Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa
Abstract
1. Introduction
2. Oxidative Stress and Retinitis Pigmentosa
2.1. Animal Models of RP
2.2. Synoptic Aspects of Oxidation and Antioxidation
2.3. Oxidative Stress and RP
3. Mesenchymal Cells: Therapeutic Strategies in Retinitis Pigmentosa
- Adipose-derived stem cells (ADSCs)
- Adult adipocytes
- Platelets
- Cell differentiation and trans-differentiation for lost/damaged cell replacement
- Paracrine action for cell repair and functional stimulation
- Exosomes and microvesicle secretion
- Modulation of host immune responses in inflammation site
3.1. Transdifferentation
3.2. Paracrine Effect
3.3. Extracellular Vesicles
| MSC Effects | Mechanisms | Comments |
|---|---|---|
| Transdifferentiation | Ability to differentiate into the three germ leyers cells. | Ectoderm: epithelial cell, neuron Mesoderm: condrocyte, adipocyte, osteocyte, connective stromal cell Endoderm: muscle cell, gut epithelial cell, lung cell |
| Cell fusion | Ability to fuse with another cell forming a heterokaryon (i.e. multinuclear cell). | |
| Mitochondrial transfer | Ability to transfer mitochondria in damaged cells to increase activity of the respiratory chain complex and ATP levels. | MSC makes contact with the targeted cell and builds a gap junctional channel to transfer mitochondria. |
| Extracellular vesicles | Ability to release microvesicles and/or exososomes containing bioactive molecules, RNA, microRNA, lipids and proteins for intercellular communication. | The interaction of extracellular vesicles with the targeted cell leads to fusion, release and transfer of the vesicles’ components. |
| Paracrine effect | Ability to secrete bioactive cytokines and chemokines that act on immunomodulation, angiogenesis/arteriogenesis, antiapoptosis, antioxidation and cell migration/stimulation. | Examples: IL-6; HGF; IDO; HO-1; TGF; NO; HLA-G5; PGE2; VEGF; FGF; IGF; MCP1; SDF1; PIGF; IL-6; Bcl-2; Akt; STC1; GM-CSF; TNF; GDNF; SCF; LIF; CCL; CXCL. |
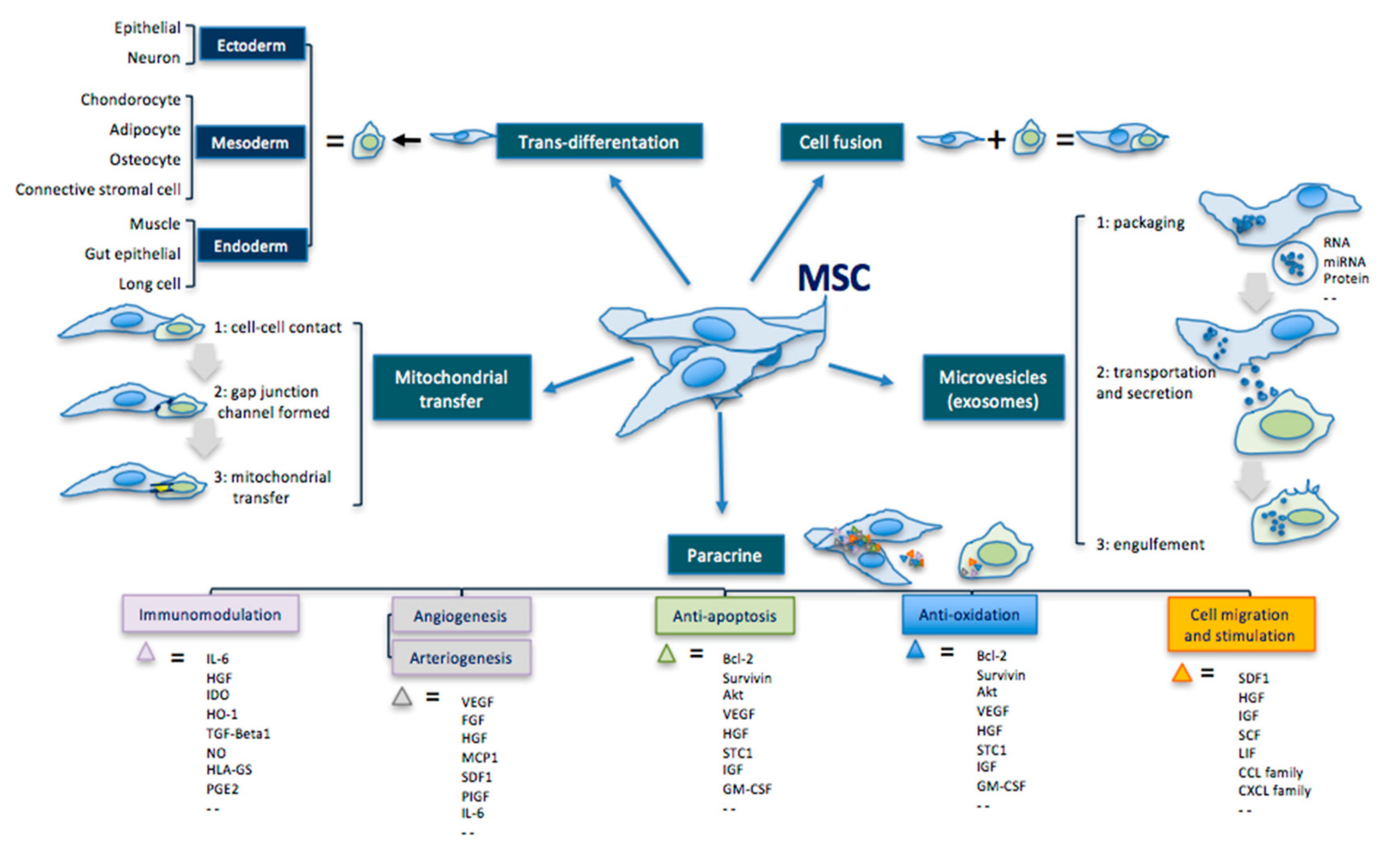
4. Cell-Mediated Biomolecular and Antioxidative Mechanisms in RP
- Hemorheological activity
- Antioxidant activity
- Anti-inflammatory activity
- Anti-apoptotic activity
- Cytoprotective activity
4.1. Hemorheological Activity
4.2. Antioxidant Activity
4.3. Anti-inflammatory Activity
4.4. Antiapoptotic Activity
4.5. Cytoprotective Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADSCs | Adipose Derived Stem Cells |
| AMD | Age Macular Disease |
| ASCs | Adipose Stromal Cells |
| BCEA | Bivariate Contour Ellipse Area |
| BCVA | Best Corrected Visual Acuity |
| BDNF | Brain-Derived Neurotrophic Factor |
| bFGF | Basic Fibroblast Growth Factor |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| CASPasis | Cysteine Aspartate-Specific Proteinases |
| cERG | Cone ERG or Photopic ERG |
| CNS | Central Nervous System |
| CNTF | Ciliary Neurotrophic Factor |
| EGF | Epidermal Growth Factor |
| ER | endoplasmic reticulum |
| ERG | ElectroretinoGram |
| ESCs | Embrionic Stem Cells |
| GAP-43 | Growth-Associated Protein-43 |
| GDNF | Glial Derived Neurotrophic Factor |
| GF | Growth Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HGF | Hepatocyte Growth Factor |
| HIF-1alpha | Hypoxia-Inducible Factor-1alpha |
| IAP | Inhibitor of Apoptosis Protein |
| IFN-β | Interferon-β |
| IGF-1 | Insulin-like Growth Factor-1 |
| IL-1RA | IL-1 Receptor Antagonist |
| IL | Interleukin |
| IRD | Inherited Retinal Disease |
| M-CSF | Macrophage Colony-Stimulating Factor |
| MAPK1 | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MSCs | Mesenchymal Stem Cells |
| PDGF | Platelet-Derived Growth Factor |
| PDAF | Platelet-Derived Angiogenesis Factor |
| PEDF | Pigment-Epithelium-Derived Factor |
| PGE2R | Prostaglandin E2 Receptor |
| PI3-K | Phosphatidylinositol-3-Kinase |
| PlGF | Placental Growth Factor |
| POS | Photoreceptor Segments |
| PRP | Platelet-Rich Plasma |
| PRDX2 | Peroxiredoxin 2 |
| RdCVF | Rod Cone Viability Factor |
| RGC | Retinal Ganglion Cell |
| RMG | Retinal Müller Glia |
| ROS | Reactive Oxygen Species |
| RP | Retinitis Pigmentosa |
| RPE | Retinal Pigment Epithelium |
| SOD | Superoxide Dismutase |
| SVF | Stromal Vascular Fraction |
| TGF- | Transforming Growth Factor- |
| TNF-alpha | Tumoral Necrosis Factor—alpha |
| TSP | Thrombospondin |
| UPR | Unfolded Protein Response |
| VEGF | Vascular Endothelial Growth Factor |
References
- Pagon, R.A. Retinitis pigmentosa. Surv. Ophthalmol. 1988, 33, 137–177. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Hamel, C.P. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ikeda, Y.; Nakatake, S.; Miller, J.W.; Vavvas, D.G.; Sonoda, K.H.; Ishibashi, T. Necrotic cone photoreceptor cell death in retinitis pigmentosa. Cell Death Dis. 2015, 6, e2038. [Google Scholar] [CrossRef]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-Derived Cone Viability Factor Promotes Cone Survival by Stimulating Aerobic Glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Yang, Y.J.; Peng, J.; Ying, D.; Peng, Q. A Brief Review on the Pathological Role of Decreased Blood Flow Affected in Retinitis Pigmentosa. J. Ophthalmol. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.-W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Strauss, R.W.; Lu, L.; Hafiz, G.; Wolfson, Y.; Shah, S.M.; Sophie, R.; Mir, T.A.; Scholl, H.P. Is There Excess Oxidative Stress and Damage in Eyes of Patients with Retinitis Pigmentosa? Antioxid. Redox Signal. 2015, 23, 643–648. [Google Scholar] [CrossRef]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of Daylight Vision in Retinal Degeneration: Are Oxidative Stress and Metabolic Dysregulation to Blame? J. Biol. Chem. 2014, R111, 304428. [Google Scholar] [CrossRef]
- Moreno, M.-L.; Mérida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy Dysfunction and Oxidative Stress, Two Related Mechanisms Implicated in Retinitis Pigmentosa. Front. Physiol. 2018, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Nicocia, G.; D’Angelo, R.; Sidoti, A. Retracted Article: Role of oxidative stress in Retinitis pigmentosa: New involved pathways by an RNA-Seq analysis. Cell Cycle 2018, 18, 84–104. [Google Scholar] [CrossRef]
- Agbaga, M.-P.; Merriman, D.K.; Brush, R.S.; Lydic, T.A.; Conley, S.M.; Naash, M.I.; Jackson, S.; Woods, A.S.; Reid, G.E.; Busik, J.V.; et al. Differential composition of DHA and very-long-chain PUFAs in rod and cone photoreceptors. J. Lipid Res. 2018, 59, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Birtel, J.; Gliem, M.; Oishi, A.; Müller, P.; Herrmann, P.; Holz, F.G.; Mangold, E.; Knapp, M.; Bolz, H.J.; Issa, P.C. Genetic testing in patients with retinitis pigmentosa: Features of unsolved cases. Clin. Exp. Ophthalmol. 2019, 47, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Mohand-Saïd, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Demontant, V.; Boyard, F.; Antonio, A.; Méjécase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Donato, L.; Esposito, T.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. A novel RLBP1 gene geographical area-related mutation present in a young patient with retinitis punctata albescens. Hum. Genom. 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Utz, V.M.; Coussa, R.G.; Antaki, F.; Traboulsi, E.I. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018, 39, 671–677. [Google Scholar] [CrossRef]
- Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Beutelspacher, S.C.; Serbecic, N.; Barash, H.; Burgansky-Eliash, Z.; Grinvald, A.; Krastel, H.; Jonas, J.B. Retinal blood flow velocity measured by retinal function imaging in retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1855–1858. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow–derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-Q.; Aleman, T.S.; Dejneka, N.S.; Dudus, L.; Fisher, K.J.; Maguire, A.M.; Jacobson, S.G.; Bennett, J. Long-Term Protection of Retinal Structure but Not Function Using RAAV.CNTF in Animal Models of Retinitis Pigmentosa. Mol. Ther. 2001, 4, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Novelli, E.; Strettoi, E. Environmental enrichment reduces photoreceptor degeneration and retinal inflammation in a mouse model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4261. [Google Scholar]
- He, Y.; Zhang, Y.; Liu, X.; Ghazaryan, E.; Li, Y.; Xie, J.; Su, G. Recent Advances of Stem Cell Therapy for Retinitis Pigmentosa. Int. J. Mol. Sci. 2014, 15, 14456–14474. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.A.; Mullins, R.F.; Stone, E.M. Stem cells for investigation and treatment of inherited retinal disease. Hum. Mol. Genet. 2014, 23, R9–R16. [Google Scholar] [CrossRef]
- Xue, C.; Rosen, R.B.; Jordan, A.; Hu, D.-N. Management of Ocular Diseases Using Lutein and Zeaxanthin: What Have We Learned from Experimental Animal Studies? J. Ophthalmol. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar] [CrossRef]
- Lavail, M.M.; Nishikawa, S.; Steinberg, R.H.; Naash, M.I.; Duncan, J.L.; Trautmann, N.; Matthes, M.T.; Yasumura, D.; Lau-Villacorta, C.; Chen, J.; et al. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp. Eye Res. 2018, 167, 56–90. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyoshi, H.; Verma, I.M.; Gage, F.H. Rescue from Photoreceptor Degeneration in therd Mouse by Human Immunodeficiency Virus Vector-Mediated Gene Transfer. J. Virol. 1999, 73, 7812–7816. [Google Scholar] [CrossRef]
- Ali, R.R.; Sarra, G.-M.; Stephens, C.; De Alwis, M.; Bainbridge, J.W.; Munro, P.M.; Fauser, S.; Reichel, M.B.; Kinnon, C.; Hunt, D.M.; et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 2000, 25, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Feng, W.; Duncan, J.L.; Yasumura, D.; D’Cruz, P.M.; Chappelow, A.; Matthes, M.T.; Kay, M.A.; Lavail, M.M. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. USA 2001, 98, 12584–12589. [Google Scholar] [CrossRef] [PubMed]
- Acland, G.M.; Aguirre, G.D.; Ray, J.; Zhang, Q.; Aleman, T.S.; Cideciyan, A.V.; Pearce-Kelling, S.E.; Anand, V.; Zeng, Y.; Maguire, A.M.; et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001, 28, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nölting, R.; López-Pedrajas, R.; Ekström, P.; Bosch-Morell, F.; Van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free. Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Usui, S.; Shen, J.; Rogers, B.S.; Campochiaro, P.A. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008, 45, 905–912. [Google Scholar] [CrossRef]
- Sanz, M.; Johnson, L.; Ahuja, S.P.; Ekström, P.; Romero, J.; Van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller Cell Alterations during Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Esquiva, G.; Pinilla, I.; Lax, P.; Cuenca, N. Retinal Vascular Degeneration in the Transgenic P23H Rat Model of Retinitis Pigmentosa. Front. Neuroanat. 2018, 12. [Google Scholar] [CrossRef]
- Frasson, M.; Picaud, S.; Léveillard, T.; Simonutti, M.; Mohand-Said, S.; Dreyfus, H.; Hicks, D.; Sabel, J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2724–2734. [Google Scholar]
- Bush, R.A.; Lei, B.; Tao, W.; Raz, D.; Chan, C.-C.; Cox, T.A.; Santos-Muffley, M.; Sieving, P.A. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: Dose-dependent effects on ERG and retinal histology. Investig. Opthalmol. Vis. Sci. 2004, 45, 2420–2430. [Google Scholar] [CrossRef]
- Uteza, Y.; Rouillot, J.-S.; Kobetz, A.; Marchant, D.; Pecqueur, S.; Arnaud, E.; Prats, H.; Honiger, J.; Dufier, J.-L.; Abitbol, M.; et al. Intravitreous transplantation of encapsulated fibroblasts secreting the human fibroblast growth factor 2 delays photoreceptor cell degeneration in Royal College of Surgeons rats. Proc. Natl. Acad. Sci. USA 1999, 96, 3126–3131. [Google Scholar] [CrossRef]
- Frasson, M.; Sahel, J.-A.; Fabre, M.; Simonutti, M.; Dreyfus, H.; Picaud, S. Retinitis pigmentosa: Rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat. Med. 1999, 5, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E. Bone marrow–derived stem cells preserve cone vision in retinitis pigmentosa. J. Clin. Investig. 2004, 114, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, M.; Todorova, V.; Gougoulakis, L.; Barben, M.; Nötzli, S.; Klee, K.; Storti, F.; Gubler, A.; Imsand, C.; Grimm, C. Light stress affects cones and horizontal cells via rhodopsin-mediated mechanisms. Exp. Eye Res. 2019, 186, 107719. [Google Scholar] [CrossRef] [PubMed]
- Rohowetz, L.J.; Kraus, J.G.; Koulen, P. Reactive Oxygen Species-Mediated Damage of Retinal Neurons: Drug Development Targets for Therapies of Chronic Neurodegeneration of the Retina. Int. J. Mol. Sci. 2018, 19, 3362. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Nedić, O.; Rattan, S.I.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic. Res. 2013, 47, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Kajdanek, J.; Morawiec, J.; Pawlowska, E.; Blasiak, J. PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis. Int. J. Mol. Sci. 2018, 19, 2317. [Google Scholar] [CrossRef]
- Honda, S.; Hjelmeland, L.M.; Handa, J.T. Oxidative stress—Induced single-strand breaks in chromosomal telomeres of human retinal pigment epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2139–2144. [Google Scholar]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Koskela, A.; Felszeghy, S.; Kivinen, N.; Salminen, A.; Kauppinen, A. Fatty acids and oxidized lipoproteins contribute to autophagy and innate immunity responses upon the degeneration of retinal pigment epithelium and development of age-related macular degeneration. Biochimie 2019, 159, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Zhang, H. Redox Regulation and Its Emerging Roles in Stem Cells and Stem-Like Cancer Cells. Antioxid. Redox Signal. 2009, 11, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef]
- Saccà, S.S.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Nicocia, G.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants 2020, 9, 416. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta BBA Bioenerg. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Wang, M.; Zhang, S.X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527. [Google Scholar] [CrossRef]
- Anderson, P.J.; Kedersha, N. Stress granules. Curr. Biol. 2009, 19, R397–R398. [Google Scholar] [CrossRef]
- Kunchithapautham, K.; Rohrer, B. Apoptosis and Autophagy in Photoreceptors Exposed to Oxidative Stress. Autophagy 2007, 3, 433–441. [Google Scholar] [CrossRef]
- Lin, W.-J.; Kuang, H.Y. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 2014, 10, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Langham, M.E.; Kramer, T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye 1990, 4, 374–381. [Google Scholar] [CrossRef]
- Murakami, Y.; Ikeda, Y.; Akiyama, M.; Fujiwara, K.; Yoshida, N.; Nakatake, S.; Notomi, S.; Nabeshima, T.; Hisatomi, T.; Enaida, H.; et al. Correlation between macular blood flow and central visual sensitivity in retinitis pigmentosa. Acta Ophthalmol. 2015, 93, e644–e648. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B. Retinal Remodeling in Inherited Photoreceptor Degenerations. Mol. Neurobiol. 2003, 28, 139–148. [Google Scholar] [CrossRef]
- Peng, Q.; Zhu, W.; Li, C. A research on the mechanism of pigmentary degeneration of retina belonging to deficiency complicated with blood stasis. Jiangsu Tradit. Chin. Med. 1990, 1, 39–41. [Google Scholar]
- Ayton, L.N.; Guymer, R.; Luu, C.D. Choroidal thickness profiles in retinitis pigmentosa. Clin. Exp. Ophthalmol. 2012, 41. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Anselmi, G.M.; Marangoni, D.; D’Esposito, F.; Fadda, A.; Di Renzo, A.; Campos, E.C.; Riva, C.E. Subfoveal Choroidal Blood Flow and Central Retinal Function in Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2011, 52, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Bill, A.; Sperber, G.; Ujiie, K. Physiology of the choroidal vascular bed. Int. Ophthalmol. 1983, 6, 101–107. [Google Scholar] [CrossRef]
- Lieberthal, W.; Triaca, V.; Koh, J.S.; Pagano, P.J.; Levine, J.S. Role of superoxide in apoptosis induced by growth factor withdrawal. Am. J. Physiol. Content 1998, 275, F691–F702. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor Death, Trophic Factor Expression, Retinal Oxygen Status, and Photoreceptor Function in the P23H Rat. Investig. Opthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Jain, S.; Thakkar, N.; Chhatai, J.; Bhadra, M.P.; Bhadra, U. Long non-coding RNA: Functional agent for disease traits. RNA Biol. 2016, 14, 522–535. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Transcriptome Analyses of lncRNAs in A2E-Stressed Retinal Epithelial Cells Unveil Advanced Links between Metabolic Impairments Related to Oxidative Stress and Retinitis Pigmentosa. Antioxidants 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Reactive Oxygen Species and Cell Signaling in Lung Ischemia. Cell Signal. Vasc. Inflamm. 2007, 31, 125–135. [Google Scholar] [CrossRef]
- Türksever, C.; Valmaggia, C.; Orgül, S.; Schorderet, D.F.; Flammer, J.; Todorova, M. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Nicocia, G.; Denaro, L.; Robledo, R.; Sidoti, A.; D’Angelo, R. GLO1 gene polymorphisms and their association with retinitis pigmentosa: A case–control study in a Sicilian population. Mol. Biol. Rep. 2018, 45, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Cerman, E.; Akkoc, T.; Eraslan, M.; Şahin, Ö.; Ozkara, S.; Aker, F.V.; Subaşı, C.; Karaoz, E.; Akkoç, T. Correction: Retinal Electrophysiological Effects of Intravitreal Bone Marrow Derived Mesenchymal Stem Cells in Streptozotocin Induced Diabetic Rats. PLoS ONE 2016, 11, e0165219. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Zeiss, C.; Johnson, E.A. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Investig. Opthalmol. Vis. Sci. 2004, 45, 971–976. [Google Scholar] [CrossRef]
- Gupta, N.; Brown, K.E.; Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003, 76, 463–471. [Google Scholar] [CrossRef]
- Zeng, H.-Y.; Zhu, X.-A.; Zhang, C.; Yang, L.-P.; Wu, L.-M.; Tso, M.O.M. Identification of Sequential Events and Factors Associated with Microglial Activation, Migration, and Cytotoxicity in Retinal Degeneration inrdMice. Investig. Opthalmol. Vis. Sci. 2005, 46, 2992–2999. [Google Scholar] [CrossRef]
- Detrick, B.; Hooks, J.J. The RPE Cell and the Immune System. In Retinal Pigment Epithelium in Health and Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 101–114. [Google Scholar]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M.; Arora, P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xiao, J.; Wang, K.; So, K.F.; Tipoe, G.L.; Lin, B. Suppression of Microglial Activation Is Neuroprotective in a Mouse Model of 21. Human Retinitis Pigmentosa. J. Neurosci. 2014, 34, 8139–8150. [Google Scholar] [CrossRef] [PubMed]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef]
- Klassen, H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin. Biol. Ther. 2015, 16, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef]
- Huo, D.-M.; Dong, F.-T.; Yu, W.-H.; Gao, F. Differentiation of mesenchymal stem cell in the microenviroment of retinitis pigmentosa. Int. J. Ophthalmol. 2010, 3, 216–219. [Google Scholar]
- Zarbin, M. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med. 2016, 22, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal injection of autologous bone marrow–derived mononuclear cells for hereditary retinal dystrophy. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Opthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Darevskaya, A.N.; Merzlikina, N.V.; Buravkova, L.B. Mesenchymal Stem Cells from Human Bone Marrow and Adipose Tissue: Isolation, Characterization, and Differentiation Potentialities. Bull. Exp. Biol. Med. 2005, 140, 138–143. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2010, 7, 269–291. [Google Scholar] [CrossRef]
- Öner, A.; Sevim, D.G. Complications of stem cell-based therapies in retinal diseases. Stem Cell Res. Open Library 2017, 1, 1–7. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise Review: Bone Marrow-Derived Mesenchymal Stem Cells Change Phenotype Following In Vitro Culture: Implications for Basic Research and the Clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef]
- Baddour, J.A.; Sousounis, K.; Tsonis, P.A. Organ repair and regeneration: An overview. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ruster, B.; Gottig, S.; Ludwig, R.J.; Bistrian, R.; Muller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Broek, I.V.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef]
- Luo, S.; Hao, L.; Li, X.; Yu, N.; Diao, Z.; Ren, L.; Xu, H. Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J. Exp. Med. 2013, 231, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-L.; Li, N.; Wei, X.; Tang, L.; Wang, T.-H.; Chen, X.-M. Neuroprotective effects of BDNF and GDNF in intravitreally transplanted mesenchymal stem cells after optic nerve crush in mice. Int. J. Ophthalmol. 2017, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 2008, 216, 3–13. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Nakagami, H.; Morishita, R.; Maeda, K.; Kikuchi, Y.; Ogihara, T.; Kaneda, Y. Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J. Atheroscler. Thromb. 2006, 13, 77–81. [Google Scholar] [CrossRef]
- Schäffler, A.; Buchler, C.H. Concise Review: Adipose Tissue-Derived Stromal Cells-Basic and Clinical Implications for Novel Cell-Based Therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and Biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef]
- Mishra, A.; Velotta, J.; Brinton, T.J.; Wang, X.; Chang, S.; Palmer, O.; Sheikh, A.; Chung, J.; Yang, P.C.-M.; Robbins, R.; et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc. Revascularization Med. 2011, 12, 158–163. [Google Scholar] [CrossRef]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and Phospho-Proteomic Profile of Human Platelets in Basal, Resting State: Insights into Integrin Signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.; Sanderson, J.; Martin, K.R. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells 2017, 36, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.P.; Poveshchenk, O.V.; Surovtseva, M.A.; Stanishevskaya, O.M.; Chernykh, D.V.; Arben’Eva, N.S.; Bratko, V. Autologous Plasma Enriched with Platelet Lysate for the Treatment of Idiopathic Age-Related Macular Degeneration: A Prospective Study. Ann. Russ. Acad. Med. Sci. 2018, 73, 40–48. [Google Scholar] [CrossRef]
- Arslan, U.; Özmert, E.; Demirel, S.; Örnek, F.; Şermet, F. Effects of subtenon-injected autologous platelet-rich plasma on visual functions in eyes with retinitis pigmentosa: Preliminary clinical results. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 893–908. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Gurgel, V.P.; Simões, B.P.; Scott, I.U.; Jorge, R. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol. 2014, 93, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Öner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef]
- Kahraman, N.S.; Öner, A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int J Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Morales, M.U.; Nebbioso, M.; Limoli, C. Preliminary Study on Electrophysiological Changes after Cellular Autograft in Age-Related Macular Degeneration. Medicine 2014, 93, e355. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 2019, 7, 94. [Google Scholar] [CrossRef]
- Limoli, P.G.; Limoli, C.S.S.; Morales, M.U.; Vingolo, E.M. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restor. Neurol. Neurosci. 2020, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Limoli, P.G.; Limoli, C.; Vingolo, E.M.; Scalinci, S.Z.; Nebbioso, M. Cell surgery and growth factors in dry age-related macular degeneration: Visual prognosis and morphological study. Oncotarget 2016, 7, 46913–46923. [Google Scholar] [CrossRef] [PubMed]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Scalinci, S.Z.; Nebbioso, M. Regenerative Therapy by Suprachoroidal Cell Autograft in Dry Age-related Macular Degeneration: Preliminary In Vivo Report. J. Vis. Exp. 2018, 12, e56469. [Google Scholar] [CrossRef] [PubMed]
- Öner, A. Stem Cell Treatment in Retinal Diseases: Recent Developments. Turk. J. Ophthalmol. 2018, 48, 33–38. [Google Scholar] [CrossRef]
- Mok, P.L.; Leong, C.F.; Cheong, S.-K. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays. J. Pathol. 2013, 35, 17–32. [Google Scholar]
- Kim, K.-S.; Park, J.-M.; Kong, T.; Kim, C.; Bae, S.-H.; Kim, H.W.; Moon, J. Retinal Angiogenesis Effects of TGF-β1 and Paracrine Factors Secreted from Human Placental Stem Cells in Response to a Pathological Environment. Cell Transplant. 2016, 25, 1145–1157. [Google Scholar] [CrossRef]
- Zhao, P.-T.; Zhang, L.-J.; Shao, H.; Bai, L.-L.; Yu, B.; Su, C.; Dong, L.-J.; Liu, X.; Li, X.; Zhang, X. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int. J. Ophthalmol. 2016, 9, 1381–1389. [Google Scholar] [CrossRef]
- Salehi, H.; Amirpour, N.; Razavi, S.; Esfandiari, E.; Zavar, R. Overview of retinal differentiation potential of mesenchymal stem cells: A promising approach for retinal cell therapy. Ann. Anat. Anat. Anz. 2017, 210, 52–63. [Google Scholar] [CrossRef]
- Moraes, L.; Vasconcelos-Dos-Santos, A.; Santana, F.C.; Godoy, M.A.; Rosado-De-Castro, P.H.; Jasmin; Azevedo-Pereira, R.L.; Cintra, W.M.; Gasparetto, E.L.; Santiago, M.F.; et al. Neuroprotective effects and magnetic resonance imaging of mesenchymal stem cells labeled with SPION in a rat model of Huntington’s disease. Stem Cell Res. 2012, 9, 143–155. [Google Scholar] [CrossRef]
- Kyurkchiev, D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2013, 137, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Giordano, C.; Galderisi, U.; Luongo, L.; Alessio, N.; Di Bernardo, G.; De Novellis, V.; Rossi, F.; Maione, S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell. Mol. Life Sci. 2009, 67, 655–669. [Google Scholar] [CrossRef]
- Rezanejad, H.; Soheili, Z.-S.; Haddad, F.; Matin, M.M.; Samiei, S.; Manafi, A.; Ahmadieh, H. In vitro differentiation of adipose-tissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. Cell Tissue Res. 2014, 356, 65–75. [Google Scholar] [CrossRef]
- Emre, E.; Yüksel, N.; Duruksu, G.; Pirhan, D.; Subaşi, C.; Erman, G.; Karaöz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow–derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Garcia, T.B.; Hollborn, M.; Bringmann, A. Expression and signaling of NGF in the healthy and injured retina. Cytokine Growth Factor Rev. 2017, 34, 43–57. [Google Scholar] [CrossRef]
- Sluch, V.M.; Zack, D.J. Stem cells, retinal ganglion cells and glaucoma. Dev. Ophthalmol. 2014, 53, 111–121. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Zaverucha-Do-Valle, C.; Rosado-De-Castro, P.H.; Silva-Junior, A.J.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Santiago, M.F. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016, 2016, 5078619. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Wyse, R.D.; Dunbar, G.L.; Rossignol, J. Use of Genetically Modified Mesenchymal Stem Cells to Treat Neurodegenerative Diseases. Int. J. Mol. Sci. 2014, 15, 1719–1745. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, srep34562. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.A.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, J.C.; Shou, M.; Lee, C.; Barr, S.; Fuchs, S.; Epstein, S. Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion Through Paracrine Mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Gao, F.; Hou, H.; Liang, H.; Weinreb, R.N.; Wang, H.; Wang, Y. Bone marrow-derived cells in ocular neovascularization: Contribution and mechanisms. Angiogenesis 2016, 19, 107–118. [Google Scholar] [CrossRef]
- Sheibani, N.; Sorenson, C.M.; Cornelius, L.A.; Frazier, W.A. Thrombospondin-1, a Natural Inhibitor of Angiogenesis, Is Present in Vitreous and Aqueous Humor and Is Modulated by Hyperglycemia. Biochem. Biophys. Res. Commun. 2000, 267, 257–261. [Google Scholar] [CrossRef]
- Carron, J.A.; Hiscott, P.; Hagan, S.; Sheridan, C.M.; Magee, R.; Gallagher, J.A. Cultured human retinal pigment epithelial cells differentially express thrombospondin-1, -2, -3, and -4. Int. J. Biochem. Cell Biol. 2000, 32, 1137–1142. [Google Scholar] [CrossRef]
- Chu, L.-Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, S.; Alex, J.C. Current Applications of Platelet Gels in Facial Plastic Surgery. Facial Plast. Surg. 2002, 18, 027–034. [Google Scholar] [CrossRef]
- Mammoto, T.; Jiang, A.; Jiang, E.; Mammoto, A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc. Res. 2013, 89, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative Therapeutic Potential of Adipose Stromal Cells in Early Stage Diabetic Retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, N.; Xu, W.; Xu, G. Mesenchymal stem cells attenuate hydrogen peroxide-induced oxidative stress and enhance neuroprotective effects in retinal ganglion cells. Vitr. Cell. Dev. Biol. Anim. 2016, 53, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.S.; Subbiah, S.K.; Khan, M.S.A.; Farhana, A.; Mok, P.L. Empowering Mesenchymal Stem Cells for Ocular Degenerative Disorders. Int. J. Mol. Sci. 2019, 20, 1784. [Google Scholar] [CrossRef] [PubMed]
- Whone, A.L.; Kemp, K.; Sun, M.; Wilkins, A.; Scolding, N.J. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012, 1431, 86–96. [Google Scholar] [CrossRef]
- Yamada, H.; Yamada, E.; Ando, A.; Esumi, N.; Bora, N.; Saikia, J.; Sung, C.-H.; Zack, D.J.; Campochiaro, P.A. Fibroblast Growth Factor-2 Decreases Hyperoxia-Induced Photoreceptor Cell Death in Mice. Am. J. Pathol. 2001, 159, 1113–1120. [Google Scholar] [CrossRef][Green Version]
- Hauck, S.M.; Kinkl, N.; Deeg, C.A.; Lange, M.S.-D.; Schöffmann, S.; Ueffing, M. GDNF Family Ligands Trigger Indirect Neuroprotective Signaling in Retinal Glial Cells. Mol. Cell. Biol. 2006, 26, 2746–2757. [Google Scholar] [CrossRef]
- Yang, Y.; Mohand-Said, S.; Danan, A.; Simonutti, M.; Fontaine, V.; Clérin, E.; Picaud, S.; Léveillard, T.; Sahel, J.-A. Functional Cone Rescue by RdCVF Protein in a Dominant Model of Retinitis Pigmentosa. Mol. Ther. 2009, 17, 787–795. [Google Scholar] [CrossRef]
- Byrne, L.C.; Dalkara, D.; Luna, G.; Fisher, S.K.; Clérin, E.; Sahel, J.-A.; Léveillard, T.; Flannery, J.G. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Investig. 2014, 125, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of Microglia-Mediated Neuroinflammation to Retinal Degenerative Diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Hermankova, B.; Krulova, M.; Zajicova, A. Cytokine interplay among the stem cell-based therapy. World J. Stem Cells 2019, 11, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteom. 2013, 13, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Poston, J.N.; Dreixler, J.C.; Torres, L.; Lopez, J.; Zelkha, R.; Balyasnikova, I.; Lesniak, M.S.; Roth, S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1581–1592. [Google Scholar] [CrossRef]
- Hooks, J.J.; Nagineni, C.N.; Hooper, L.C.; Hayashi, K.; Detrick, B. IFN-beta provides immuno-protection in the retina by inhibiting ICAM-1 and CXCL9 in retinal pigment epithelial cells. J. Immunol. 2008, 180, 3789–3796. [Google Scholar] [CrossRef]
- Nemunaitis, J. Macrophage function activating cytokines: Potential clinical application. Crit. Rev. Oncol. 1993, 14, 153–171. [Google Scholar] [CrossRef]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef]
- Lavail, M.M.; Unoki, K.; Yasumura, D.; Matthes, M.T.; Yancopoulos, G.D.; Steinberg, R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl. Acad. Sci. USA 1992, 89, 11249–11253. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Samuel, W.; Nagineni, S.; Pardhasaradhi, K.; Wiggert, B.; Detrick, B.; Hooks, J.J. Transforming growth factor-β induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003, 197, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Kutty, V.; Detrick, B.; Hooks, J.J. Expression of PDGF and their receptors in human retinal pigment e Bpithelial cells and fibroblasts: Regulation by TGF-beta. J. Cell. Phys. 2005, 203, 35–43. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, A.; Zouq, N.; Gilmore, A.P. Anoikis. Biochem. Soc. Trans. 2004, 32, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J.; Benowitz, L.; Crowston, J.; Huberman, A.; Johnson, E.; Lu, R.; Pekny, M.; Sappington, R.M.; Zack, D.; Calkins, D.J.; et al. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp. Eye Res. 2017, 157, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Magaki, T.; Takeda, M.; Kajiwara, Y.; Hanaya, R.; Sugiyama, K.; Arita, K.; Nishimura, M.; Kato, Y.; Kurisu, K. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci. Lett. 2008, 430, 109–114. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Reed, J.C. Mitochondria and Apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Q.; Qin, X.; Shen, L.; Cheng, L.; Su, Y.; Phillips, M.I. Paracrine Action Enhances the Effects of Autologous Mesenchymal Stem Cell Transplantation on Vascular Regeneration in Rat Model of Myocardial Infarction. Ann. Thorac. Surg. 2005, 80, 229–237. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Dixit, V.; Ferrara, N. Vascular Endothelial Growth Factor Induces Expression of the Antiapoptotic Proteins Bcl-2 and A1 in Vascular Endothelial Cells. J. Biol. Chem. 1998, 273, 13313–13316. [Google Scholar] [CrossRef]
- Ilić, D.; Almeida, E.A.; Schlaepfer, D.D.; Dazin, P.; Aizawa, S.; Damsky, C.H. Extracellular Matrix Survival Signals Transduced by Focal Adhesion Kinase Suppress p53-mediated Apoptosis. J. Cell Biol. 1998, 143, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Ganju, R.K.; Wang, J.F.; Schweitzer, K.; Weksler, B.; Avraham, S.; Groopman, J.E. Characterization of signal transduction pathways in human bone marrow endothelial cells. Blood J. Am. Soc. Hematol. 1997, 90, 2253–2259. [Google Scholar]
- Lobo, M.; Zachary, I. Nuclear Localization and Apoptotic Regulation of an Amino-Terminal Domain Focal Adhesion Kinase Fragment in Endothelial Cells. Biochem. Biophys. Res. Commun. 2000, 276, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Sternfeld, M.D.; Robertson, J.E.; Shipley, G.D.; Tsai, J.; Rosenbaum, J.T. Cultured human retinal pigment epithelial cells express basic fibroblast growth factor and its receptor. Curr. Eye Res. 1989, 8, 1029–1037. [Google Scholar] [CrossRef]
- Tanihara, H.; Yoshida, M.; Matsumoto, M.; Yoshimura, N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1993, 34, 413–419. [Google Scholar]
- Adamis, A.; Shima, D.; Yeo, K.; Yeo, T.; Brown, L.; Berse, B.; D’Amore, P.A.; Folkman, J. Synthesis and Secretion of Vascular Permeability Factor/Vascular Endothelial Growth Factor by Human Retinal Pigment Epithelial Cells. Biochem. Biophys. Res. Commun. 1993, 193, 631–638. [Google Scholar] [CrossRef]
- Wahlin, K.J.; Campochiaro, P.A.; Zack, D.J.; Adler, R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Investig. Ophthalmol. Vis. Sci. 2000, 41, 927–936. [Google Scholar]
- Bringmann, A. Role of Muller cells in retinal degenerations. Front. Biosci. 2001, 6, e77–e92. [Google Scholar] [CrossRef]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar] [CrossRef]
- Ortín-Martínez, A.; Valiente-Soriano, F.J.; García-Ayuso, D.; Alarcon-Martinez, L.; Jiménez-López, M.; Bernal-Garro, J.M.; Nieto-López, L.; Nadal-Nicolás, F.M.; Villegas-Pérez, M.P.; Wheeler, L.A.; et al. A Novel In Vivo Model of Focal Light Emitting Diode-Induced Cone-Photoreceptor Phototoxicity: Neuroprotection Afforded by Brimonidine, BDNF, PEDF or bFGF. PLoS ONE 2014, 9, e113798. [Google Scholar] [CrossRef] [PubMed]
- Othberg, A.; Odin, P.; Ballagi, A.; Funa, K.; Lindvall, O. Specific effects of platelet derived growth factor (PDGF) on fetal rat and human dopaminergic neurons in vitro. Exp. Brain Res. 1995, 105, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mocanu, C.; Mcleod, D.S.; Bhutto, I.A.; Merges, C.; Eid, M.; Tong, P.; Lutty, G.A. Expression of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp. Eye Res. 2003, 77, 433–445. [Google Scholar] [CrossRef]
- Tsang, C.K.; Qi, H.; Liu, L.F.; Zheng, X.F.S. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov. Today 2007, 12, 112–124. [Google Scholar] [CrossRef]
- Chung, S.; Rho, S.; Kim, G.; Kim, S.-R.; Baek, K.-H.; Kang, M.; Lew, H. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int. J. Mol. Med. 2016, 37, 1170–1180. [Google Scholar] [CrossRef]
- Zack, D.J. Neurotrophic Rescue of Photoreceptors. Neuron 2000, 26, 285–286. [Google Scholar] [CrossRef][Green Version]
- Slomiany, M.G.; Rosenzweig, S.A. Autocrine effects of IGF-I-induced VEGF and IGFBP-3 secretion in retinal pigment epithelial cell line ARPE-19. Am. J. Physiol. Physiol. 2004, 287, C746–C753. [Google Scholar] [CrossRef]
- Gasperi, M.; Castellano, A.E. Growth hormone/insulin-like growth factor I axis in neurodegenerative diseases. J. Endocrinol. Investig. 2010, 33, 587–591. [Google Scholar] [CrossRef]
- Na, L.; Xiao-Rong, L.; Jia-Qin, Y.; Li, N.; Li, X.; Yuan, J.-Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 247, 503–514. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
| Disease | Cell Source | Delivery | WHO Identifier | References |
|---|---|---|---|---|
| AMD (GA), RP and ischaemic retinopathy | Autologous BMHSC | Intravitreal injection | NCT01560715 NCT01518127 NCT01518842 | [103,104] |
| AMD (GA), RP, RVO and DR | Autologous BMHSC | Intravitreal injection | NCT01736059 | [105] |
| RP | Autologous ADMSC | Subretinal application | Not registered | [128] |
| AMD (GA), RP, OA | Autologous ADMSC And PRP | Suprachoroidal application | Not registered | [131,132,133,134] |
| RP | Autologous PRP | Subtenon injection | Not registered | [126] |
| RP | Eterologous UC-MSCs | Suprachoroidal application | Ministry of Health 56733164/203 | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants 2020, 9, 983. https://doi.org/10.3390/antiox9100983
Limoli PG, Vingolo EM, Limoli C, Nebbioso M. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants. 2020; 9(10):983. https://doi.org/10.3390/antiox9100983
Chicago/Turabian StyleLimoli, Paolo Giuseppe, Enzo Maria Vingolo, Celeste Limoli, and Marcella Nebbioso. 2020. "Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa" Antioxidants 9, no. 10: 983. https://doi.org/10.3390/antiox9100983
APA StyleLimoli, P. G., Vingolo, E. M., Limoli, C., & Nebbioso, M. (2020). Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants, 9(10), 983. https://doi.org/10.3390/antiox9100983






