The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Sample and Reagents
2.2. Cell Culture
2.3. In Vitro Digestion of Manuka Honey and Sample Preparation
2.4. Quantification and Identification of Phenolic Compounds of Manuka Honey and Digested Manuka Honey
2.4.1. Estimation of Total Phenolic Content
2.4.2. Estimation of Total Flavonoid Content
2.4.3. Quantification of the Total Antioxidant Capacity
FRAP Assay
TEAC Assay
DPPH Assay
2.5. Individual Phenolic Compounds by HPLC-ESI-MS/MS
2.6. Cell Viability Assay
2.7. Determination of Intracellular ROS Levels Throught the Tali® Image-Based Cytometer
2.8. Determination of Apoptotic Cells Throught the Tali® Image-Based Cytometer
2.9. Cell Cycle Analysis Throught the Tali® Image-Based Cytometer
2.10. Colony Formation Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Quantification and Identification of Phenolic Compounds of Manuka Honey and Digested Manuka Honey
3.1.1. Total Phenolic Compounds and Antioxidant Activity
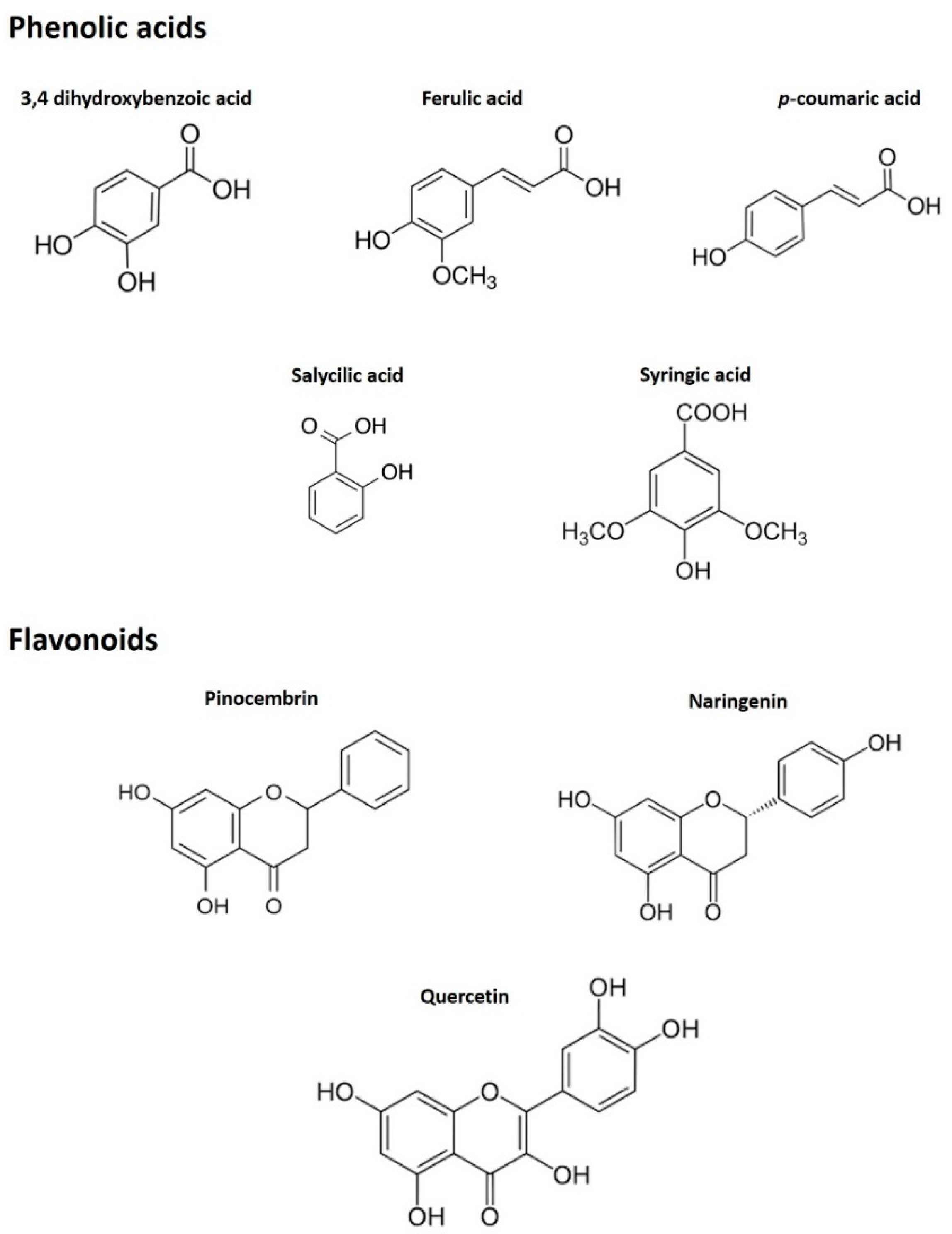
3.1.2. Phenolic Profiling by HPLC-ESI-MS/MS
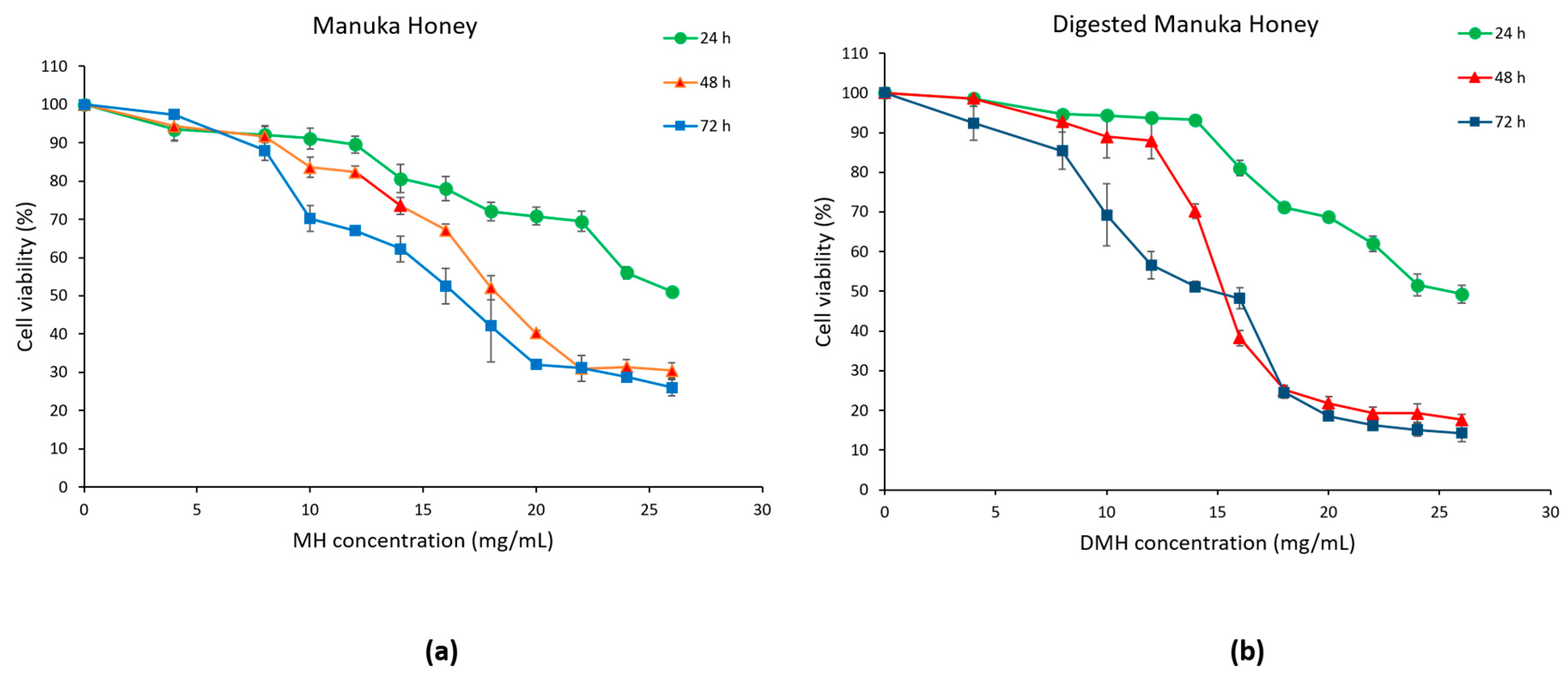
3.2. Antiproliferative Effect of MH and DMH on HCT-116 Cells
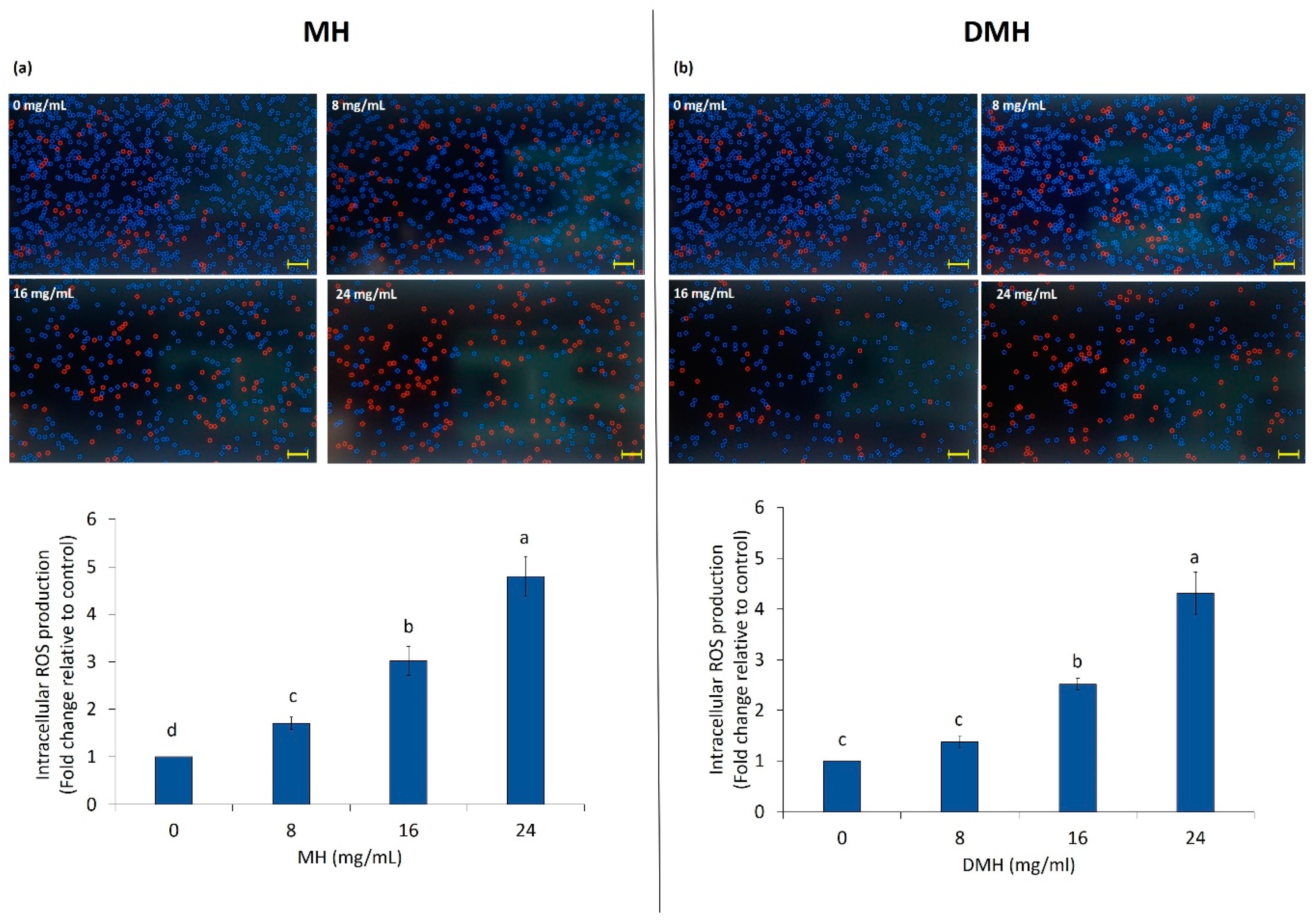
3.3. Effect of MH and DMH on Intracellular ROS Production
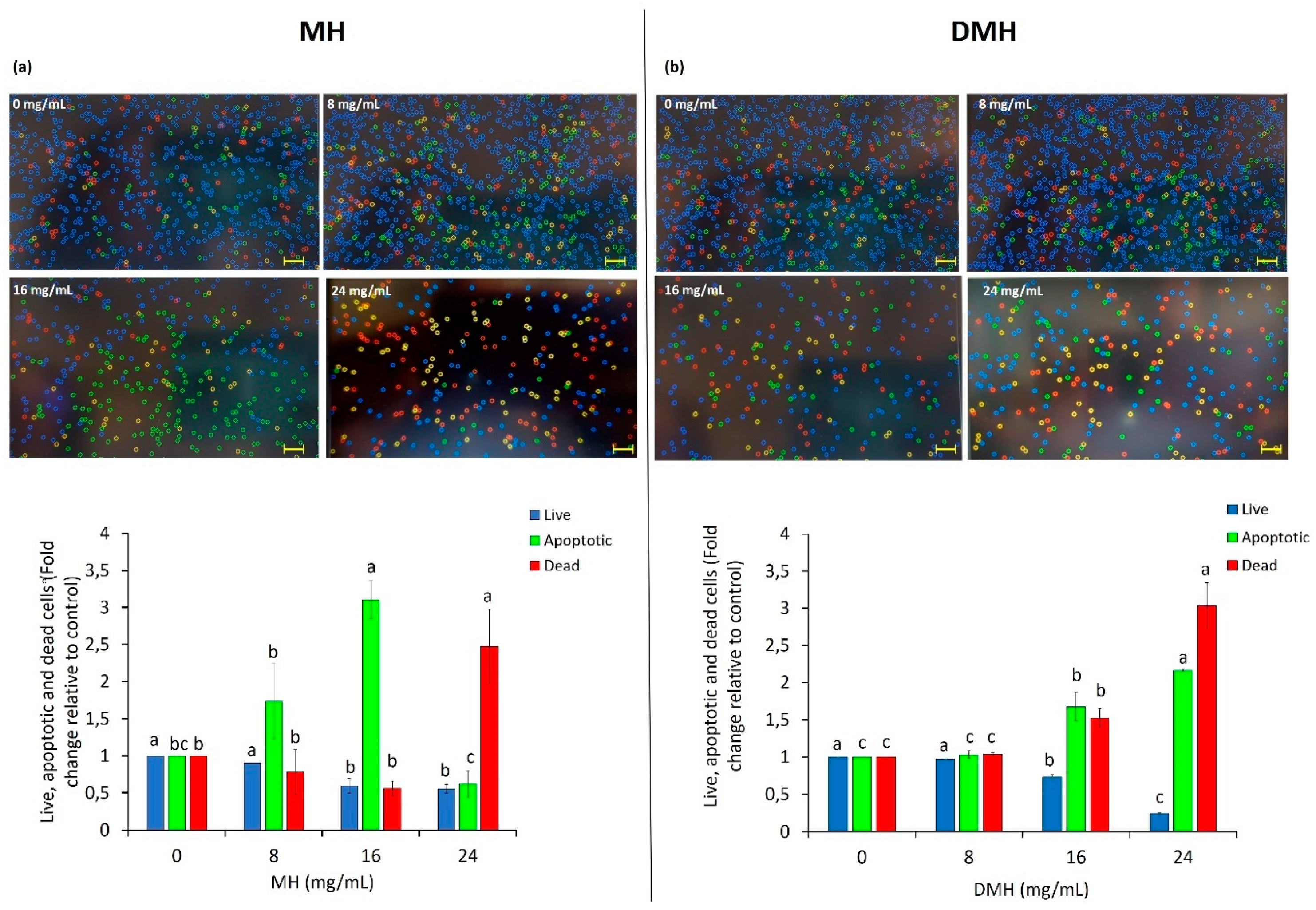
3.4. Effect of MH and DMH on Apoptosis
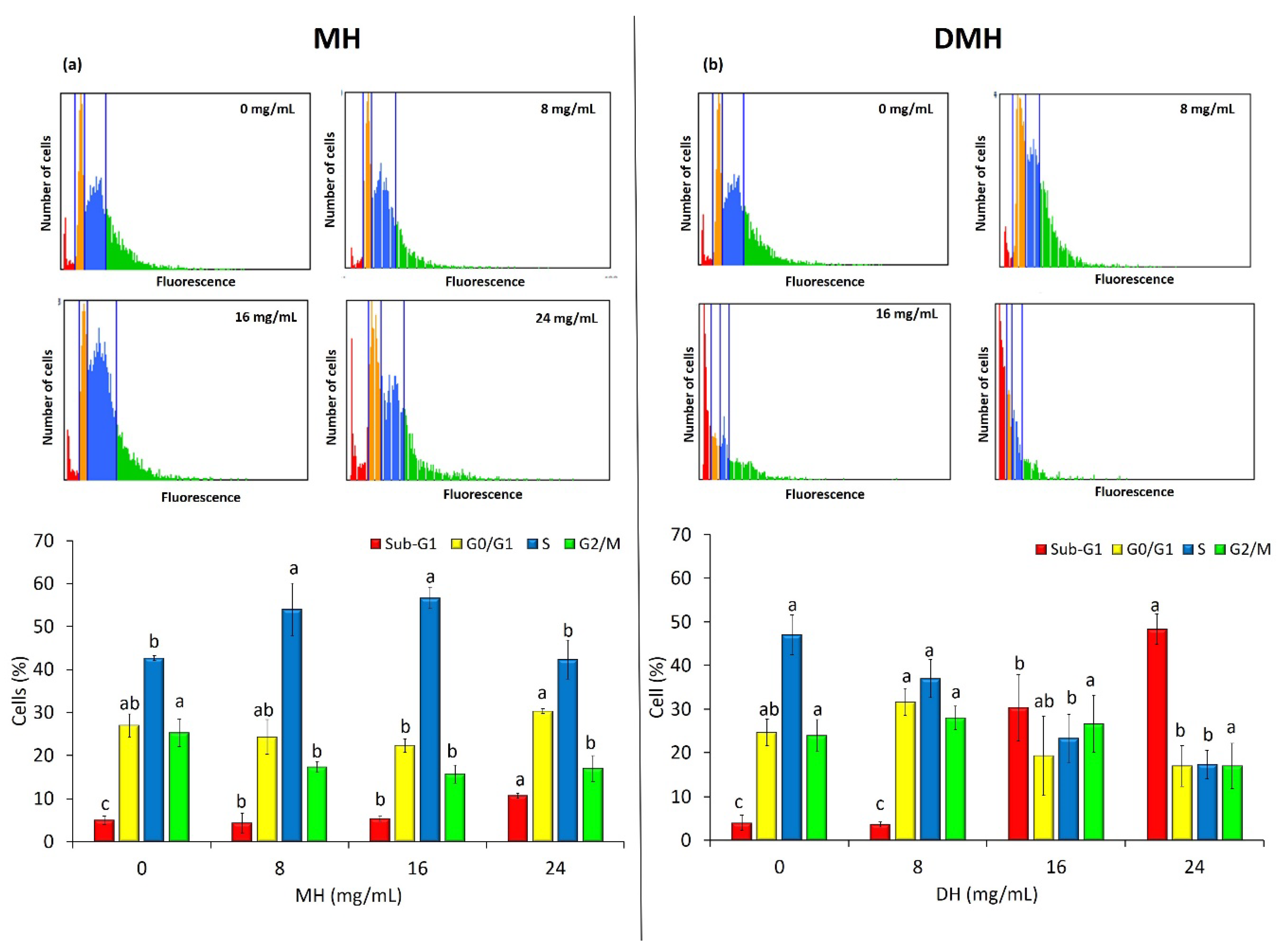
3.5. MH and DMH Induce Cell Cycle Arrest
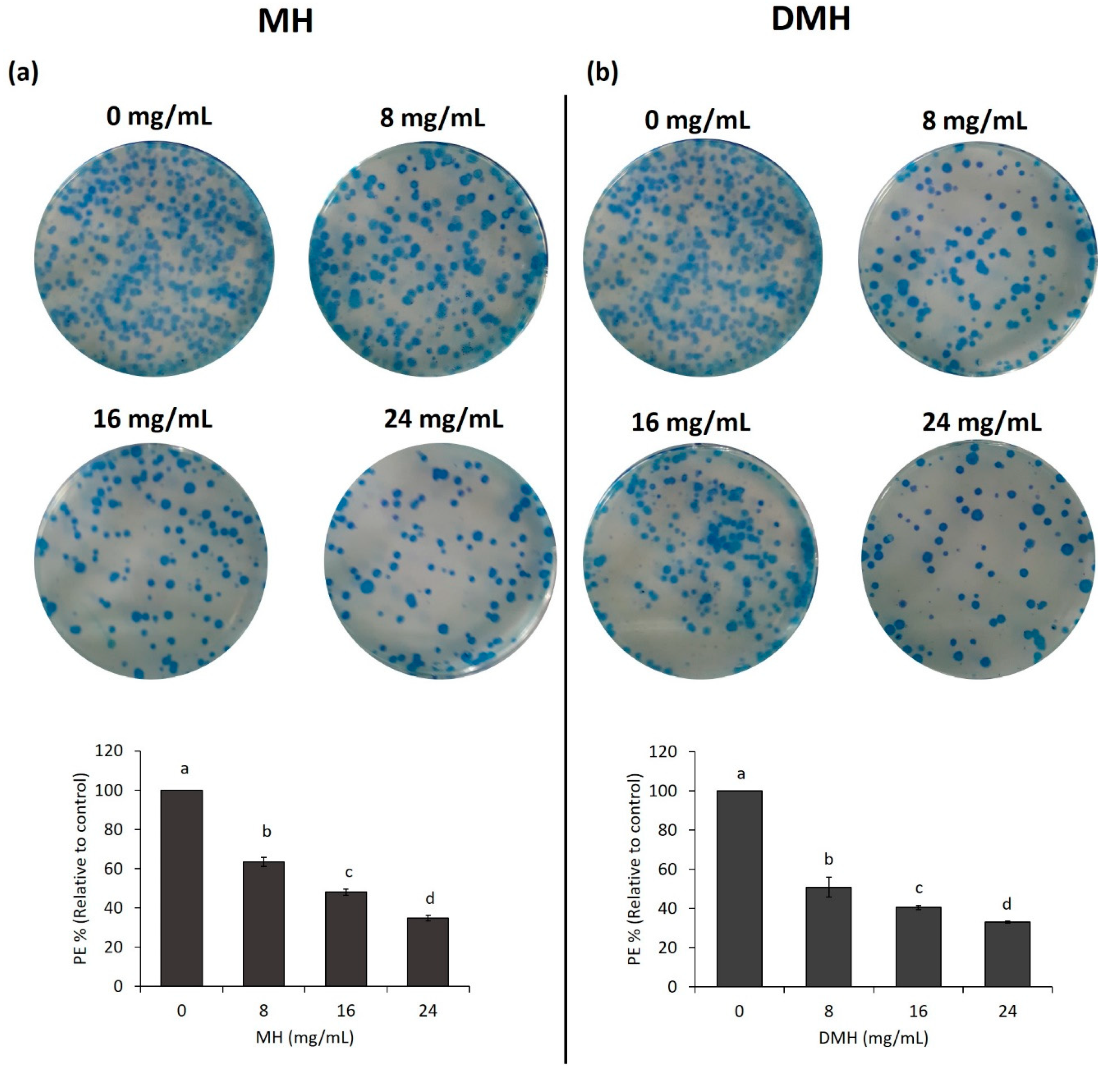
3.6. MH and DMH Reduced Colony Formation Ability of HCT-116
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baena, R.; Salinas, P. Diet and colorectal cancer. Maturitas 2015, 80, 258–264. [Google Scholar] [CrossRef]
- Thanikachalam, C.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. Chem. 2019, 59, 893–920. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo, L.; Martínez, F.S.; Agudo, T.P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 15, 568. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Mazzoni, L.; Forbes-Hernández, T.Y.; Giampieri, F.; Afrin, S.; Gasparrini, M.; Soria, C.; Martínez-Ferri, E.; Battino, M.; et al. Strawberry Achenes Are an Important Source of Bioactive Compounds for Human Health. Int. J. Mol. Sci. 2016, 17, 1103. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Sci. Techol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Waghela, B.N.; Sharma, A.; Dhumale, S.; Pandey, S.M.; Pathak, C. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS ONE 2015, 10, e0117526. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Connor, T.P.; O’Brien, N.M. Comparison of the Antioxidant Activity of Commercial Honeys, Before and After In-Vitro Digestion. Pol. J. Food Nutr. Sci. 2013, 63, 167–171. [Google Scholar] [CrossRef]
- Jara-Palacios, J.M.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef]
- Pavan, V.; Soriano Sancho, A.R.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Mtolo, M.; Gerrano, A.; Mellem, J. Effect of simulated gastrointestinal digestion on the phenolic compound content and in vitro antioxidant capacity of processed Cowpea (V. unguiculata) cultivars. CyTA J. Food 2017, 15, 391–399. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Z.; Dai, M.; Cao, X.; Xiong, X.; He, S.; Yuan, Y.; Zhang, M.; Dong, L.; Zhang, R.; et al. Effects of simulated digestion on the phenolic composition and antioxidant activity of different cultivars of lychee pericarp. BMC Chem. 2019, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, D.-C.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods 2014, 7, 506–516. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of in vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah grape pomace extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Parker, T.L.; Miller, S.A.; Myers, L.E.; Miguez, F.E.; Engeseth, N.J. Evaluation of synergistic antioxidant potential of complex mixtures using oxygen radical absorbance capacity (ORAC) and electron paramagnetic resonance (EPR). J. Agric. Food Chem. 2010, 58, 209–217. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; Al-Ramadi, B.K. Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS ONE 2013, 8, e55993. [Google Scholar] [CrossRef] [PubMed]
- Hashim, F.; Ismail, W.I.; Ali, A.M. Combinatorial Cytotoxic Effects of Gelam Honey and 5-Fluorouracil against Human Adenocarcinoma Colon Cancer HT-29 Cells In Vitro. Int. J. Cell Biol. 2019, 2019, 3059687. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N. Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Ariza, M.T.; Forbes-Hernández, T.Y.; Reboredo-Rodríguez, P.; Afrin, S.; Gasparrini, M.; Cervantes, L.; Soria, C.; Martínez-Ferri, E.; Battino, M.; Giampieri, F. Strawberry and Achenes Hydroalcoholic Extracts and Their Digested Fractions Efficiently Counteract the AAPH-Induced Oxidative Damage in HepG2 Cells. Int. J. Mol. Sci. 2018, 19, 2180. [Google Scholar] [CrossRef]
- Kubow, S.; Iskandar, M.M.; Melgar-Bermudez, E.; Sleno, L.; Sabally, K.; Azadi, B.; How, E.; Prakash, S.; Burgos, G.; Felde, T.Z. Effects of Simulated Human Gastrointestinal Digestion of Two Purple-Fleshed Potato Cultivars on Anthocyanin Composition and Cytotoxicity in Colonic Cancer and Non-Tumorigenic Cells. Nutrients 2017, 9, 953. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct. 2018, 9, 2158–2170. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Investig. New Drugs 2010, 28, 624–633. [Google Scholar] [CrossRef]
- Kocyigit, A.; Aydogdu, G.; Balkan, E.; Yenigun, V.B.; Guler, E.; Bulut, H.; Koktasoglu, F.; Gören, A.C.; Atayoglu, A.T. Quercus pyrenaica Honeydew Honey with High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells. Integr. Cancer Ther. 2019, 18, 1534735419876334. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.; Xu, Y.; Jin, C. In vitro gastrointestinal digestion promotes the protective effect of blackberry extract against acrylamide-induced oxidative stress. Sci. Rep. 2017, 7, 40514. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Nair, M.; Hema, P.S.; Mohan, J.; Santhoshkumar, T.R. Pinocembrin triggers Bax-dependent mitochondrial apoptosis in colon cancer cells. Mol. Carcinog. 2007, 46, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Pathi, S.; Jutooru, I.; Chadalapaka, G.; Nair, V.; Lee, S.O.; Safe, S. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PLoS ONE 2012, 7, e48208. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Haza, A.I. Antiproliferative and apoptotic effects of spanish honeys. Pharmacogn. Mag. 2013, 9, 231–237. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Honey induces apoptosis in renal cell carcinoma. Pharmacogn. Mag. 2011, 7, 46–52. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef]
- Sadeghi-Aliabadi, H.; Hamzeh, J.; Mirian, M. Investigation of Astragalus honey and propolis extract’s cytotoxic effect on two human cancer cell lines and their oncogen and proapoptotic gene expression profiles. Adv. Biomed. Res. 2015, 4, 42. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Boninsegna, A.; Bellovino, D.; Lucarini, M.; Monastra, G.; Gaetani, S. The growth-inhibitory effects of tomatoes digested in vitro in colon adenocarcinoma cells occur through down regulation of cyclin D1, Bcl-2 and Bcl-xL. Br. J. Nutr. 2007, 98, 789–795. [Google Scholar] [CrossRef]
- Cilla, A.; Gonzàles-Sarrìas, A.; Tomàs-Barberàn, F.A.; Espìn, J.C.; Barberà, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Meeran, S.Y.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Rasul, A.; Zhao, C.; Millimouno, F.M.; Tsuji, I.; Yamamura, T.; Iqbal, R.; Malhi, M. Antiproliferative and apoptotic effects of pinocembrin in human prostate cancer cells. Bangladesh J. Pharmacol. 2013, 8. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, S.; Hosur Shrungeswara, A.; Basu Mallik, S.; Hipolith Viji, M.; Elizabeth Mathew, J.; Mathew, J.; Nandakumar, K.; Lobo, R. Pinocembrin enriched fraction of Elytranthe parasitica (L.) Danser induces apoptosis in HCT 116 colorectal cancer cells. J. Infect. Chemother. 2017, 23, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Atashpour, S.; Fouladdel, S.; Komeili Movahhed, T.; Barzegar, E.; Hossein Ghahremani, M.; Nasser Ostad, S. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015, 18, 635–643. [Google Scholar] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Reddy, B.D.; Padukudru, M.A.; Kumari Chitturi, C.M.; Vimalambike, M.G.; Madhunapantula, S.V. Naturally occurring benzoic acid derivatives retard cancer cell growth by inhibiting histone deacetylases (HDAC). Cancer Biol. Ther. 2017, 18, 492–504. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, I.; Li, Q.; Yang, S.; Zhang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell. Int. 2018, 18, 102. [Google Scholar] [CrossRef]
- Aryappalli, P.; Al-Qubaisi, S.S.; Attoub, S.; George, J.A.; Arafat, K.; Ramadi, K.B.; Mohamed, Y.A.; Al-Dhaheri, M.M.; Al-Sbiei, A.; Fernandez-Cabezudo, M.J.; et al. The IL-6/STAT3 Signaling Pathway Is an Early Target of Manuka Honey-Induced Suppression of Human Breast Cancer Cells. Front. Oncol. 2017, 7, 67. [Google Scholar] [CrossRef]
- Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Soares, A.E.; Tone, L.G. Antiproliferative effects of Tubi-bee propolis in glioblastoma cell lines. Genet. Mol. Biol. 2011, 34, 310–314. [Google Scholar] [CrossRef]

| Samples | TPC (mg GAEq/g Honey) | TFC (mg CatEq/Kg Honey) | TAC | ||
|---|---|---|---|---|---|
| FRAP (µmol FEq/100 g Honey) | TEAC (µmol TEq/100 g Honey) | DPPH (µmol TEq/100 g Honey | |||
| MH | 1.27 ± 0.08 a | 48.99 ± 1.44 a | 261.73 ± 3.65 a | 251.89 ± 4.74 a | 86.479 ± 2.60 a |
| DMH | 0.203 ± 0.01 b | 6.49 ± 0.96 b | 119.81 ± 0.55 b | 114.67 ± 4.66 b | 11.821 ± 2.25 b |
| Compounds | Quantification Ion | Retention Time (min) | Concentration of Phenolic Compounds | |
|---|---|---|---|---|
| Manuka Honey | Digested Manuka Honey | |||
| Phenolic acid | ||||
| 3,4 dihydroxybenzoic acid | 109 | 14.29 | 17.10 ± 1.01 | 15.73 ± 1.36 |
| Ferulic acid | 89 | 13.77 | 3.00 ± 0.18 | 0.60 ± 0.10 |
| p-coumaric acid | 119 | 13.10 | 11.20 ± 0.66 | 1.70 ± 0.20 |
| Salicylic acid | 93 | 14.43 | 42.40 ± 2.50 | 56.23 ± 7.91 |
| Syringic acid | 155 | 12.42 | 1.70 ± 0.10 | 0.43 ± 0.15 |
| Σ phenolic acids | 75 | 75 | ||
| Flavonoids | ||||
| Pinocembrin | 153 | 16.36 | 102.20 ± 6.04 | 0.47 ± 0.06 |
| Naringenin | 151 | 15.25 | 6.10 ± 0.36 | n.d. |
| Quercetin | 150 | 14.50 | 7.50 ± 0.44 | n.d |
| Σ flavonoids | 116 | 1 | ||
| Total phenolics | 191 | 76 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey. Antioxidants 2020, 9, 64. https://doi.org/10.3390/antiox9010064
Cianciosi D, Forbes-Hernández TY, Afrin S, Gasparrini M, Quiles JL, Gil E, Bompadre S, Simal-Gandara J, Battino M, Giampieri F. The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey. Antioxidants. 2020; 9(1):64. https://doi.org/10.3390/antiox9010064
Chicago/Turabian StyleCianciosi, Danila, Tamara Yuliett Forbes-Hernández, Sadia Afrin, Massimiliano Gasparrini, José L. Quiles, Emilio Gil, Stefano Bompadre, Jesus Simal-Gandara, Maurizio Battino, and Francesca Giampieri. 2020. "The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey" Antioxidants 9, no. 1: 64. https://doi.org/10.3390/antiox9010064
APA StyleCianciosi, D., Forbes-Hernández, T. Y., Afrin, S., Gasparrini, M., Quiles, J. L., Gil, E., Bompadre, S., Simal-Gandara, J., Battino, M., & Giampieri, F. (2020). The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey. Antioxidants, 9(1), 64. https://doi.org/10.3390/antiox9010064











