Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Alignment, Network, and Phylogenetic Reconstructions
2.2. Axenic Culture and Treatment of T. thermophila
2.3. Isolation of Total RNA and Preparation of Cellular Extract for Enzymatic Activity
2.4. Experimental Validation of the Putative Automated mRNA Predictions
2.5. Determination of Total and Selenium-Dependent GPx Activity
2.6. Statistics
3. Results
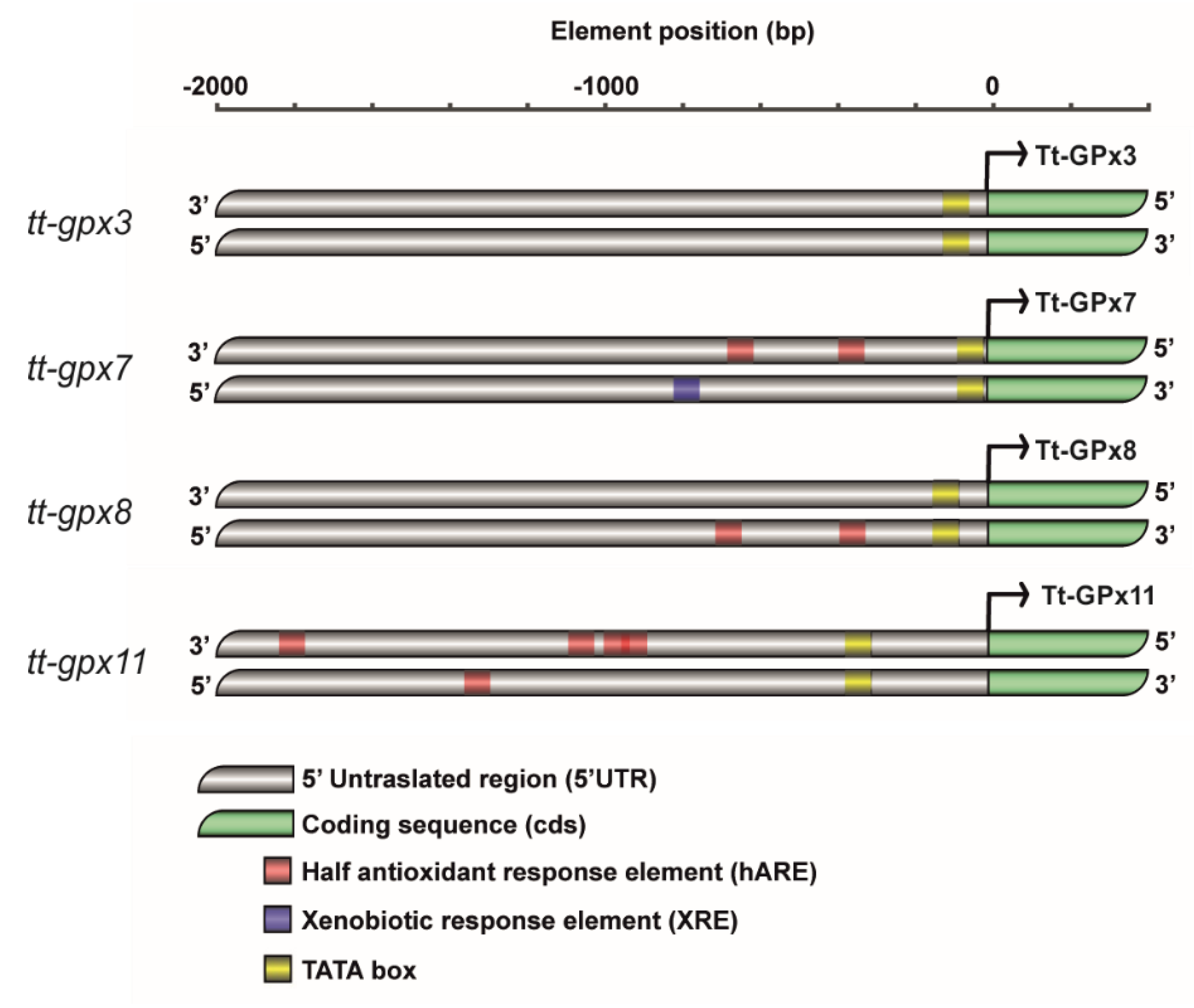
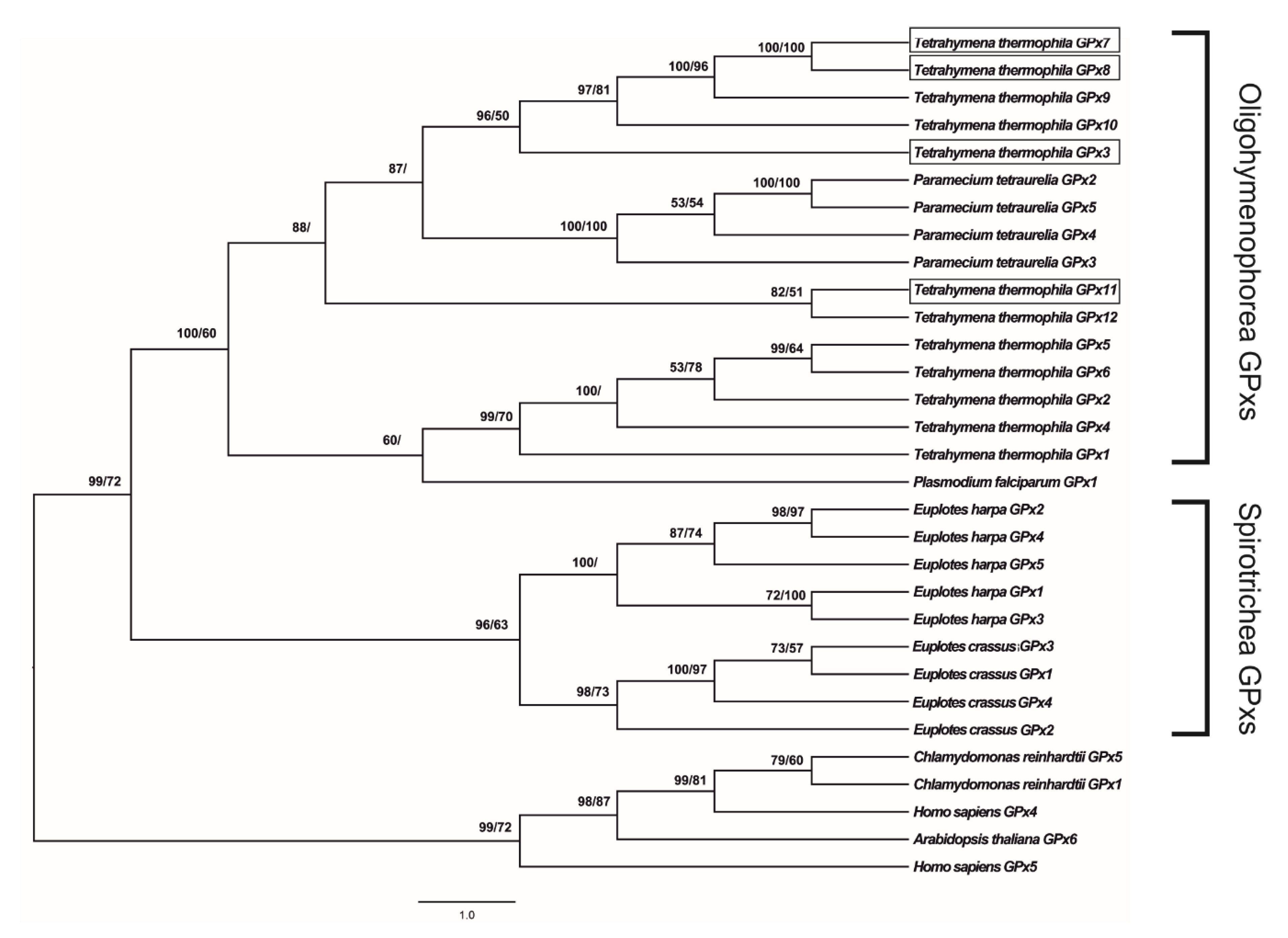
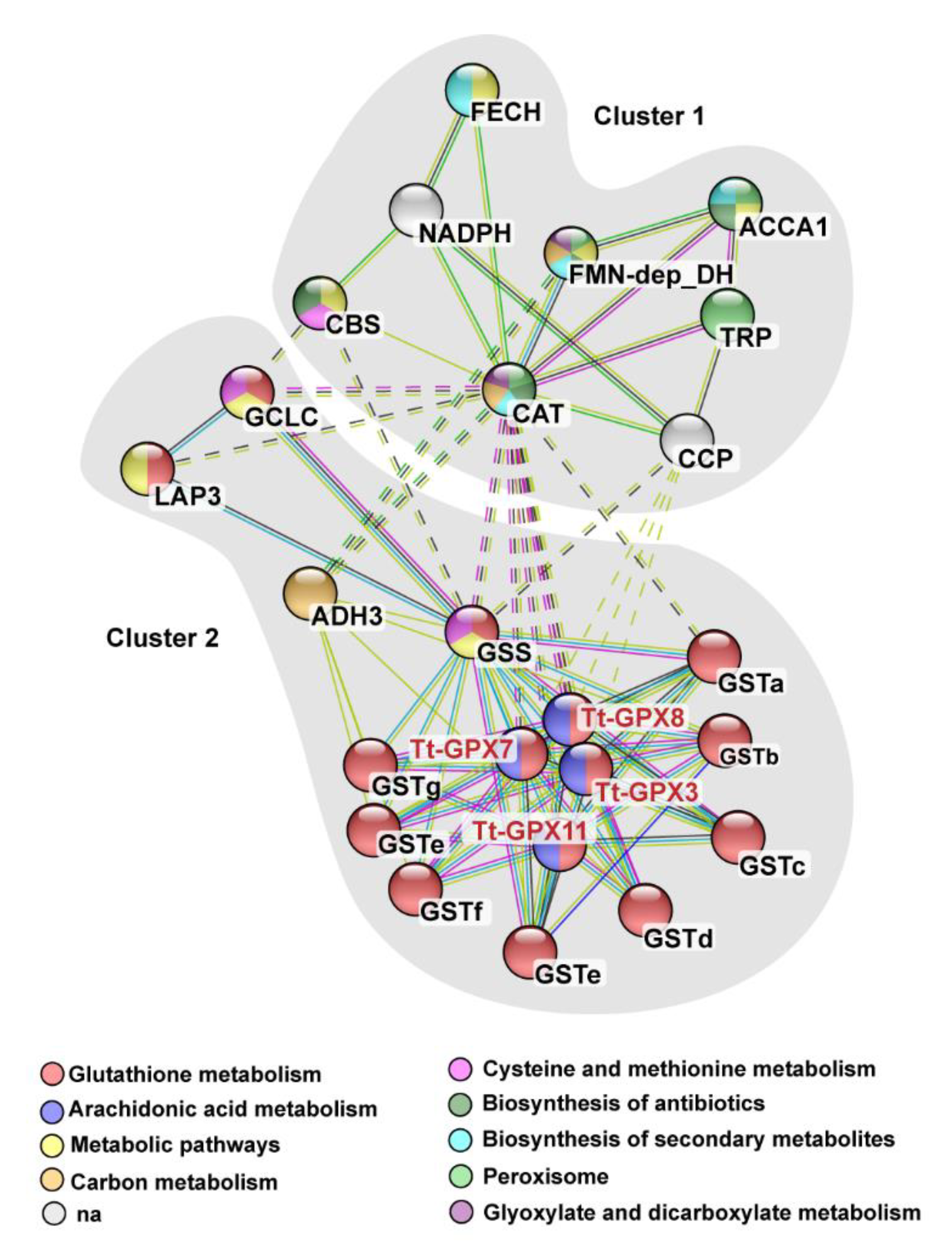
3.1. Gene Characterization, Evolution, and Protein–Protein Interaction Network
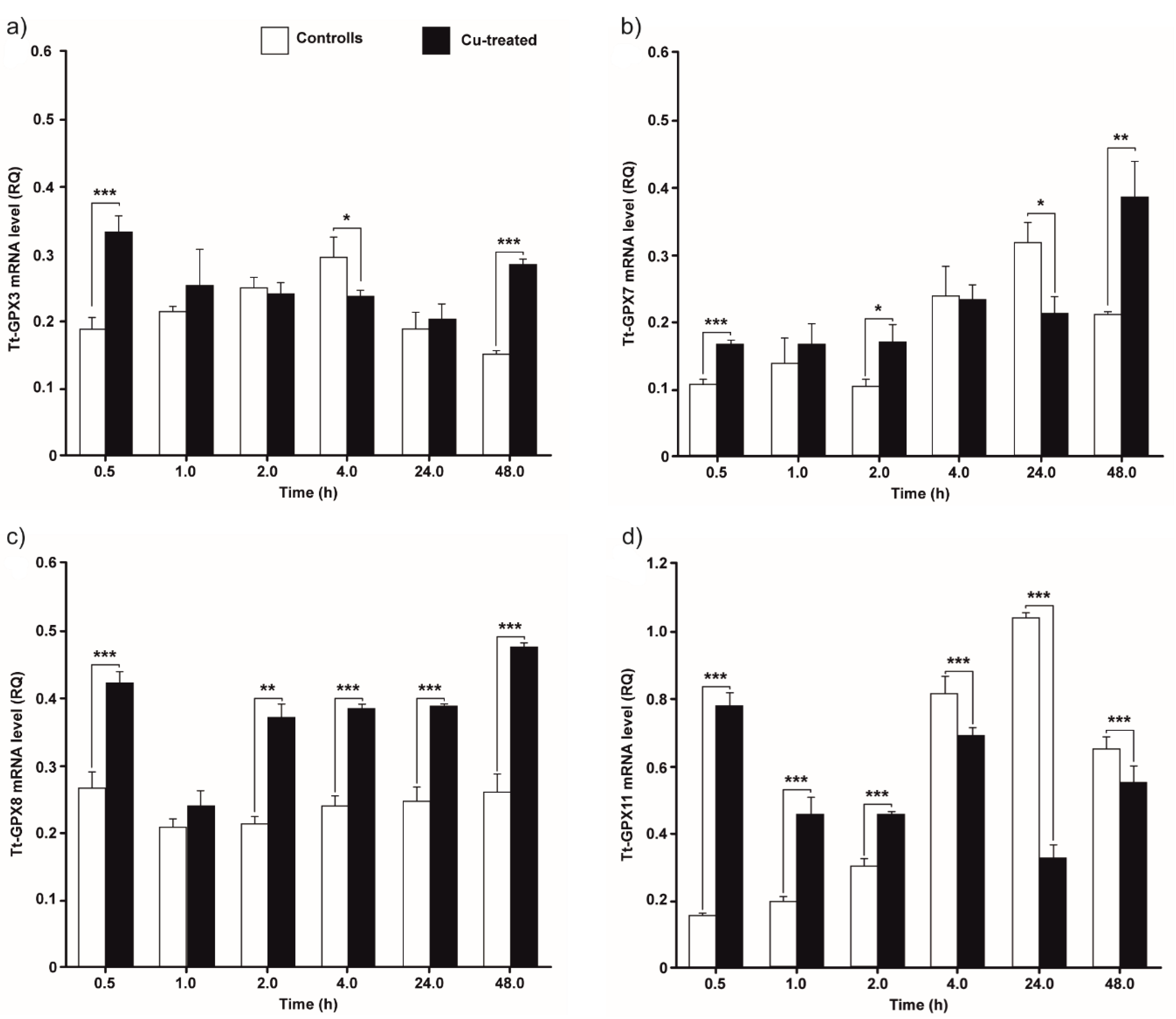
3.2. GPx mRNA Levels
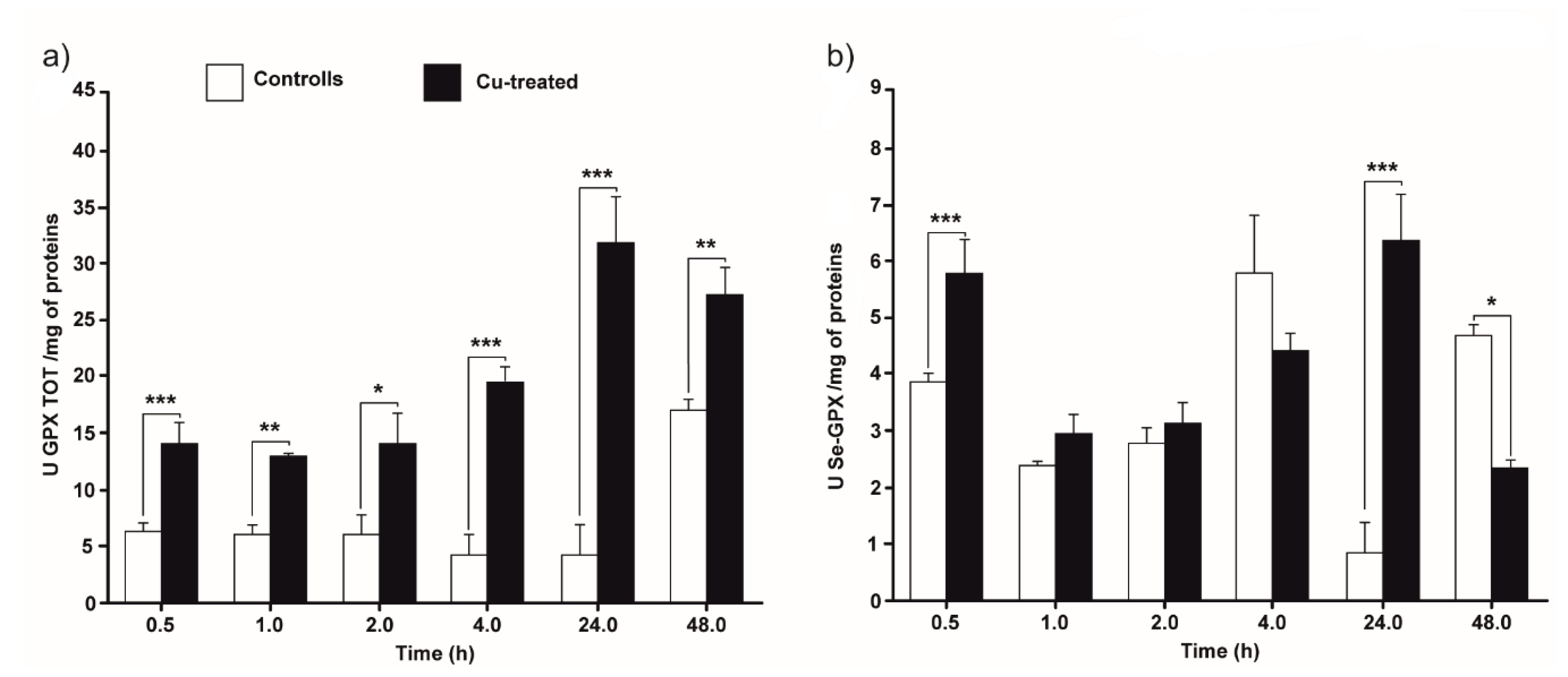
3.3. Total and Selenium-Dependent GPx Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is a question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Schlecker, T.; Comini, M.A.; Melchers, J.; Ruppert, T.; Krauth-Siegel, R.L. Catalytic mechanism of the glutathione peroxidase-type tryparedoxin peroxidase of Trypanosoma brucei. Biochem. J. 2007, 405, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Seward, J.R.; Hamblen, E.L.; Wayne Schultz, T. Regression comparisons of Tetrahymena pyriformis and Poecilia reticulata toxicity. Chemosphere 2002, 47, 93–101. [Google Scholar] [CrossRef]
- Sinks, G.D.; Schultz, T.W. Correlation of Tetrahymena and Pimephales toxicity: Evaluation of 100 additional compounds. Environ. Toxicol. Chem. 2001, 20, 917–921. [Google Scholar] [CrossRef]
- Gallego, A.; Martín-González, A.; Ortega, R.; Gutiérrez, J.C. Flow cytometry assessment of cytotoxicity and reactive oxygen species generation by single and binary mixtures of cadmium, zinc and copper on populations of the ciliated protozoan Tetrahymena thermophila. Chemosphere 2007, 68, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Swart, E.C.; Serra, V.; Petroni, G.; Nowacki, M. Genetic codes with no dedicated stop codon: Context-dependent translation termination. Cell 2016, 166, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, R.K.; Lobanov, A.V.; Xu, X.M.; Rao, M.; Carlson, B.A.; Mahadeo, D.C.; Parent, C.A.; Gladyshev, V.N.; Hatfield, D.L. Selenocysteine tRNA identification in the model organisms Dictyostelium discoideum and Tetrahymena thermophila. Biochem. Biophys. Res. Commun. 2005, 329, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Eisen, J.A.; Coyne, R.S.; Wu, M.; Wu, D.; Thiagarajan, M.; Wortman, J.R.; Badger, J.H.; Ren, Q.; Amedeo, P.; Jones, K.M.; et al. Macronuclear Genome Sequence of the Ciliate Tetrahymena thermophila, a Model Eukaryote. PLoS Biol. 2006, 4, e286. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; De Pittà, C.; Boldrin, F.; Cattalini, F.; Pucciarelli, S.; Miceli, C.; Santovito, G. Cu,Zn Superoxide Dismutases from Tetrahymena thermophila: Molecular Evolution and Gene Expression of the First Line of Antioxidant Defenses. Protist 2015, 166, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef]
- Ziouzenkova, O.; Sevanian, A.; Abuja, P.M.; Ramos, P.; Esterbauer, H. Copper can promote oxidation of LDL by markedly different mechanisms. Free Radic. Biol. Med. 1998, 24, 607–623. [Google Scholar] [CrossRef]
- Santovito, G.; Boldrin, F.; Irato, P. Metal and metallothionein distribution in different tissues of the Mediterranean clam Venerupis philippinarum during copper treatment and detoxification. Comp. Biochem. Physiol. C 2015, 174–175, 46–53. [Google Scholar] [CrossRef]
- Lee, Y.M.; Friedman, D.J.; Ayala, F.J. Superoxide dismutase: An evolutionary puzzle. Proc. Natl. Acad. Sci. USA 1985, 82, 824–828. [Google Scholar] [CrossRef]
- Ferro, K.; Ferro, D.; Corrà, F.; Bakiu, R.; Santovito, G.; Kurtz, J. Cu,Zn superoxide dismutase genes in Tribolium castaneum: Evolution, molecular characterisation, and gene expression during immune priming. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Doron-Faigenboim, A.; Pupko, T. A combined empirical and mechanistic codon model. Mol. Biol. Evol. 2007, 24, 388–397. [Google Scholar] [CrossRef]
- Löytynoja, A. Phylogeny-aware alignment with PRANK. Methods Mol. Biol. 2014, 1079, 155–170. [Google Scholar] [CrossRef]
- Ferro, D.; Franchi, N.; Bakiu, R.; Ballarin, L.; Santovito, G. Molecular characterization and metal induced gene expression of the novel glutathione peroxidase 7 from the chordate invertebrate Ciona robusta. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 1–7. [Google Scholar] [CrossRef]
- Wasserman, W.W.; Fahl, W.E. Functional antioxidant responsive elements. Med. Sci. 1997, 94, 5361–5366. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Gaona, L.L.; de Francisco, P.; Martín-González, A.; Gutiérrez, J.C. Tetrahymena glutathione peroxidase family: A comparative analysis of these antioxidant enzymes and differential gene expression to metals and oxidizing agents. Microorganisms 2020, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Allmang, C.; Wurth, L.; Krol, A. The selenium to selenoproteinpathway in eukaryotes: More nolecular partners than anticipated. Biochim. Biophys. Acta 2009, 1790, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Formigari, A.; Boldrin, F.; Santovito, G.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. Functional characterization of the 5’-upstream region of MTT5 metallothionein gene from Tetrahymena thermophila. Protist 2010, 161, 71–77. [Google Scholar] [CrossRef]
- Kosiol, C.; Vinar, T.; da Fonseca, R.R.; Hubisz, M.J.; Bustamante, C.D.; Nielsen, R.; Siepel, A. Patterns of positive selection in six mammalian genomes. PLoS Genet. 2008, 4, e1000144. [Google Scholar] [CrossRef]
- Bakiu, R.; Korro, K.; Santovito, G. Positive selection effects on the biochemical properties of mammal pyroglutamylated RFamide peptide receptor (QRFPR). Ital. J. Zool. 2015, 82, 309–326. [Google Scholar] [CrossRef]
- Bakiu, R.; Tolomeo, A.M.; Santovito, G. Positive selection effects on the biochemical properties of fish pyroglutamylated RFamide peptide receptor (QRFPR). Ital. J. Zool. 2015, 82, 460–472. [Google Scholar] [CrossRef][Green Version]
- Luisi, P.; Alvarez-Ponce, D.; Pybus, M.; Fares, M.A.; Bertranpetit, J.; Laayouni, H. Recent positive selection has acted on genes encoding proteins with more interactions within the whole human interactome. Genome Biol. Evol. 2015, 7, 1141–1154. [Google Scholar] [CrossRef]
- Lapouge, K. Structure of the TPR Domain of p67phox in Complex with Rac·GTP. Mol. Cell 2000, 6, 899–907. [Google Scholar] [CrossRef]
- Galbis-Estrada, C.; Pons-Vázquez, S.; Gallego-Pinazo, R.; Lleó-Perez, A.; Garcia-Medina, J.J.; Bou, V.V.; Sanz-Solana, P.; Pinazo-Durán, M.D. Glutathione-dependent formaldehyde dehydrogenase (ADH3) and low km mitochondrial aldehyde dehydrogenase (ALDH2). New evidence for differential expression in the rat retina in response to oxidative stress. Free Radic. Res. 2012, 46, 77–84. [Google Scholar] [CrossRef]
- Bakiu, R.; Santovito, G. New insights into the molecular evolution of metazoan peroxiredoxins. Acta Zool. Bulg. 2015, 67, 305–317. [Google Scholar]
- Tolomeo, A.M.; Carraro, A.; Bakiu, R.; Toppo, S.; Garofalo, F.; Pellegrino, D.; Gerdol, M.; Ferro, D.; Place, S.P.; Santovito, G. Molecular characterization of novel mitochondrial peroxiredoxins from the Antarctic emerald rockcod and their gene expression in response to environmental warming. Comp. Biochem. Physiol. C 2019, 255, 108580. [Google Scholar] [CrossRef] [PubMed]
- Al-Asadi, S.; Malik, A.; Bakiu, R.; Santovito, G.; Schuller, K. Characterization of the peroxiredoxin 1 subfamily from Tetrahymena thermophila. Cell. Mol. Life Sci. 2019, 76, 4745–4768. [Google Scholar] [CrossRef]
- Fox, J.H.; Kama, J.A.; Lieberman, G.; Chopra, R.; Dorsey, K.; Chopra, V.; Volitakis, I.; Cherny, R.A.; Bush, A.I.; Hersch, S. Mechanisms of copper ion mediated Huntington’s disease progression. PLoS ONE 2007, 2, e334. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Manto, M. Abnormal copper homeostasis: Mechanisms and roles in neurodegeneration. Toxics 2014, 2, 327–345. [Google Scholar] [CrossRef]
- Rossi, L.; Lombardo, M.F.; Ciriolo, M.R.; Rotilio, G. Mitochondrial Dysfunction in Neurodegenerative Diseases Associated with Copper Imbalance. Neurochem. Res. 2004, 29, 493–504. [Google Scholar] [CrossRef]
- Jones, D.P. Redox theory of aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef]
- Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Qiao, X.; Li, X.; Li, Y.; Zhang, Y.; Chen, C. The decay of Redox-stress Response Capacity is a substantive characteristic of aging: Revising the redox theory of aging. Redox Biol. 2017, 11, 365–374. [Google Scholar] [CrossRef]
- Corbit, K.C.; Soh, J.W.; Yoshida, K.; Eves, E.M.; Weinstein, I.B.; Rosner, M.R. Different protein kinase C isoforms determine growth factor specificity in neuronal cells. Mol. Cell. Biol. 2000, 20, 5392–5403. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.B.; Das, K.P.; Rooney, J.; Abbott, B.; Lau, C.; Corton, J.C. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017, 387, 95–107. [Google Scholar] [CrossRef]
- Lavut, A.; Raveh, D. Sequestration of highly expressed mRNAs in cytoplasmic granules, P-bodies, and stress granules enhances cell viability. PLoS Genet. 2012, 8, e1002527. [Google Scholar] [CrossRef]
- Olszewska, M.; Bujarski, J.J.; Kurpisz, M. P-bodies and their functions during mRNA cell cycle: Mini-review. Cell Biochem. Funct. 2012, 30, 177–182. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Bisaccia, P.; Corrà, F.; Bonato, M.; Irato, P.; Manuto, L.; Toppo, S.; Bakiu, R.; Santovito, G. Copper/zinc superoxide dismutase from the crocodile icefish Chionodraco hamatus: Antioxidant defense at constant sub-zero temperature. Antioxidants 2020, 9, 325. [Google Scholar] [CrossRef]
| Gene | NCBI Identifier | Tetrahymena Genome Database ID | Uniprot ID | Predicted Isoforms Number 1 |
|---|---|---|---|---|
| tt-gpx3 | XM_001030317.2 | TTHERM_01099010 | EAR82654.2 | GPx3 |
| tt-gpx7 | XM_001014606.1 | TTHERM_00046110 | EAR94658.1 | GPx7 |
| tt-gpx8 | XM_001014604.1 | TTHERM_00046090 | EAR94389.1 | GPx8 |
| tt-gpx11 | XM_001020136.1 | TTHERM_00661720 | EAR99891.1 | GPx11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, D.; Bakiu, R.; Pucciarelli, S.; Miceli, C.; Vallesi, A.; Irato, P.; Santovito, G. Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants 2020, 9, 949. https://doi.org/10.3390/antiox9100949
Ferro D, Bakiu R, Pucciarelli S, Miceli C, Vallesi A, Irato P, Santovito G. Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants. 2020; 9(10):949. https://doi.org/10.3390/antiox9100949
Chicago/Turabian StyleFerro, Diana, Rigers Bakiu, Sandra Pucciarelli, Cristina Miceli, Adriana Vallesi, Paola Irato, and Gianfranco Santovito. 2020. "Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila" Antioxidants 9, no. 10: 949. https://doi.org/10.3390/antiox9100949
APA StyleFerro, D., Bakiu, R., Pucciarelli, S., Miceli, C., Vallesi, A., Irato, P., & Santovito, G. (2020). Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants, 9(10), 949. https://doi.org/10.3390/antiox9100949










