Purification and Identification of Antioxidant Alcalase-Derived Peptides from Sheep Plasma Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic Hydrolysis of Sheep Plasma
2.3. Fractionation of Hydrolysates by Ultrafiltration Separation
2.4. Fractionation by Semi-Preparation Reverse-Phase High-Performance Liquid Chromatography
2.5. Fractionation by AKTA Protein Rapid Separation and Purification System
2.6. Determination of Antioxidant Properties
2.7. Peptide Identification by LC-MS/MS
2.8. Statistical Analysis
3. Results and Discussion
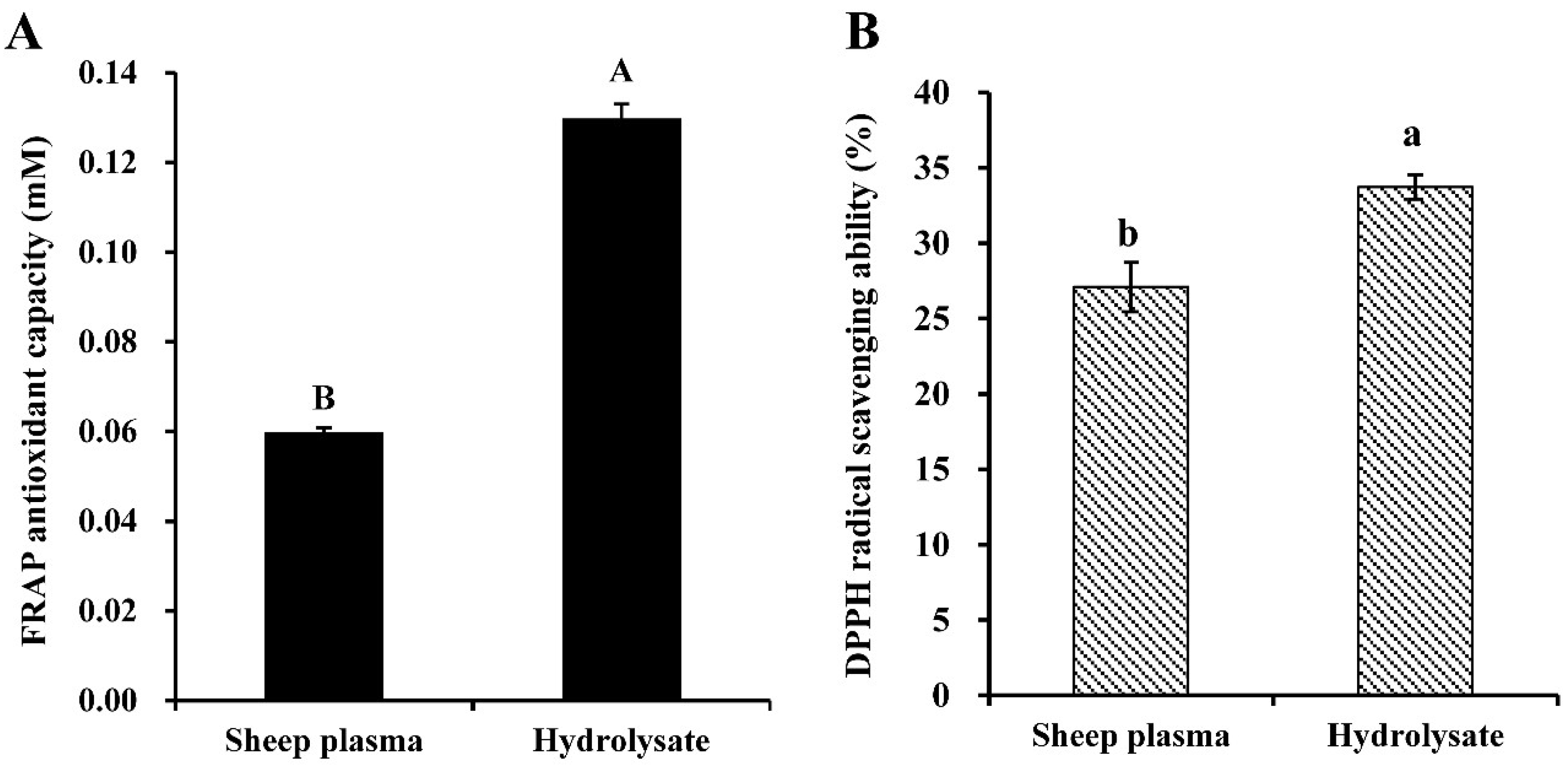
3.1. Antioxidant Properties of Alcalase-Hydrolysate Prepared from Sheep Plasma
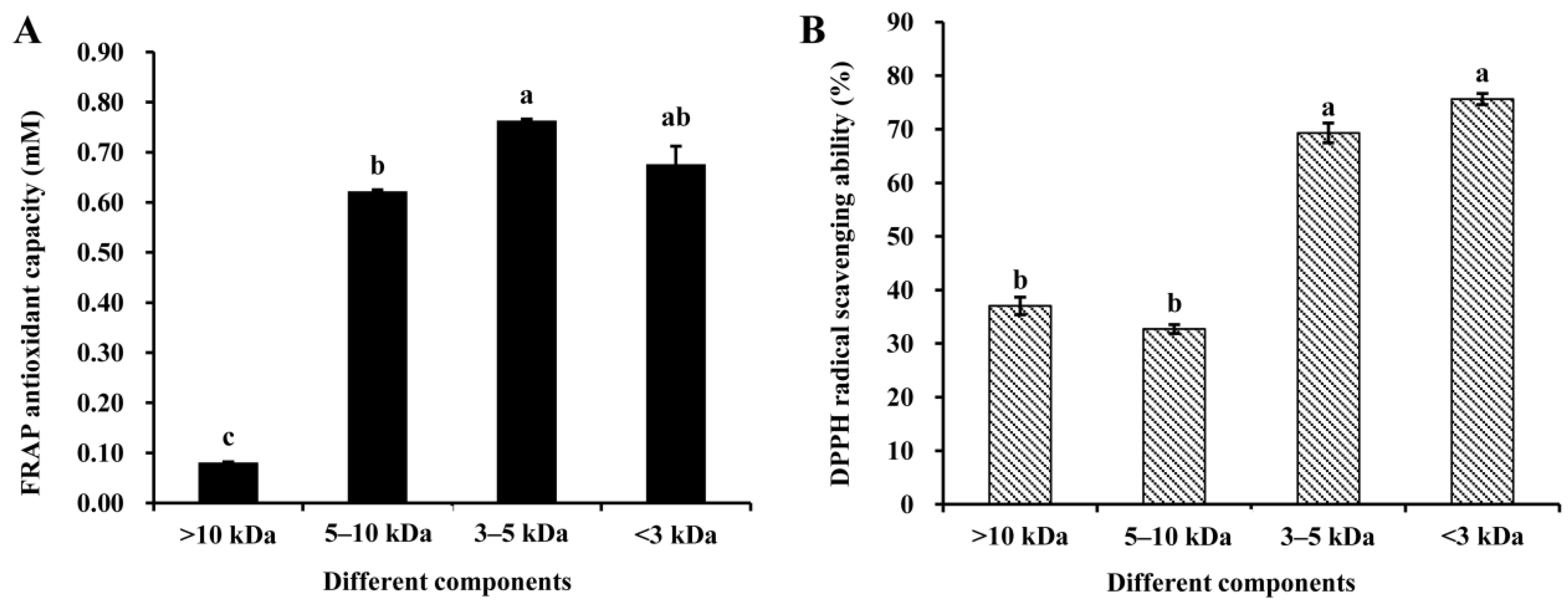
3.2. Antioxidant Properties of Peptide Fractions Obtained by Ultrafiltration
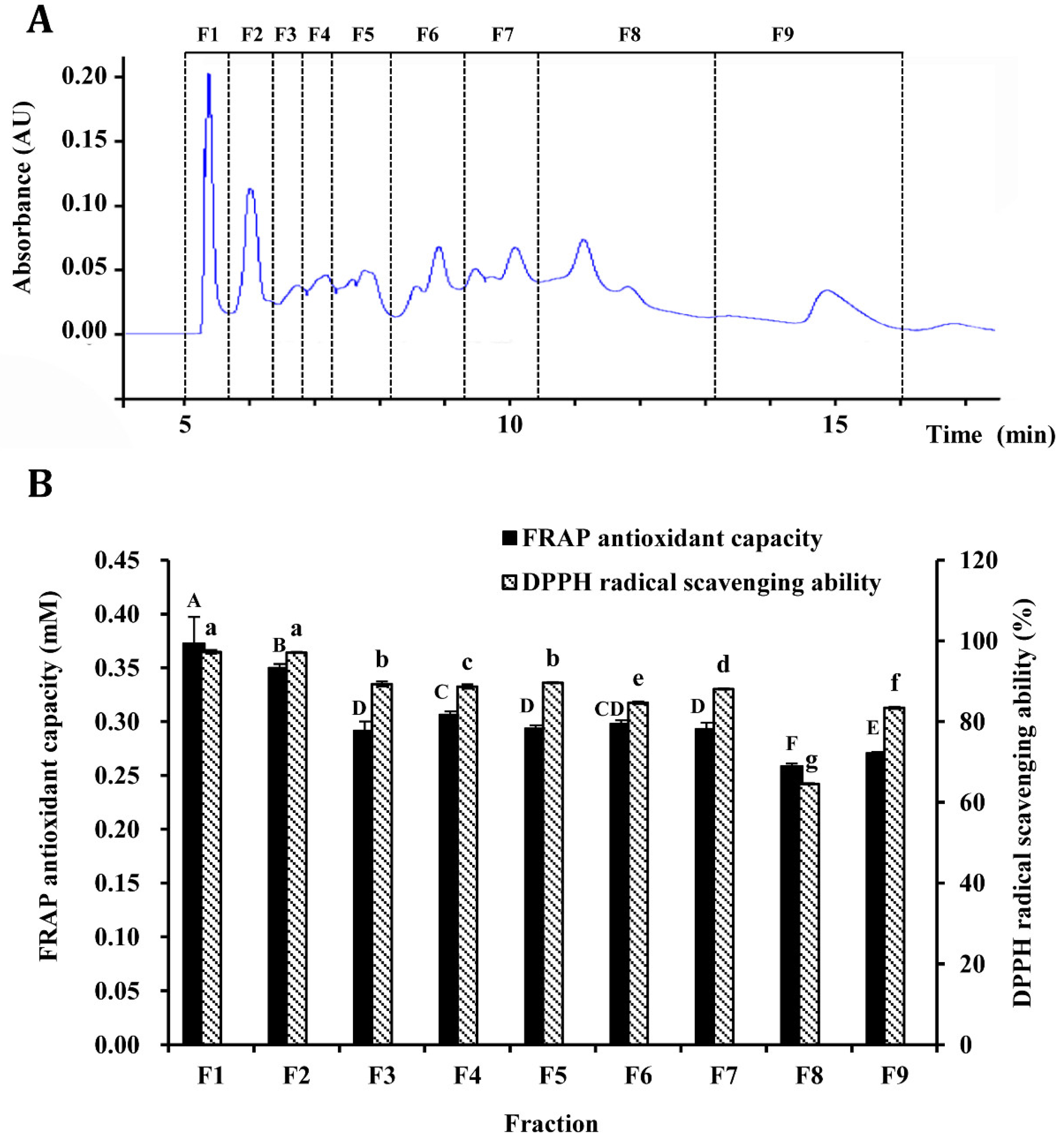
3.3. Purification of Antioxidant Peptides by Semi-Preparation RP-HPLC
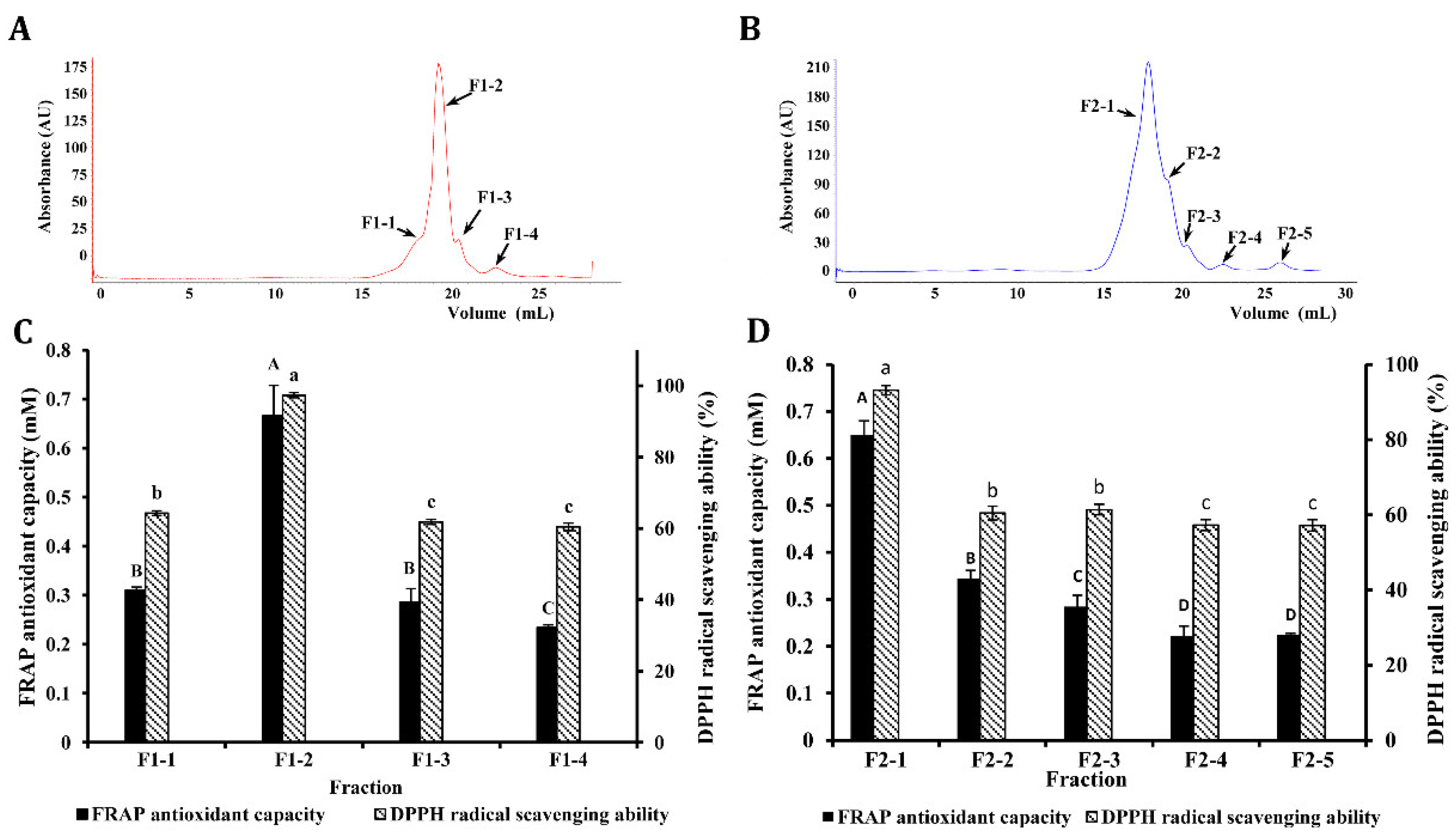
3.4. Purification of Antioxidant Peptides by AKTA Protein Rapid Separation and Purification System
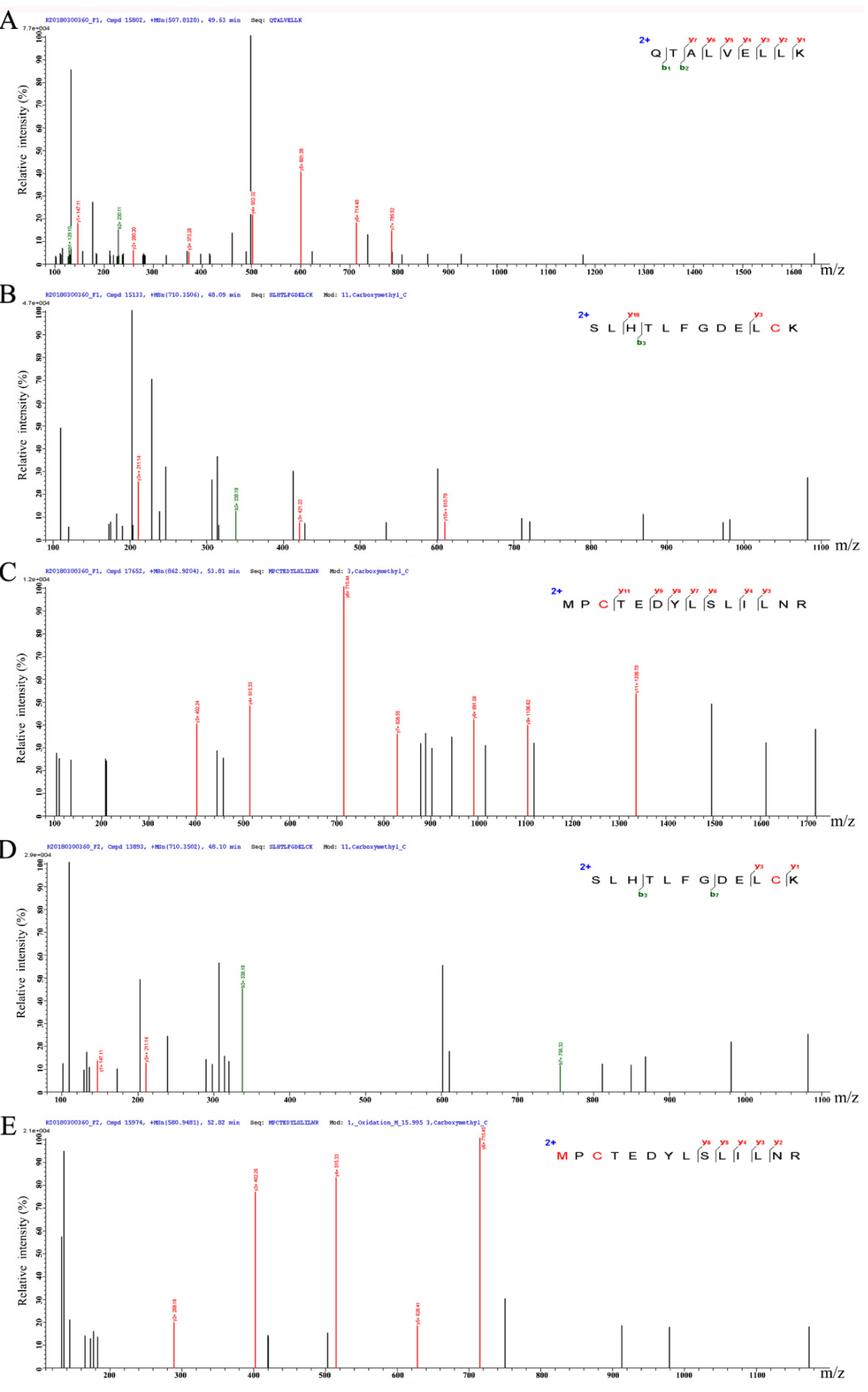
3.5. Peptide Characterization and Identification of Their Sequence
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Zhu, W.; Chen, Z. The determinants of mutton consumption-at-home in urban China using an IHS double-hurdle model. Br. Food J. 2018, 120, 952–968. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Available online: http://data.stats.gov.cn/english/ (accessed on 22 July 2019).
- Bah, C.S.; Bekhit, A.E.-D.A.; Carne, A.; McConnell, M.A. Composition and biological activities of slaughterhouse blood from red deer, sheep, pig and cattle. J. Sci. Food Agric. 2016, 96, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sorapukdee, S.; Narunatsopanon, S. Comparative study on compositions and functional properties of porcine, chicken and duck blood. Korean J. Food Sci. Anim. Resour. 2017, 37, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Assessment of physico-chemical, antioxidant and antimicrobial activity of porcine blood protein hydrolysate in pork emulsion stored under aerobic packaging condition at 4 ± 1 °C. Lwt Food Sci. Technol. 2018, 88, 71–79. [Google Scholar] [CrossRef]
- Del Hoyo, P.; Rendueles, M.; Díaz, M. Effect of processing on functional properties of animal blood plasma. Meat Sci. 2008, 78, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Ofori, J.A.; Hsieh, Y.H.P. The Use of Blood and Derived Products as Food Additives; El-Samragy, Y., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Malomo, S.A.; Alashi, A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Purification and hypotensive activity of rapeseed protein-derived renin and angiotensin converting enzyme inhibitory peptides. J. Funct. Foods 2013, 5, 781–789. [Google Scholar] [CrossRef]
- Thammarat, K.; Leena, N.; Punnanee, S.; Soottawat, B. Functional and antioxidative properties of Bambara groundnut (Voandzeia subterranea) protein hydrolysates. Int. Food Res. J. 2015, 256, 105–111. [Google Scholar]
- Wu, L.; Hou, C.; Zhao, M.; Zhang, D. Preparation and process optimization of sheep plasma protein antioxidant peptide. Food Sci. Technol. 2018, 43, 144–150. [Google Scholar]
- Guimarães Drummond e Silva, F.; Miralles, B.; Hernández-Ledesma, B.; Amigo, L.; Iglesias, A.H.; Reyes Reyes, F.G.; Netto, F.M. Influence of protein–phenolic complex on the antioxidant capacity of flaxseed (Linum usitatissimum L.) products. J. Agric. Food Chem. 2017, 65, 800–809. [Google Scholar]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009, 113, 196–201. [Google Scholar] [CrossRef]
- Silva, F.G.D.e.; Hernández-Ledesma, B.; Amigo, L.; Netto, F.M.; Miralles, B. Identification of peptides released from flaxseed (Linum usitatissimum) protein by Alcalase® hydrolysis: Antioxidant activity. Lwt Food Sci. Technol. 2017, 76, 140–146. [Google Scholar] [CrossRef]
- Delange, R.J.; Smith, E.L. Subtilisin Carlsberg. I. Amino acid composition; isolation and composition of peptides from the tryptic hydrolysate. J. Biol. Chem. 1968, 243, 2134–2142. [Google Scholar] [PubMed]
- Wu, L.; Hou, C.; Xi, B.; Aude Ingrid Boga, L.; Zhang, D. Sheep plasma hydrolysate inhibits lipid and protein oxidation to improve color stability in mutton patties. Food Sci. Technol. Res. 2018, 24, 661–668. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.; Li, L. Relationship between primary structure or spatial conformation and functional activity of antioxidant peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Baron, C.P.; Nielsen, N.S.; Otte, J.; Jacobsen, C. Antioxidant activity of yoghurt peptides: Part 2—Characterisation of peptide fractions. Food Chem. 2010, 123, 1090–1097. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014, 156, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Li, L.H.; Duan, Z.H.; Yang, X.Q.; Shang, J.; Chen, S.J. Application of response surface methodology to optimise preparation high antioxidant activity product from pinctada fucata muscle. Adv. Mater. Res. 2012, 396-398, 1341–1348. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar]
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int. J. Food Prop. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Le Maux, S.; Nongonierma, A.B.; Murray, B.; Kelly, P.M.; FitzGerald, R.J. Identification of short peptide sequences in the nanofiltration permeate of a bioactive whey protein hydrolysate. Food Res. Int. 2015, 77, 534–539. [Google Scholar] [CrossRef]
- Dontha, S. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar]
- Zheng, L.; Lin, L.; Su, G.; Zhao, Q.; Zhao, M. Pitfalls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its susceptibility to interference and low reactivity towards peptides. Food Res. Int. 2015, 76, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.; Gilman, J.T.; Sussmane, J.; Raszynski, A.; Wolfsdorf, J. Changes in plasma levels of oxygen radical scavenging enzymes during extracorporeal membrane oxygenation in a lamb model. Biol. Neonate 1993, 64, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid antioxidants: How they may act in biological systems. Br. J. Cancer Suppl. 1987, 8, 153–157. [Google Scholar]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; Carne, A.; McConnell, M.A. Production of bioactive peptide hydrolysates from deer, sheep and pig plasma using plant and fungal protease preparations. Food Chem. 2015, 176, 54–63. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Wei, J.T.; Beenhuang, C. Bioactive peptide production by hydrolysis of porcine blood proteins in a continuous enzymatic membrane reactor. J. Sci. Food Agric. 2009, 89, 372–378. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; McConnell, M.A.; Carne, A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016, 213, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Hu, F.; Ci, A.T.; Wang, H.; Zhang, Y.Y.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Jung, W.K.; Nam, K.S.; Shahidi, F.; Kim, S.K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001, 78, 651–656. [Google Scholar] [CrossRef]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ 2018, 6, e5337. [Google Scholar] [CrossRef]
- Yu, P.; Linden, D.S.v.d.; Sugiarto, H.; Anderson, R.C. Antimicrobial peptides isolated from the blood of farm animals. Anim. Prod. Sci. 2010, 50, 660–669. [Google Scholar] [CrossRef]
- Chai, H.J.; Chan, Y.L.; Li, T.L.; Shiau, C.Y.; Wu, C.J. Evaluation of lanternfish (Benthosema pterotum) hydrolysates as antioxidants against hydrogen peroxide induced oxidative injury. Food Res. Int. 2013, 54, 1409–1418. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Yongsawatdigul, J. Production and purification of antioxidant peptides from a mungbean meal hydrolysate by Virgibacillus sp. SK37 proteinase. Food Chem. 2013, 141, 992–999. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, X.S.; Yang, X.Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Albumin-an important extracellular antioxidant? Biochem. Pharmacol. 1988, 37, 569–571. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. Febs Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
| Fraction | Sequence | MW (Da) | pI | Special Amino Acids Ratio (%) | ||
|---|---|---|---|---|---|---|
| Hydrophobic Amino Acids | Acid Amino Acids | Basic Amino Acid | ||||
| F1-2 | QTALVELLK | 1014.23 | 6.00 | 55.56 | 11.11 | 11.11 |
| SLHTLFGDELCK | 1362.56 | 5.30 | 33.33 | 16.67 | 16.67 | |
| MPCTEDYLSLILNR | 1667.96 | 4.37 | 50.00 | 14.29 | 7.14 | |
| F2-1 | SLHTLFGDELCK | 1362.56 | 5.30 | 33.33 | 16.67 | 16.67 |
| MPCTEDYLSLILNR | 1667.96 | 4.37 | 50.00 | 14.29 | 7.14 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Wu, L.; Wang, Z.; Saguer, E.; Zhang, D. Purification and Identification of Antioxidant Alcalase-Derived Peptides from Sheep Plasma Proteins. Antioxidants 2019, 8, 592. https://doi.org/10.3390/antiox8120592
Hou C, Wu L, Wang Z, Saguer E, Zhang D. Purification and Identification of Antioxidant Alcalase-Derived Peptides from Sheep Plasma Proteins. Antioxidants. 2019; 8(12):592. https://doi.org/10.3390/antiox8120592
Chicago/Turabian StyleHou, Chengli, Liguo Wu, Zhenyu Wang, Elena Saguer, and Dequan Zhang. 2019. "Purification and Identification of Antioxidant Alcalase-Derived Peptides from Sheep Plasma Proteins" Antioxidants 8, no. 12: 592. https://doi.org/10.3390/antiox8120592
APA StyleHou, C., Wu, L., Wang, Z., Saguer, E., & Zhang, D. (2019). Purification and Identification of Antioxidant Alcalase-Derived Peptides from Sheep Plasma Proteins. Antioxidants, 8(12), 592. https://doi.org/10.3390/antiox8120592






