A Combination of Rosa Canina Extracts and Gold Complex Favors Apoptosis of Caco-2 Cells by Increasing Oxidative Stress and Mitochondrial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation, Purification and Fractioning of R. canina Acidic Polyphenols
2.2. HPLC Analysis of Polyphenols of Rosehips
2.3. Synthesis of (Au(C≡C-2-NC5H4)(PTA))
2.4. Cell Culture
2.5. Cell Treatment
2.6. Cell Viability Assay
2.7. Measurements of Apoptosis
2.8. Analysis of the Activation of Caspase 3
2.9. Cell Cycle Analysis
2.10. Determination of RIP-1 Protein Amount by Flow Cytometry
2.11. Measurement of Autophagosome Formation
2.12. Determination of Intracellular Levels of Reactive Oxygen Species (ROS)
2.13. Flow Cytometry Mitochondrial Membrane Potential Assay
2.14. Determination of Lysosome Alkalization
2.15. Statistical Analysis
3. Results and Discussion
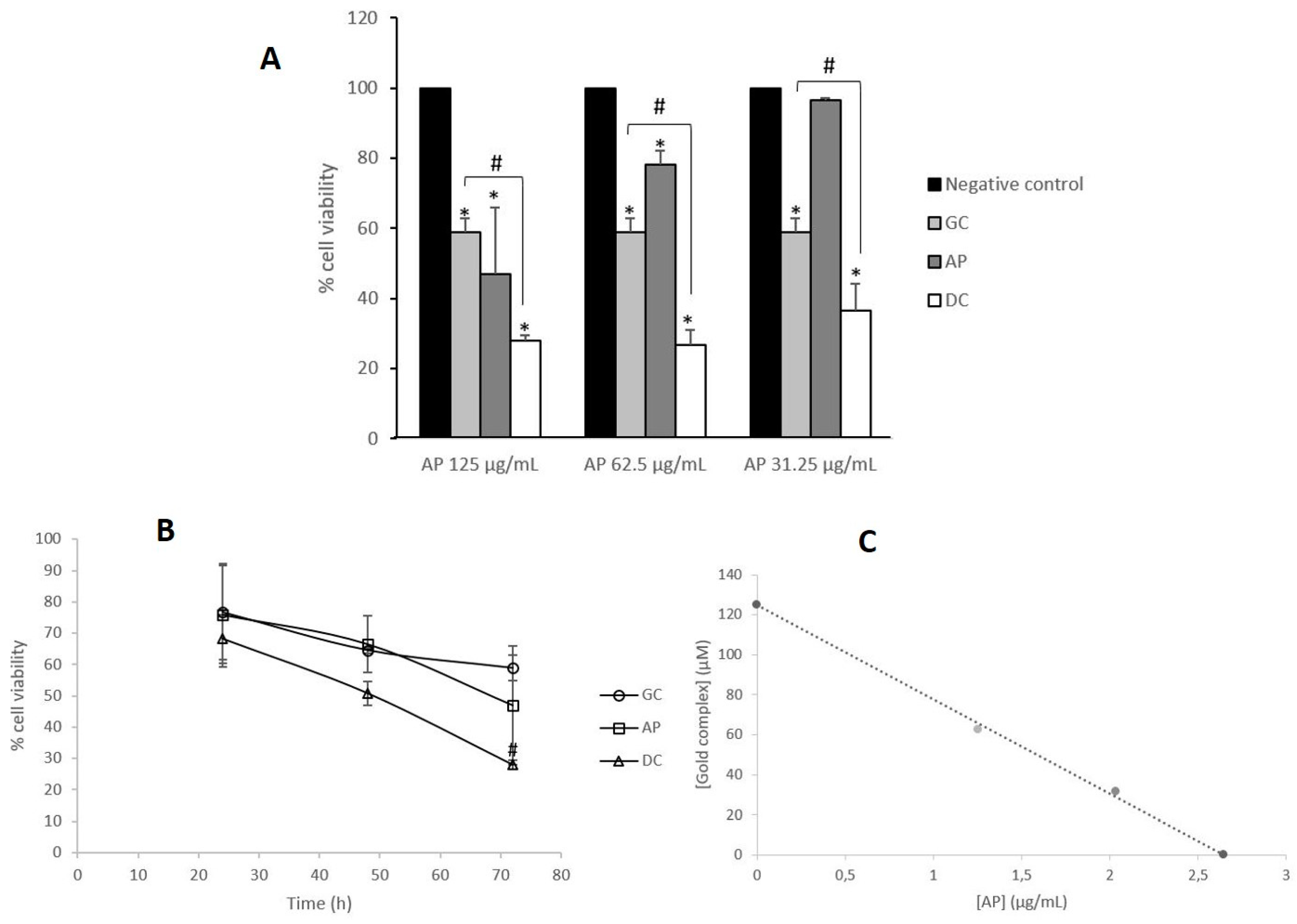
3.1. Acidic Polyphenols from R. canina Enhance the Antiproliferative Effect of Gold Complex
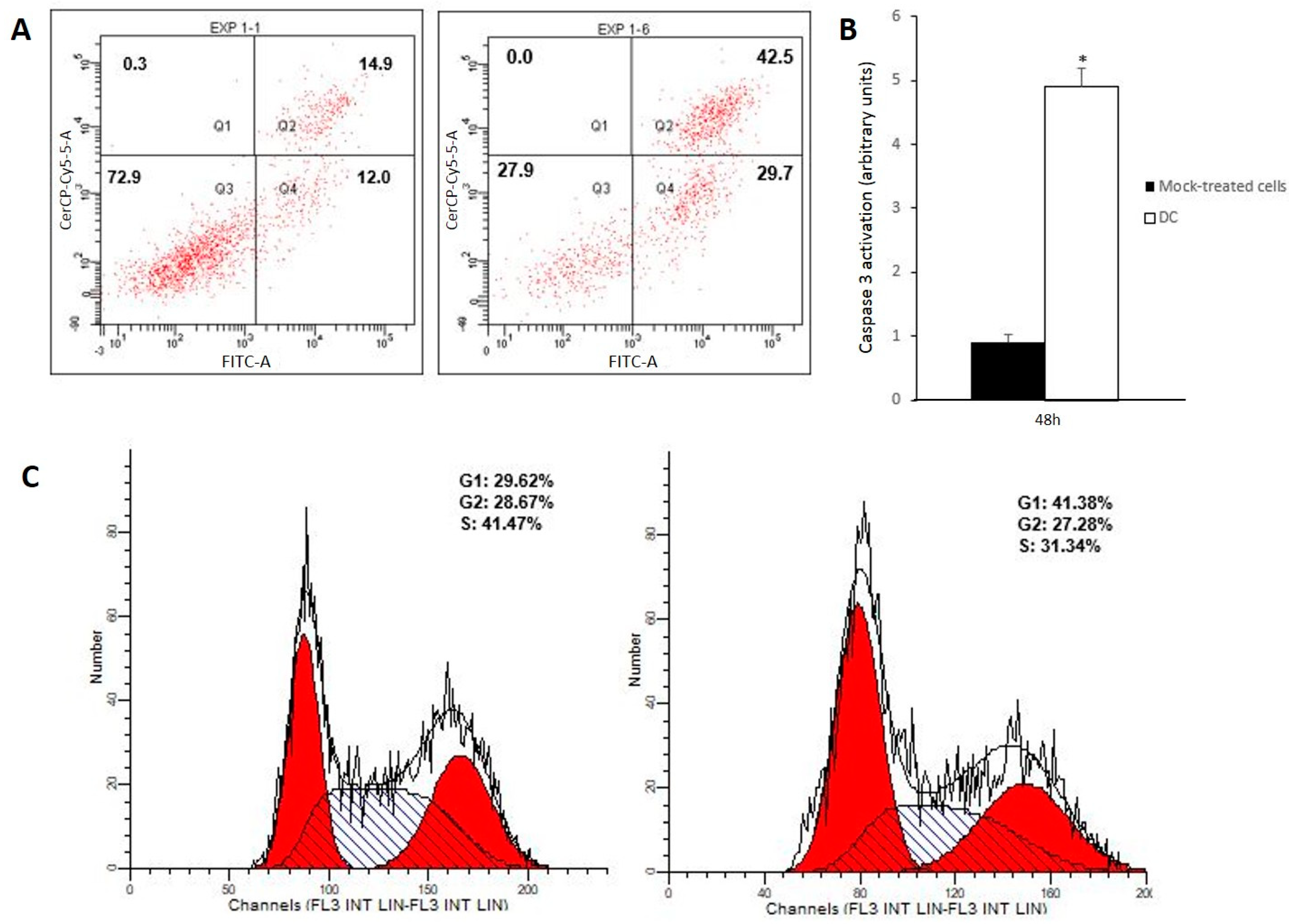
3.2. Drug Combination Triggers Apoptosis on Caco-2 Cells
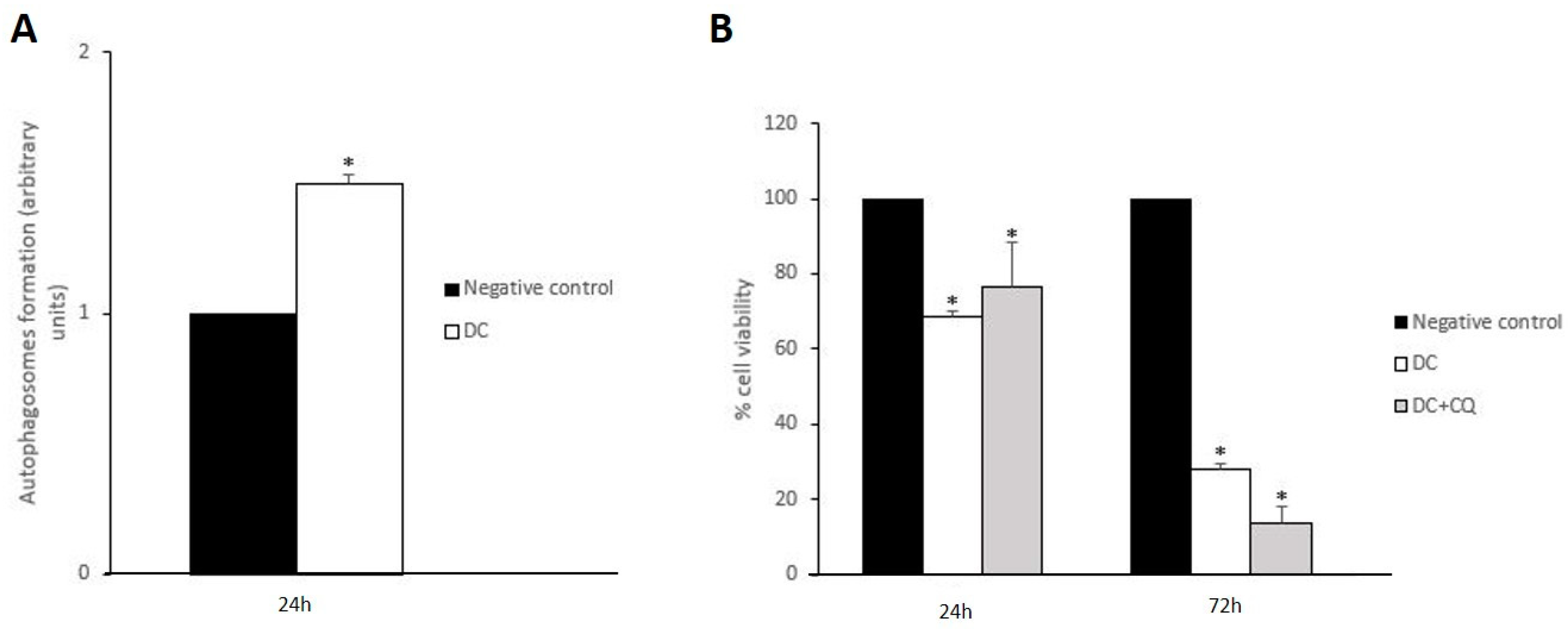
3.3. Drug Combination Disrupts Redox Homeostasis, Which Induces Mitochondrial Disturbances along with Lysosomal Dysfunction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, P.Y. Polyphenols in Health and Disease. Cell Biochem. Biophys. 2015, 73, 649–664. [Google Scholar] [CrossRef]
- Ci, Y.; Qiao, J.; Han, M. Molecular mechanisms and metabolomics of natural polyphenols interfering with breast cancer metastasis. Molecules 2016, 21, 1634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Han, L.; Zhou, Y.; Sun, S. Green tea polyphenol EGCG reverse cisplatin resistance of A549/DDP cell line through candidate genes demethylation. Biomed. Pharmacother. 2015, 69, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Yan, L.; Zhu, L.; Jiao, D.M.; Chen, J.; Chen, Q.Y. Salvianolic acid A reverses cisplatin resistance in lung cancer A549 cells by targeting c-met and attenuating Akt/mTOR pathway. J. Pharmacol. Sci. 2017, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, D.; Ayed, Y.; Hfaiedh, M.; Bouaziz, C.; Mansour, H.B.; Zourgui, L.; Bacha, H. Protective effect of cactus cladode extract against cisplatin induced oxidative stress, genotoxicity and apoptosis in balb/c mice: Combination with phytochemical composition. BMC Complement. Altern. Med. 2012, 12, 111. [Google Scholar] [CrossRef]
- Divya, M.K.; Lincy, L.; Raghavamenon, A.C.; Babu, T.D. Ameliorative effect of Apodytes dimidiata on cisplatin-induced nephrotoxicity in Wistar rats. Pharm. Biol. 2016, 54, 2149–2157. [Google Scholar] [CrossRef]
- Czyzowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-de-Diego, C.; Jimenez-Moreno, N.; Ancin-Azpilicueta, C.; Rodriguez-Yoldi, M.J. Therapeutic applications of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Marmol, I.; Quero, J.; Rodriguez Yoldi, M.J. Modifiable and non-modifiable risk factors of colorectal cancer. In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; Volume 7, pp. 67–117. [Google Scholar]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar] [PubMed]
- Marmol, I.; Quero, J.; Rodriguez-Yoldi, M.J.; Cerrada, E. Gold as a possible alternative to platinum-based chemotherapy for colon cancer. Cancers 2019, 11, 780–816. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Combinations of plant polyphenols & anti-cancer molecules: A novel treatment strategy for cancer chemotherapy. Anticancer Ag. Med. Chem. 2013, 13, 281–295. [Google Scholar]
- Vergara, E.; Cerrada, E.; Casini, A.; Zava, O.; Laguna, M.; Dyson, P.J. Antiproliferative activity of gold(I) alkyne complexes containing-water soluble phosphane ligands. Organometallics 2010, 29, 2596–2603. [Google Scholar] [CrossRef]
- Marmol, I.; Virumbrales-Munoz, M.; Quero, J.; Sanchez-de-Diego, C.; Fernandez, L.; Ochoa, I.; Cerrada, E.; Rodríguez-Yoldi, M.J. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef]
- Jimenez, S.; Gascon, S.; Luquin, A.; Laguna, M.; Ancin-Azpilicueta, C.; Rodriguez-Yoldi, M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 cell culture practices. Toxicol. In Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef]
- Zhao, L.; Au, J.L.; Wientjes, M.G. Comparison of methods for evaluating drug-drug interaction. Front. Biosci. 2010, 2, 241–249. [Google Scholar]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Sanchez-de-Diego, C.; Marmol, I.; Perez, R.; Gascon, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold(I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef]
- Sambuy, Y.; Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zeller, P.; Bricks, T.; Vidal, G.; Jacques, S.; Anton, P.M.; Leclerc, E. Multiparametric temporal analysis of the Caco-2/TC7 demonstrated functional and differentiated monolayers as early as 14 days of culture. Eur. J. Pharm. Sci. 2015, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Semkova, S.; Aoki, I.; Bakalova, R. Resveratrol modulates the redox-status and Cytotoxicity of anticancer drugs by sensitizing leukemic lymphocytes and protecting normal lymphocytes. Anticancer Res. 2019, 39, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Yarijani, Z.; Godini, A.; Madani, S.H.; Najafi, H. Reduction of cisplatin-induced renal and hepatic side effects in rat through antioxidative and anti-inflammatory properties of Malva sylvestris L. extract. Biomed. Pharmacother. 2018, 106, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Elwy, H.M.; Badawi, A.M. Fenugreek seed extract attenuates cisplatin-induced testicular damage in Wistar rats. Andrologia 2016, 48, 211–221. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Zhang, L. Necroptosis: An alternative cell death program defending against cancer. Biochim. Biophys. Acta 2016, 1865, 228–236. [Google Scholar] [CrossRef]
- Shen, H.M.; Codogno, P. Autophagy is a survival force via suppression of necrotic cell death. Exp. Cell Res. 2012, 318, 1304–1308. [Google Scholar] [CrossRef]
- Matsuzawa-Ishimoto, Y.; Shono, Y.; Gomez, L.E.; Hubbard-Lucey, V.M.; Cammer, M.; Neil, J.; Dewan, M.Z.; Lieberman, S.R.; Lazrak, A.; Marinis, J.M.; et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J. Exp. Med. 2017, 214, 3687–3705. [Google Scholar] [CrossRef]
- Kim, H.J.; Hwang, K.E.; Park, D.S.; Oh, S.H.; Jun, H.Y.; Yoon, K.H.; Jeong, E.T.; Kim, H.R.; Kim, Y.S. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J. Transl. Med. 2017, 15, 123. [Google Scholar] [CrossRef]
- Honscheid, P.; Datta, K.; Muders, M.H. Autophagy: Detection, regulation and its role in cancer and therapy response. Int. J. Radiat. Biol. 2014, 90, 628–635. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Shi, X.; Zhou, M.; Zhao, Y.; Pan, S.; Zhao, C.; Guo, X.; Wang, M.; Li, X.; Qin, R. Alantolactone induces apoptosis and improves chemosensitivity of pancreatic cancer cells by impairment of autophagy-lysosome pathway via targeting TFEB. Toxicol. Appl. Pharmacol. 2018, 356, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Stamelos, V.A.; Fisher, N.; Bamrah, H.; Voisey, C.; Price, J.C.; Farrell, W.E.; Redman, C.W.; Richardson, A. The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization. PLoS ONE 2016, 11, e0150696. [Google Scholar] [CrossRef] [PubMed]
- Demers-Lamarche, J.; Guillebaud, G.; Tlili, M.; Todkar, K.; Belanger, N.; Grondin, M.; Nguyen, A.P.; Michel, J.; Germain, M. Loss of mitochondrial function impairs lysosomes. J. Biol. Chem. 2016, 291, 10263–10276. [Google Scholar] [CrossRef] [PubMed]
| % Cell viability | |
|---|---|
| Control | 100 ± 0.00 |
| DC | 69.27 ± 0.64* |
| DC+Nec-1 | 72.14 ± 6.61* |
| % Cells with Functional (Acid) Lysosomes | ||
| 24 h | 48 h | |
| Negative control | 85.50 ± 3.11 | 94.10 ± 2.26 |
| DC | 85.55 ± 1.62 | 74.95 ± 1.62* |
| % Cells with Conserved ψm | ||
| 24 h | 48 h | |
| Negative control | 100.00 ± 0.00 | 100.00 ± 0.00 |
| DC | 61.98 ± 10.68* | 27.25 ± 8.52* |
| ROS levels (Arbitrary Units) | ||
| 1 h | 24 h | |
| Negative control | 1 ± 0.00 | 1 ± 0.00 |
| DC | 1.32 ± 0.10* | 1.31 ± 0.04* |
| % Cell Viability | ||
| 24 h | 72 h | |
| Negative control | 100 ± 0.00 | 100 ± 0.00 |
| DC | 68.13 ± 7.73* | 27.94 ± 1.50* |
| DC + NAC | 72.42 ± 0.47* | 80.16 ± 2.52*# |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mármol, I.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Osada, J.; Cerrada, E.; Rodríguez-Yoldi, M.J. A Combination of Rosa Canina Extracts and Gold Complex Favors Apoptosis of Caco-2 Cells by Increasing Oxidative Stress and Mitochondrial Dysfunction. Antioxidants 2020, 9, 17. https://doi.org/10.3390/antiox9010017
Mármol I, Jiménez-Moreno N, Ancín-Azpilicueta C, Osada J, Cerrada E, Rodríguez-Yoldi MJ. A Combination of Rosa Canina Extracts and Gold Complex Favors Apoptosis of Caco-2 Cells by Increasing Oxidative Stress and Mitochondrial Dysfunction. Antioxidants. 2020; 9(1):17. https://doi.org/10.3390/antiox9010017
Chicago/Turabian StyleMármol, Inés, Nerea Jiménez-Moreno, Carmen Ancín-Azpilicueta, Jesús Osada, Elena Cerrada, and María Jesús Rodríguez-Yoldi. 2020. "A Combination of Rosa Canina Extracts and Gold Complex Favors Apoptosis of Caco-2 Cells by Increasing Oxidative Stress and Mitochondrial Dysfunction" Antioxidants 9, no. 1: 17. https://doi.org/10.3390/antiox9010017
APA StyleMármol, I., Jiménez-Moreno, N., Ancín-Azpilicueta, C., Osada, J., Cerrada, E., & Rodríguez-Yoldi, M. J. (2020). A Combination of Rosa Canina Extracts and Gold Complex Favors Apoptosis of Caco-2 Cells by Increasing Oxidative Stress and Mitochondrial Dysfunction. Antioxidants, 9(1), 17. https://doi.org/10.3390/antiox9010017











