Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Collection and Laboratory Measurements
2.3. Redox Assays
2.4. Myeloperoxidase Activity
2.5. Protein Glycoxidation
2.6. Protein Oxidative Damage
2.7. Nitrosative/Nitrative Stress
2.8. Statistics
3. Results
3.1. General Characteristics
3.2. Myeloperoxidase Activity
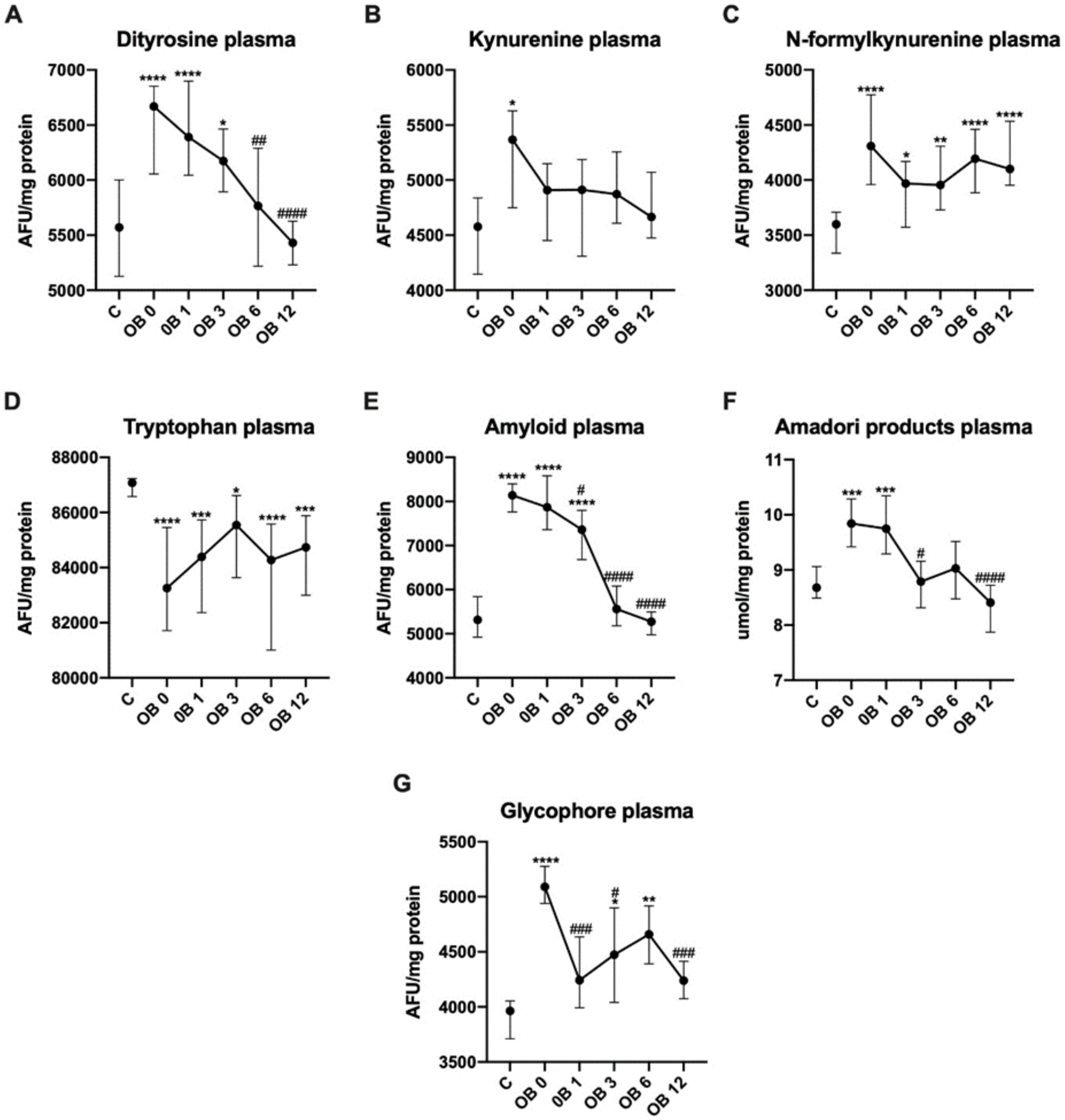
3.3. Protein Glycoxidation
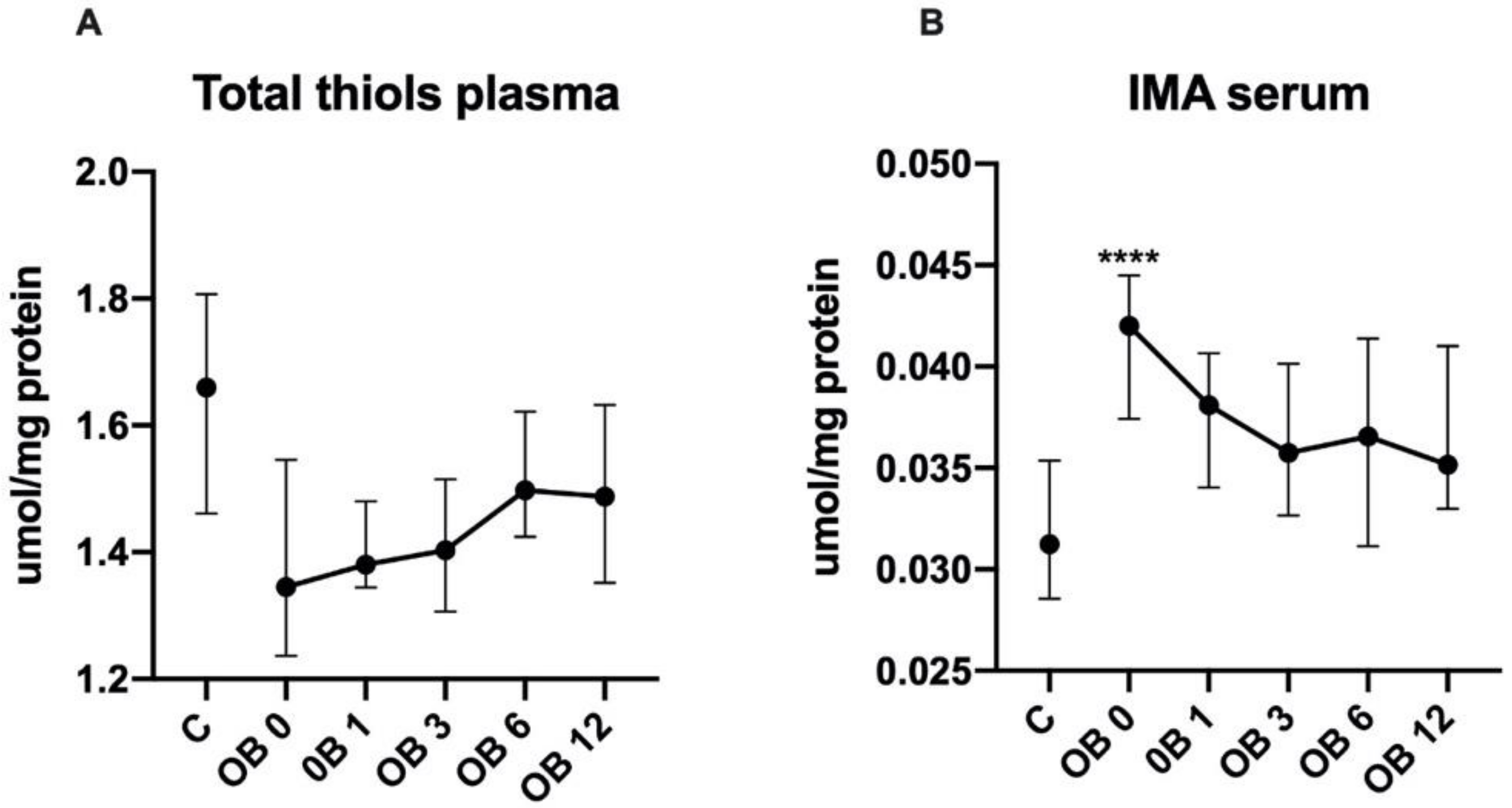
3.4. Protein Oxidative Damage
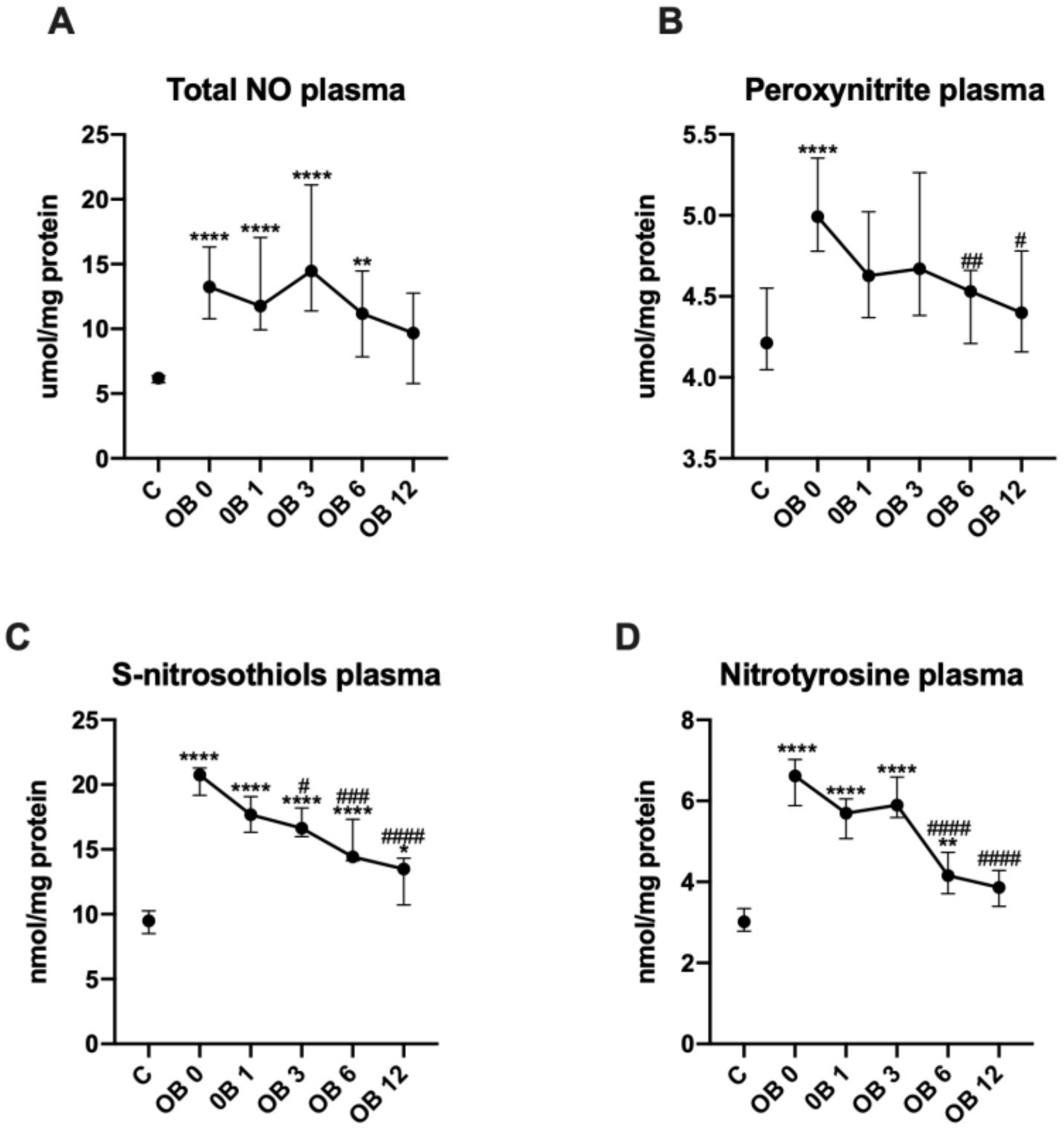
3.5. Nitrosative Stress
3.6. Comparison between MS− and MS+
3.7. ROC Analysis
3.8. Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015. [Google Scholar] [CrossRef]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, leptin and breast cancer: Epidemiological evidence and proposed mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Iwan-Zietek, I.; Ruszkowska-Ciastek, B.; Michalska, M.; Overskaug, E.; Goralczyk, K.; Dabrowiecki, S.; Rosc, D. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. J. Physiol. Pharmacol. 2016, 67, 555–561. [Google Scholar]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Żendzian-Piotrowska, M.; Dadan, J.; Zalewska, A.; Maciejczyk, M. Impact of Weight Loss on the Total Antioxidant/Oxidant Potential in Patients with Morbid Obesity—A Longitudinal Study. Antioxidants 2020, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases With the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Free Radic. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Taranta-Janusz, K.; Wasilewska, A.; Kossakowska, A.; Zalewska, A. A Case-Control Study of Salivary Redox Homeostasis in Hypertensive Children. Can Salivary Uric Acid be a Marker of Hypertension? J. Clin. Med. 2020, 9, 837. [Google Scholar] [CrossRef]
- Pawlukianiec, C.; Gryciuk, M.E.; Mil, K.M.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. A New Insight into Meloxicam: Assessment of Antioxidant and Anti-Glycating Activity in In Vitro Studies. Pharmaceuticals 2020, 13, 240. [Google Scholar] [CrossRef]
- Klimiuk, A.; Maciejczyk, M.; Choromańska, M.; Fejfer, K.; Waszkiewicz, N.; Zalewska, A. Salivary Redox Biomarkers in Different Stages of Dementia Severity. J. Clin. Med. 2019, 8, 840. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef]
- El-Bikai, R.; Welman, M.; Margaron, Y.; Côté, J.F.; Macqueen, L.; Buschmann, M.D.; Fahmi, H.; Shi, Q.; Maghni, K.; Fernandes, J.C.; et al. Perturbation of adhesion molecule-mediated chondrocyte-matrix interactions by 4-hydroxynonenal binding: Implication in osteoarthritis pathogenesis. Arthritis Res. Ther. 2010. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Wasilewska, A.; Zalewska, A. Salivary Gland Dysfunction, Protein Glycooxidation and Nitrosative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2020, 9, 1285. [Google Scholar] [CrossRef]
- Hady, R.H.; Zbucki, R.; Łuba, M.E.; Gołaszewski, P.; Ładny, R.J.; Dadan, J.W. Obesity as a social disease and the influence of environmental factors on BMI in own material. Adv. Clin. Exp. Med. 2010. [Google Scholar]
- Peterli, R.; Wolnerhanssen, B.K.; Peters, T.; Vetter, D.; Kroll, D.; Borbely, Y.; Schultes, B.; Beglinger, C.; Drewe, J.; Schiesser, M.; et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss in patients with morbid obesity the sm-boss randomized clinical trial. JAMA J. Am. Med. Assoc. 2018. [Google Scholar] [CrossRef]
- Adil, M.T.; Jain, V.; Rashid, F.; Al-taan, O.; Whitelaw, D.; Jambulingam, P. Meta-analysis of the effect of bariatric surgery on physical function. Br. J. Surg. 2018, 105, 1107–1118. [Google Scholar] [CrossRef]
- Sudlow, A.; Le Roux, C.; Pournaras, D. The metabolic benefits of different bariatric operations: What procedure to choose? Endocr. Connect. 2020, 9, R28–R35. [Google Scholar] [CrossRef]
- Breusing, N.; Grune, T. Biomarkers of protein oxidation from a chemical, biological and medical point of view. Exp. Gerontol. 2010, 45, 733–737. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Toczewska, J.; Konopka, T.; Zalewska, A.; Maciejczyk, M. Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 259. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Van Duijn, W.; Mieremet-Ooms, M.A.C.; Van Hogezand, R.A.; Lamers, C.B.H.W.; Verspaget, H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J. Pathol. 2003. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jablonska, E.; Zaleski, P.; Waszkiel, D.; Ladny, J.R.; Zukowski, P.; et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- LeVine, H. Quantification of Beta-Sheet Amyloid Fibril Structures with Thioflavin T. Methods Enzym. 1999, 309, 274–284. [Google Scholar] [CrossRef]
- Johnson, R.; Baker, J. Assay of serum fructosamine: Internal vs. external standardization. Clin. Chem. 1987, 33, 1955–1956. [Google Scholar] [CrossRef]
- Kalousová, M.; Zima, T.; Tesař, V.; Dusilová-Sulková, S.; Škrha, J. Advanced glycoxidation end products in chronic diseases—Clinical chemistry and genetic background. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 37–46. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J. Emerg. Med. 2000. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.R.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione Metabolism, Mitochondria Activity, and Nitrosative Stress in Patients Treated for Mandible Fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef]
- Beckman, J.S.; Ischiropoulos, H.; Zhu, L.; Van der Woerd, M.; Smith, C.; Chen, J.; Harrison, J.; Martin, J.C.; Tsai, M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 438–445. [Google Scholar] [CrossRef]
- Wink, D.A.; Kim, S.; Coffin, D.; Cook, J.C.; Vodovotz, Y.; Chistodoulou, D.; Jourd’heuil, D.; Grisham, M.B. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999, 301, 201–211. [Google Scholar] [CrossRef]
- Salminen, P.; Helmio, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss at 5 years among patients with morbid obesity the SLEEVEPASS randomized clinical trial. JAMA J. Am. Med. Assoc. 2018. [Google Scholar] [CrossRef]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Dadan, J.; Zalewska, A.; Maciejczyk, M. The Impact of Hypertension and Metabolic Syndrome on Nitrosative Stress and Glutathione Metabolism in Patients with Morbid Obesity. Oxid. Med. Cell. Longev. 2020, 2020, 1057570. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Megías-Vericat, J.; Tortajada-Girbés, M.; Alonso-Iglesias, E. Nitric oxide production is increased in severely obese children and related to markers of oxidative stress and inflammation. Atherosclerosis 2011. [Google Scholar] [CrossRef]
- Román-Pintos, L.M.; Villegas-Rivera, G.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. Diabetic polyneuropathy in type 2 diabetes mellitus: Inflammation, oxidative stress, and mitochondrial function. J. Diabetes Res. 2016. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Skutnik-Radziszewska, A.; Zieniewska, I.; Matczuk, J.; Domel, E.; Waszkiel, D.; Żendzian-Piotrowska, M.; Szarmach, I.; Zalewska, A. Antioxidant Defense, Oxidative Modification, and Salivary Gland Function in an Early Phase of Cerulein Pancreatitis. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Reddy, P.Y.; Giridharan, N.V.; Reddy, G.B. Activation of sorbitol pathway in metabolic syndrome and increased susceptibility to cataract in Wistar-Obese rats. Mol. Vis. 2012, 18, 495. [Google Scholar]
- Yazıcı, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 277–304. [Google Scholar]
- Griffiths, H.R.; Dias, I.H.K.; Willetts, R.S.; Devitt, A. Redox regulation of protein damage in plasma. Redox Biol. 2014, 2, 430–435. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Vissers, M.C.M.; Kettle, A.J. Myeloperoxidase. Curr. Opin. Hematol. 2000, 7, 53–58. [Google Scholar] [CrossRef]
- Brovkovych, V.; Gao, X.P.; Ong, E.; Brovkovych, S.; Brennan, M.L.; Su, X.; Hazen, S.L.; Malik, A.B.; Skidgel, R.A. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008. [Google Scholar] [CrossRef]
- Bender, S.B.; Herrick, E.K.; Lott, N.D.; Klabunde, R.E. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes. Metab. 2007. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Matsuura, H.; Chayama, K.; Oshima, T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am. J. Hypertens. 2001. [Google Scholar] [CrossRef]
- Gruber, H.J.; Mayer, C.; Mangge, H.; Fauler, G.; Grandits, N.; Wilders-Truschnig, M. Obesity reduces the bioavailability of nitric oxide in juveniles. Int. J. Obes. 2008. [Google Scholar] [CrossRef]
- Kone, B.C.; Kuncewicz, T.; Zhang, W.; Yu, Z.Y. Protein interactions with nitric oxide synthases: Controlling the right time, the right place, and the right amount of nitric oxide. Am. J. Physiol. Ren. Physiol. 2003, 285, F178–F190. [Google Scholar] [CrossRef]
- Salvolini, E.; Vignini, A.; Sabbatinelli, J.; Lucarini, G.; Pompei, V.; Sartini, D.; Cester, A.M.; Ciavattini, A.; Mazzanti, L.; Emanuelli, M. Nitric oxide synthase and VEGF expression in full-term placentas of obese women. Histochem. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Baker, P.R.S.; Freeman, B.A. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003, 28, 646–654. [Google Scholar] [CrossRef]
- Jourd’Heuil, D.; Hallén, K.; Feelisch, M.; Grisham, M.B. Dynamic state of S-nitrosothiols in human plasma and whole blood. Free Radic. Biol. Med. 2000. [Google Scholar] [CrossRef]
- Zalewska, A.; Kossakowska, A.; Taranta-Janusz, K.; Zięba, S.; Fejfer, K.; Salamonowicz, M.; Kostecka-Sochoń, P.; Wasilewska, A.; Maciejczyk, M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. J. Clin. Med. 2020, 9, 548. [Google Scholar] [CrossRef]
- Cournot, M.; Burillo, E.; Saulnier, P.J.; Planesse, C.; Gand, E.; Rehman, M.; Ragot, S.; Rondeau, P.; Catan, A.; Gonthier, M.P.; et al. Circulating concentrations of redox biomarkers do not improve the prediction of adverse cardiovascular events in patients with type 2 diabetes mellitus. J. Am. Heart Assoc. 2018. [Google Scholar] [CrossRef]
- Pieme, C.A.; Tatangmo, J.A.; Simo, G.; Nya, B.P.C.; Moor, A.V.J.; Moukette, B.; Nzufo, F.T.; Nono, B.L.N.; Sobngwi, E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes 2017. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasilewska, A.; Zalewska, A. Salivary FRAP as A Marker of Chronic Kidney Disease Progression in Children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef]
- Gerreth, P.; Maciejczyk, M.; Zalewska, A.; Gerreth, K.; Hojan, K. Comprehensive Evaluation of the Oral Health Status, Salivary Gland Function, and Oxidative Stress in the Saliva of Patients with Subacute Phase of Stroke: A Case-Control Study. J. Clin. Med. 2020, 9, 2252. [Google Scholar] [CrossRef]
- Ding, L.; Fan, Y.; Li, H.; Zhang, Y.; Qi, D.; Tang, S.; Cui, J.; He, Q.; Zhuo, C.; Liu, M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes. Rev. 2020. [Google Scholar] [CrossRef]
- Kodama, S.; Fujihara, K.; Horikawa, C.; Harada, M.; Ishiguro, H.; Kaneko, M.; Furukawa, K.; Matsubayashi, Y.; Matsunaga, S.; Shimano, H.; et al. Network meta-analysis of the relative efficacy of bariatric surgeries for diabetes remission. Obes. Rev. 2018, 19, 1621–1629. [Google Scholar] [CrossRef]
- Sánchez, E.; Baena-Fustegueras, J.A.; De la Fuente, M.C.; Gutiérrez, L.; Bueno, M.; Ros, S.; Lecube, A. Advanced glycation end-products in morbid obesity and after bariatric surgery: When glycemic memory starts to fail. Endocrinol. Diabetes Nutr. 2017. [Google Scholar] [CrossRef]
- Koliaki, C.; Roden, M. Alterations of Mitochondrial Function and Insulin Sensitivity in Human Obesity and Diabetes Mellitus. Annu. Rev. Nutr. 2016, 36, 337–367. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef]

| C | OB 0 | OB 1 | OB 3 | OB 6 | OB 12 | ANOVA | |
|---|---|---|---|---|---|---|---|
| Age | 45 | 45 | >0.9999 | ||||
| (41–49) | (41–49) | ||||||
| Weight (kg) | 62 | 124 | 113 | 100.5 | 90 ****#### | 81.5 ***#### | <0.0001 |
| (60.32–63) | (121.5–131.1) | (106.4–115.8) | (96.6–104.9) | (86.4–93.2) | (78–84.8) | ||
| BMI (kg/m2) | 23 | 46 **** | 41 **** | 37 ****### | 34 ****#### | 30 ***#### | <0.0001 |
| (23–23) | (45–48) | (40–43) | (36–40) | (32–35) | (29–32) | ||
| Weight loss (kg) | 13 | 24 | 33 | 41.5 | <0.0001 | ||
| (10.39–13.09) | (21.52–25.3) | (30–34.34) | (38.72–44.03) | ||||
| WHR | 0.72 | 0.97 **** | 0.98 **** | 0.97 **** | 0.96 **** | 0.92 ****### | <0.0001 |
| (0.71–0.72) | (0.96–0.99) | (0.96–0.99) | (0.94–0.99) | (0.93–0.97) | (0.91–0.94) | ||
| SBP (mmHg) | 120 | 130 **** | 130 **** | 130 **** | 128 *** | 125 ** | <0.0001 |
| (110–120) | (125–140) | (125–140) | (125–135) | (120–135) | (120–130) | ||
| DBP (mmHg) | 80 | 85 **** | 85 *** | 80 * | 80 * | 80 | <0.0001 |
| (70–80) | (80–90) | (80–90) | (80–90) | (80–85) | (80–85) | ||
| Glucose (mg/dL) | 76 | 101 **** | 98 **** | 93 ****# | 93 **** | 87 ****#### | <0.0001 |
| (73–78) | (95–106) | (91–99) | (90–97) | (87–96) | (85–92) | ||
| Insulin (μIU/mL) | 7.6 | 19 **** | 13 ***#### | 9 #### | 8.6 #### | 7.8 #### | <0.0001 |
| (7.4–7.8) | (17–22) | (9.8–15) | (7.3–11) | (7.5–9.2) | (6.9–8.5) | ||
| HOMA–IR | 1.4 | 4.4 **** | 3 ****## | 2 ***#### | 1.9 *#### | 1.7 #### | <0.0001 |
| (1.3–1.5) | (4–5.4) | (2.4–3.5) | (1.7–2.4) | (1.7–2.2) | (1.5–1.9) | ||
| Cholesterol (mg/dL) | 175 | 198 **** | 185 # | 177 ## | 184 | 175 #### | <0.0001 |
| (170–178) | (186–209) | (175–192) | (173–184) | (174–191) | (167–180) | ||
| HDL (mg/dL) | 60 | 46 **** | 45 **** | 47 **** | 50 *** | 55 # | <0.0001 |
| (59–62) | (42–54) | (39–49) | (43–49) | (48–54) | (51–57) | ||
| TG (mg/dL) | 134 | 135 | 126 | 115 | 116 **## | 98 ****#### | <0.0001 |
| (130–135) | (125–151) | (107–139) | (103–135) | (99–124) | (85–106) | ||
| LDL (mg/dL) | 118 | 137 * | 115 #### | 112 #### | 109 #### | 103 **#### | <0.0001 |
| (116–120) | (128–148) | (110–120) | (104–119) | (99–118) | (99–113) | ||
| ALT (IU/L) | 25 | 27 | 28 | 22 | 20 ### | 18 #### | <0.0001 |
| (22–27) | (24–30) | (21–35) | (19–24) | (17–21) | (17–20) | ||
| AST (IU/L) | 23 | 20 | 27 | 19 | 18 ** | 18 | <0.0001 |
| (22–26) | (18–24) | (22–32) | (17–21) | (16–20) | (16–25) | ||
| UA (mg/dL) | 3.9 | 6.4 **** | 5.9 **** | 5.1 ****## | 4.8 *#### | 4.4 #### | <0.0001 |
| (3.8–4.3) | (5.7–7.2) | (5.4–6.2) | (4.8–5.5) | (4.5–5) | (4.1–4.7) | ||
| Urea (mg/dL) | 25 | 29 * | 26 | 23 ## | 25 | 25 # | 0.0053 |
| (22–27) | (25–32) | (22–28) | (22–26) | (22–27) | (22–27) | ||
| Crea (mg/dL) | 0.74 | 0.73 | 0.76 | 0.72 | 0.72 | 0.75 | 0.2102 |
| (0.71–0.76) | (0.71–0.75) | (0.72–0.8) | (0.7–0.76) | (0.69–0.75) | (0.7–0.79) | ||
| CRP (mg/L) | 5.5 | 10 * | 6 | 6.7 | 5.3 ### | 5.1 #### | <0.0001 |
| (5.3–5.7) | (6.7–12) | (5.1–7.8) | (6–7.1) | (5–6.3) | (4.9–5.3) | ||
| WBC (103/μL) | 7.5 | 8.8 ** | 6.8 ### | 6.1 #### | 6.7 #### | 6.1 #### | <0.0001 |
| (6.8–7.8) | (8.1–9.8) | (6.5–7.6) | (5.8–6.9) | (5.9–7.9) | (5.5–7) | ||
| RBC (106/μL) | 4.6 | 4.6 | 4.7 | 4.7 | 4.7 | 4.6 | 0.3807 |
| (4.5–4.7) | (4.4–4.9) | (4.6–4.9) | (4.5–4.8) | (4.4–4.8) | (4.5–4.8) | ||
| HGB (g/dL) | 14 | 13 | 14 | 13 | 13 | 14 | 0.0355 |
| (14–14) | (13–14) | (13–14) | (13–14) | (13–14) | (13–14) | ||
| PLT (103/μL) | 289 | 258 | 237 **** | 255 ** | 261 | 221 ****# | <0.0001 |
| (278–298) | (231–299) | (213–254) | (225–277) | (238–307) | (197–230) |
| MS− 0 | MS− 1 | MS− 3 | MS− 6 | MS− 12 | MS+ 0 | MS+ 1 | MS+ 3 | MS+ 6 | MS+ 12 | |
|---|---|---|---|---|---|---|---|---|---|---|
| n = 25 | n = 25 | |||||||||
| Dityrosine | 5433 | 6267 | 6144 | 5592 | 5385 | 5631 | 6624 | 6174 | 5871 | 5587 |
| (AFU/mg Protein) | (4879−6002) | (5820-7069) | (5489-6487) | (5059-6570) | (4912–5647) | (5160–6172) | (5863–7042) | (5901–6578) | (5087–6166) | (4943–5851) |
| Kynurenine | 5525 | 4927 | 5104 | 4996 | 4665 | 5032 | 4813 | 4402 | 4802 | 4619 |
| (AFU/mg protein) | (4881−5743) | (4238−5231) | (4514−5614) | (4156−5841) | (4501−5842) | (4157–5628) | (3866–5329) | (3916–5173) | (3114–5240) | (3378–5136) |
| N–formyl-kynurenine | 4257 | 3903 | 3864 | 4130 | 4147 | 4343 | 4072 | 4136 | 4258 | 4061 |
| (AFU/mg protein) | (3858−4774) | (3472−4169) | (3429−4352) | (3775−4454) | (3757−4577) | (3929–4982) | (3431–4533) | (3780–4402) | (3704–4721) | (3932–5162) |
| Tryptophan | 83957 | 82467 | 86026 | 85097 | 84737 | 82126 | 85232 | 84260 | 83275 | 84671 |
| (AFU/mg protein) | (81854–86712) | (80908–85730) | (84293–87229) | (79487–89554) | (82142–87014) | (78363–85510) | (81778–87357) | (82824–86703) | (80369–85412) | (82712–86292) |
| Amyloid | 8220 | 7359 | 7404 | 5564 | 5135 | 8095 | 8570 | 7210 | 5557 | 5303 |
| (AFU/mg protein) | (7594–8760) | (6627–8162) | (6284–7800) | (5262–6083) | (4859–5495) | (7498–8528) | (7563–8712) | (6620–8025) | (5034–6389) | (4977–5820) |
| Amadori products | 9.5 | 9.4 | 8.5 | 8.5 | 8 | 10 | 10 # | 8.9 | 9.8 ~~ | 8.6 |
| (µmol/mg protein) | (8.6–0) | (8.7–9.7) | (8.1–9.1) | (8.2–8.9) | (7–8.9) | (9.5–11) | (9.7–11) | (8.4–10) | (9.2–11) | (8.1–9.1) |
| Glycophore | 5237 | 4137 | 4799 | 4688 | 4233 | 5039 | 4339 | 4228 ^ | 4451 | 4245 |
| (AFU/mg protein) | (4512–5333) | (3919–4601) | (3852–5329) | (4478–4974) | (3805–4551) | (4939–5282) | (3792–4954) | (3848–4637) | (3987–4948) | (4058–4525) |
| Total thiols | 1.4 | 1.5 | 1.4 | 1.5 | 1.5 | 1.3 | 1.3 # | 1.4 | 1.5 | 1.5 |
| (µmol/mg protein) | (1.2–1.7) | (1.4–1.6) | (1.3–1.7) | (1.4–1.6) | (1.4–1.7) | (1.2–1.5) | (1.2–1.4) | (1.3–1.6) | (1.4–1.8) | (1.2–1.7) |
| IMA | 0.038 | 0.038 | 0.034 | 0.037 | 0.042 | 0.043 | 0.038 | 0.036 | 0.034 | 0.034 |
| (µmol/mg protein) | (0.035–0.044) | (0.032–0.044) | (0.031–0.041) | (0.032–0.042) | (0.033–0.051) | (0.039–0.045) | (0.034–0.041) | (0.032–0.052) | (0.03–0.042) | (0.03–0.037) |
| MPO | 0.12 | 0.12 | 0.12 | 0.11 | 0.11 | 0.12 | 0.12 | 0.12 | 0.12 | 0.11 |
| (mU/mg protein) | (0.11–0.13) | (0.11–0.12) | (0.11–0.12) | (0.11–0.12) | (0.1–0.12) | (0.11–0.13) | (0.11–0.12) | (0.11–0.12) | (0.11–0.13) | (0.1–0.12) |
| Total NO | 12 | 11 | 12 | 9.3 | 6.4 | 16 | 15 | 16 | 14 ~ | 12 |
| (µmol/mg protein) | (11–20) | (8.6–17) | (5.8–23) | (4.5–13) | (3.7–13) | (9.8–27) | (9.5–21) | (8.2–22) | (7.9–20) | (5.8–17) |
| Peroxy-nitrite | 4.8 | 4.6 | 4.8 | 4.4 | 4.5 | 5.8 **** | 4.6 | 4.6 | 4.6 | 4.3 |
| (µmol/mg protein) | (4.4–5.1) | (4.4–5.2) | (4.1–5.7) | (4.1–4.8) | (4.1–5.2) | (5–6.4) | (4.2–5.3) | (4.4–5.7) | (4.2–4.8) | (4.1–4.8) |
| S–nitroso-thiols | 20 | 18 | 16 | 14 | 13 | 22 *** | 18 | 17 | 17 | 14 |
| (nmol/mg protein) | (16–21) | (16–21) | (14–18) | (14–16) | (7.9–16) | (20–23) | (16–20) | (16–19) | (13–18) | (9.7–15) |
| Nitro-tyrosine | 6.1 | 5.7 | 5.9 | 4.1 | 4 | 6.7 | 5.6 | 6.2 | 4.2 | 3.7 |
| (nmol/mg protein) | (5.3–7.2) | (5.2–6.7) | (4.9–6.7) | (3.5–4.8) | (3.1–4.3) | (5.9–7.1) | (4.7–6.4) | (5.7–6.9) | (3.6–4.8) | (3.1–4.3) |
| AUC | 95% CI | P Value | Cut off | Sensitivity% | 95% CI | Specificity% | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Dityrosine | 0.54 | 0.38 to 0.70 | 0.6208 | >5571 | 60 | 41% to 77% | 60 | 41% to 77% |
| (AFU/mg protein) | ||||||||
| Kynurenine | 0.65 | 0.49 to 0.80 | 0.0833 | <5270 | 58 | 39% to 76% | 62 | 43% to 79% |
| (AFU/mg protein) | ||||||||
| N-formyl-kynurenine | 0.55 | 0.39 to 0.72 | 0.5222 | >4287 | 56 | 37% to 73% | 54 | 35% to 72% |
| (AFU/mg protein) | ||||||||
| Tryptophan | 0.6 | 0.44 to 0.76 | 0.2225 | <83402 | 60 | 41% to 77% | 58 | 39% to 76% |
| (AFU/mg protein) | ||||||||
| Amyloid | 0.57 | 0.40 to 0.73 | 0.4187 | <8155 | 56 | 37% to 74% | 54 | 35% to 72% |
| (AFU/mg protein) | ||||||||
| Amadori products | 0.64 | 0.48 to 0.80 | 0.099 | >9.921 | 58 | 39% to 76% | 62 | 43% to 79% |
| (µmol/mg protein) | ||||||||
| Glycophore | 0.52 | 0.35 to 0.68 | 0.8538 | >5061 | 48 | 30% to 66% | 44 | 27% to 63% |
| (AFU/mg protein) | ||||||||
| Total thiols | 0.59 | 0.43 to 0.76 | 0.2745 | <1.345 | 58 | 39% to 76% | 58 | 39% to 76% |
| (µmol/mg protein) | ||||||||
| IMA | 0.59 | 0.43 to 0.75 | 0.2836 | >0.0415 | 62 | 43% to 79% | 58 | 39% to 76% |
| (µmol/mg protein) | ||||||||
| MPO | 0.5 | 0.34 to 0.67 | 0.9507 | <0.1194 | 50 | 31% to 69% | 50 | 31% to 69% |
| (mU/mg protein) | ||||||||
| Total NO | 0.56 | 0.40 to 0.73 | 0.4354 | >13.28 | 54 | 35% to 72% | 56 | 37% to 73% |
| (µmol/mg protein) | ||||||||
| Peroxy-nitrite | 0.83 | 0.71 to 0.95 | 0.0001 | >4.984 | 72 | 52% to 86% | 73 | 52% to 87% |
| (µmol/mg protein) | ||||||||
| S-nitroso-thiols | 0.78 | 0.65 to 0.91 | 0.0011 | >20.51 | 64 | 45% to 80% | 64 | 43% to 80% |
| (nmol/mg protein) | ||||||||
| Nitro-tyrosine | 0.52 | 0.35 to 0.69 | 0.8084 | >6.400 | 60 | 41% to 77% | 56 | 37% to 73% |
| (nmol/mg protein) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Dadan, J.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients. Antioxidants 2020, 9, 1087. https://doi.org/10.3390/antiox9111087
Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Myśliwiec H, Choromańska K, Dadan J, Żendzian-Piotrowska M, Zalewska A, Maciejczyk M. Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients. Antioxidants. 2020; 9(11):1087. https://doi.org/10.3390/antiox9111087
Chicago/Turabian StyleChoromańska, Barbara, Piotr Myśliwiec, Magdalena Łuba, Piotr Wojskowicz, Hanna Myśliwiec, Katarzyna Choromańska, Jacek Dadan, Małgorzata Żendzian-Piotrowska, Anna Zalewska, and Mateusz Maciejczyk. 2020. "Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients" Antioxidants 9, no. 11: 1087. https://doi.org/10.3390/antiox9111087
APA StyleChoromańska, B., Myśliwiec, P., Łuba, M., Wojskowicz, P., Myśliwiec, H., Choromańska, K., Dadan, J., Żendzian-Piotrowska, M., Zalewska, A., & Maciejczyk, M. (2020). Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients. Antioxidants, 9(11), 1087. https://doi.org/10.3390/antiox9111087








