Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatments
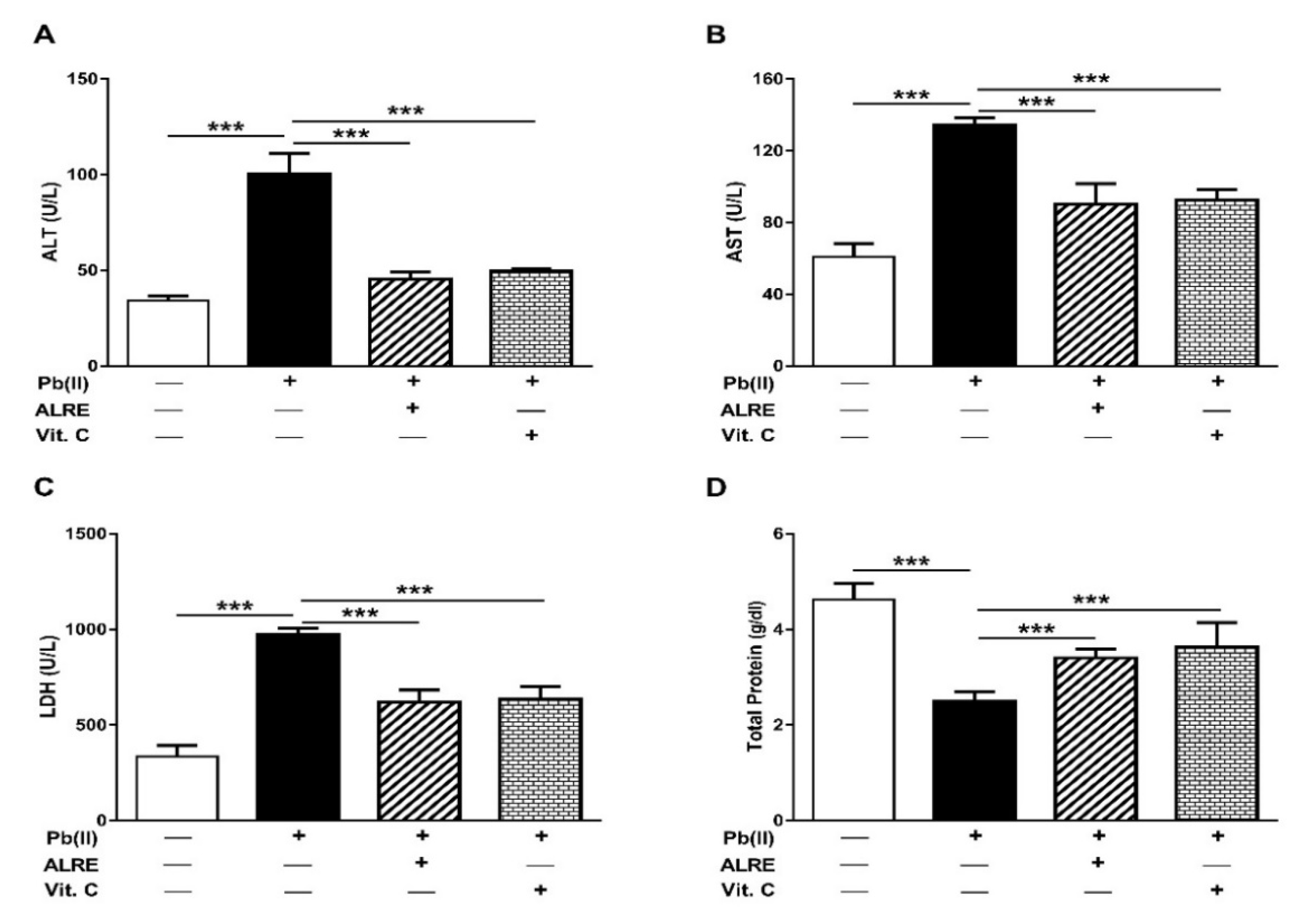
2.2. Assay of Liver Function
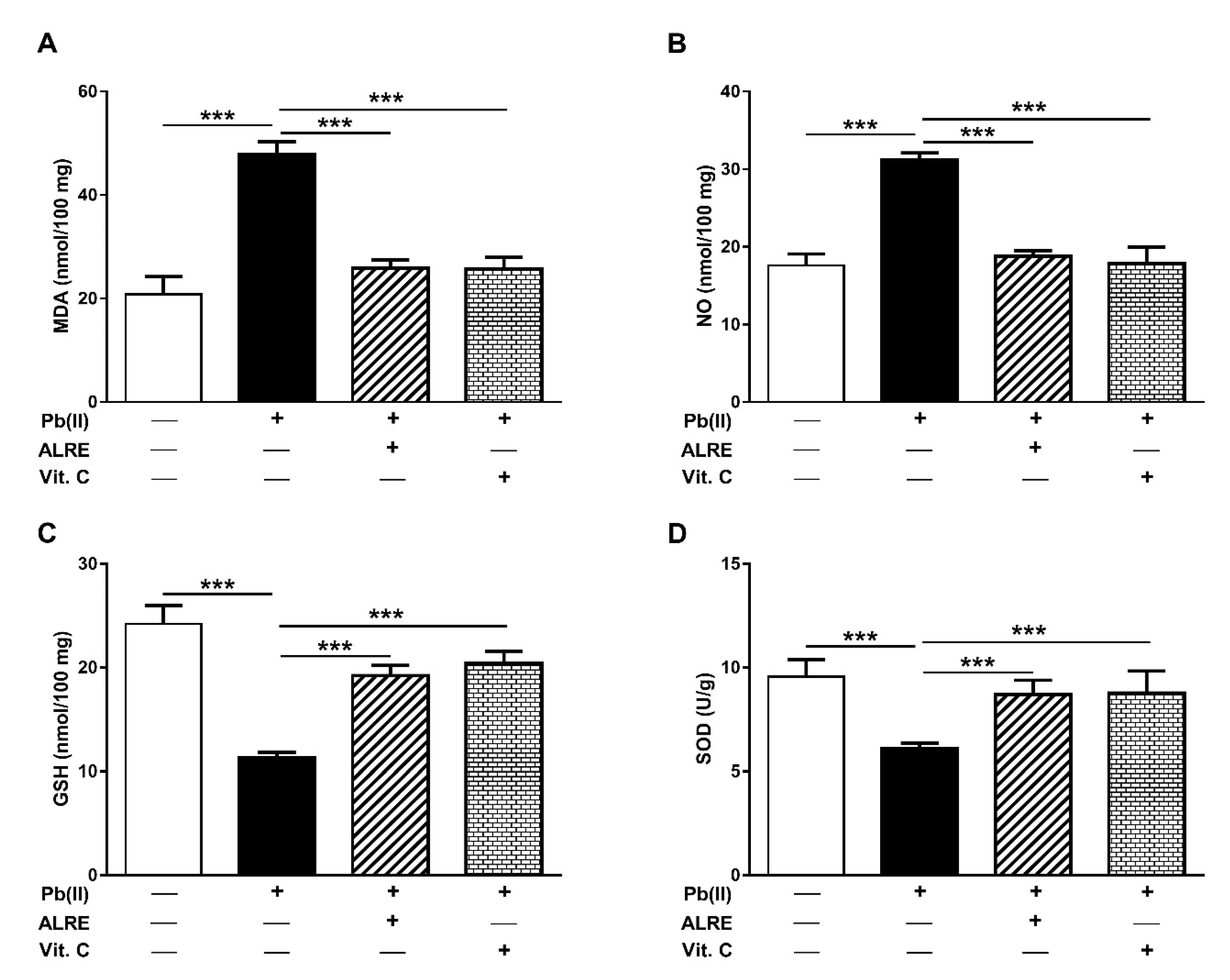
2.3. Assay of Oxidative Stress Markers and Antioxidants
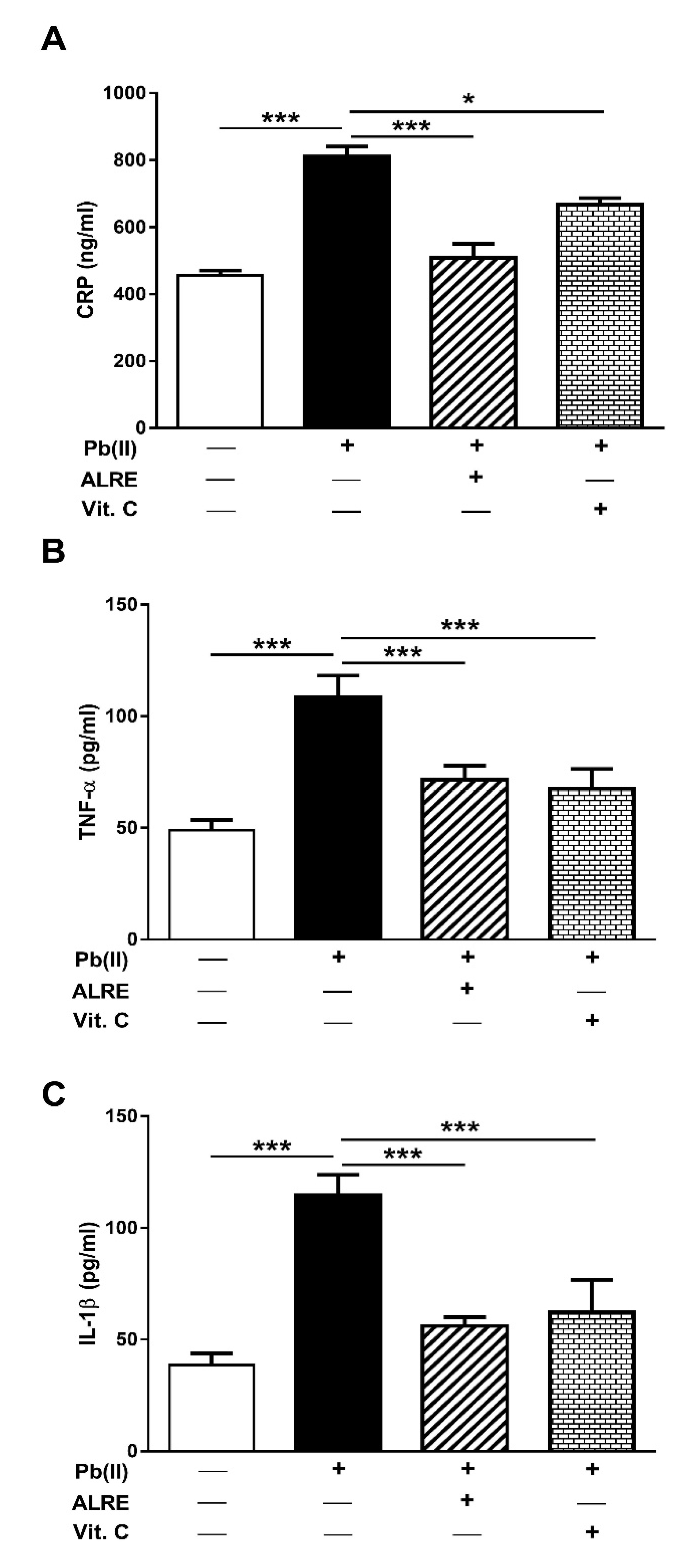
2.4. Assay of C-Reactive Protein (CRP), Pro-Inflammatory Cytokines, and Caspase-3
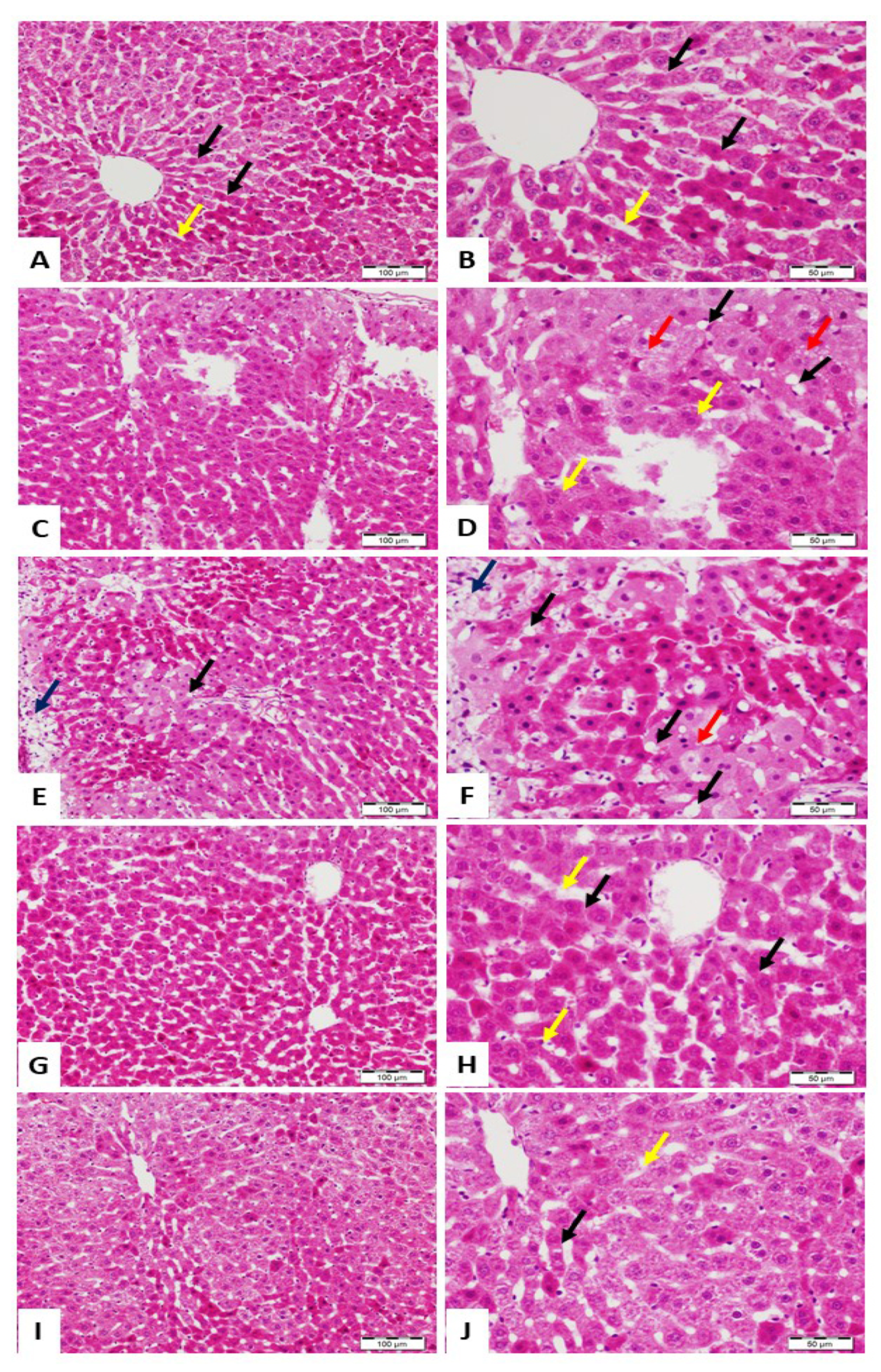
2.5. Histological Examination
2.6. Western Blot
2.7. DNA Fragmentation Assay
2.8. Statistical Analysis
3. Results
3.1. ALRE Attenuates Pb(II)-Induced Liver Injury
3.2. ALRE Prevents Pb(II)-Induced Oxidative Stress in Liver of Rats
3.3. ALRE Attenuates Inflammation in Pb(II)-Induced Rats
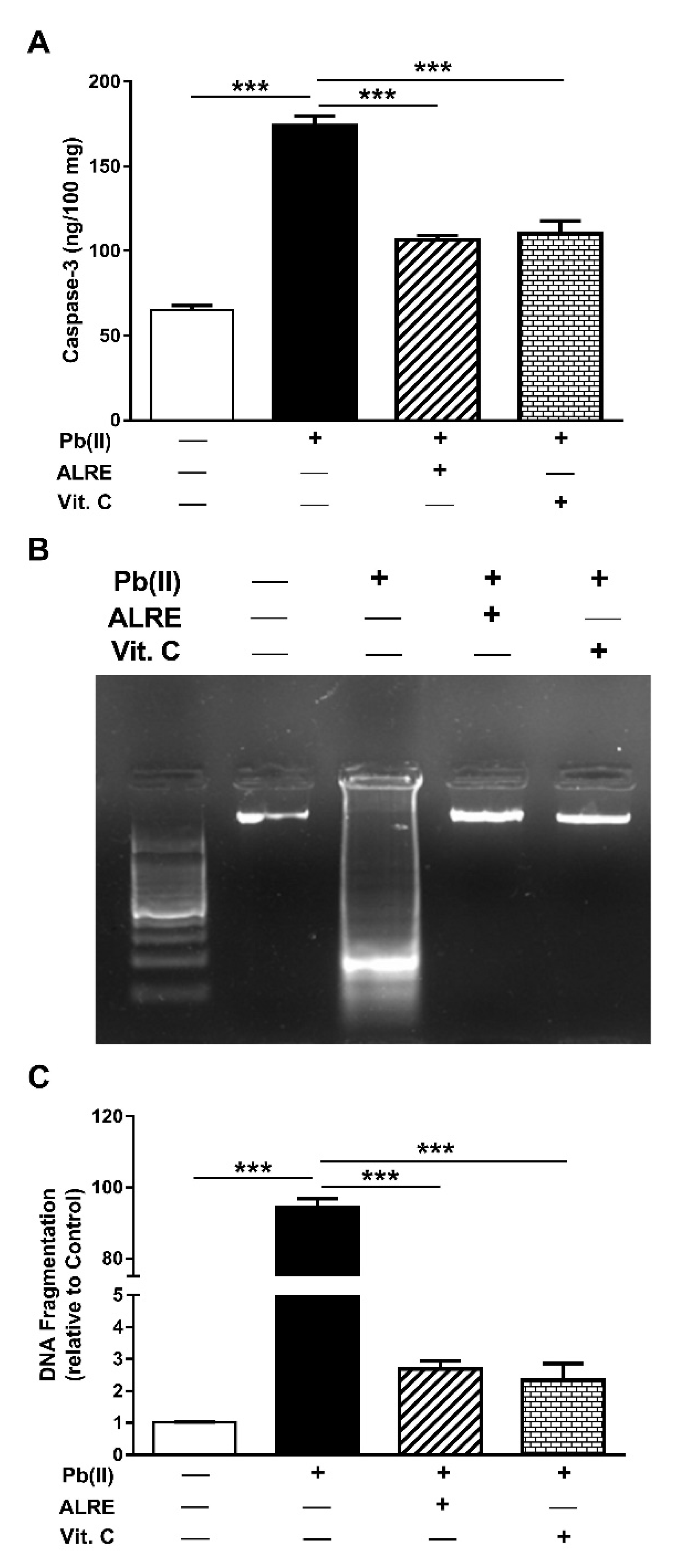
3.4. ALRE Suppresses Caspase-3 and DNA Fragmentation in Liver of Pb(II)-Induced Rats
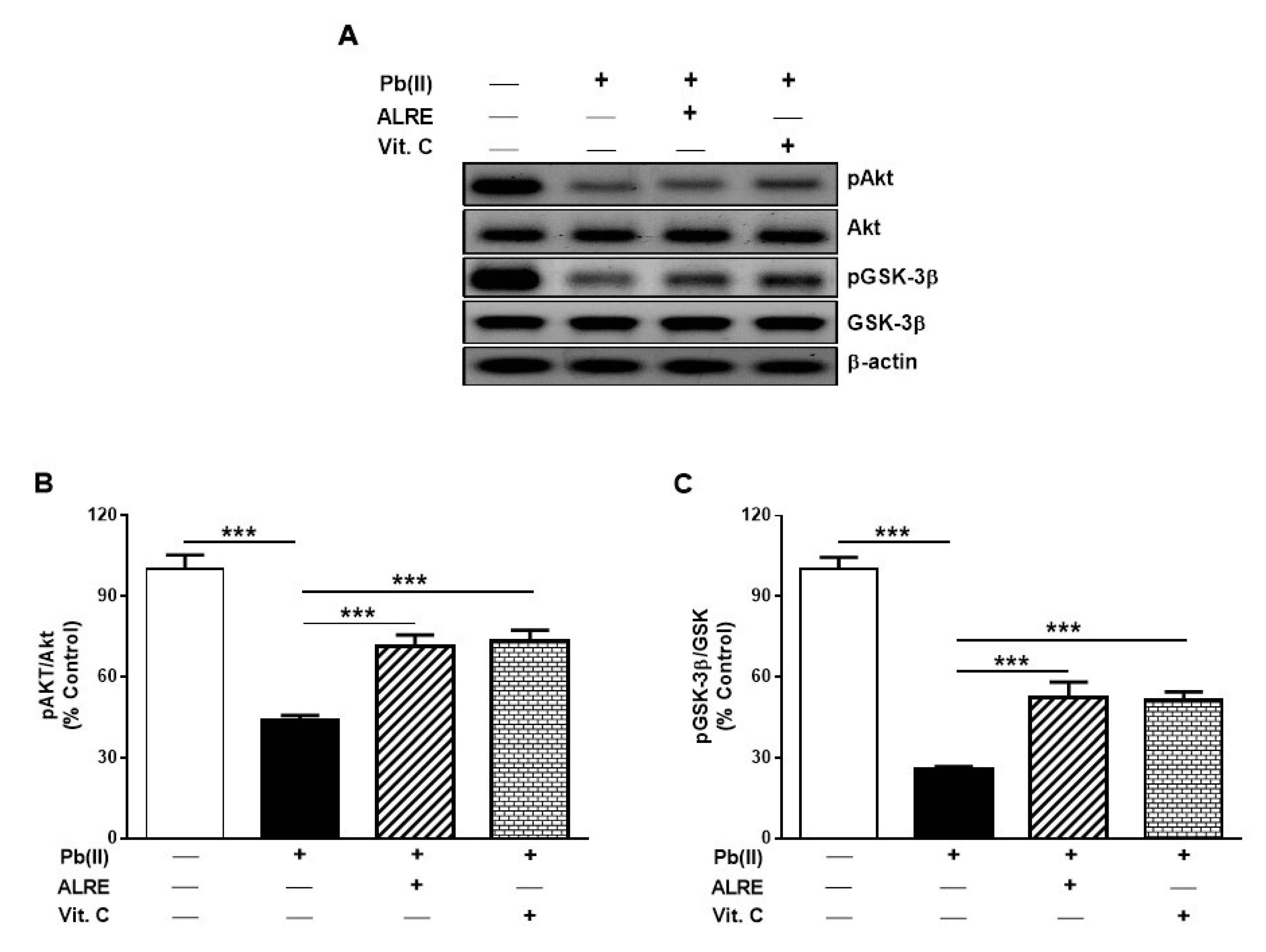
3.5. ALRE Activates Akt/GSK-3β Signaling in Liver of Pb(II)-Induced Rats
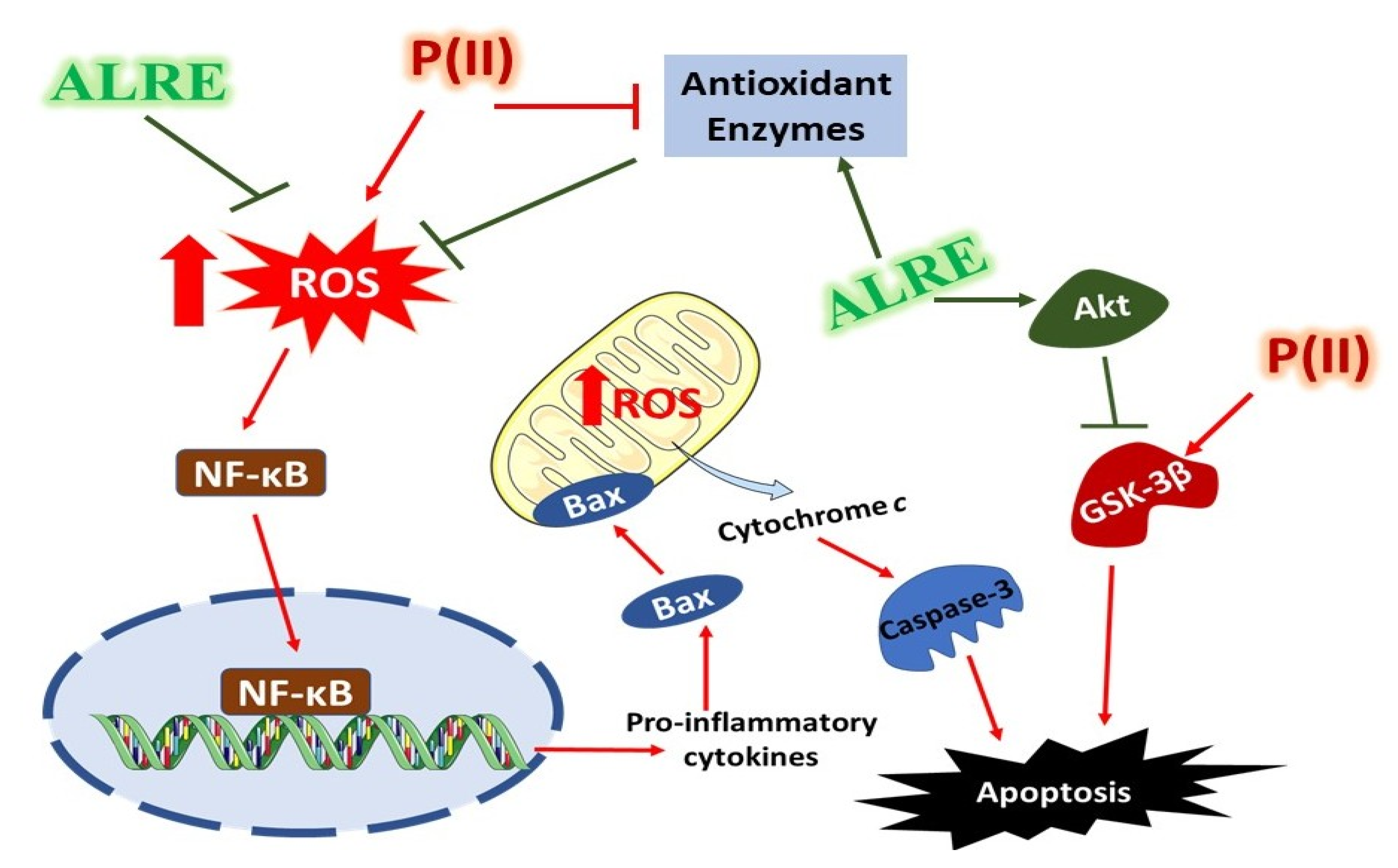
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. [Google Scholar] [PubMed]
- World Health Organization. Lead Poisoning and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 22 November 2019).
- The New Top Six Toxic Threats: A Priority List for Remediation, World’s Worst Pollution Problems Report; Pure Earth: New York, NY, USA, 2015.
- Alissa, E.M.; Ferns, G.A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar] [CrossRef]
- Khan, D.A.; Qayyum, S.; Saleem, S.; Khan, F.A. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol. Ind. Health 2008, 24, 611–618. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H.; Yoo, D.H.; Kang, D.; Cho, S.H.; Hong, Y.C. Gstm1 and tnf-alpha gene polymorphisms and relations between blood lead and inflammatory markers in a non-occupational population. Mutat. Res. 2007, 629, 32–39. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Flora, G.; Saxena, G. Environmental occurrence, health effects and management of lead poisoning. In Lead Chemistry, Analytical Aspects, Environmental Impacts and Health Effects; Cascas, S.B., Sordo, J., Eds.; Elsevier Publication: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Adegbesan, B.O.; Adenuga, G.A. Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol. Trace Elem. Res. 2007, 116, 219–225. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; El-Kady, A.A.; Soliman, M.S.; Hassan, N.S.; Abdel-Wahhab, M.A. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 2209–2215. [Google Scholar] [CrossRef]
- Jia, Q.; Ha, X.; Yang, Z.; Hui, L.; Yang, X. Oxidative stress: A possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol. Mech. Methods 2012, 22, 705–710. [Google Scholar] [CrossRef]
- El-Tantawy, W.H. Antioxidant effects of spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complement. Med. 2016, 6, 327–331. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.A.C.; Gardinassi, L.G.; Silveira, I.M.G.; Gallucci, M.G.; Tomé, M.A.; Oliveira, J.F.D.; Moreira, M.R.A.; Meirelles, A.F.G.; Faccioli, L.H.; Tefé-Silva, C.; et al. Arctium lappa extract suppresses inflammation and inhibits melanoma progression. Medicines 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Kan, J.; Wu, X.; Zhang, X.; Tang, S.; Sun, R.; Liu, J.; Qian, C.; Jin, C. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from arctium lappa. Int. J. Biol. Macromol. 2019, 135, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Z.; Kuang, P.; Shi, X.; Wang, Z.; Guo, L. Regulation of lipid metabolism in diabetic rats by arctium lappa l. Polysaccharide through the pkc/nf-κb pathway. Int. J. Biol. Macromol. 2019, 136, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hyam, S.R.; Lee, I.A.; Gu, W.; Kim, K.A.; Jeong, J.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the pi3k/akt pathway and polarizing m1 macrophages to m2-like macrophages. Eur. J. Pharmacol. 2013, 708, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.J.; Chang, C.T.; Wang, G.J.; Lee, T.H.; Chang, S.F.; Lu, S.C.; Kuo, Y.C. Arctigenin from arctium lappa inhibits interleukin-2 and interferon gene expression in primary human t lymphocytes. Chin. Med. 2011, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, N.; Huang, B.; Wang, Y.; Hu, Y.; Zhu, Y. Effect of anti-influenza virus of arctigenin In Vivo. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2005, 28, 1012–1014. [Google Scholar]
- Jang, Y.P.; Kim, S.R.; Choi, Y.H.; Kim, J.; Kim, S.G.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J. Neurosci. Res. 2002, 68, 233–240. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chung, T.-C.; Lin, C.-C.; Ueng, T.-H.; Lin, Y.-H.; Lin, S.-Y.; Wang, L.-Y. Hepatoprotective effects of arctium lappa on carbon tetrachloride- and acetaminophen-induced liver damage. Am. J. Chin. Med. 2000, 28, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Woodgett, J.R. Gsk-3: Functional insights from cell biology and animal models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Jope, R.S. The paradoxical pro- and anti-apoptotic actions of gsk3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006, 79, 173–189. [Google Scholar] [CrossRef] [PubMed]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of gsk3 plays in insulin and wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Martin, M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011, 53, 130–140. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, L.; Zhang, X.; Shi, H.; Wen, T.; Bai, L.; Zheng, S.; Chen, Y.; Chen, D.; Li, L.; et al. Inhibition of glycogen synthase kinase 3β promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor α. Cell Death Dis. 2016, 7, e2151. [Google Scholar] [CrossRef]
- Bhushan, B.; Poudel, S.; Manley, M.W., Jr.; Roy, N.; Apte, U. Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am. J. Pathol. 2017, 187, 543–552. [Google Scholar] [CrossRef]
- Soliman, M.M.; Baiomy, A.A.; Yassin, M.H. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in wistar rats. Biol. Trace Elem. Res. 2015, 167, 91–102. [Google Scholar] [CrossRef]
- Suliman Al-Gebaly, A. Ameliorative effect of arctium lappa against cadmium genotoxicity and histopathology in kidney of wistar rat. Pak. J. Biol. Sci. PJBS 2017, 20, 314–319. [Google Scholar] [CrossRef][Green Version]
- Farshid, A.A.; Tamaddonfard, E.; Ranjbar, S. Oral administration of vitamin c and histidine attenuate cyclophosphamide-induced hemorrhagic cystitis in rats. Indian J. Pharmacol. 2013, 45, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Jarrell, S.T.; Scheckenbach, R.; Lieberman, S.; Anderson, R.A. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in shr. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hickey, E.J.; Raje, R.R.; Reid, V.E.; Gross, S.M.; Ray, S.D. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free Radic. Biol. Med. 2001, 31, 139–152. [Google Scholar] [CrossRef]
- Fu, H.; Xu, H.; Chen, H.; Li, Y.; Li, W.; Zhu, Q.; Zhang, Q.; Yuan, H.; Liu, F.; Wang, Q.; et al. Inhibition of glycogen synthase kinase 3 ameliorates liver ischemia/reperfusion injury via an energy-dependent mitochondrial mechanism. J. Hepatol. 2014, 61, 816–824. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating akt/gsk-3beta signaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med. Res. 2007, 126, 518–527. [Google Scholar]
- Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Abu Bakar Zakaria, M.Z. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.E.; Meki, A.R.M.A.; Fazili, K.M. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp. Toxicol. Pathol. 2012, 64, 291–295. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.F.; Bin-Meferij, M.M. Use of arctium lappa extract against acetaminophen-induced hepatotoxicity in rats. Curr. Ther. Res. Clin. Exp. 2015, 77, 73–78. [Google Scholar] [CrossRef] [PubMed]
- de Souza Predes, F.; da Silva Diamante, M.A.; Foglio, M.A.; Camargo Cde, A.; Aoyama, H.; Miranda, S.C.; Cruz, B.; Gomes Marcondes, M.C.; Dolder, H. Hepatoprotective effect of arctium lappa root extract on cadmium toxicity in adult wistar rats. Biol. Trace Elem. Res. 2014, 160, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Sirivarasai, J.; Wananukul, W.; Kaojarern, S.; Chanprasertyothin, S.; Thongmung, N.; Ratanachaiwong, W.; Sura, T.; Sritara, P. Association between inflammatory marker, environmental lead exposure, and glutathione s-transferase gene. BioMed Res. Int. 2013, 2013, 474963. [Google Scholar] [CrossRef]
- Guo, T.L.; Mudzinski, S.P.; Lawrence, D.A. The heavy metal lead modulates the expression of both tnf-alpha and tnf-alpha receptors in lipopolysaccharide-activated human peripheral blood mononuclear cells. J. Leukoc. Biol. 1996, 59, 932–939. [Google Scholar] [CrossRef]
- Lin, S.C.; Lin, C.H.; Lin, C.C.; Lin, Y.H.; Chen, C.F.; Chen, I.C.; Wang, L.Y. Hepatoprotective effects of arctium lappa linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J. Biomed. Sci. 2002, 9, 401–409. [Google Scholar] [CrossRef]
- Predes, F.S.; Ruiz, A.L.; Carvalho, J.E.; Foglio, M.A.; Dolder, H. Antioxidative and in vitro antiproliferative activity of arctium lappa root extracts. BMC Complement. Altern. Med. 2011, 11, 25. [Google Scholar] [CrossRef]
- Lin, C.C.; Lu, J.M.; Yang, J.J.; Chuang, S.C.; Ujiie, T. Anti-inflammatory and radical scavenge effects of arctium lappa. Am. J. Chin. Med. 1996, 24, 127–137. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Alipoor, B.; Abed, R.; Eftekhar Sadat, B.; Mesgari-Abbasi, M.; Asghari Jafarabadi, M. Effects of arctium lappa l. (burdock) root tea on inflammatory status and oxidative stress in patients with knee osteoarthritis. Int. J. Rheum. Dis. 2016, 19, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Y.; Mi, S.; Fan, Q.; Sun, X.; Deng, B.; Wu, G.; Li, Y.; Zhou, Q.; Ruan, Z. Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct. Foods 2019, 62, 103540. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hong, C.O.; Lee, G.P.; Kim, C.T.; Lee, K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of perilla frutescens, against t-bhp-induced oxidative liver damage. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Maleki Dizaj, S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin b1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Hong, S.S.; Kwon, S.W.; Lee, H.Y.; Sung, H.; Lee, I.J.; Hwang, B.Y.; Song, S.; Lee, C.K.; Chung, D.; et al. Diarctigenin, a lignan constituent from arctium lappa, down-regulated zymosan-induced transcription of inflammatory genes through suppression of DNA binding ability of nuclear factor-kappab in macrophages. J. Pharmacol. Exp. Ther. 2008, 327, 393–401. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting cox-2 expression via nf-kappab pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Koppula, S.; Shim, D.-W.; In, E.-J.; Kwak, S.-B.; Kim, M.-K.; Yu, S.-H.; Lee, K.-H.; Kang, T.-B. Inhibitory effect and mechanism of arctium lappa extract on nlrp3 inflammasome activation. Evid.-Based Complement. Altern. Med. 2018, 2018, 6346734. [Google Scholar] [CrossRef]
- Maurer, U.; Preiss, F.; Brauns-Schubert, P.; Schlicher, L.; Charvet, C. Gsk-3—At the crossroads of cell death and survival. J. Cell Sci. 2014, 127, 1369–1378. [Google Scholar] [CrossRef]
- Pap, M.; Cooper, G.M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/akt cell survival pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef]
- Linseman, D.A.; Butts, B.D.; Precht, T.A.; Phelps, R.A.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Florez-McClure, M.L.; Heidenreich, K.A. Glycogen synthase kinase-3beta phosphorylates bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 2004, 24, 9993–10002. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of nrf2 and pparγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Singh, R.; Lefkowitch, J.H.; Rigoli, R.M.; Czaja, M.J. Tumor necrosis factor-induced toxic liver injury results from jnk2-dependent activation of caspase-8 and the mitochondrial death pathway. J. Biol. Chem. 2006, 281, 15258–15267. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al Dera, H.S. 18β-glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of pparγ and nrf2 upregulation. Genes Nutr. 2015, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Mahmoud, A.M. Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of pparγ and attenuation of oxidative stress, inflammation, and apoptosis. Oxid. Med. Cell. Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhusaini, A.; Fadda, L.; Hasan, I.H.; Ali, H.M.; El Orabi, N.F.; Badr, A.M.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling. Antioxidants 2019, 8, 582. https://doi.org/10.3390/antiox8120582
Alhusaini A, Fadda L, Hasan IH, Ali HM, El Orabi NF, Badr AM, Zakaria E, Alenazi AM, Mahmoud AM. Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling. Antioxidants. 2019; 8(12):582. https://doi.org/10.3390/antiox8120582
Chicago/Turabian StyleAlhusaini, Ahlam, Laila Fadda, Iman H. Hasan, Hanaa M. Ali, Naglaa F. El Orabi, Amira M. Badr, Enas Zakaria, Abeer M. Alenazi, and Ayman M. Mahmoud. 2019. "Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling" Antioxidants 8, no. 12: 582. https://doi.org/10.3390/antiox8120582
APA StyleAlhusaini, A., Fadda, L., Hasan, I. H., Ali, H. M., El Orabi, N. F., Badr, A. M., Zakaria, E., Alenazi, A. M., & Mahmoud, A. M. (2019). Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling. Antioxidants, 8(12), 582. https://doi.org/10.3390/antiox8120582





