Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals
Abstract
1. Chemical Basis of Antioxidant Action
2. Are Antioxidants Needed for Human Life?
3. When Does Antioxidant Intake Become Beneficial in Human Life?
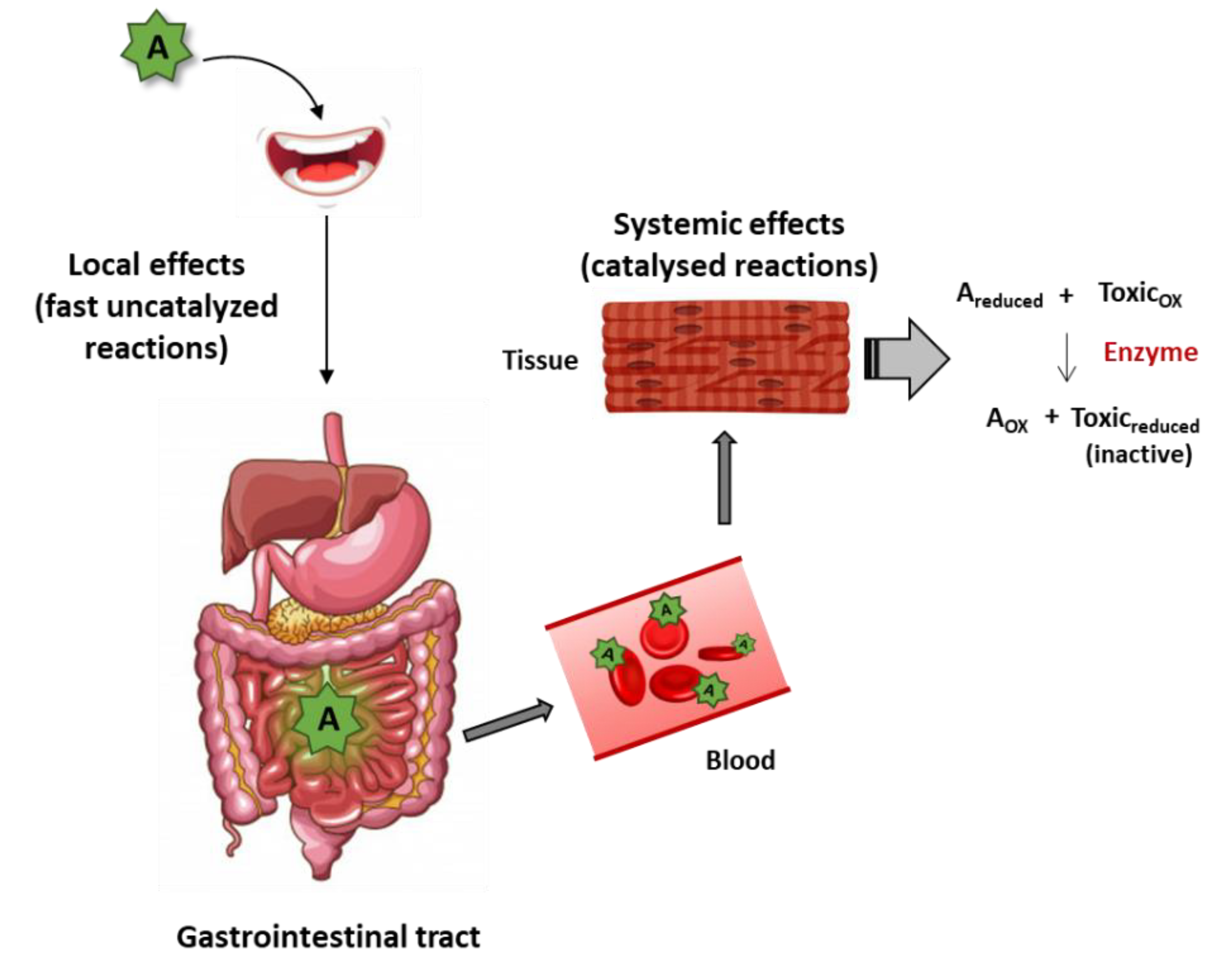
4. Direct Mechanisms of Plant Antioxidant/Nutraceutical Action
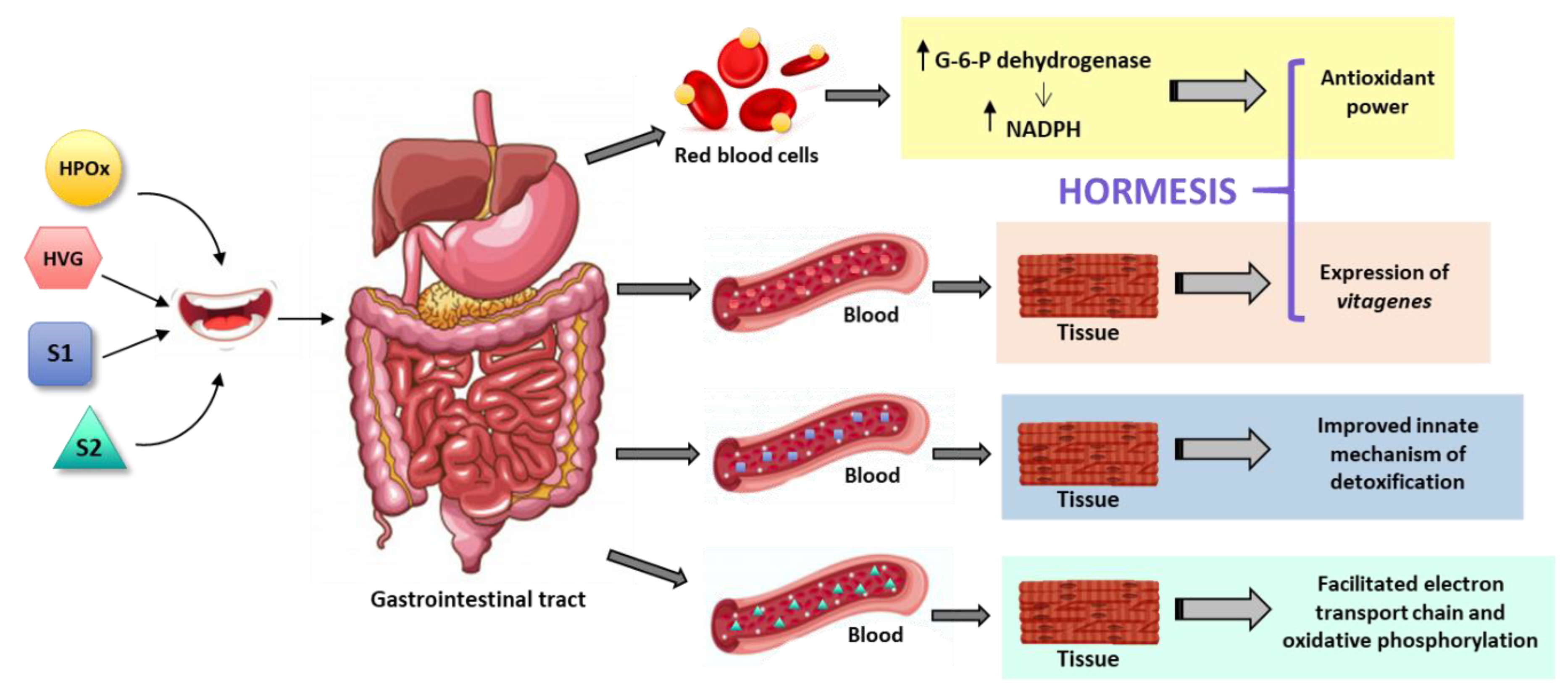
5. Hormetic and Replenishment Mechanisms of Indirect Antioxidant/Nutraceutical Action
6. Mitochondria-Related Mechanisms of Plant Antioxidant/Nutraceutical Action
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franco, R.; Martínez-Pinilla, E. Chemical rules on the assessment of antioxidant potential in food and food additives aimed at reducing oxidative stress and neurodegeneration. Food Chem. 2017, 235, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Buckley, S.; Collins, M.J.; Estalrrich, A.; Brothwell, D.; Copeland, L.; García-Tabernero, A.; García-Vargas, S.; de la Rasilla, M.; Lalueza-Fox, C.; et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften 2012, 99, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, L.S.; Duchene, S.; Soubrier, J.; Arriola, L.; Llamas, B.; Breen, J.; Morris, A.G.; Alt, K.W.; Caramelli, D.; Dresely, V.; et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 2017, 544, 357–361. [Google Scholar] [CrossRef]
- Milton, K. Nutritional characteristics of wild primate foods: Do the diets of our closest living relatives have lessons for us? Nutrition 1999, 15, 488–498. [Google Scholar] [CrossRef]
- Milton, K. Back to basics: Why foods of wild primates have relevance for modern human health. Nutrition 2000, 16, 480–483. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Gonzaga, G.; Muñoz-Jiménez, I.; Osorio-Alonso, H.; Iroz, A.; Vecchio, M.; Tapia, E.; Roncal-Jiménez, C.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Antioxidant supplements as a novel mean for blocking recurrent heat stress-induced kidney damage following rehydration with fructose-containing beverages. Free Radic. Biol. Med. 2019, 141, 182–191. [Google Scholar] [CrossRef]
- Girard, A.; Madani, S.; Boukortt, F.; Cherkaoui-Malki, M.; Belleville, J.; Prost, J. Fructose-enriched diet modifies antioxidant status and lipid metabolism in spontaneously hypertensive rats. Nutrition 2006, 22, 758–766. [Google Scholar] [CrossRef]
- Song, M.; Schuschke, D.A.; Zhou, Z.; Chen, T.; Pierce, W.M.; Wang, R.; Johnson, W.T.; McClain, C.J. High fructose feeding induces copper deficiency in Sprague-Dawley rats: A novel mechanism for obesity related fatty liver. J. Hepatol. 2012, 56, 433–440. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef]
- Bray, G.A. Energy and Fructose From Beverages Sweetened With Sugar or High-Fructose Corn Syrup Pose a Health Risk for Some People. Adv. Nutr. 2013, 4, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Fine, E.J. Fructose in perspective. Nutr. Metab. 2013, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, V.J.; Tappy, L.; Brouns, F.J.P.H. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr. Res. Rev. 2014, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Mu, W.; Roncal, C.; Sautin, Y.Y.; Abdelmalek, M.; Reungjui, S.; Le, M.; Nakagawa, T.; Lan, H.Y.; Yu, X.; et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur. J. Nutr. 2010, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Massa, M.L.; Arbeláez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 137, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cydylo, M.A.; Davis, A.T.; Kavanagh, K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity 2017, 25, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.A.; Stanhope, K.L.; Graham, J.L.; Cummings, B.P.; Wang, W.; Saville, B.R.; Havel, P.J. Fructose-fed rhesus monkeys: A nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin. Transl. Sci. 2011, 4, 243–252. [Google Scholar] [CrossRef]
- Schwitzer, C.; Polowinsky, S.Y.; Solman, C. Fruits as foods-common misconceptions about frugivory. In Zoo Animal Nutrition IV; Clauss, M., Fidgett, A., Janssens, J., Hatt, J.-M., Huisman, T., Hummel, J., Nijboer, J., Plowman, A., Eds.; Filander Verlag: Fürth, Germany, 2008; pp. 131–168. [Google Scholar]
- Nagase, M.; Yamamoto, Y.; Matsumoto, N.; Arai, Y.; Hirose, N. Increased oxidative stress and coenzyme Q10 deficiency in centenarians. J. Clin. Biochem. Nutr. 2018, 63, 129–136. [Google Scholar] [CrossRef]
- Rembold, C.M. Coenzyme Q10 Supplementation in Orthostatic Hypotension and Multiple-System Atrophy: A Report on 7 Cases. Am. J. Med. 2018, 131, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Harcourt, B.E.; Tang, P.H.; Morley, A.L.; Huynh, K.; Penfold, S.A.; Coughlan, M.T.; Cooper, M.E.; Nguyen, T.-V.; Ritchie, R.H.; et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic. Biol. Med. 2012, 52, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Chen, X.-Q.; Oliver Chen, C.-Y. Ubiquinol is superior to ubiquinone to enhance Coenzyme Q10 status in older men. Food Funct. 2018, 9, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Rahimlou, M.; Asadi, M.; Banaei Jahromi, N.; Mansoori, A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: A Systematic Review and meta- analysis. Clin. Nutr. ESPEN 2019, 32, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, R.; Verhoeven, A.J.; Roos, D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014, 67, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol. Oncol. Clin. North Am. 2016, 30, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Cheng, M.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—from oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007, 12, 109–118. [Google Scholar] [CrossRef]
- Baker, M.A.; Bosia, A.; Pescarmona, G.; Turrini, F.; Arese, P. Mechanism of Action of Divicine in a Cell-free System and in Glucose-6-phosphate Dehydrogenase-deficient Red Cells. Toxicol. Pathol. 1984, 12, 331–336. [Google Scholar] [CrossRef]
- Vural, N.; Sardas, S. Biological activities of broad bean (Vicia faba L.) extracts cultivated in South Anatolia in favism sensitive subjects. Toxicology 1984, 31, 175–179. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Simsek, S.; Ozcan, M.M.; Al Juhaimi, F.; ElBabiker, E.; Ghafoor, K. Amino Acid and Sugar Contents of Wild and Cultivated Carob (Ceratonia siliqua) Pods Collected in Different Harvest Periods. Chem. Nat. Compd. 2017, 53, 1008–1009. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.; Kwon, O. Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022. [Google Scholar] [CrossRef] [PubMed]
- Cayman Chemical Co. Kaempferol Data Sheet. Available online: https://www.caymanchem.com/product/11852 (accessed on 26 August 2019).
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, S44–S55. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Barone Lumaga, R.; Ferracane, R.; Sellitto, S.; Morelló, J.R.; Reguant Miranda, J.; Shimoni, E.; Fogliano, V. Human bioavailability of flavanols and phenolic acids from cocoa-nut creams enriched with free or microencapsulated cocoa polyphenols. Br. J. Nutr. 2013, 109, 1832–1843. [Google Scholar] [CrossRef]
- Vitaglione, P.; Barone Lumaga, R.; Ferracane, R.; Radetsky, I.; Mennella, I.; Schettino, R.; Koder, S.; Shimoni, E.; Fogliano, V. Curcumin Bioavailability from Enriched Bread: The Effect of Microencapsulated Ingredients. J. Agric. Food Chem. 2012, 60, 3357–3366. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Parmar, S.S.; Jaiwal, A.; Dhankher, O.P.; Jaiwal, P.K. Coenzyme Q10 production in plants: Current status and future prospects. Crit. Rev. Biotechnol. 2015, 35, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. The threshold vs LNT showdown: Dose rate findings exposed flaws in the LNT model part 1. The Russell-Muller debate. Environ. Res. 2017, 154, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Sakai, K.; Sanbongi, C.; Sugiura, K. Dietary whey protein downregulates fatty acid synthesis in the liver, but upregulates it in skeletal muscle of exercise-trained rats. Nutrition 2005, 21, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Hormesis and the Antiaging Action of Dietary Restriction. Exp. Gerontol. 1998, 33, 61–66. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. The Nature of Gerontogenes and Vitagenes: Antiaging Effects of Repeated Heat Shock on Human Fibroblasts. Ann. N. Y. Acad. Sci. 1998, 854, 54–60. [Google Scholar] [CrossRef]
- Rattan, S.I. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem. Mol. Biol. Int. 1998, 45, 753–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thorpe, G.W.; Reodica, M.; Davies, M.J.; Heeren, G.; Jarolim, S.; Pillay, B.; Breitenbach, M.; Higgins, V.J.; Dawes, I.W. Superoxide radicals have a protective role during H2O2 stress. Mol. Biol. Cell 2013, 24, 2876–2884. [Google Scholar] [CrossRef]
- Mane, N.R.; Gajare, K.A.; Deshmukh, A.A. Mild heat stress induces hormetic effects in protecting the primary culture of mouse prefrontal cerebrocortical neurons from neuropathological alterations. IBRO Rep. 2018, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Brielmann, R.M.; Neto, M.F.; Lin, Y.-F.; Haynes, C.M.; Morimoto, R.I. Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging. Cell Rep. 2017, 21, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Bartling, B.; Friedrich, I.; Silber, R.E.; Simm, A. Ischemic preconditioning is not cardioprotective in senescent human myocardium. Ann. Thorac. Surg. 2003, 76, 105–111. [Google Scholar] [CrossRef]
- Simm, A.; Müller, B.; Nass, N.; Hofmann, B.; Bushnaq, H.; Silber, R.E.; Bartling, B. Protein glycation—Between tissue aging and protection. Exp. Gerontol. 2015, 68, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Snoek, L.B.; Riksen, J.A.G.; Bevers, R.P.; Kammenga, J.E. Genetic variation for stress-response hormesis in C. elegans lifespan. Exp. Gerontol. 2012, 47, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals Rosmarinic Acid, Ampelopsin, and Amorfrutin-A Can Modulate Age-Related Phenotype of Serially Passaged Human Skin Fibroblasts in vitro. Front. Genet. 2019, 10, 81. [Google Scholar] [CrossRef]
- Uchitomi, R.; Nakai, S.; Matsuda, R.; Onishi, T.; Miura, S.; Hatazawa, Y.; Kamei, Y. Genistein, daidzein, and resveratrols stimulate PGC-1β-mediated gene expression. Biochem. Biophys. Rep. 2019, 17, 51–55. [Google Scholar] [CrossRef]
- Atlante, A.; Bobba, A.; Paventi, G.; Pizzuto, R.; Passarella, S. Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochem. Pharmacol. 2010, 79, 758–767. [Google Scholar] [CrossRef][Green Version]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Wood dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; A. Villena, J.; Lima Ribeiro, M. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef] [PubMed]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int. J. Cancer 2002, 98, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.; Shukla, F.; Wala, H.; Pillai, P. Mitochondrial Electron Transport Chain Complex Dysfunction in MeCP2 Knock-Down Astrocytes: Protective Effects of Quercetin Hydrate. J. Mol. Neurosci. 2019, 67, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Adedara, I.A.; Ajayi, B.O.; Ayepola, O.R.; Egbeme, E.E. Kolaviron, a Natural Antioxidant and Anti-Inflammatory Phytochemical Prevents Dextran Sulphate Sodium-Induced Colitis in Rats. Basic Clin. Pharmacol. Toxicol. 2013, 113, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.B.; Amoo, Z.A.; Saliu, I.O.; Olaleye, M.T.; Farombi, E.O.; Akinmoladun, A.C. Neurotherapeutic potential of kolaviron on neurotransmitter dysregulation, excitotoxicity, mitochondrial electron transport chain dysfunction and redox imbalance in 2-VO brain ischemia/reperfusion injury. Biomed. Pharmacother. 2019, 111, 859–872. [Google Scholar] [CrossRef] [PubMed]
| Phytochemicals | Sources | Mechanism of Action | Effects | References |
|---|---|---|---|---|
| Fructose | Cultivated fruits or sweeteners | Increases in plasma triglycerides, insulin resistance, high blood pressure, etc. | Fatty liver, insulin resistance, dyslipidemia, etc. | [8,9,12,13,14,15,16,17,18,19,20] |
| Coenzyme Q10 | Oranges, spinach, broccoli, soybeans, nuts, sesame seeds, etc. | Correct function of the electron chain transport in mitochondria | Improvement in orthostatic hypotension, renal alterations in type II diabetes | [21,22,23,24] |
| Lipoic acid | Potatoes, spinach, broccoli, carrots, tomatoes, rice bran, etc. | Correct function of different enzymatic systems | To combat oxidative stress (by mechanisms not known) | [25] |
| Vicine and convicine | Fava beans | “Hormetic” maintenance of high levels of glucose-6-phosphate dehydrogenase | Maintenance hemoglobin in a functional state and the innate antioxidant mechanism of red blood cells | [26,27,28,29,30] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. https://doi.org/10.3390/antiox8090373
Franco R, Navarro G, Martínez-Pinilla E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants. 2019; 8(9):373. https://doi.org/10.3390/antiox8090373
Chicago/Turabian StyleFranco, Rafael, Gemma Navarro, and Eva Martínez-Pinilla. 2019. "Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals" Antioxidants 8, no. 9: 373. https://doi.org/10.3390/antiox8090373
APA StyleFranco, R., Navarro, G., & Martínez-Pinilla, E. (2019). Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants, 8(9), 373. https://doi.org/10.3390/antiox8090373






