Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives
Abstract
1. Introduction
2. Radical Interactions and Antioxidant Properties Assessment
2.1. Radicals Formation and Antioxidant Action
2.2. Antioxidant Assays
In Vitro Antioxidant Assays
| Antioxidant In Vitro Assay a,b | Outcome of the Assay | Reference |
|---|---|---|
| DPPH assay | Production of reduced DPPH (Colorimetry) | [48] |
| 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) ABTS*+ | Production of reduced ABTS | [61] |
| Ferric reducing antioxidant power (FRAP) | Colorimetric method. Production of reduced Fe2+ (TPTZ (2,4,6-tri(2-pyridyl)- 1,3,5-triazine) | [45] |
| Cupric ion reducing antioxidant capacity (CUPRAC) assay | Reduction of cupric ions to cuprous ions | [62] |
| Oxygen radical absorbance capacity (ORAC)(HAT) | Fluorescence generation | [63] |
| Thiobarbituric acid reactive substance (TBARS) assay | Fluorescence generation | [56] |
| Lipid peroxidation inhibition capacity (LPIC) | Fluorescence generation | [57] |
| Hydroxyl radical scavenging activity | Scavenging activity of antioxidants measured spectrophotometrically | [55] |
| NO free radical scavenging activity | Spectrophotometric method | [58] |
| Metal chelating activity | Decolorization of iron-ferrozine complex with fungal extracts | [64] |
3. Class of Marine Fungi Derived Antioxidant Molecules: Activities and Extraction Methodologies
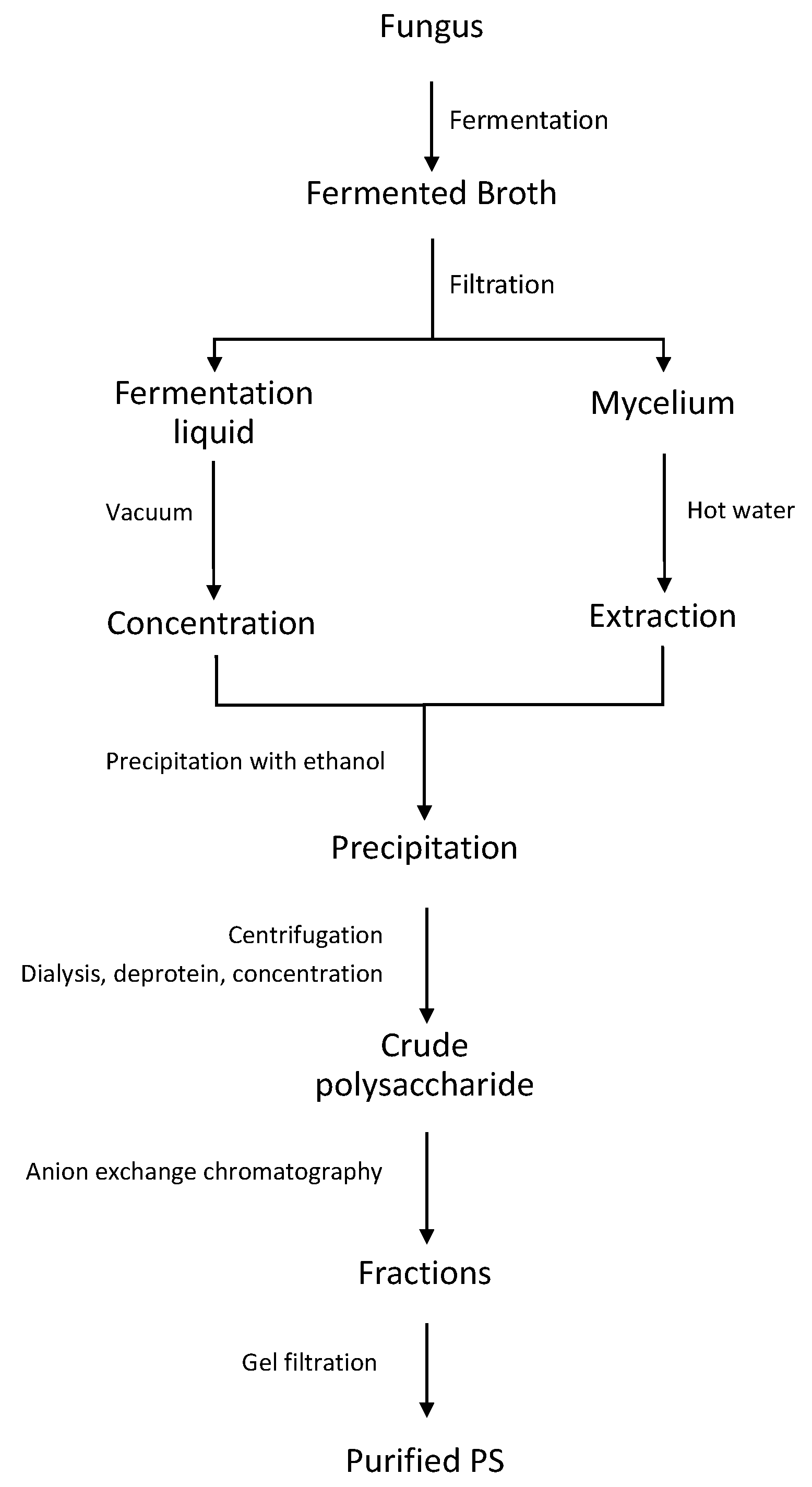
3.1. Carbohydrates
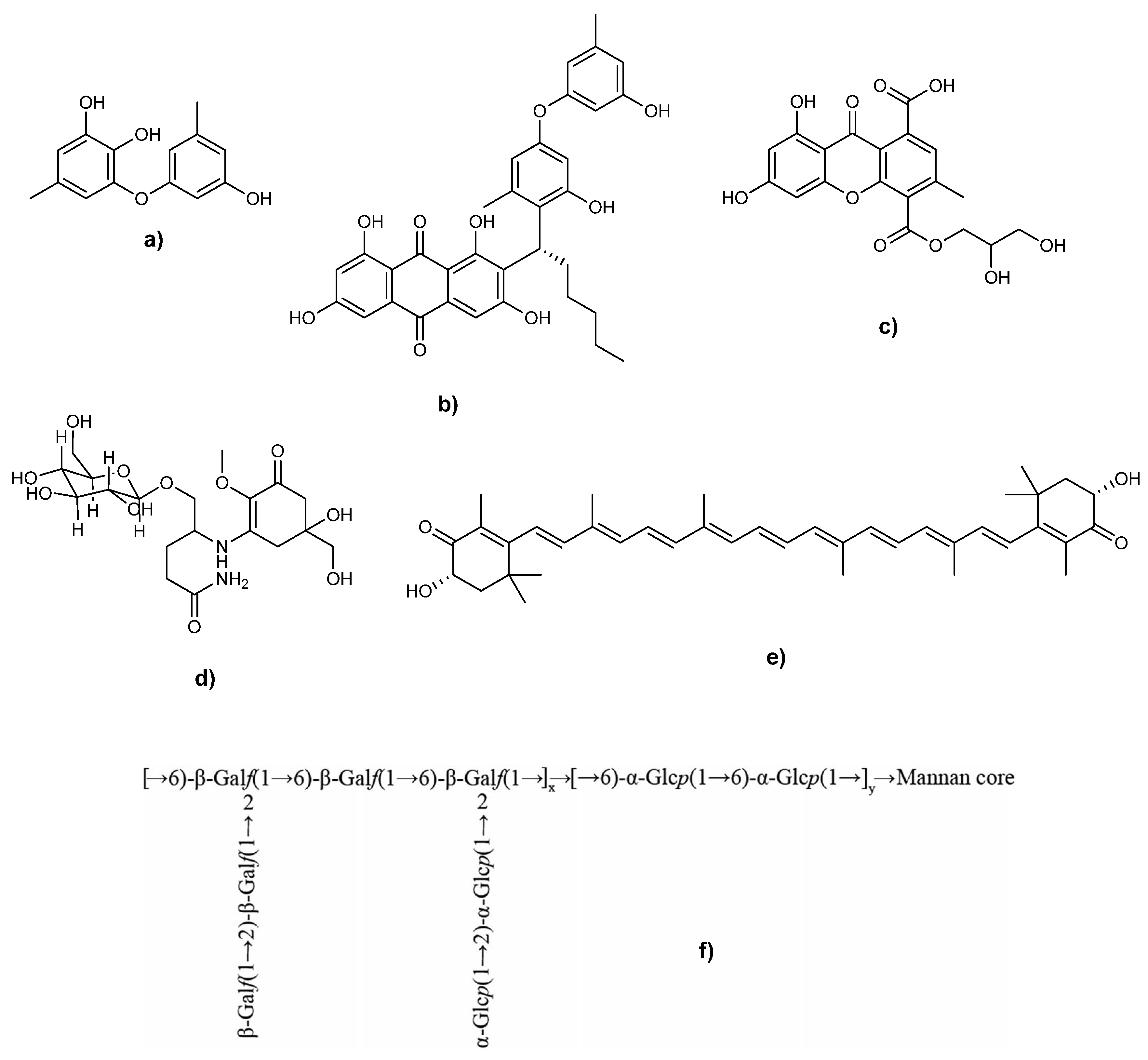
3.2. Phenolic Compounds
3.3. Carotenoids
3.4. Anthraquinones and Xanthones
3.5. Other Molecules
| Source | Strain | Antioxidant Molecule/s | Extraction Methodologies a | Ref. |
|---|---|---|---|---|
| Phenolic Compounds | ||||
| Callyspongia siphonella (Sponge) | Penicillium brevicompactum | syringic acid, acetosyringone, sinapic acid | E.b. extraction: EtOAc | [121] |
| Hippospongia communis (Sponge) | Gymnascella dankaliensis, Nigrospora oryzae, Chaetomium globosum, Engyodontium album | Crude extracts | Mycelium extraction: EtOAc | [117] |
| Deep-sea sediments (depth of 3002 m) | Aspergillus versicolor | fumalic acid, 1-methylpyrogallol, cordyol C, lecanoric acid | Solid culture serial extractions: EtOAc, n-BuOH (active fraction) and H2O | [112] |
| Deep-sea sediments (depth of 2300-2400 m) | Aspergillus versicolor SCSIO 41502 | 6-methylbenzene-1,2,4-triol, cordyol C, sydowiol B-D | E.b. extraction: XAD-16 resin and elution with EtOH. Mycelium extraction: acetone. The two extracts were combined together. | [68] |
| Sargassum sp. (Brown algae) | Aspergillus wentii EN-48 | 4-(3,4-dihydroxybenzamido) butanoate | E.b. extraction: EtOAc. Mycelium extraction: acetone. | [123] |
| Polysiphonia urceolata (red algae) | Chaetomium globosum | Chaetopyramin, isotetrahydroauroglaucin, 2-(2′,3-epoxy-1′,3′-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde | E.b. and mycelium homogenized extraction: MeOH and EtOAc. | [120] |
| Lomentaria catenate (red algae) | Microsporum sp. | flavoglaucin and isodihydroauroglaucin | E.b. extraction: EtOAc. Mycelium extraction: CH2Cl2:MeOH 1:1. The two extracts were combined together. | [120] |
| Fucus vesuculosus (brown algae) | Epicoccum sp. | 4,5,6-trihydroxy-7-methylphtalide, (-)-(3R)-5-hydroxymellein | E.b. and mycelium homogenized extraction: EtOAc. | [124] |
| Carpopeltis cornea (red algae) | Aspergillus parasiticus | gentisyl alcohol, 3-chloro-4,5-dihydroxybenzyl alcohol | E.b. extraction: EtOAc. Mycelium extraction: CH2Cl2:MeOH 1:1. The two extracts were combined together. | [125] |
| Sargassum ringgoldium (brown algae) | Chrysosporium synchronum | 1-O-(a-D-mannopyranosyl)chlorogentisyl alcohol from chlorogentisyl alcohol | E.b. extraction: EtOAc. | [119] |
| Algae (species not specified) | Acremonium sp. | 7-isopropenylbicyclo[4.2.0] octa-1,3,5-triene-2,5-diol, Gliomastin C, Gliomastin D, F-11334A1 | E.b. and mycelium homogenized extraction: EtOAc. | [128] |
| Marine sediment | Penicillium sp. | farnesylhydroquinone | Mycelium extraction: CH2Cl2:MeOH 1:1. | [129] |
| Chondria crassicualis (red algae) | Dothideomycete sp. | 5-bromotoluhydroquinone, 4-O-methyltoluhydroquinone, toluhydroquinone, gentisyl alcohol | E.b. extraction: EtOAc. | [118] |
| Anthraquinones and Xanthones | ||||
| Deep-sea sediments (depth of 3002 m) | Aspergillus versicolor (A-21-2-7) | aspergiol A, aspergiol B, averythrin, averantin, and methylaverantin | Solid culture serial extractions: EtOAc, n-BuOH (active fraction) and H2O. | [112] |
| Deep-sea sediments (depth of 3002 m) | Aspergillus versicolor (A-21-2-7) | versicolorin B, UCT1072M1, averantin, methylaverantin, averythrin, averufanin, averufine, nidurufin, oxisterigmatocystin D, oxisterigmatocystin C, sterigmatocystine, and dihydrosterigmatocystine | Solid culture serial extractions: EtOAc, n-BuOH (active fraction) and H2O. | [70] |
| Deep-sea sediments (depth of 2300-2400 m) | Aspergillus versicolor SCSIO 41502 | aspergilol G, aspergilol H, aspergilol I, aspergilol A, aspergilol B, SC3-22-3, coccoquinone A, averufin, methylaverantin, and versiconol | E.b. extraction: XAD-16 resin and elution with EtOH. Mycelium extraction: acetone. The two extracts were combined together. | [68] |
| Xestospongia testudinaria (sponge) | Aspergillus europaeus WZXY-SX-4-1 | euroxanthone A and euroxanthone B, calyxanthone, yicathin A, yicathin B, yicathin C, new 1-O-demethylvariecolorquinone A, variecolorquinone A, dermolutein, methylemodin, 1-methoxy-14-dehydroxywentiquinone C and wentiquinone C | Solid culture extraction: EtOAc. | [189] |
| Carotenoids | ||||
| Marine environement | Phaffia rhodozyma | Astaxanthin | The mycelium was treated with Lactic acid 5.5 mol/L at 30 °C, then extracted with a EtOH. | [135] |
| Marine environement | Phaffia rhodozyma | Astaxanthin | Mycelium extraction: n-hexane:EtOAc (1:1) | [208] |
| Marine environment | Rhodotorula glutinis YS-185 | Astaxanthin | Mycelium extraction: EtOAc. | [140] |
| Calanus finmarchius (copepods) | Rhodosporidium babjevae (Golubev) | Torularhodin, Torulene, β -carotene. | Cell suspensions added to 10% 1 M KOH in MeOH and neutralized with 1M HCl at 37 °C and extracted into ether | [143] |
| Deep sea sediments | Sporobolomyces ruberrimus | Carotenoids (torularhodin, torulene, β-carotene and ɤ-carotene) | Mycelium extraction: EtOH. | [146] |
| Macrocystis pyrifera (large brown algae) | Rhodotorula mucilginosa | Carotenoids (lycopene, β -carotene, astaxanthin) | Mycelium extraction: MeOH:CHCl3 (2:1). | [145] |
| Marine environment | Rhodotorula sp. RY1801 | Carotenoids | Mycelium extraction: DMSO:Acetone (2:5) | [144] |
| Tanegashima, Kagoshima Prefecture, Japan | Fusarium Strain T-1 | neurosporaxanthin β -D-glucopyranoside, neurosporax- anthin, β -carotene, γ-carotene, and torulene | Mycelium extraction: acetone | [147]. |
| Other Molecules | ||||
| Halichondria okadai (sponge) | Pichia membranifaciens | Tryptophol, 2-(1H-indol-3-yl)ethyl 2-hydroxypropanoate, 2-(1H-indol-3-yl)ethyl 5-hydroxypentanoate | E.b. (pH 3.0) extraction: EtOAc. Then the extract was dried over Na2SO4 and filtered | [197] |
| Sargassum sp. (brown algae) | Aspergillus sp. SpD081030G1f1 | JBIR-81, JBIR-82, terpeptin | Solid culture extraction: Acetone:H2O 8:2 | [69] |
| Sargassum kjellmanianum (brown algae) | Aspergillus ochraceus | 2-Hydroxycircumdatin C | E.b. and mycelium homogenized extraction: EtOAc and MeOH. | [199] |
| Lomentaria catenata | Aspergillus sp. | Golmaenone, neochinulin A | E.b. extraction: EtOAc. | [200] |
| Agarum cribrosum (brown-algae) | Pseudallescheria sp. | gliotoxin | E.b. extraction: EtOAc. | [201] |
| Carpopeltis cornea (red algae) | Aspergillus parasiticus | parasitenone | E.b. extraction: EtOAc. Mycelium extraction: CH2Cl2:MeOH 1:1 from mycelium, then combined together | [125] |
| Enteromorpha sp. (green algae) | Wardomyces anomalus | 5-(hydroxymethyl)-2-furanocarboxylic acid | E.b. and mycelium homogenized extraction: EtOAc. | [187] |
| Sponge (species not specified) | Acremonium strictum | Acremostrictin | E.b. extraction: EtOAc. | [202] |
| Marine source (not specified) | Phaeotheca triangularis, Trimmatostroma salinum, Hortaea werneckii, Aureobasidium pullulans and Cryptococcus liquefaciens | mycosporine–glutaminol–glucoside, mycosporine–glutamicol–glucoside | The Lyophilized mycelium is pulverized under liquid nitrogen and extracted with 0.2% aqueous acetic acid supplemented with 0.5% MeOH (v/v) for 12h at 4 °C. [209] | [203] |
4. Trends and Perspective on the Market for Marine Fungal Antioxidants
4.1. Food Industry
4.2. Cosmetic and Cosmeceutical Industries
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauritano, C.; Ianora, A. Grand Challenges in Marine Biotechnology: Overview of Recent EU-Funded Projects. In Grand Challenges in Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 425–449. [Google Scholar]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.; Martiny, J.B.; Sogin, M.; Boetius, A.; Ramette, A. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Coppola, D.; Russo, R.; Denaro, R.; Giuliano, L.; Lauro, F.M.; di Prisco, G.; Verde, C. Marine Microbial Secondary Metabolites: Pathways, Evolution and Physiological Roles. Adv. Microb. Physiol. 2015, 66, 357–428. [Google Scholar] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2009, 26, 338–362. [Google Scholar] [CrossRef]
- Penesyan, A.; Kjelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Stocker, R. Marine microbes see a sea of gradients. Science 2012, 338, 628–633. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Pons, L.N.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological applications of bioactive peptides from marine sources. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 73, pp. 171–220. [Google Scholar]
- Heidelberg, K.B.; Gilbert, J.A.; Joint, I. Marine genomics: At the interface of marine microbial ecology and biodiscovery. Microb. Biotechnol. 2010, 3, 531–543. [Google Scholar] [CrossRef]
- Massana, R.; Pedrós-Alió, C. Unveiling new microbial eukaryotes in the surface ocean. Curr. Opin. Microbiol. 2008, 11, 213–218. [Google Scholar] [CrossRef]
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal diversity in deep-sea hydrothermal ecosystems. Appl. Environ. Microbiol. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Paz, Z.; Komon-Zelazowska, M.; Druzhinina, I.; Aveskamp, M.; Shnaiderman, A.; Aluma, Y.; Carmeli, S.; Ilan, M.; Yarden, O. Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Divers. 2010, 42, 17–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, J.; Feng, Y.; Kang, Y.; Zhang, J.; Gu, P.-J.; Wang, Y.; Ma, L.-F.; Zhu, Y.-H. Broad-spectrum antimicrobial epiphytic and endophytic fungi from marine organisms: Isolation, bioassay and taxonomy. Mar. Drugs 2009, 7, 97–112. [Google Scholar] [CrossRef]
- Wiese, J.; Ohlendorf, B.; Blümel, M.; Schmaljohann, R.; Imhoff, J.F. Phylogenetic identification of fungi isolated from the marine sponge Tethya aurantium and identification of their secondary metabolites. Mar. Drugs 2011, 9, 561–585. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi-Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Zhu, P. Phylogenetic diversity of culturable fungi associated with the Hawaiian sponges Suberites zeteki and Gelliodes fibrosa. Antonie Van Leeuwenhoek 2008, 93, 163–174. [Google Scholar] [CrossRef]
- Höller, U.; Wright, A.D.; Matthee, G.F.; Konig, G.M.; Draeger, S.; Hans-Jürgen, A.; Schulz, B. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycol. Res. 2000, 104, 1354–1365. [Google Scholar] [CrossRef]
- Gulder, T.A.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.F.; Gross, H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef]
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, M.A.; Albang, R.; Albermann, K.; Badger, J.H.; Daran, J.-M.; Driessen, A.J.; Garcia-Estrada, C.; Fedorova, N.D.; Harris, D.M.; Heijne, W.H. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008, 26, 1161. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.M. Biotechnological potential of marine microbes. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 651–661. [Google Scholar]
- Fenical, W.; Jensen, P.R. Marine microorganisms: A new biomedical resource. In Pharmaceutical and Bioactive Natural Products; Springer: Berlin/Heidelberg, Germany, 1993; pp. 419–457. [Google Scholar]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Juranek, I.; Bezek, S. Controversy of free radical hypothesis: Reactive oxygen species-cause or consequence of tissue injury? Gen. Physiol. Biophys. 2005, 24, 263. [Google Scholar] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Madhavi, D.L.; Deshpande, S.; Salunkhe, D.K. Food Antioxidants: Technological: Toxicological and Health Perspectives; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. Int. Food Res. Int. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D. Mechanism of lipid peroxidation in meat and meat products-A review. Food Sci. Biotechnol. Adv. 2005, 14, 152–163. [Google Scholar]
- Zuo, L.; Zhou, T.; Pannell, B.; Ziegler, A.; Best, T.M. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox roles of reactive oxygen species in cardiovascular diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Retsky, K.L.; Freeman, M.W.; Frei, B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti- rather than prooxidant activity of vitamin C in the presence of transition metal ions. J. Biol. Chem. 1993, 268, 1304–1309. [Google Scholar]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369s–373s. [Google Scholar] [CrossRef]
- Mescic Macan, A.; Gazivoda Kraljevic, T.; Raic-Malic, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Dontha, S. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Niscair Online Period. Repos. 2013, 42, 556–564. [Google Scholar]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi. Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; DiLabio, G.A.; Ingold, K. Kinetics of the oxidation of quercetin by 2, 2-diphenyl-1-picrylhydrazyl (dpph•). Org. Lett. 2011, 13, 4826–4829. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Naguib, Y.M. A fluorometric method for measurement of oxygen radical-scavenging activity of water-soluble antioxidants. Anal. Biochem. 2000, 284, 93–98. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Krastanov, A.; Stoilova, I.; Denev, P.; Georgiev, M.; Trifonova, D.; Dimitrova, S. Scavenge Radical Ability and Inhibition of Lipid Autooxidation of Extracts from Antarctic Yeast Strain Sporobolomyces Salmonicolor Al1 Biomass. Eur. J. Biomed. Pharm. Sci. 2016, 3, 95–99. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.; Nabavi, S.; Nabavi, S. Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak. J. Biol. Sci. 2009, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Demirata, B.; Apak, R. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Methods 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Ravindran, C.; Varatharajan, G.R.; Rajasabapathy, R.; Vijayakanth, S.; Kumar, A.H.; Meena, R.M. A role for antioxidants in acclimation of marine derived pathogenic fungus (NIOCC 1) to salt stress. Microb. Pathog. 2012, 53, 168–179. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Giada, M. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power; Intech: London, UK, 2013; pp. 87–112. [Google Scholar]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorganic Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, M.; Hashimoto, J.; Takagi, M.; Shin-ya, K. Isolation of two new terpeptin analogs—JBIR-81 and JBIR-82—from a seaweed-derived fungus, Aspergillus sp. SpD081030G1f1. J. Antibiot. 2010, 63, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Liu, D.; Xu, Y.; Chen, J.-L.; Lin, W.-H. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin. J. Nat. Med. 2018, 16, 219–224. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Yadav, H.; Karthikeyan, C. 1-Natural polysaccharides: Structural features and properties. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–17. [Google Scholar]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Zhang, L.; Reddy, N.; Koyyalamudi, S.R. Chapter 5-Isolation, Characterization, and Biological Activities of Polysaccharides from Medicinal Plants and Mushrooms. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 42, pp. 117–151. [Google Scholar]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.O.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell Longev. 2016, 2016, 1–13. [Google Scholar]
- Chun-hui, L.; Chang-hai, W.; Zhi-liang, X.; Yi, W. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-H.; Mao, W.-J.; Jiao, J.-Y.; Xu, J.-C.; Li, H.-Y.; Chen, Y.; Qi, X.-H.; Chen, Y.-L.; Xu, J.; Zhao, C.-Q.; et al. Structural Characterization of Extracellular Polysaccharides Produced by the Marine Fungus Epicoccum nigrum JJY-40 and Their Antioxidant Activities. Mar. Biotechnol. 2011, 13, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, X.; Han, F.; Tan, R. Sulfation of a polysaccharide produced by a marine filamentous fungus Phoma herbarum YS4108 alters its antioxidant properties in vitro. Biochim. Et Biophys. Acta 2005, 1725, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Shi, M.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef]
- Madla, S.; Methacanon, P.; Prasitsil, M.; Kirtikara, K. Characterization of biocompatible fungi-derived polymers that induce IL-8 production. Carbohydr. Polym. 2005, 59, 275–280. [Google Scholar] [CrossRef]
- Yadava, K.L.; Rahi, D.K.; Soni, S.K.; Rahi, S. Diversity of exopolysaccharide producing fungi from foot hills of shivalik ranges of chandigarh capital region. Res. Biotechnol. 2012, 3, 4. [Google Scholar]
- Chen, Y.; Mao, W.; Gao, Y.; Teng, X.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr. Polym. 2013, 93, 478–483. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; BeMiller, J.N. Methods in Carbohydrate Chemistry: General Polysaccharides; Academic Press: Cambridge, MA, USA, 1965; Volume 5. [Google Scholar]
- Guo, S.; Mao, W.; Han, Y.; Zhang, X.; Yang, C.; Chen, Y.; Chen, Y.; Xu, J.; Li, H.; Qi, X. Structural characteristics and antioxidant activities of the extracellular polysaccharides produced by marine bacterium Edwardsiella tarda. Bioresour. Technol. 2010, 101, 4729–4732. [Google Scholar] [CrossRef]
- Shu, C.-H.; Lung, M.-Y. Effect of pH on the production and molecular weight distribution of exopolysaccharide by Antrodia camphorata in batch cultures. Process Biochem. 2004, 39, 931–937. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Mao, W.-J.; Tao, H.-W.; Zhu, W.-M.; Yan, M.-X.; Liu, X.; Guo, T.-T.; Guo, T. Preparation and characterization of a novel extracellular polysaccharide with antioxidant activity, from the mangrove-associated fungus Fusarium oxysporum. Mar. Biotechnol. 2015, 17, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; Stingele, F.; Petruccioli, M. Exopolysaccharide production by filamentous fungi: The example of Botryosphaeria rhodina. Antonie Van Leeuwenhoek 2003, 84, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-X.; Mao, W.-J.; Liu, X.; Wang, S.-Y.; Xia, Z.; Cao, S.-J.; Li, J.; Qin, L.; Xian, H.-L. Extracellular polysaccharide with novel structure and antioxidant property produced by the deep-sea fungus Aspergillus versicolor N2bc. Carbohydr. Polym. 2016, 147, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, W.-J.; Yan, M.-X.; Liu, X.; Wang, S.-Y.; Xia, Z.; Xiao, B.; Cao, S.-J.; Yang, B.-Q.; Li, J. Purification, Chemical Characterization, and Bioactivity of an Extracellular Polysaccharide Produced by the Marine Sponge Endogenous Fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, W.; Chen, Z.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Yan, M.; Liu, X.; Guo, T. Purification, structural characterization and antioxidant property of an extracellular polysaccharide from Aspergillus terreus. Process Biochem. 2013, 48, 1395–1401. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Yang, Y.; Teng, X.; Zhu, W.; Qi, X.; Chen, Y.; Zhao, C.; Hou, Y.; Wang, C.; et al. Structure and antioxidant activity of an extracellular polysaccharide from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Carbohydr. Polym. 2012, 87, 218–226. [Google Scholar] [CrossRef]
- Sun, H.-H.; Mao, W.-J.; Chen, Y.; Guo, S.-D.; Li, H.-Y.; Qi, X.-H.; Chen, Y.-L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Justyna, S.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New Bioactive Fungal Molecules with High Antioxidant and Antimicrobial Capacity Isolated from Cerrena unicolor Idiophasic Cultures. Biomed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef]
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014, 143, 33–39. [Google Scholar] [CrossRef]
- Reddy, C.; Dande, S.; Manchala, R. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int.-Food Res. Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [CrossRef] [PubMed]
- Ndakidemi, P.A.; Dakora, F.D. Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Funct. Plant Biol. 2003, 30, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Park, S.-W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Polyphenols in the Resistance Mechanisms of Plants Against Fungal Pathogens and Insects. Phytochemistry 2006, 37, 23–67. [Google Scholar]
- Cle, C.; Hill, L.M.; Niggeweg, R.; Martin, C.R.; Guisez, Y.; Prinsen, E.; Jansen, M.A. Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 2008, 69, 2149–2156. [Google Scholar] [CrossRef]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol 2003, 2, 13–18. [Google Scholar]
- Lee, S.K.; Lee, H.J.; Min, H.Y.; Park, E.J.; Lee, K.M.; Ahn, Y.H.; Cho, Y.J.; Pyee, J.H. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Morton, L.W.; Abu-Amsha Caccetta, R.; Puddey, I.B.; Croft, K.D. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2000, 27, 152–159. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Antioxidative phenolic compounds from a marine-derived fungus Aspergillus versicolor. Tetrahedron 2016, 72, 50–57. [Google Scholar] [CrossRef]
- Gupta, C. Functional foods enhanced with Microbial antioxidants. Acad. J. Nutr. 2013, 2, 10–18. [Google Scholar]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Bok, J.W.; Chiang, Y.-M.; Szewczyk, E.; Reyes-Dominguez, Y.; Davidson, A.D.; Sanchez, J.F.; Lo, H.-C.; Watanabe, K.; Strauss, J.; Oakley, B.R. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 2009, 5, 462. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, C.-L.; Chen, M.; Liu, Q.-A.; Wang, C.-Y. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 2014, 55, 4888–4891. [Google Scholar] [CrossRef]
- Abdel-Monem, N.; Abdel-Azeem, A.; El Ashry, E.S.; Ghareeb, D.; Nabil, A. Assessment of Secondary Metabolites from Marine-Derived Fungi as Antioxidant. Open J. Med. Chem. 2013, 03, 60–73. [Google Scholar] [CrossRef][Green Version]
- Leutou, A.S.; Yun, K.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. New production of 5-bromotoluhydroquinone and 4-O-methyltoluhydroquinone from the marine-derived fungus Dothideomycete sp. J. Microbiol. Biotechnol. 2012, 22, 80–83. [Google Scholar] [CrossRef]
- Yun, K.; Kondempudi, C.M.; Choi, H.D.; Kang, J.S.; Son, B.W. Microbial mannosidation of bioactive chlorogentisyl alcohol by the marine-derived fungus Chrysosporium synchronum. Chem. Pharm. Bull. 2011, 59, 499–501. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Li, X.-M.; Teuscher, F.; Li, D.-L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.-G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, Y.; Xu, X.-Y.; Qi, S.-H. Diverse deep-sea fungi from the South China Sea and their antimicrobial activity. Curr. Microbiol. 2013, 67, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.-M.; Xu, G.-M.; Li, C.-S.; Wang, B.-G. Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48. Phytochem. Lett. 2014, 7, 120–123. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; König, G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar]
- Son, B.W.; Choi, J.S.; Kim, J.C.; Nam, K.W.; Kim, D.-S.; Chung, H.Y.; Kang, J.S.; Choi, H.D. Parasitenone, a new epoxycyclohexenone related to gabosine from the marine-derived fungus Aspergillus parasiticus. J. Nat. Prod. 2002, 65, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Nenkep, V.N.; Yun, K.; Li, Y.; Choi, H.D.; Kang, J.S.; Son, B.W. New production of haloquinones, bromochlorogentisylquinones A and B, by a halide salt from a marine isolate of the fungus Phoma herbarum. J. Antibiot. 2010, 63, 199–201. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Son, B.-W. Antibacterial and radical scavenging epoxycyclohexenones and aromatic polyols from a marine isolate of the fungus Aspergillus. Nat. Prod. Sci. 2005, 11, 136–138. [Google Scholar]
- Abdel-Lateff, A.; König, G.M.; Fisch, K.M.; Höller, U.; Jones, P.G.; Wright, A.D. New antioxidant hydroquinone derivatives from the algicolous marine fungus Acremonium sp. J. Nat. Prod. 2002, 65, 1605–1611. [Google Scholar] [CrossRef]
- Son, B.W.; Kim, J.C.; Choi, H.D.; Kang, J.S. A radical scavenging Farnesylhydroquinone from a marine-derived fungus Penicillium sp. Arch. Pharmacal Res. 2002, 25, 77–79. [Google Scholar] [CrossRef]
- Echavarri-Erasun, C.; Johnson, E.A. Fungal carotenoids. In Applied Mycology and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 2, pp. 45–85. [Google Scholar]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant. 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of marine carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Sedmak, J.J.; Weerasinghe, D.K.; Jolly, S.O. Extraction and quantitation of astaxanthin from Phaffia rhodozyma. Biotechnol. Tech. 1990, 4, 107–112. [Google Scholar] [CrossRef]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Hiu, S. Utilization of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, in rainbow trout, Oncorhynchus mykiss: Effects of enzymatic cell wall disruption and feed extrusion temperature. Aquaculture 2004, 236, 391–403. [Google Scholar] [CrossRef]
- Ni, H.; Chen, Q.-H.; He, G.-Q.; Wu, G.-B.; Yang, Y.-F. Optimization of acidic extraction of astaxanthin from Phaffia rhodozyma. J. Zhejiang Univ. Sci. B 2008, 9, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.; Xu, X.; Zhuang, H.; Shen, W. Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 2008, 109, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernández-De Jesús, M.L.; Barragán-Huerta, B.E. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.-L.; Ni, H.; Zhu, M.-J. Disruption of Phaffia rhodozyma cells and preparation of microencapsulated astaxanthin with high water solubility. Food Sci. Biotechnol. 2019, 28, 111–120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, J.; Qin, S.; Yu, J.; Sun, M. Identification of an astaxanthin-producing marine yeast strain YS-185 and optimization of its fermentation conditions. Prog. Fish. Sci. 2011, 32, 93–101. [Google Scholar]
- Mannazzu, I.; Landolfo, S.; Da Silva, T.L.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M.J. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed]
- Sperstad, S.; Lutnæs, B.F.; Stormo, S.K.; Liaaen-Jensen, S.; Landfald, B. Torularhodin and torulene are the major contributors to the carotenoid pool of marine Rhodosporidium babjevae (Golubev). J. Ind. Microbiol. Biotechnol. 2006, 33, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.; Shene, C.J. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef]
- Cardoso, L.; Jäckel, S.; Karp, S.; Framboisier, X.; Chevalot, I.; Marc, I. Improvement of Sporobolomyces ruberrimus carotenoids production by the use of raw glycerol. Bioresour. Technol. 2016, 200, 374–379. [Google Scholar] [CrossRef]
- Sakaki, H.; Kaneno, H.; Sumiya, Y.; Tsushima, M.; Miki, W.; Kishimoto, N.; Fujita, T.; Matsumoto, S.; Komemushi, S.; Sawabe, A. A new carotenoid glycosyl ester isolated from a marine microorganism, Fusarium strain T-1. J. Nat. Prod. 2002, 65, 1683–1684. [Google Scholar] [CrossRef]
- Suen, Y.L.; Tang, H.; Huang, J.; Chen, F. Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J. Agric. Food Chem. 2014, 62, 12392–12398. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A.N. Chapter 11-Anthraquinones: An Overview. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 313–338. [Google Scholar]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Fouillaud, M.; Caro, Y.; Venkatachalam, M.; Grondin, I.; Dufossé, L. Anthraquinones. In Phenolic Compounds in Food Characterization and Analysis; Nollet, L.M.L., Gutiérrez-Uribe, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 130–170. [Google Scholar]
- Motallebi, S.; Maghsoodi, S. Anthraquinone Dye-Containing Material, Composition Including the Same, Camera Including the Same, and Associated Methods. U.S. Patent 8,492,569, 23 July 2013. [Google Scholar]
- Räisänen, R.; Nousiainen, P.; Hynninen, P.H. Emodin and dermocybin natural anthraquinones as high-temperature disperse dyes for polyester and polyamide. Text. Res. J. 2001, 71, 922–927. [Google Scholar] [CrossRef]
- Perumal, K.; Stalin, V.; Chandrasekarenthiran, S.; Sumathi, E.; Saravanakumar, A. Extraction and characterization of pigment from Sclerotinia sp. and its use in dyeing cotton. Text. Res. J. 2009, 79, 1178–1187. [Google Scholar] [CrossRef]
- Shrestha, J.P.; Subedi, Y.P.; Chen, L.; Chang, C.-W.T. A mode of action study of cationic anthraquinone analogs: A new class of highly potent anticancer agents. MedChemComm 2015, 6, 2012–2022. [Google Scholar] [CrossRef]
- Khan, K.; Karodi, R.; Siddiqui, A.; Thube, S.; Rub, R. Development of anti-acne gel formulation of anthraquinones rich fraction from Rubia cordifolia (Rubiaceae). Int. J. Appl. Res. Nat. Prod. 2011, 4, 28–36. [Google Scholar]
- Wuthi-udomlert, M.; Kupittayanant, P.; Gritsanapan, W. In vitro evaluation of antifungal activity of anthraquinone derivatives of Senna alata. J. Health Res. 2010, 24, 117–122. [Google Scholar]
- Fosso, M.Y.; Chan, K.Y.; Gregory, R.; Chang, C.-W.T. Library synthesis and antibacterial investigation of cationic anthraquinone analogs. Acs Comb. Sci. 2012, 14, 231–235. [Google Scholar] [CrossRef]
- Barnard, D.L.; Fairbairn, D.W.; O’Neill, K.L.; Gage, T.L.; Sidwell, R.W. Anti-human cytomegalovirus activity and toxicity of sulfonated anthraquinones and anthraquinone derivatives. Antivir. Res. 1995, 28, 317–329. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef]
- Hanson, J.R. Natural Products: The Secondary Metabolites; Royal Society of Chemistry: London, UK, 2003; Voluime 17. [Google Scholar]
- Miethbauer, S.; Haase, S.; Schmidtke, K.-U.; Günther, W.; Heiser, I.; Liebermann, B. Biosynthesis of photodynamically active rubellins and structure elucidation of new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry 2006, 67, 1206–1213. [Google Scholar] [CrossRef]
- Gatenbeck, S. Incorporation of Labelled Acetate in Emodin in. Acta Chem. Scand 1958, 12, 6. [Google Scholar] [CrossRef]
- Hsieh, D.P.; Singh, R.; Yao, R.C.; Bennett, J.W. Anthraquinones in the biosynthesis of sterigmatocystin by Aspergillus versicolor. Appl. Environ. Microbiol. 1978, 35, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ikekawa, T. Metabolic Products of Fungi. XX. The Biosynthesis of Rugulosin. Chem. Pharm. Bull. 1963, 11, 368–372. [Google Scholar] [CrossRef][Green Version]
- Zheng, C.-J.; Shao, C.-L.; Guo, Z.-Y.; Chen, J.-F.; Deng, D.-S.; Yang, K.-L.; Chen, Y.-Y.; Fu, X.-M.; She, Z.-G.; Lin, Y.-C.; et al. Bioactive Hydroanthraquinones and Anthraquinone Dimers from a Soft Coral-Derived Alternaria sp. Fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Wakuliński, W.; Kachlicki, P.; Sobiczewski, P.; Schollenberger, M.; Zamorski, C.; Łotocka, B.; Sarova, J. Catenarin Production by Isolates of Pyrenophora tritici-repentis (Died.) Drechsler and its Antimicrobial Activity. J. Phytopathol. 2003, 151, 74–79. [Google Scholar] [CrossRef]
- Li, D.-L.; Li, X.-M.; Wang, B.-G. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: Structural elucidation and DPPH radical scavenging activity. J. Microbiol. Biotechnol. 2009, 19, 675–680. [Google Scholar] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Cefarelli, G.; Uzzo, P.; Monaco, P. Natural dibenzoxazepinones from leaves of Carex distachya: Structural elucidation and radical scavenging activity. Bioorganic Med. Chem. Lett. 2007, 17, 636–639. [Google Scholar] [CrossRef]
- Heo, S.-J.; Kim, J.-P.; Jung, W.-K.; Lee, N.-H.; Kang, H.-S.; Jun, E.-M.; Park, S.-H.; Kang, S.-M.; Lee, Y.-J.; Park, P.-J. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar]
- Rietjens, I.M.C.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Miethbauer, S.; Heiser, I.; Liebermann, B. The phytopathogenic fungus Ramularia collo-cygni produces biologically active rubellins on infected barley leaves. J. Phytopathol. 2003, 151, 665–668. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; de Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Masters, K.-S.; Bräse, S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Chhouk, K.; Quitain, A.T.; Gaspillo, P.D.; Maridable, J.B.; Sasaki, M.; Shimoyama, Y.; Goto, M. Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from Garcinia Mangostana pericarp. J. Supercrit. Fluids 2016, 110, 167–175. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Kang, K.; Jho, E.H.; Chin, Y.-W.; Kim, J.; Nho, C.W. α- and γ-Mangostin inhibit the proliferation of colon cancer cells via β-catenin gene regulation in Wnt/cGMP signalling. Food Chem. 2011, 129, 1559–1566. [Google Scholar] [CrossRef]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Asai, F.; Kobayashl, Y.; Shimano, R.; Miyauchi, K.-I. Antibacterial Activity of Xanthones from Guttiferaeous Plants against Methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmacol. 1996, 48, 861–865. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W.; Thanuhiranlert, J.; Ratananukul, P.; Chimnoi, N.; Suksamrarn, A. Antimycobacterial Activity of Prenylated Xanthones from the Fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef]
- Adnan, A.; Allaudin, Z.N.; Hani, H.; Loh, H.-S.; Khoo, T.-J.; Ting, K.N.; Abdullah, R. Virucidal activity of Garcinia parvifolia leaf extracts in animal cell culture. BMC Complementary Altern. Med. 2019, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateff, A.; Klemke, C.; König, G.M.; Wright, A.D. Two new xanthone derivatives from the algicolous marine fungus Wardomyces anomalus. J. Nat. Prod. 2003, 66, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.; Chua, C. The potential of xanthones as a therapeutic option in macrophage-associated inflammatory diseases. Pharm. Rev. 2019, 13, 28. [Google Scholar]
- Du, X.; Liu, D.; Huang, J.; Zhang, C.; Proksch, P.; Lin, W. Polyketide derivatives from the sponge associated fungus Aspergillus europaeus with antioxidant and NO inhibitory activities. Fitoterapia 2018, 130, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Kijjoa, A. Naturally-Occurring Xanthones: Recent Developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Miura, I. A New Xanthone Diglucoside from Swertia perennisL. Helv. Chim. Acta 1977, 60, 262–264. [Google Scholar] [CrossRef]
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Flörke, U.; Kurtán, T.; Pescitelli, G.; Di Bari, L.; Draeger, S.; et al. Xanthones and Oxepino[2, 3-b]chromones from Three Endophytic Fungi. Chem. Eur. J. 2009, 15, 12121–12132. [Google Scholar] [CrossRef]
- Yuan, M.-X.; Qiu, Y.; Ran, Y.-Q.; Feng, G.-K.; Deng, R.; Zhu, X.-F.; Lan, W.-J.; Li, H.-J. Exploration of indole alkaloids from marine fungus Pseudallescheria boydii F44-1 using an amino acid-directed strategy. Mar. Drugs 2019, 17, 77. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Kalinovsky, A.I.; Khudyakova, Y.V.; Pivkin, M.V.; Dmitrenok, P.S.; Fedorov, S.N.; Ji, H.; Kwak, J.-Y.; Kuznetsova, T.A. Indole alkaloids produced by a marine fungus isolate of Penicillium janthinellum Biourge. J. Nat. Prod. 2007, 70, 906–909. [Google Scholar] [CrossRef]
- Zhou, R.; Liao, X.; Li, H.; Li, J.; Feng, P.; Zhao, B.; Xu, S. Isolation and synthesis of misszrtine A: A novel indole alkaloid from marine sponge-associated Aspergillus sp. SCSIO XWS03F03. Front. Chem. 2018, 6, 212. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Arnao, M.B. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal. Bioanal. Chem. 2003, 376, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Ito, Y.; Suzuki, M.; Hirota, A. Indole derivatives from a marine sponge-derived yeast as DPPH radical scavengers. J. Nat. Prod. 2009, 72, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Hashimoto, J.; Inaba, S.; Khan, S.T.; Komaki, H.; Nagai, A.; Takagi, M.; Shin-ya, K. New sesquiterpenes, JBIR-27 and-28, isolated from a tunicate-derived fungus, Penicillium sp. SS080624SCf1. J. Antibiot. 2009, 62, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Li, C.S.; Sun, H.F.; Gao, S.S.; Wang, B.G. Benzodiazepine Alkaloids from Marine-Derived Endophytic Fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Kim, S.-K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a new diketopiperazine alkaloid from the marine-derived fungus Aspergillus sp. Chem. Pharm. Bull. 2004, 52, 375–376. [Google Scholar] [CrossRef]
- Li, X.; Kim, S.-K.; Nam, K.W.; Kang, J.S.; Choi, H.D.; Son, B.W. A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. 2006, 59, 248–250. [Google Scholar] [CrossRef]
- Julianti, E.; Oh, H.; Jang, K.H.; Lee, J.K.; Lee, S.K.; Oh, D.-C.; Oh, K.-B.; Shin, J. Acremostrictin, a highly oxygenated metabolite from the marine fungus Acremonium strictum. J. Nat. Prod. 2011, 74, 2592–2594. [Google Scholar] [CrossRef]
- Kogej, T.; Gostinčar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N. Mycosporines in extremophilic fungi—novel complementary osmolytes? Environ. Chem. 2006, 3, 105–110. [Google Scholar] [CrossRef]
- De la Coba Luque, F.A.A.; José, L.F. Use of a Mycosporin-Type Amino Acid (Shinorine) as an Antioxidant. Intl Patent WO2007/026038A2, 24 May 2007. [Google Scholar]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Suh, H.J.; Lee, H.W.; Jung, J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Martínez-Cárdenas, A.; Chávez-Cabrera, C.; Vasquez-Bahena, J.M.; Flores-Cotera, L.B. A common mechanism explains the induction of aerobic fermentation and adaptive antioxidant response in Phaffia rhodozyma. In Microb Cell Fact; BMC: London, UK, 2018; Volume 17, p. 53. [Google Scholar]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Vaya, J.; Aviram, M. Nutritional antioxidants mechanisms of action, analyses of activities and medical applications. Curr. Med. Chem.-Immunol. Endocr. Metab. Agents 2001, 1, 99–117. [Google Scholar] [CrossRef]
- Shahidi, F. Oxidative Stability of Edible Oils as Affected by Their Fatty Acid Composition and Minor Constituents. In Freshness and Shelf Life of Foods; American Chemical Society: New York, NY, USA, 2002; Volume 836, pp. 201–211. [Google Scholar]
- Shebis, Y.; Iluz, D.; Kinel-Tahan, Y.; Dubinsky, Z.; Yehoshua, Y. Natural Antioxidants: Function and Sources. Food Nutr. Sci. 2013, 04, 643–649. [Google Scholar] [CrossRef]
- Trott, P. Innovation Management and New Product Development; Pearson Education: London, UK, 2008. [Google Scholar]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhang, M.; Wang, X. Improvement of oxidative stability of trimethylolpropane trioleate lubricant. Thermochim. Acta 2013, 569, 112–118. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Hu, D.; Luo, Y.; Jia, D.J. Reinforcement and reinforcing mechanism of styrene–butadiene rubber by antioxidant-modified silica. Compos. Part A Appl. Sci. Manuf. 2015, 78, 303–310. [Google Scholar] [CrossRef]
- Pokorný, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food: Practical Applications; Woodhead Publishing Ltd.: Cambridge, MA, USA, 2001. [Google Scholar]
- Gutteridge, J.M.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef]
- Botterweck, A.; Verhagen, H.; Goldbohm, R.; Kleinjans, J.; Van den Brandt, P. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands cohort study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- Sarafian, T.A.; Kouyoumjian, S.; Tashkin, D.; Roth, M.D. Synergistic cytotoxicity of Δ9-tetrahydrocannabinol and butylated hydroxyanisole. Toxicol. Lett. 2002, 133, 171–179. [Google Scholar] [CrossRef]
- Saito, M.; Sakagami, H.; Fujisawa, S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Anticancer Res. 2003, 23, 4693. [Google Scholar] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT (1). Int. J. Toxicol. 2002, 21, 19. [Google Scholar] [PubMed]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ambati, D.R.R.; Phang, S.-M.; Ravi, S.; Gokare, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Igene Biotechnology, I. Available online: https://www.dnb.com/business-directory/company-profiles.igene_biotechnology_inc.f79a3612c3029e9a9ec86d4f2014dc97.html#:~:text=IGENE%20Biotechnology%20makes%20vitamins%20for,feed%20under%20the%20AstaXin%20brand (accessed on 13 November 2020).
- Lehman, A.; Fitzhugh, O.; Nelson, A.; Woodard, G. The pharmacological evaluation of antioxidants. Adv. Food Res. 1951, 3, 197–208. [Google Scholar]
- Coppen, P.; Allen, J.; Hamilton, R. Rancidity in Foods; Elseiver Applied Science: London, UK, 1989. [Google Scholar]
- Thorat, I.D.; Jagtap, D.D.; Mohapatra, D.; Joshi, D.; Sutar, R.; Kapdi, S. Antioxidants, their properties, uses in food products and their legal implications. Int. J. Food Stud. 2013, 2. [Google Scholar] [CrossRef]
- Warner, C.R.; Brumley, W.; Daniels, D.; Joe, F., Jr.; Fazio, T. Reactions of antioxidants in foods. Food Chem. Toxicol. 1986, 24, 1015–1019. [Google Scholar] [CrossRef]
- Nenadis, N.; Zafiropoulou, I.; Tsimidou, M. Commonly used food antioxidants: A comparative study in dispersed systems. Food Chem. 2003, 82, 403–407. [Google Scholar] [CrossRef]
- Cuppett, S. The Use of Natural Antioxidants in Food Products of Animal Origin; Elsevier: Amsterdam, The Netherlands, 2001; pp. 285–310. [Google Scholar]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Santos Sánchez, N.; Salas-Coronado, R.; Valadez-Blanco, R.; Hernandez-Carlos, B.; Guadarrama, P. Natural antioxidant extracts as food preservatives. Acta Sci. Polonorum. Technol. Aliment. 2017, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance. EFSA J. 2005, 3, 223. [Google Scholar]
- Martins, S.; Mussatto, S.; Martinez, G.; Montañez-Saenz, J.; Aguilar, C.; Teixeira, J. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bolumar, T.; Andersen, M.; Orlien, V. Antioxidant active packaging for chicken meat processed. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Jindal, N.; Khattar, J.I.S. Microbial Polysaccharides in Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Broakway, B. Marine derived ingredients for personal care. Mar. Ingred. 2012, 4, 70–73. [Google Scholar]
- Waites, M.J.; Morgan, N.L.; Rockey, J.S.; Higton, G. Industrial Microbiology: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Cambon-Bonavita, M.A.; Raguenes, G.; Jean, J.; Vincent, P.; Guezennec, J. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J. Appl. Microbiol. 2002, 93, 310–315. [Google Scholar] [CrossRef]
- Martins, A.; Tenreiro, T.; Andrade, G.; Gadanho, M.; Chaves, S.; Abrantes, M.; Calado, P.; Tenreiro, R.; Vieira, H. Photoprotective bioactivity present in a unique marine bacteria collection from Portuguese deep sea hydrothermal vents. Mar. Drugs 2013, 11, 1506–1523. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. https://doi.org/10.3390/antiox9121183
Vitale GA, Coppola D, Palma Esposito F, Buonocore C, Ausuri J, Tortorella E, de Pascale D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants. 2020; 9(12):1183. https://doi.org/10.3390/antiox9121183
Chicago/Turabian StyleVitale, Giovanni Andrea, Daniela Coppola, Fortunato Palma Esposito, Carmine Buonocore, Janardhan Ausuri, Emiliana Tortorella, and Donatella de Pascale. 2020. "Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives" Antioxidants 9, no. 12: 1183. https://doi.org/10.3390/antiox9121183
APA StyleVitale, G. A., Coppola, D., Palma Esposito, F., Buonocore, C., Ausuri, J., Tortorella, E., & de Pascale, D. (2020). Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants, 9(12), 1183. https://doi.org/10.3390/antiox9121183








