Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatments
2.3. Cell Viability Assay
2.4. Determination of the Mitotic Index
2.5. Cell Growth Assays
2.6. Measurement of Intracellular ROS Generation
2.7. The Mitochondrial Membrane Potential
2.8. Ca2+-Retention Capacity of Mitochondria in Permeabilized Cells
2.9. Immunoblotting Analysis
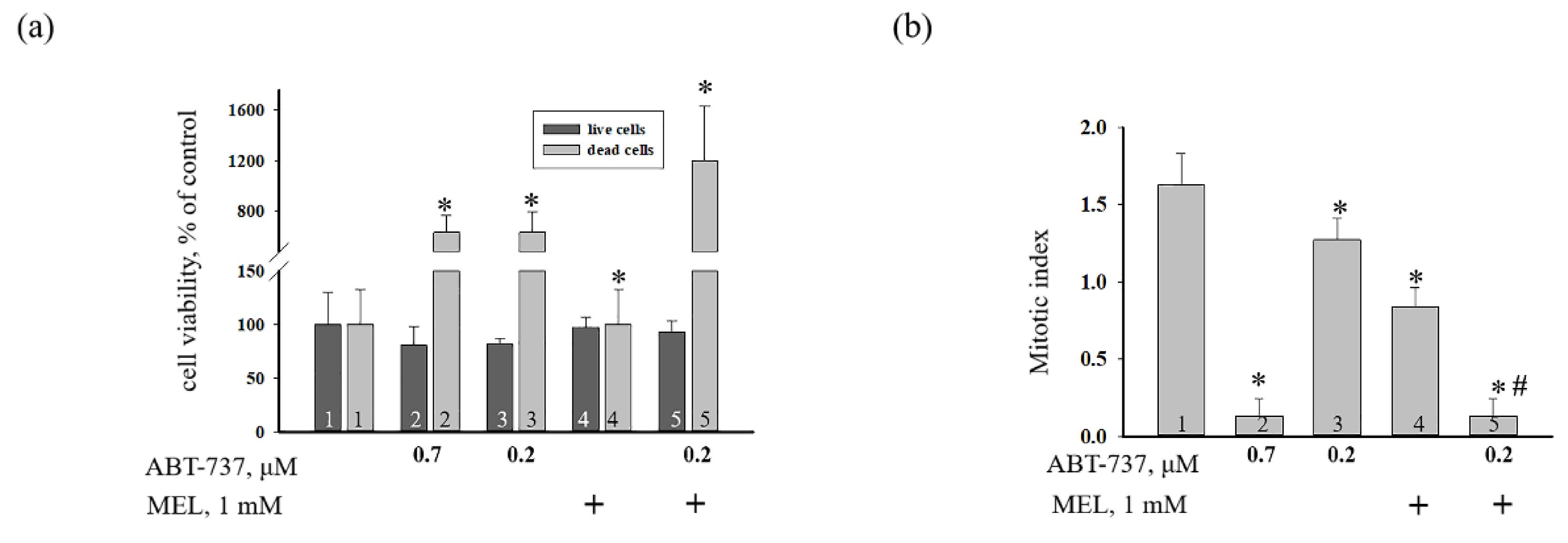
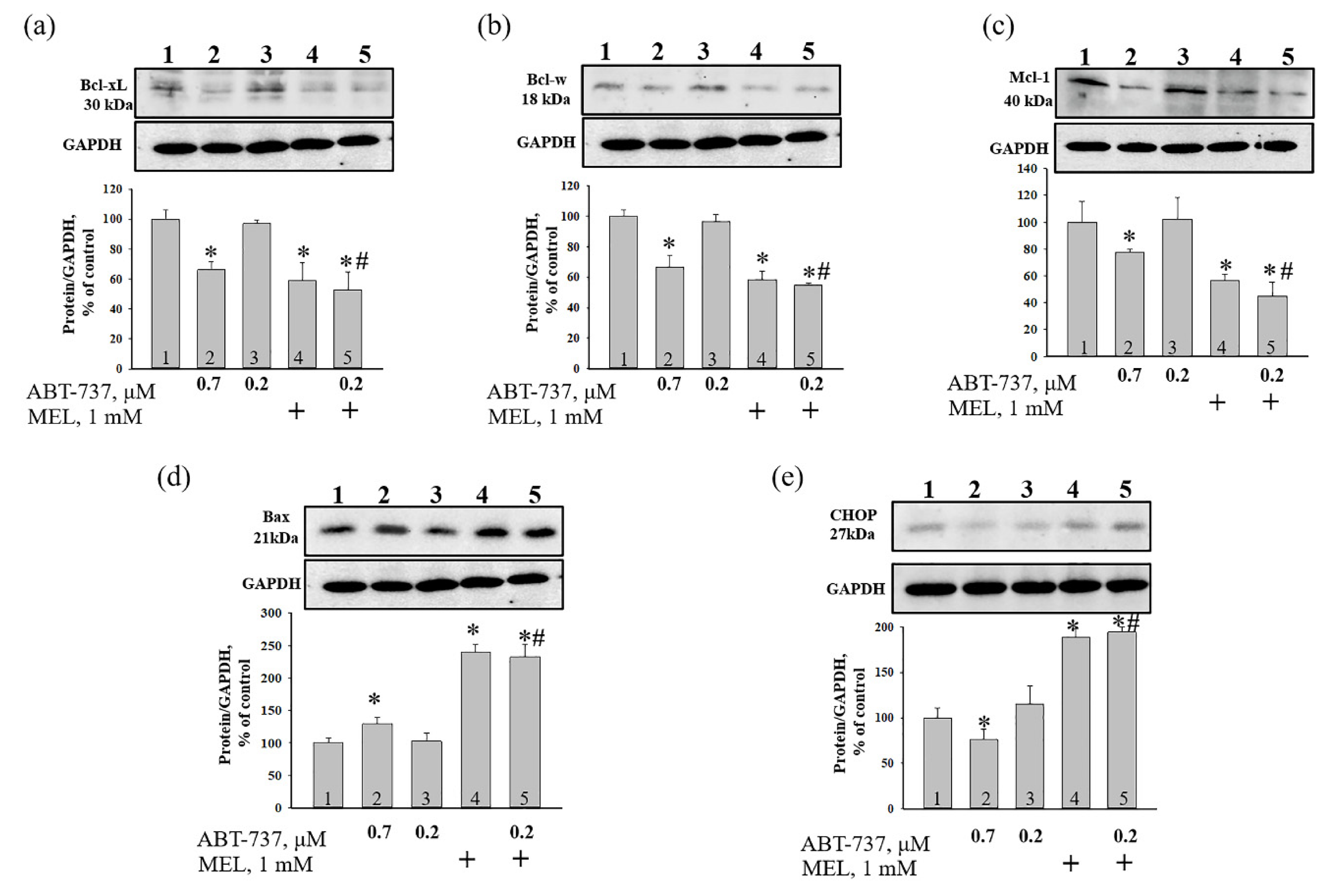
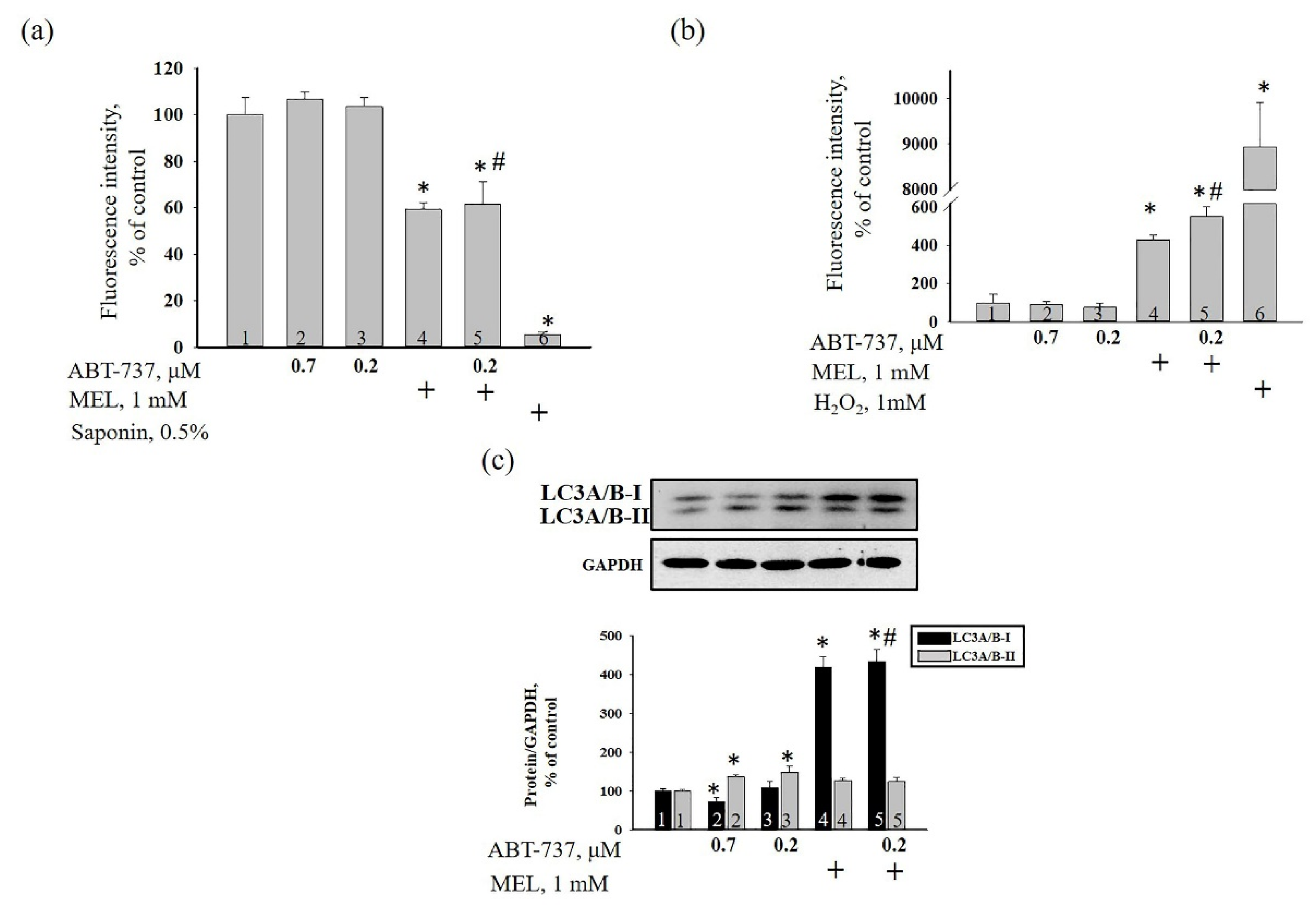
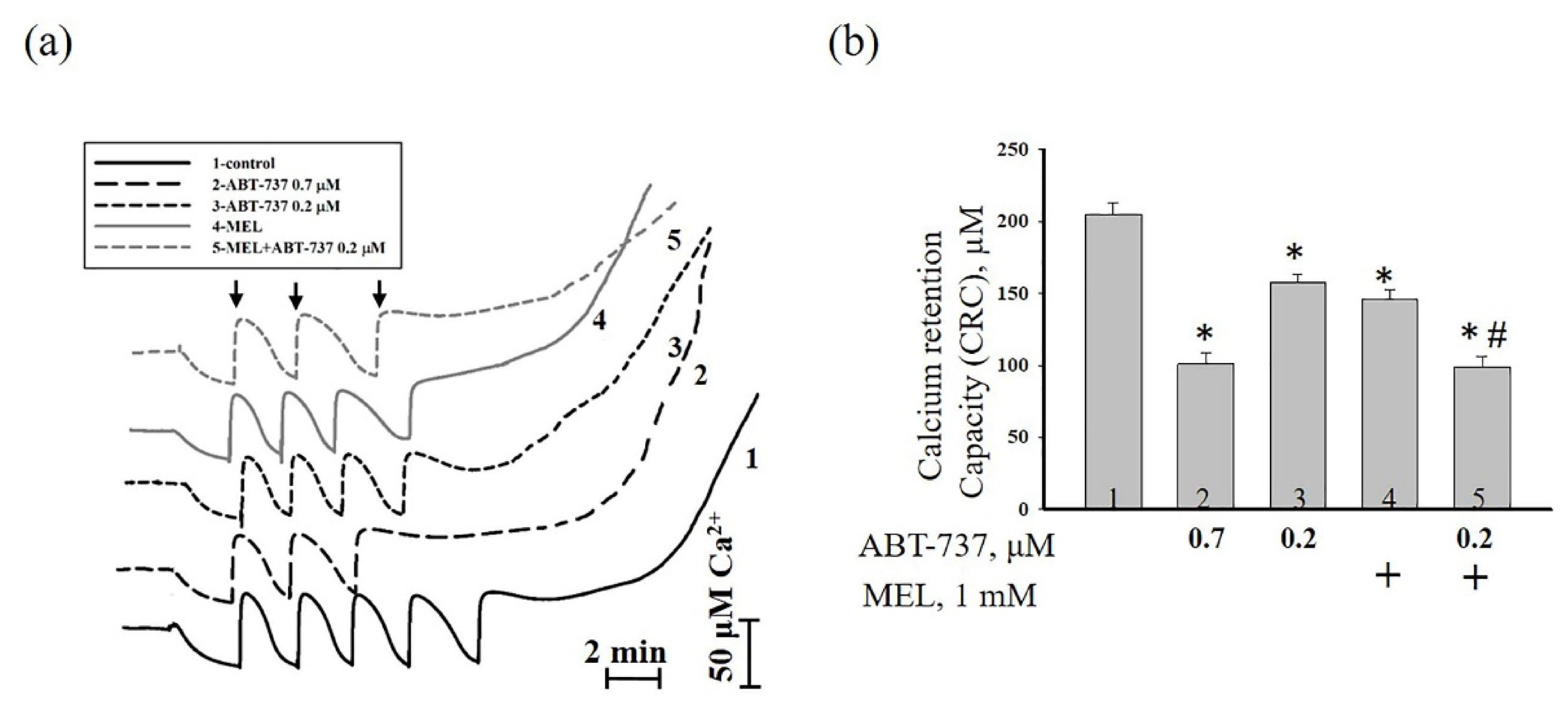
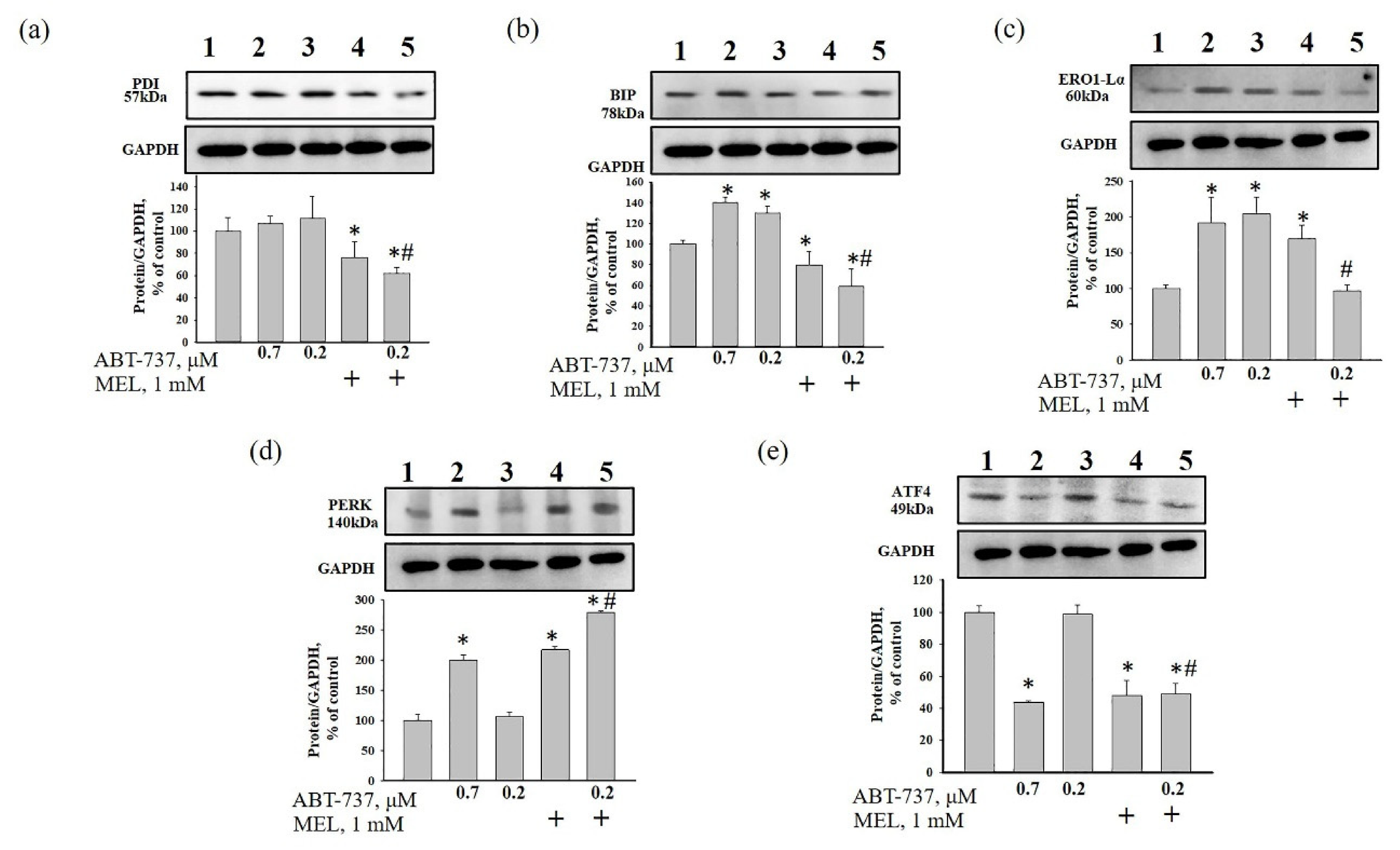
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. CMLS 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar]
- Fernandez-Mar, M.I.; Mateos, R.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Baburina, Y.L.; Odinokova, I.V.; Krestinina, O.V. The proapoptotic effect of melatonin on the functioning of the nonspecific mitochondrial pore (mptp) in rat mitochondria. Neurochem. J. 2019, 13, 156–163. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Therapeutic actions of melatonin in cancer: Possible mechanisms. Integr. Cancer Ther. 2008, 7, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International union of basic and clinical pharmacology. Lxxv. Nomenclature, classification, and pharmacology of g protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Rivera-Bermudez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef]
- Crompton, M.; Costi, A. A heart mitochondrial ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that adp facilitates pore interconversion between the closed and open states. Biochem. J. 1990, 266, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, N.; Matsuo, N.; Tanaka, T. Oxidative damage to mitochondria is mediated by the ca(2+)-dependent inner-membrane permeability transition. Biochem. J. 1993, 294, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Karmazyn, M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell. Physiol. Biochem. 2007, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Deniaud, A.; Sharaf el dein, O.; Maillier, E.; Poncet, D.; Kroemer, G.; Lemaire, C.; Brenner, C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 2008, 27, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, K.; Kaufman, R.J. The unfolded protein response--a stress signaling pathway of the endoplasmic reticulum. J. Chem. Neuroanat. 2004, 28, 79–92. [Google Scholar] [CrossRef]
- Schroder, M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008, 65, 862–894. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q.; Sun, Y.; Jin, Y.; Zhang, J.; Wu, J. Melatonin induces anti-inflammatory effects via endoplasmic reticulum stress in raw264.7 macrophages. Mol. Med. Rep. 2018, 17, 6122–6129. [Google Scholar] [CrossRef]
- Moreira, A.J.; Ordonez, R.; Cerski, C.T.; Picada, J.N.; Garcia-Palomo, A.; Marroni, N.P.; Mauriz, J.L.; Gonzalez-Gallego, J. Melatonin activates endoplasmic reticulum stress and apoptosis in rats with diethylnitrosamine-induced hepatocarcinogenesis. PLoS ONE 2015, 10, e0144517. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, S.; Lee, J.H.; Lee, S.H. Melatonin promotes apoptosis of colorectal cancer cells via superoxide-mediated er stress by inhibiting cellular prion protein expression. Anticancer Res. 2018, 38, 3951–3960. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Nair, U.; Yang, Z.; Klionsky, D.J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006, 281, 30299–30304. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the bcl-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Lee, S.H.; Meng, X.W.; Vincelette, N.D.; Knorr, K.L.; Ding, H.; Nowakowski, G.S.; Dai, H.; Kaufmann, S.H. Emerging understanding of bcl-2 biology: Implications for neoplastic progression and treatment. Biochim. Biophys. Acta 2015, 1853, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Velankar, M.; Brahmandam, M.; Hideshima, T.; Podar, K.; Richardson, P.; Schlossman, R.; Ghobrial, I.; Raje, N.; Munshi, N.; et al. A novel bcl-2/bcl-x(l)/bcl-w inhibitor abt-737 as therapy in multiple myeloma. Oncogene 2007, 26, 2374–2380. [Google Scholar] [CrossRef]
- Krestinina, O.; Fadeev, R.; Lomovsky, A.; Baburina, Y.; Kobyakova, M.; Akatov, V. Melatonin can strengthen the effect of retinoic acid in hl-60 cells. Int. J. Mol. Sci. 2018, 19, 2873. [Google Scholar] [CrossRef]
- Lomovsky, A.I.; Baburina, Y.L.; Kobyakova, M.I.; Fadeev, R.S.; Akatov, V.S.; Krestinina, O.V. Melatonin strengthens a chemotherapeutic effect of cytarabin in hl-60 cells. Biol. Membr. 2020, 37, 103–109. [Google Scholar]
- Cui, C.; Lin, T.; Gong, Z.; Zhu, Y. Relationship between autophagy, apoptosis and endoplasmic reticulum stress induced by melatonin in osteoblasts by septin7 expression. Mol. Med. Rep. 2020, 21, 2427–2434. [Google Scholar] [CrossRef]
- Quintana, C.; Cabrera, J.; Perdomo, J.; Estevez, F.; Loro, J.F.; Reiter, R.J.; Quintana, J. Melatonin enhances hyperthermia-induced apoptotic cell death in human leukemia cells. J. Pineal Res. 2016, 61, 381–395. [Google Scholar] [CrossRef]
- Franco, L.; Terrinca, J.; Rodriguez, A.B.; Espino, J.; Pariente, J.A. Extracellular heat shock proteins protect u937 cells from h2o2-induced apoptotic cell death. Mol. Cell. Biochem. 2016, 412, 19–26. [Google Scholar] [CrossRef]
- Bejarano, I.; Redondo, P.C.; Espino, J.; Rosado, J.A.; Paredes, S.D.; Barriga, C.; Reiter, R.J.; Pariente, J.A.; Rodriguez, A.B. Melatonin induces mitochondrial-mediated apoptosis in human myeloid hl-60 cells. J. Pineal Res. 2009, 46, 392–400. [Google Scholar] [CrossRef]
- Nishitoh, H. Chop is a multifunctional transcription factor in the er stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Shamsizadeh, A.; Hayes, A.W.; Reiter, R.J.; Karimi, G. Melatonin as an endogenous regulator of diseases: The role of autophagy. Pharmacol. Res. 2018, 133, 265–276. [Google Scholar] [CrossRef]
- San-Miguel, B.; Crespo, I.; Sanchez, D.I.; Gonzalez-Fernandez, B.; Ortiz de Urbina, J.J.; Tunon, M.J.; Gonzalez-Gallego, J. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J. Pineal Res. 2015, 59, 151–162. [Google Scholar] [CrossRef]
- Vega-Naredo, I.; Caballero, B.; Sierra, V.; Garcia-Macia, M.; de Gonzalo-Calvo, D.; Oliveira, P.J.; Rodriguez-Colunga, M.J.; Coto-Montes, A. Melatonin modulates autophagy through a redox-mediated action in female syrian hamster harderian gland controlling cell types and gland activity. J. Pineal Res. 2012, 52, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Tokarchuk, A.V.; Panteleeva, A.A.; Mulkidjanian, A.Y.; Skulachev, V.P.; Chernyak, B.V. Induction of autophagy by depolarization of mitochondria. Autophagy 2018, 14, 921–924. [Google Scholar] [CrossRef]
- Banerjee, A.; Banerjee, V.; Czinn, S.; Blanchard, T. Increased reactive oxygen species levels cause er stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget 2017, 8, 26142–26153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Reiter, R.J. Melatonin uses in oncology: Breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin. Investig. Drugs 2012, 21, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Paternoster, L.; De Nicola, M.; Cerella, C.; Ammendola, S.; Bedini, A.; Tarzia, G.; Aquilano, K.; Ciriolo, M.; Ghibelli, L. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol. Appl. Pharmacol. 2009, 239, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Estevez, F.; Cabrera, J.; Reiter, R.J.; Loro, J.; Quintana, J. Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid hl-60 cells. J. Pineal Res. 2007, 42, 131–138. [Google Scholar] [CrossRef]
- Trubiani, O.; Recchioni, R.; Moroni, F.; Pizzicannella, J.; Caputi, S.; Di Primio, R. Melatonin provokes cell death in human b-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J. Pineal Res. 2005, 39, 425–431. [Google Scholar] [CrossRef]
- Casado-Zapico, S.; Rodriguez-Blanco, J.; Garcia-Santos, G.; Martin, V.; Sanchez-Sanchez, A.M.; Antolin, I.; Rodriguez, C. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human ewing sarcoma cancer cells: Potentiation of the extrinsic apoptotic pathway. J. Pineal Res. 2010, 48, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.D.; Vandenberg, C.J.; Scott, C.L.; Wei, A.H.; Cory, S.; Huang, D.C.; Roberts, A.W. In Vivo efficacy of the bcl-2 antagonist abt-737 against aggressive myc-driven lymphomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17961–17966. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.F.; Wei, A.H.; Mason, K.D.; Vandenberg, C.J.; Chen, L.; Czabotar, P.E.; Willis, S.N.; Scott, C.L.; Day, C.L.; Cory, S.; et al. The bh3 mimetic abt-737 targets selective bcl-2 proteins and efficiently induces apoptosis via bak/bax if mcl-1 is neutralized. Cancer Cell 2006, 10, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Pinton, P. The mitochondrial permeability transition pore and cancer: Molecular mechanisms involved in cell death. Front. Oncol. 2014, 4, 302. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin protects sh-sy5y neuronal cells against methamphetamine-induced endoplasmic reticulum stress and apoptotic cell death. Neurotox. Res. 2017, 31, 1–10. [Google Scholar] [CrossRef]
- de Luxan-Delgado, B.; Potes, Y.; Rubio-Gonzalez, A.; Caballero, B.; Solano, J.J.; Fernandez-Fernandez, M.; Bermudez, M.; Rodrigues Moreira Guimaraes, M.; Vega-Naredo, I.; Boga, J.A.; et al. Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 2016, 61, 108–123. [Google Scholar] [CrossRef]
- Song, H.S.; Jang, S.; Kang, S.C. Bavachalcone from cullen corylifolium induces apoptosis and autophagy in hepg2 cells. Phytomedicine 2018, 40, 37–47. [Google Scholar] [CrossRef]
- Li, M.; Pi, H.; Yang, Z.; Reiter, R.J.; Xu, S.; Chen, X.; Chen, C.; Zhang, L.; Yang, M.; Li, Y.; et al. Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor eb-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 2016, 61, 353–369. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef]
- Prieto-Dominguez, N.; Ordonez, R.; Fernandez, A.; Mendez-Blanco, C.; Baulies, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C.; Mauriz, J.L.; Gonzalez-Gallego, J. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J. Pineal Res. 2016, 61, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishitoh, H. Stress responses from the endoplasmic reticulum in cancer. Front. Oncol. 2015, 5, 93. [Google Scholar] [CrossRef]
- Berridge, M.J. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium 2002, 32, 235–249. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Pinton, P. The endoplasmic reticulum-mitochondria connection: One touch, multiple functions. Biochim. Biophys. Acta 2014, 1837, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef]
- Fernandez, A.; Ordonez, R.; Reiter, R.J.; Gonzalez-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef]
- Borges, J.C.; Ramos, C.H. Protein folding assisted by chaperones. Protein Pept. Lett. 2005, 12, 257–261. [Google Scholar] [CrossRef]
- Ali Khan, H.; Mutus, B. Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front. Chem. 2014, 2, 70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomovsky, A.; Baburina, Y.; Odinokova, I.; Kobyakova, M.; Evstratova, Y.; Sotnikova, L.; Krestinin, R.; Krestinina, O. Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells. Antioxidants 2020, 9, 1143. https://doi.org/10.3390/antiox9111143
Lomovsky A, Baburina Y, Odinokova I, Kobyakova M, Evstratova Y, Sotnikova L, Krestinin R, Krestinina O. Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells. Antioxidants. 2020; 9(11):1143. https://doi.org/10.3390/antiox9111143
Chicago/Turabian StyleLomovsky, Alexey, Yulia Baburina, Irina Odinokova, Margarita Kobyakova, Yana Evstratova, Linda Sotnikova, Roman Krestinin, and Olga Krestinina. 2020. "Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells" Antioxidants 9, no. 11: 1143. https://doi.org/10.3390/antiox9111143
APA StyleLomovsky, A., Baburina, Y., Odinokova, I., Kobyakova, M., Evstratova, Y., Sotnikova, L., Krestinin, R., & Krestinina, O. (2020). Melatonin Can Modulate the Effect of Navitoclax (ABT-737) in HL-60 Cells. Antioxidants, 9(11), 1143. https://doi.org/10.3390/antiox9111143




