Scholar] [CrossRef]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Baarsma, H.A.; Königshoff, M. WNT Signaling in Lung Aging and Disease. Ann. Am. Thorac. Soc. 2016, 13, S411–S416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Shao, R.; Li, W. Cdc42-interacting protein 4 silencing relieves pulmonary fibrosis in STZ-induced diabetic mice via the Wnt/GSK-3β/β-catenin pathway. Exp. Cell Res. 2017, 359, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Hua, X.; He, K.; Zhou, T.; Zhang, J.; Wu, H.; Ma, X.; Xia, Q.; Zhang, J. Inhibition of GSK-3β induces AP-1-mediated osteopontin expression to promote cholestatic liver fibrosis. FASEB J. 2018, 32, 4494–4503. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gupte, M.; Umbarkar, P.; Singh, A.P.; Sui, J.Y.; Force, T.; Lal, H. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J. Mol. Cell. Cardiol. 2017, 110, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Tang, L.; Wang, G.; Lin, J.; Liao, W.; Pan, W.; Xu, J. Molecular Hydrogen Protects Human Melanocytes from Oxidative Stress by Activating Nrf2 Signaling. J. Investig. Dermatol. 2020, S0022-202X, 31206–31209. [Google Scholar] [CrossRef] [PubMed]
- Kühl, M.; Sheldahl, L.C.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway. Trends Genet. 2000, 16, 279–283. [Google Scholar] [CrossRef]
- Bergmann, M.W. WNT Signaling in Adult Cardiac Hypertrophy and Remodeling. Circ. Res. 2010, 107, 1198–1208. [Google Scholar] [CrossRef]
- Uehara, S.; Udagawa, N.; Mukai, H.; Ishihara, A.; Maeda, K.; Yamashita, T.; Murakami, K.; Nishita, M.; Nakamura, T.; Kato, S.; et al. Protein kinase N3 promotes bone resorption by osteoclasts in response to Wnt5a-Ror2 signaling. Sci. Signal. 2017, 10, eaan0023. [Google Scholar] [CrossRef]
- Mulligan, K.A.; Cheyette, B.N.R. Wnt Signaling in Vertebrate Neural Development and Function. J. Neuroimmune Pharmacol. 2012, 7, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, Y.-G. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef]
- Santos, A.; Bakker, A.D.; de Blieck-Hogervorst, J.M.A.; Klein-Nulend, J. WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase Rock. Cytotherapy 2010, 12, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Lu, J.; Wang, P.; Chen, J.; Guo, Y.; Han, F.; Wang, J.; Li, W.; Liu, P. sFRP1 protects H9c2 cardiac myoblasts from doxorubicin-induced apoptosis by inhibiting the Wnt/PCP-JNK pathway. Acta Pharmacol. Sin. 2020, 41, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Sheldahl, L.C.; Slusarski, D.C.; Pandur, P.; Miller, J.R.; Kühl, M.; Moon, R.T. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 2003, 161, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, W.; Chen, H.; Han, H.; Liu, D.; Wang, G.; Yu, Y. Hydrogen-rich medium attenuated lipopolysaccharide-induced monocyte-endothelial cell adhesion and vascular endothelial permeability via rho-associated coiled-coil protein kinase. Shock 2015, 44, 58–64. [Google Scholar] [CrossRef]
- Yang, T.; Wang, L.; Sun, R.; Chen, H.; Zhang, H.; Yu, Y.; Wang, Y.; Wang, G.; Yu, Y.; Xie, K. Hydrogen-rich medium ameliorates lipopolysaccharide-induced barrier dysfunction via rhoa-mdia1 signaling in caco-2 cells. Shock 2016, 45, 228–237. [Google Scholar] [CrossRef]
- Kawamura, T.; Huang, C.S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation 2010, 90, 1344–1351. [Google Scholar] [CrossRef]
- Cai, J.; Kang, Z.; Liu, W.W.; Luo, X.; Qiang, S.; Zhang, J.H.; Ohta, S.; Sun, X.; Xu, W.; Tao, H.; et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia–ischemia rat model. Neurosci. Lett. 2008, 441, 167–172. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Z.; Cai, J.; Liu, W.; Liu, Y.; Zhang, J.H.; Denoble, P.J.; Tao, H.; Sun, X. Hydrogen-Rich Saline Protects Myocardium Against Ischemia/Reperfusion Injury in Rats. Exp. Biol. Med. 2009, 234, 1212–1219. [Google Scholar] [CrossRef]
- Chen, H.; Xie, K.; Han, H.; Li, Y.; Liu, L.; Yang, T.; Yu, Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int. Immunopharmacol. 2015, 28, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, K.; Chen, H.; Dong, X.; Li, Y.; Yu, Y.; Wang, G.; Yu, Y. Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Res. 2014, 1589, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Y.; Bian, Y.; Li, Y.; Liu, L.; Zhang, H.; Xie, K.; Wang, G.; Yu, Y. Hydrogen Gas Protects Against Intestinal Injury in Wild Type But Not NRF2 Knockout Mice With Severe Sepsis by Regulating HO-1 and HMGB1 Release. Shock 2017, 48, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Manaenko, A.; Zhan, Y.; Liu, W.W.; Ostrowki, R.P.; Tang, J.; Zhang, J.H. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience 2010, 169, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Németh, J.; Oláh, O.; Tóth-Szűki, V.; Kovács, V.; Remzső, G.; Domoki, F. Molecular hydrogen alleviates asphyxia-induced neuronal cyclooxygenase-2 expression in newborn pigs. Acta Pharmacol. Sin. 2018, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Hugyecz, M.; Mracskó, É.; Hertelendy, P.; Farkas, E.; Domoki, F.; Bari, F. Hydrogen supplemented air inhalation reduces changes of prooxidant enzyme and gap junction protein levels after transient global cerebral ischemia in the rat hippocampus. Brain Res. 2011, 1404, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, S.; Zhu, L.; Cai, J.; Fu, L. Hydrogen-containing saline alleviates pressure overload-induced interstitial fibrosis and cardiac dysfunction in rats. Mol. Med. Rep. 2017, 16, 1771–1778. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by Self-Digestion. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Carloni, S.; Albertini, M.C.; Galluzzi, L.; Buonocore, G.; Proietti, F.; Balduini, W. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia–ischemia: Role of protein synthesis and autophagic pathways. Exp. Neurol. 2014, 255, 103–112. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy Is Activated for Cell Survival after Endoplasmic ReticulumStress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Gomez, I.G.; Ren, S.; Hudkins, K.; Roach, A.; Alpers, C.E.; Shankland, S.J.; D’Agati, V.D.; Duffield, J.S. Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J. Am. Soc. Nephrol. 2015, 26, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, A.; Sil, S.; Tripathi, A.; Chivero, E.T.; Periyasamy, P.; Buch, S. Targeting endoplasmic reticulum stress and autophagy as therapeutic approaches for neurological diseases. Int. Rev. Cell Mol. Biol. 2020, 350, 285–325. [Google Scholar] [PubMed]
- Shi, W.; Guo, Z.; Yuan, R. Testicular Injury Attenuated by Rapamycin Through Induction of Autophagy and Inhibition of Endoplasmic Reticulum Stress in Streptozotocin- Induced Diabetic Rats. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 665–675. [Google Scholar] [CrossRef]
- Guan, G.; Yang, L.; Huang, W.; Zhang, J.; Zhang, P.; Yu, H.; Liu, S.; Gu, X. Mechanism of interactions between endoplasmic reticulum stress and autophagy in hypoxia/reoxygenation-induced injury of H9c2 cardiomyocytes. Mol. Med. Rep. 2019, 20, 350–358. [Google Scholar] [CrossRef]
- Xu, H.-D.; Qin, Z.-H. Beclin 1, Bcl-2 and Autophagy. In Autophagy: Biology and Diseases; Xu, H.D., Qin, Z.H., Eds.; Springer: Singapore, 2019; pp. 109–126. [Google Scholar]
- Nagatani, K.; Wada, K.; Takeuchi, S.; Kobayashi, H.; Uozumi, Y.; Otani, N.; Fujita, M.; Tachibana, S.; Nawashiro, H. Effect of Hydrogen Gas on the Survival Rate of Mice Following Global Cerebral Ischemia. Shock 2012, 37, 645–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Z.; Xu, J.; Tan, S.; Zhang, N.; Li, A.; Wang, L.; Wang, T. Hydrogen inhibits isoproterenol-induced autophagy in cardiomyocytes in vitro and in vivo. Mol. Med. Rep. 2017, 16, 8253–8258. [Google Scholar] [CrossRef]
- Jiang, X.; Niu, X.; Guo, Q.; Dong, Y.; Xu, J.; Yin, N.; Qi, Q.; Jia, Y.; Gao, L.; He, Q.; et al. FoxO1-mediated autophagy plays an important role in the neuroprotective effects of hydrogen in a rat model of vascular dementia. Behav. Brain Res. 2019, 356, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-Rich Saline Attenuates Acute Kidney Injury After Liver Transplantation via Activating p53-Mediated Autophagy. Transplantation 2016, 100, 563–570. [Google Scholar] [CrossRef]
- Wang, H.; Huo, X.; Chen, H.; Li, B.; Liu, J.; Ma, W.; Wang, X.; Xie, K.; Yu, Y.; Shi, K. Hydrogen-Rich Saline Activated Autophagy via HIF-1 α Pathways in Neuropathic Pain Model. Biomed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.V.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tan, J.; Zhang, Q. Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol. Int. 2015, 39, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Morten, M.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Ngo, D.T.; Sverdlov, A.L.; Knight, D.A.; et al. The Role of Pathological Aging in Cardiac and Pulmonary Fibrosis. Aging Dis. 2019, 10, 419–428. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar] [CrossRef]
- Sun, Q.; Kawamura, T.; Masutani, K.; Peng, X.; Sun, Q.; Stolz, D.B.; Pribis, J.P.; Billiar, T.R.; Sun, X.; Bermudez, C.A.; et al. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc. Res. 2012, 94, 144–153. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants 2020, 9, 1281. https://doi.org/10.3390/antiox9121281
Barancik M, Kura B, LeBaron TW, Bolli R, Buday J, Slezak J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants. 2020; 9(12):1281. https://doi.org/10.3390/antiox9121281
Chicago/Turabian StyleBarancik, Miroslav, Branislav Kura, Tyler W. LeBaron, Roberto Bolli, Jozef Buday, and Jan Slezak. 2020. "Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems" Antioxidants 9, no. 12: 1281. https://doi.org/10.3390/antiox9121281
APA StyleBarancik, M., Kura, B., LeBaron, T. W., Bolli, R., Buday, J., & Slezak, J. (2020). Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants, 9(12), 1281. https://doi.org/10.3390/antiox9121281







