Glycemic Control during Pregnancy—A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women?
Abstract
1. Introduction
2. Materials and Methods
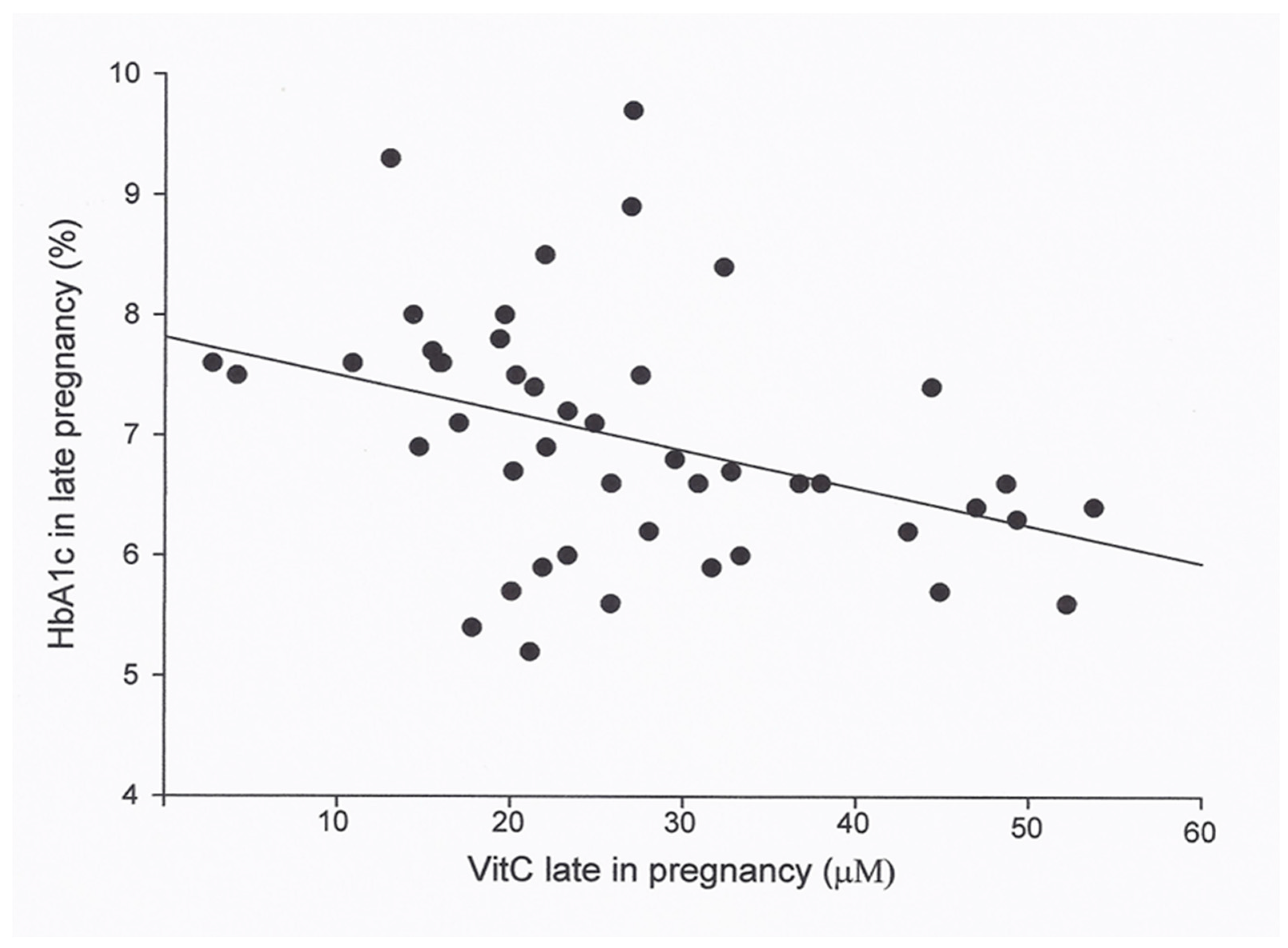
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinclair, A.J.; Girling, A.J.; Gray, L.; Le Guen, C.; Lunec, J.; Barnett, A.H. Disturbed handling of ascorbic acid in diabetic patients with and without microangiopathy during high dose ascorbate supplementation. Diabetologia 1991, 34, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Juhl, B.; Lauszus, F.F.; Lykkesfeldt, J. Is diabetes associated with lower vitamin C status in pregnant women? A prospective study. Int. J. Vit. Nutr. Res. 2016, 86, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Juhl, B.; Klein, F.; Christiansen, J.S. Vitamin C treatment reduces transcapillary escape rate of albumin in type 1 diabetes. Eur. J. Internal. Med. 2004, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Scaife, A.R.; McNeill, G.; Campbell, D.M.; Martindale, S.; Devereux, G.; Seaton, A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal levels at delivery. Br. J. Nutr. 2006, 95, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A.; Otradovec, C.L.; Russell, R.M.; Munro, H.N.; Hartz, S.C.; McGandy, R.B.; Morrow, F.D.; Sadowski, J.A. Vitamin C status and nutrient interactions in a healthy elderly population. Am. J. Clin. Nutr. 1988, 48, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Juhl, B.; Lauszus, F.F.; Lykkesfeldt, J. Poor vitamin C status late in pregnancy is associated with increased risk of complications in type 1 diabetic women: A cross sectional study. Nutrients 2017, 9, 186. [Google Scholar] [CrossRef]
- Michael, J.A.M.; Holmes, V.A.; Patterson, C.C.; Young, I.S.; Pearson, D.W.M.; Walker, J.D. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015, 38, 34–42. [Google Scholar] [CrossRef]
- Holmes, V.A.; Young, I.S.; Patterson, C.C.; Pearson, D.W.M.; Walker, J.D.; Michael, J.A.M.; McCance, D.R. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care 2011, 34, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.E.; Florkowski, J.R.C.M. Is there a role for HbA1c in pregnancy. Curr. Diab. Rep. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R. Diabetes in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Hamernyik, P.; Hutchinson, M.; Raisys, V.A.; Labbé, R.F. Ascorbic acid in lymphocytes: Cell preparation and liquid-chromatographic assay. Clin. Chem. 1982, 28, 2165–2169. [Google Scholar] [PubMed]
- Klemetti, M.M.; Laivuori, H.; Tikkanen, M.; Nuutila, M.; Hiilesmaa, V.; Teramo, K. White’s classification and pregnancy outcome in women with type 1 diabetes: A polulation-based cohort study. Diabetologia 2016, 59, 92–100. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Hare, J.W.; Cloherty, J.P.; Dunn, P.J.; Gleason, J.R.A.; Soeldner, S.S.; Kitzmiller, J.L. Elevated maternal Hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. Engl. J. Med. 1981, 304, 1331–1334. [Google Scholar] [CrossRef]
- Evers, I.M.; de Valk, H.W.; Visser, G.H. Risk of complications of pregnancy in women with type 1 diabetes: Nationwide prospective study in the Netherlands. BMJ 2004, 328, 915. [Google Scholar] [CrossRef] [PubMed]
- Heen, S.T.; Tay, S.S.; Boran, J.; Ting, L.W.; Kumar, S.D.; Fu, J.; Ling, E.A. Recent studies on neural tube defects in embryos of diabetic pregnancy: an overview. Curr. Med. Chem. 2009, 16, 2345–2354. [Google Scholar] [CrossRef]
- Cederberg, J.; Eriksson, U.K. Antioxidative treatment of pregnant diabetic rats diminished embryonic dysmorphogenesis. Birth Defects Res. A. Clin. Mol. Teratol. 2005, 3, 498–505. [Google Scholar] [CrossRef]
- Cederberg, J.; Siman, C.M.; Eriksson, U.J. Combined treatment with vitamin E and C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatric Res. 2001, 49, 755–762. [Google Scholar] [CrossRef]
- Siman, C.M.; Eriksson, U.J. Vitamin C supplementation of the maternal diet reduces the rate of malformations in the offspring of diabetic rats. Diabetologia 1997, 40, 1416–1424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; et al. Vitamin C trnaporter Slc23al links renal reabtion, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Invest. 2010, 120, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, G.; Martinoli, L.; Miceli, M.; Ciuti, M.; D’Alessandri, G.; Gironi, A.; Palmieri, L.; Anichini, R.; Bartolomei, G.; Franconi, F. Renal excretion of ascorbic acid in insulin dependent diabetes mellitus. Int. J. Vitam Nutr. Res. 1994, 64, 119–124. [Google Scholar] [PubMed]
- Hirsch, I.B.; Atchley, D.H.; Tsai, E.; Labbé, R.F.; Chait, A.J. Ascorbic acid clearance in diabetic nephropathy. Diabetes Complications 1998, 12, 259–263. [Google Scholar] [CrossRef]
- Chen, L.; Jia, R.H.; Qiu, C.J.; Ding, G. Hyperglycemia inhibits the uptake of dehydroascorbate in tubular epithelial cell. Am. J. Nephrol. 2005, 25, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Morimoto, S.; Okigaki, M.; Seo, M.; Someya, K.; Morita, T.; Matsubara, H.; Sugiura, T.; Iwasaka, T. Decreased plasma level of vitamin C in chronic kidney disease: comparison between diabetic and non-diabetic patients. Nephrol. Dial. Transplant. 2011, 26, 1252–1257. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Wamme, W.P.; et al. Normalizing mitochondrialsuperoxide production blocks three pathways of hyper-glycemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Brownlee, M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes 1994, 43, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Kallner, A.B.; Hartmann, D.; Horning, H.D. On the requirements of ascorbic acid in man: steady-state turnover and body pool in smokers. Am. J. Clin. Nutr. 1981, 34, 1347–1355. [Google Scholar] [CrossRef]
- Alberg, A. The influence of cigarette smoking on circurlaing concentrations of antioxidant micronutrients. Toxicolology 2002, 180, 121–137. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini-Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245–20253. [Google Scholar]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate is depleted by smoking and repleted by supplementation: A study in male smokers and non-smokers with matched dietary intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Viscovich, M.; Poulsen, H.E. Ascorbic acid recycling in human erythrocytes is induced by smoking in vivo. Free Radic. Biol. Med. 2003, 35, 1439–1447. [Google Scholar] [CrossRef]
- Shah, N.R.; Bracken, M.B. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am. J. Obstet. Gynecol. 2000, 182, 465–472. [Google Scholar] [CrossRef]
- Mei-Dan, E.; Walfisch, A.; Weisz, B.; Hallak, M.; Brown, R.; Shrim, A. The unborn smoker: association between smoking during pregnancy and adverse perinatal outcomes. J. Perinat. Med. 2015, 43, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Roberts, C.L.; Simpson, J.M.; March, L.M. Pregnancy Outcomes in Women with Rare Autoimmune Diseases. Arthritis Rheumatol. 2015, 67, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
| Variable | n | Value |
|---|---|---|
| Age (yr) | 46 | 28.2 (3.6) |
| Diabetes duration (yr) | 46 | 14.1 (8.9) |
| Parity | 46 | 1.8 (0.7) |
| Systolic blood pressure at entry (mmHg) | 28 | 120.0 (9.9) |
| Diastolic blood pressure at entry (mmHg) | 28 | 71.2 (6.9) |
| Retinopathy (n = No/Simplex/Proliferative) | 46 | 23/17/6 |
| BMI at delivery (kg/m²) | 31 | 28.6 (4.3) |
| Normo-/Micro-/Macro-albuminuria (n/n/n) | 46 | 37/8/1 |
| HbA1c in first trimester (%) ¹ | 45 | 7.6 (1.2) |
| HbA1c in second trimester (%) ¹ | 43 | 7.0 (0.9) |
| HbA1c in late pregnancy (%) ¹ | 46 | 7.0 (1.0) |
| HbA1c in first and second trimester combined (%) | 43 | 7.3(1.1) |
| HbA1c mean of the whole pregnancy (%) | 42 | 7.2(0.9) |
| VitC in late pregnancy (µmol/L) | 46 | 30.1(13.8) |
| VitC mean of pregnancy (µmol/L) | 37 | 34.8(14.0) |
| Complications of pregnancy (n = yes/no) ² | 46 | 19/27 |
| Creatinine clearance at entry (ml/min) | 35 | 122.1 (22.1) |
| Creatinine clearance at delivery (ml/min) | 31 | 99.2 (33.2) |
| Smoker (n = yes/no/unknown) | 46 | 15/30/1 |
| Independent Variable | r² | n | p Value |
|---|---|---|---|
| HbA1c in first trimester (%) | 0.11 | 45 | 0.03 |
| HbA1c in first and second trimester (%) | 0.14 | 43 | 0.034 |
| HbA1c of the whole pregnancy (%) | 0.18 | 42 | 0.017(0.055) *² |
| HbA1c in late pregnancy (%) | 0.15 | 46 | <0.01(0.029) *¹ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhl, B.; Lauszus, F.F.; Lykkesfeldt, J. Glycemic Control during Pregnancy—A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women? Antioxidants 2019, 8, 153. https://doi.org/10.3390/antiox8060153
Juhl B, Lauszus FF, Lykkesfeldt J. Glycemic Control during Pregnancy—A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women? Antioxidants. 2019; 8(6):153. https://doi.org/10.3390/antiox8060153
Chicago/Turabian StyleJuhl, Bente, Finn F. Lauszus, and Jens Lykkesfeldt. 2019. "Glycemic Control during Pregnancy—A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women?" Antioxidants 8, no. 6: 153. https://doi.org/10.3390/antiox8060153
APA StyleJuhl, B., Lauszus, F. F., & Lykkesfeldt, J. (2019). Glycemic Control during Pregnancy—A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women? Antioxidants, 8(6), 153. https://doi.org/10.3390/antiox8060153






