Hystrix Brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Porcupine Bezoar Extract Preparation
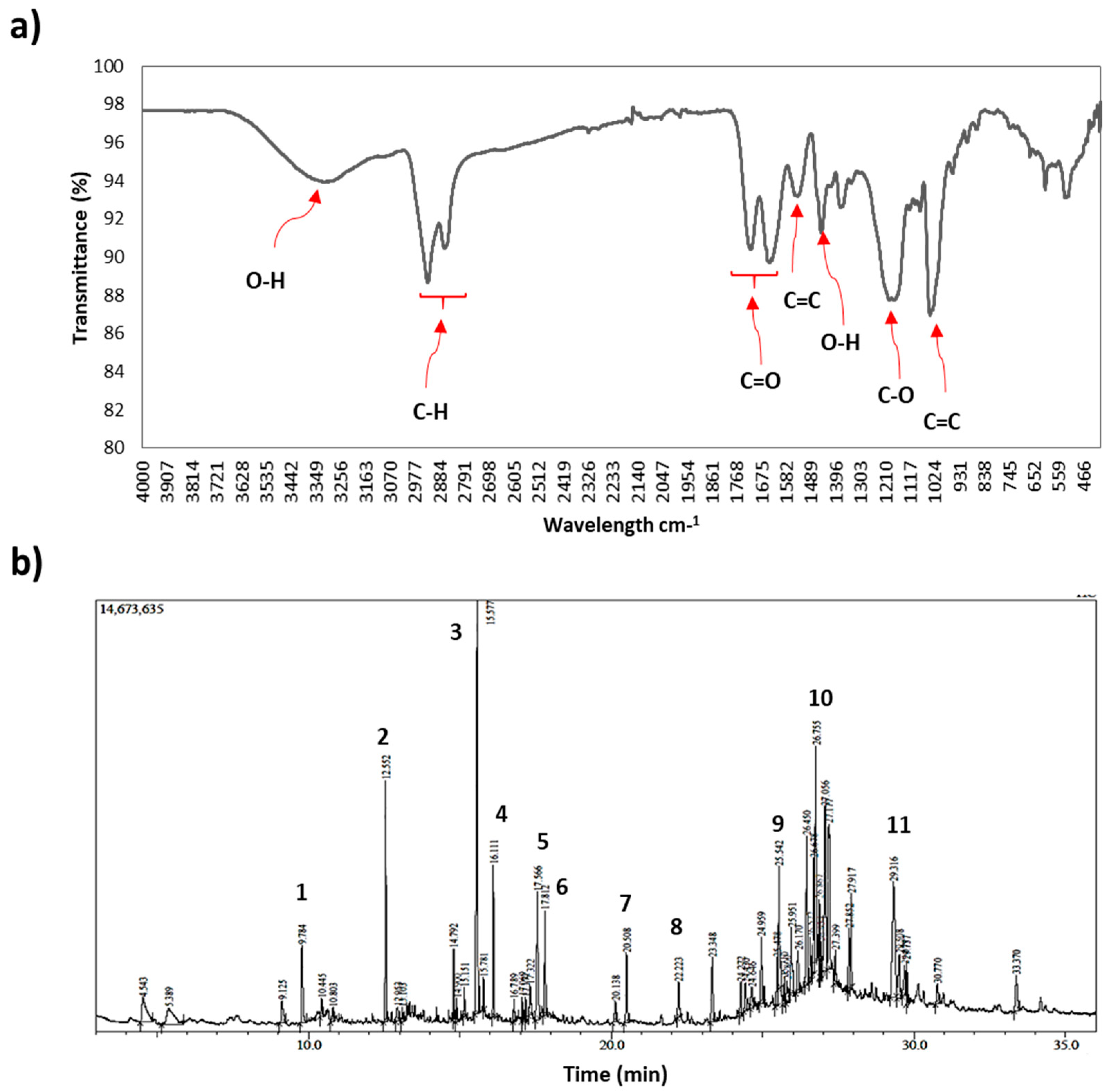
2.3. Fourier-Transform Infrared Spectroscopy Analysis Of Porcupine Bezoar
2.4. Gas Chromatography Mass Spectroscopy (GCMS) Profile Analysis of Porcupine Bezoar
2.5. Total Phenol Content (TPC)
2.6. Total Flavonoid Content (TFC)
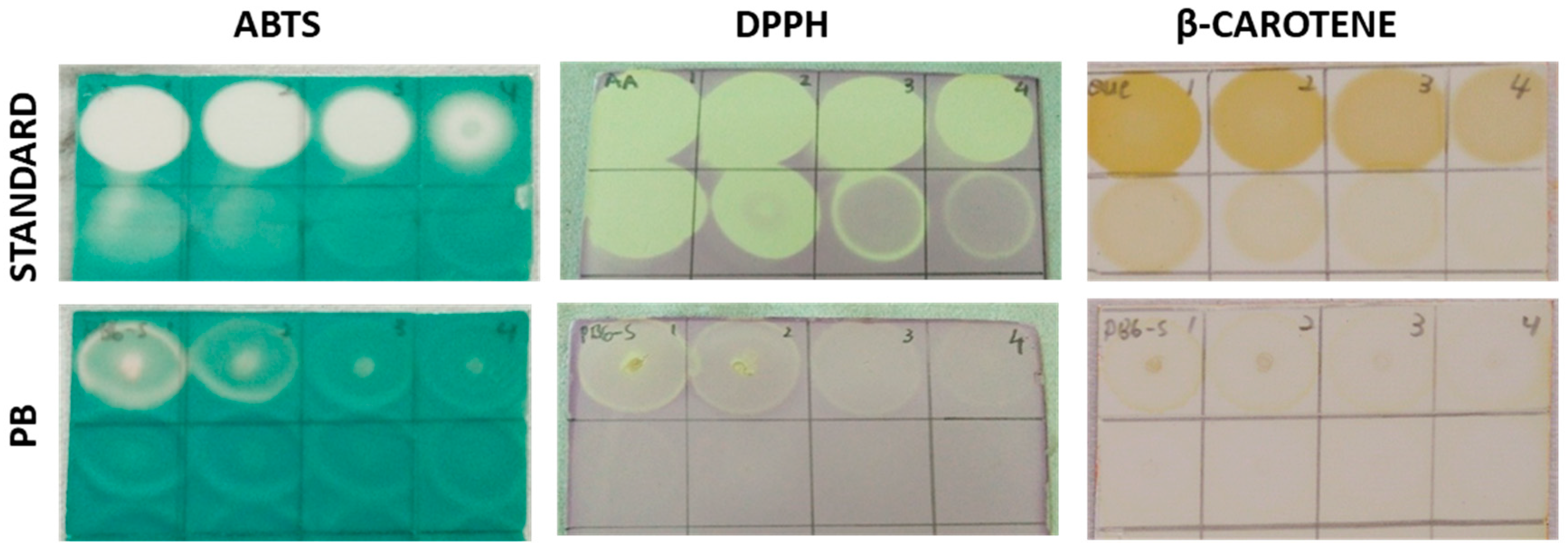
2.7. Rapid Screening of Antioxidant by Dot-Blot Assay
2.7.1. DPPH Dot-Blot Assay
2.7.2. ABTS+ Dot-Blot Assay
2.7.3. β-Carotene Dot-Blot Assay
2.8. Cell Culture Maintenance
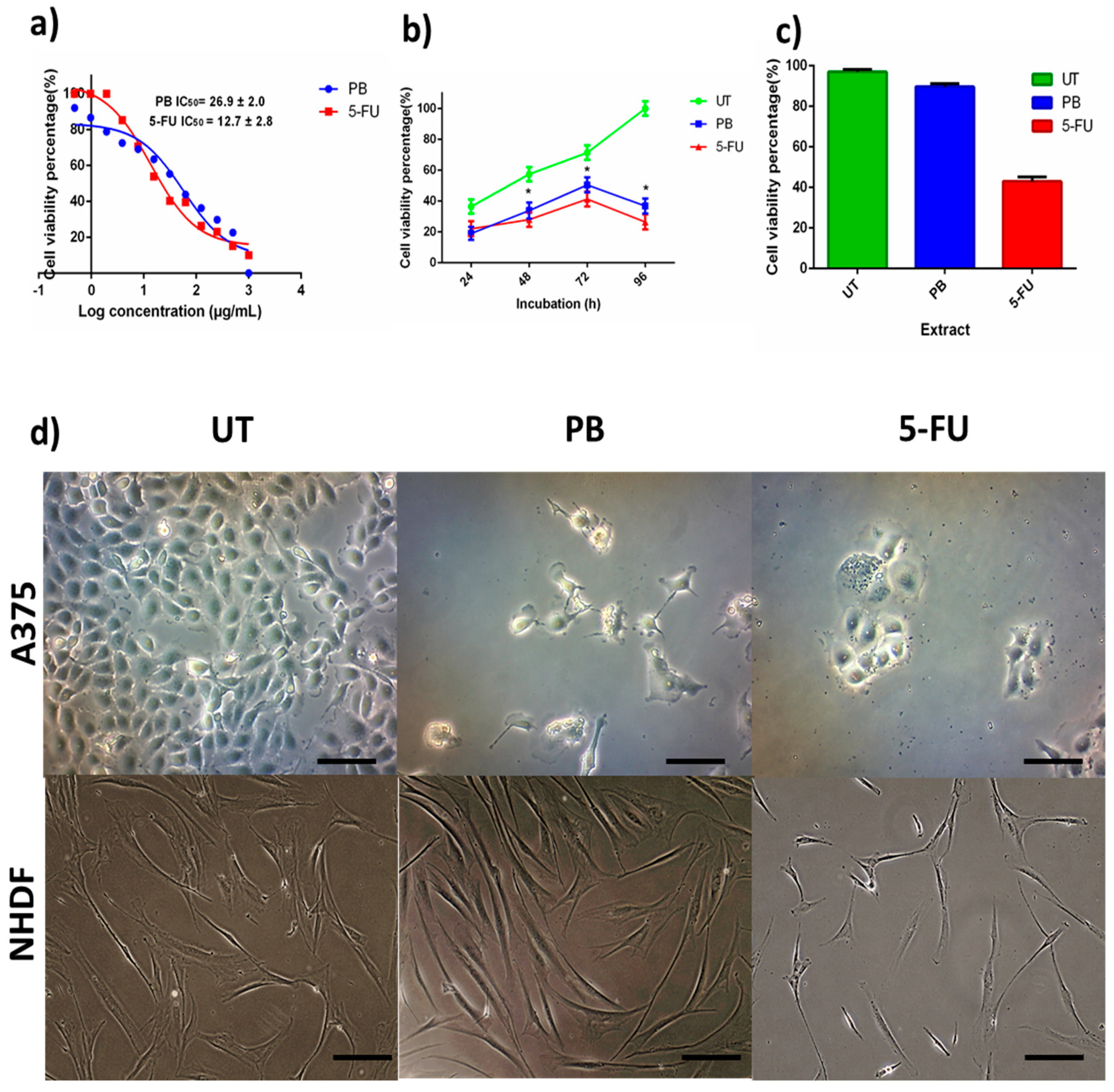
2.9. Cytotoxicity Assay of Porcupine Bezoar on Cancer Cell and Normal Cell
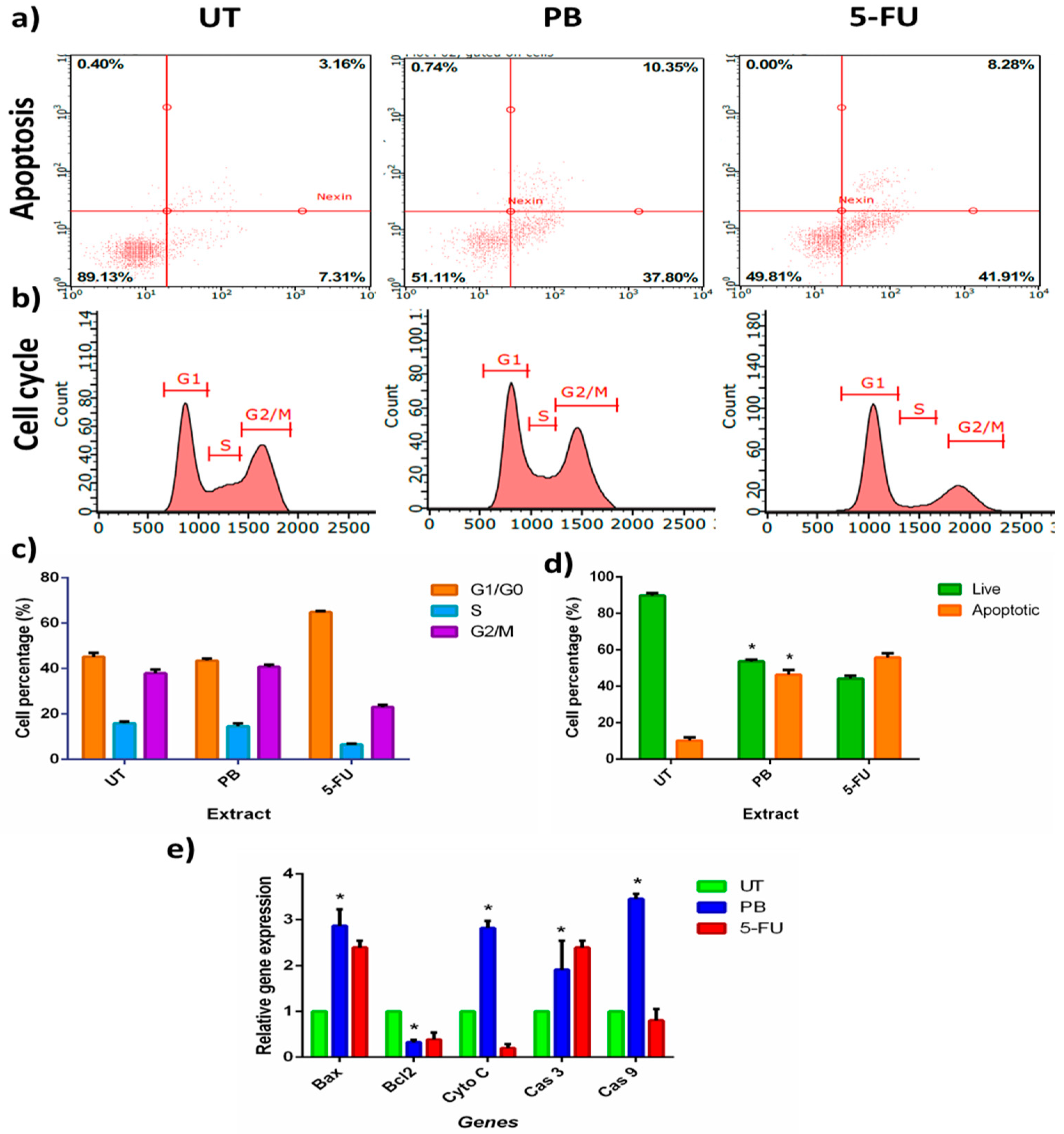
2.10. Cell Cycle Arrest Assay
2.11. Apoptosis Assay
2.12. Real Time Quantitative PCR (RT-qPCR) Analysis
2.13. Statistical Analysis
3. Results
3.1. Porcupine Bezoar Characterization
3.2. Total Phenolic Content and Total Flavonoid Content of Porcupine Bezoar
3.3. Anticancer Activity of Porcupine Bezoar Aqueous Extract on Melanoma Cell (A375)
3.4. Porcupine Bezoar Aqueous Extract Induced Apoptosis on Melanoma Cell
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 29. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Gerber, M.; du Plessis, L.; du Preez, J.; du Plessis, J. Topical Delivery of 5-Fluorouracil from PheroidTM Formulations and the In Vitro Efficacy Against Human Melanoma. AAPS PharmSciTech 2015, 16, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plant as source of anticancer agents Plants as a source of anti-cancer agents. J. Ethnopharmacol. 100 2014, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1, 1–10. [Google Scholar] [CrossRef]
- Khurana, R.K.; Jain, A.; Jain, A.; Sharma, T.; Singh, B.; Kesharwani, P. Administration of antioxidants in cancer: debate of the decade. Drug Discov. Today 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Dehghn-nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Mori, E.; Sforzi, A. Structure of phytobezoars found in the stomach of a crested porcupine, Hystrix cristata L., 1758. Folia. Zool. 2013, 62, 232–234. [Google Scholar] [CrossRef]
- Duffin, C.J. Porcupine Stones. Pharm. Hist. (Lond) 2013, 43, 13–22. [Google Scholar]
- Barroso, M.D.S. The Bezoar Stone: A Princely Antidote, The Távora Sequeira Pinto Collection—Oporto. Acta Med. Hist. Adriat. 2014, 12, 77–98. [Google Scholar]
- Beaven, L.; Lloyd, K.J. Cardinal Paluzzo Paluzzi degli Albertoni Altieri and his collection in the Palazzo Altieri The evidence of the 1698 death inventory, Part ii. J. Hist. Collect. 2018, 1–16. [Google Scholar] [CrossRef]
- Stephenson, M. From Marvelous Antidote to the Poison of Idolatry : The Transatlantic Role of Andean Bezoar Stones during the Late Sixteenth and Early Seventeeth Centuries. Hisp. Am. Hist. Rev. 2014, 90, 1–39. [Google Scholar] [CrossRef]
- Duffin, C. Bezoar stones and their mounts. Society of Jewellery Historians 2013, 16, 1–3. [Google Scholar]
- Rezaie, M.; Farhoosh, R.; Iranshahi, M.; Sharif, A.; Golmohamadzadeh, S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015, 173, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-Based Metabolite Profiling of Cosmos caudatus Leaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014, 79, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ahmed, Q.U.; Muhammad, B.Y.; Dogarai, B.B.S.; Soad, S.Z.B.M. Anti-hyperglycemic activity of the leaves of Tetracera scandens Linn. Merr. (Dilleniaceae) in alloxan induced diabetic rats. J. Ethnopharmacol. 2010, 131, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ho, Y.; Sheu, M.; Lin, Y.; Tseng, M.; Wu, S.; Huang, G.; Chang, Y. Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud. 2007, 48, 407–417. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Dewanjee, S.; Gangopadhyay, M.; Bhattabharya, N.; Khanra, R.; Dua, T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015, 5, 75–84. [Google Scholar] [CrossRef]
- Ilghami, A.; Ghanbarzadeh, S.; Hamishehkar, H. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds, Ferric Reducing Activity and Antioxidant Activity of the Beta vulgaris Using Response Surface Methodology Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds. Pharm. Sci. 2015. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Bhat, R.; Min-Tze, L.; Karim, A.A.; Mansor, S.M. Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J. Food Sci. Technol. 2012, 49, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Lu, H.; Chiang, W. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process. Biochem. 2014, 10, 4–8. [Google Scholar] [CrossRef]
- Srivastava, S.; Dubey, R. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul. 2011, 64, 1–16. [Google Scholar] [CrossRef]
- Akram, F.; Ilyas, O.; Haleem, A. Food and Feeding Habits of Indian Crested Porcupine in Pench Tiger Reserve, Madhya Pradesh, India. Ambient Sci. 2017, 4, 0–5. [Google Scholar] [CrossRef]
- Felicetti, L.A.; Shipley, L.A.; Witmer, G.W.; Robbins, C.T. Digestibility, nitrogen excretion, and mean retention time by North American porcupines (Erethizon dorsatum) consuming natural forages. Physiol. Biochem. Zool. 2000, 73, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.; Diwan, A.; Chandra, S. Flavanoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Firus Khan, A.Y.; Asuhaimi, F.A.; Jalal, T.; Natto, H.A.; Roheem, F.O.; Ahmed, Q.U.; Johan, M.F.; Abd Wahab, R. Anti-Proliferative Property of Porcupine Bezoar Extract on Melanoma( A375 ). Int. J. Allied. Heal. Sci. 2017, 1, 1–13. [Google Scholar]
- Yew, P.N.; Lee, L.W.L.; Lim, Y.Y. Antioxidant and Intracellular Reactive Oxygen Species/Reactive Nitrogen Species Scavenging Activities of Three Porcupine Bezoars from Hystrix brachyura. Pharmacogenosy Nat. Prod. 2017, 366–372. [Google Scholar] [CrossRef]
- Aydemir, S.; De Smet, S.; Miserez, B.; Sandra, P. Antioxidant components of Viburnum opulus L. determined by on-line HPLC–UV–ABTS radical scavenging and LC–UV–ESI–MS methods. Food Chem. 2015, 175, 106–114. [Google Scholar] [CrossRef]
- Schleiden, M.; Carvalho, S.; Cardoso, G.; Resende, L.V.; Gomes, M.D.S.; Roberto, L.; Albuquerque, M.; Cristini, A.; Gomes, S.; Sales, T.A.; et al. Phytochemical Screening, Extraction of Essential Oils and Antioxidant Activity of Five Species of Unconventional Vegetables. Am. J. Plant Sci. 2015, 6, 2632–2639. [Google Scholar]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J. Agric. Food Chem. 2010, 58, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Ciofani, G.; Festi, D.; Neri, M.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Antioxidant properties of ursodeoxycholic acid. Biochem. Pharmacol. 2002, 64, 1661–1667. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y.; Hail, N.; Lotan, R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Al’aina yuhainis, F.K.; Mohd Hamzah, M.N.; Tara, J.; Shakirah, R.; Habibah, H.; Faizah Abdullah, A.; Ridhwan, A.W. In Vitro Evaluation of Porcupine Bezoar Extracts as Anticancer Agent on A549 -A Preliminary Study. Adv. Biotechnol. Microbiol. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Zhang, H.; Yang, Q. The anticancer effect of Huaier (Review). Oncol. Rep. 2015, 34, 12–21. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Li, J.; Pan, C.; Yanase, T.; Nawata, H. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem. Biophys. Res. Comm. 2003, 303, 1002–1007. [Google Scholar] [CrossRef]
- Evans, L.M.; Cowey, S.L.; Siegal, G.P.; Hardy, R.W. NIH Public Access. Nutr. Cancer 2010, 61, 746–753. [Google Scholar] [CrossRef]
- Hardy, S.; El-assaad, W.; Przybytkowski, E.; Joly, E.; Prentki, M.; Langelier, Y. Saturated Fatty Acid-induced Apoptosis in MDA-MB-231 Breast Cancer Cells. J. Biol. Chem. 2003, 278, 31861–31870. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cho, J.H.; Kim, E.; Kim, Y.J. Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS ONE 2017, 12, e0181183. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, M.; Thompson, K.A.; Barton, D.; Talley, J.; Volle, K.; Stasiak, I.; Beyea, L.; Guthrie, A.; Roda, A.; Camborata, C.; et al. Gastrolithiasis in Prehensile-Tailed Porcupines (Coendou prehensilis): Nine Cases And Pathogenesis of Stone Formation. J. Zoo. Wildl. Med. 2014, 45, 883–891. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward Sequence 5′ to 3′ | Reverse Sequence 5′ to 3′ |
|---|---|---|
| ACTB | F-CGGCGCCCTATAAAACCCA | R-ATCATCCATGGTGAGCTGGC |
| GAPDH | F-GACAGTCAGCCGCATCTTCT | R-GCGCCCAATACGACCAAATC |
| BAX | F-GAACCATCATGGGCTGGACAT | R-ATGGTCACGGTCCAACCACC |
| BCL2 | F-ATGTGTGTGGAGAGCGTCAA | R-GGGCCGTACAGTTCCACAAA |
| CYTOCHROME C | F-CCCAAGAAGTACATCCCTGGAAC | R-GGCAGTGGCCAATTATTACTCA |
| CAS 3 | F-TGGTTTGAGCCTGAGCAGAG | R-TGGCAGCATCATCCACACAT |
| CAS 9 | F-TGACCCCAGAATTGACCCTG | R-AAGGATTCGCTCTTGCGTC |
| Peak | Retention Time | Area (%) | Molecular Formula | Compound | Similarity Index |
|---|---|---|---|---|---|
| 1 | 9.784 | 2.91 | C12H26O | 1-Dodecanol | 97 |
| 2 | 12.552 | 4.10 | C18H34O2 | Pentadecyl acrylate | 91 |
| 3 | 15.577 | 11.28 | C14H22N2O2 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo [1, 2-a: 1′, 2′-d] pyrazine | 81 |
| 4 | 16.11 | 2.32 | C15H30O2S | Lauryl 3-mercaptopropionate | 93 |
| 5 | 17.566 | 3.50 | C18H36O2 | Stearic acid | 93 |
| 6 | 17.812 | 2.00 | C16H33NO | Palmitamide | 94 |
| 7 | 20.508 | 2.12 | C18H37NO | Octadecanamide | 93 |
| 8 | 22.223 | 1.04 | C19H38O4 | Glyceryl 2-palmitate | 81 |
| 9 | 25.542 | 1.63 | C21H42O4 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 82 |
| 10 | 26.755 | 5.09 | C28H45ClO2 | Cholest-5-en-3-ol (3 beta)-, carbonochloridate | 81 |
| 11 | 29.316 | 8.63 | C24H40O4 | Ursodeoxycholic acid | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firus Khan, A.Y.; Abdullah Asuhaimi, F.; Jalal, T.K.; Roheem, F.O.; Natto, H.A.; Johan, M.F.; Ahmed, Q.U.; Abdul Wahab, R. Hystrix Brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study. Antioxidants 2019, 8, 39. https://doi.org/10.3390/antiox8020039
Firus Khan AY, Abdullah Asuhaimi F, Jalal TK, Roheem FO, Natto HA, Johan MF, Ahmed QU, Abdul Wahab R. Hystrix Brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study. Antioxidants. 2019; 8(2):39. https://doi.org/10.3390/antiox8020039
Chicago/Turabian StyleFirus Khan, Al’aina Yuhainis, Faizah Abdullah Asuhaimi, Tara K. Jalal, Fatimah Opeyemi Roheem, Hatim Abdullah Natto, Muhammad Farid Johan, Qamar Uddin Ahmed, and Ridhwan Abdul Wahab. 2019. "Hystrix Brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study" Antioxidants 8, no. 2: 39. https://doi.org/10.3390/antiox8020039
APA StyleFirus Khan, A. Y., Abdullah Asuhaimi, F., Jalal, T. K., Roheem, F. O., Natto, H. A., Johan, M. F., Ahmed, Q. U., & Abdul Wahab, R. (2019). Hystrix Brachyura Bezoar Characterization, Antioxidant Activity Screening, and Anticancer Activity on Melanoma Cells (A375): A Preliminary Study. Antioxidants, 8(2), 39. https://doi.org/10.3390/antiox8020039





