Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plant Materials
2.3. Drugs, Chemicals, and Reagent Kits
2.4. Overview of Methodology
2.5. Preliminary Phytochemical Screening of Extracts
2.6. Evaluation of the In vitro Anti-Oxidant Activity (DPPH Assay)
2.7. Experimental Design
2.8. Induction of Gastric Ulcer and Sample Preparation
2.9. Macroscopic Examination (Assessment of Gross Mucosal Damage)
2.10. Microscopic Histopathological Examination (Routine H and E Staining and Alcian Blue Staining)
2.10.1. Routine H and E Staining (Histopathological Examination of Stomach)
2.10.2. Alcian Blue Staining for Determination of Gastric Wall Mucus (Mucin Content)
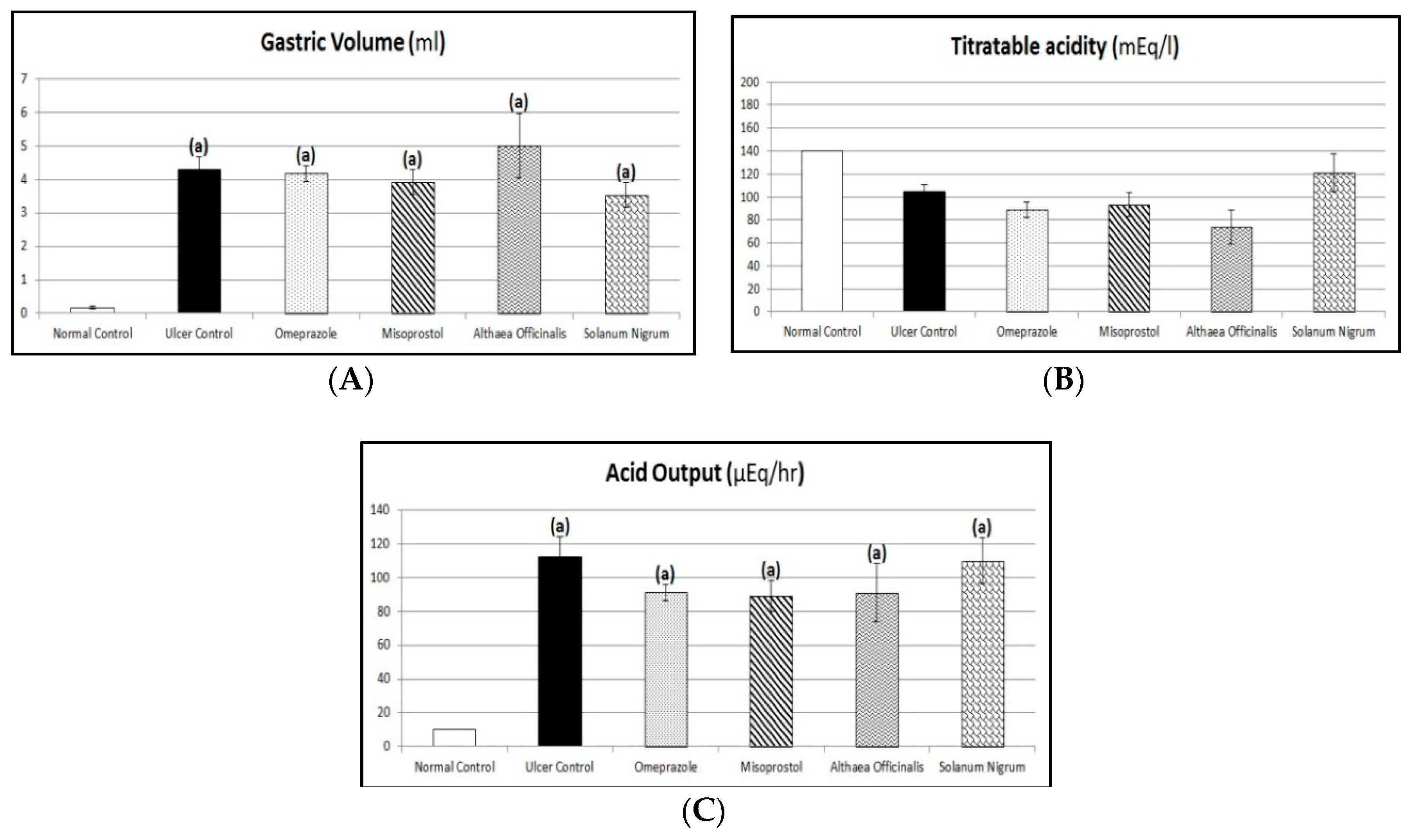
2.11. Acid Productivity Parameters (Gastric Volume, Titratable Acidity, and Acid Output Determination)
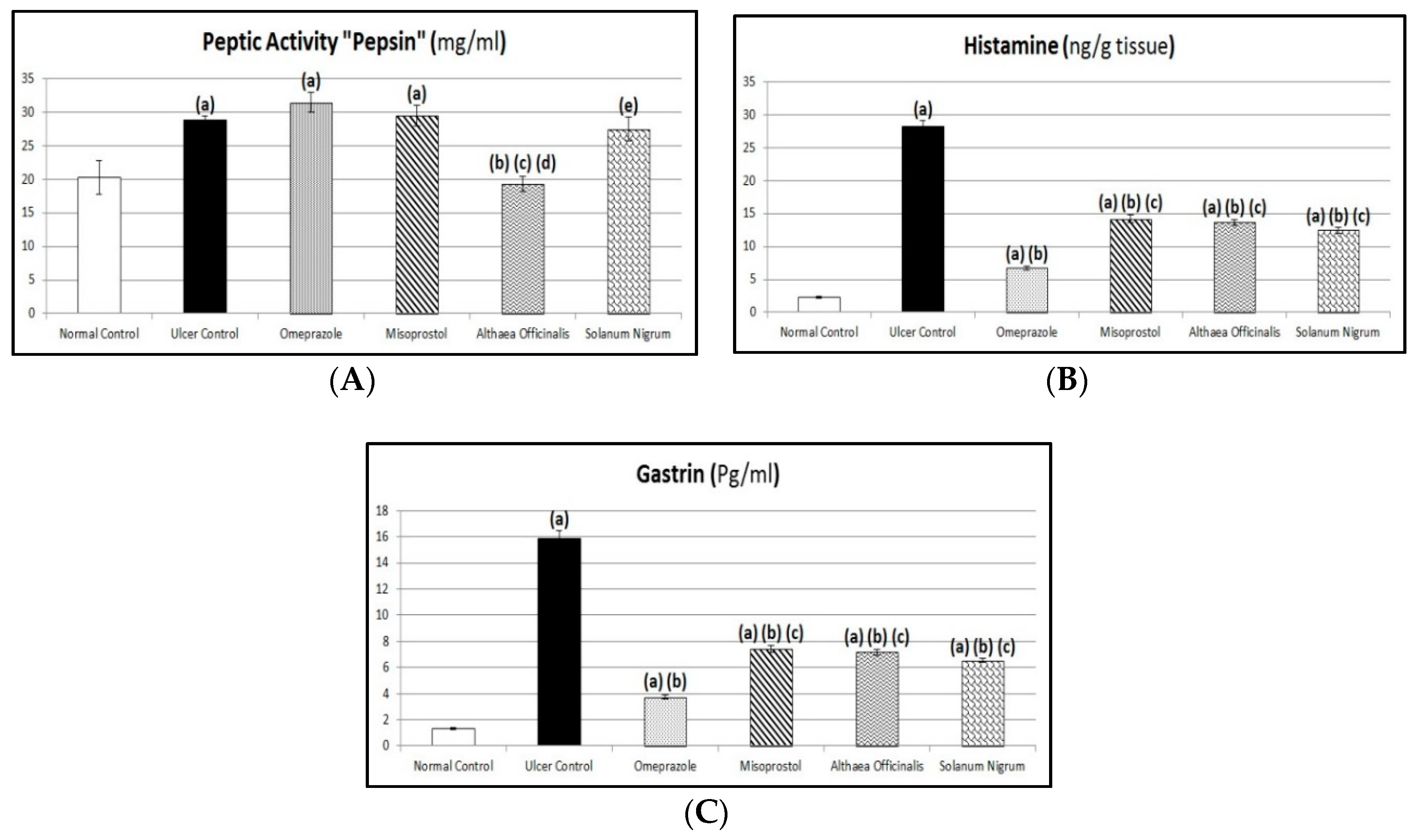
2.12. Peptic Activity Determination
2.13. Biomarkers Estimated Using ELISA Technique
2.14. Immunological Markers (Gastric Mucosal TNF-α and IL-1β Contents and CBS and HO-1 Activities) Estimated by Western Blot Analysis
2.15. Statistical Analysis
3. Results
3.1. Preliminary Phytochemical Screening of Extracts
3.2. Evaluation of the In Vitro Anti-Oxidant Activity (DPPH Assay)
3.3. Macroscopic Examination (Assessment of Gross Mucosal Damage) (Ulcer Number, Ulcer Index, and Preventive Index)
3.4. Microscopic Examination (Routine H and E Staining and Alcian Blue Staining)
3.4.1. Routine H and E Staining (Histopathological Examination of Stomach)
3.4.2. Alcian Blue Staining for Determination of Gastric Wall Mucus (Mucin Content)
3.5. Acid Productivity Parameters (Gastric Volume, Titratable Acidity, and Acid Output Determination)
3.6. Assessment of Aggressive Factors (Peptic Activity in Gastric Secretions, Gastric Mucosal Histamine, and Plasma Gastrin Contents)
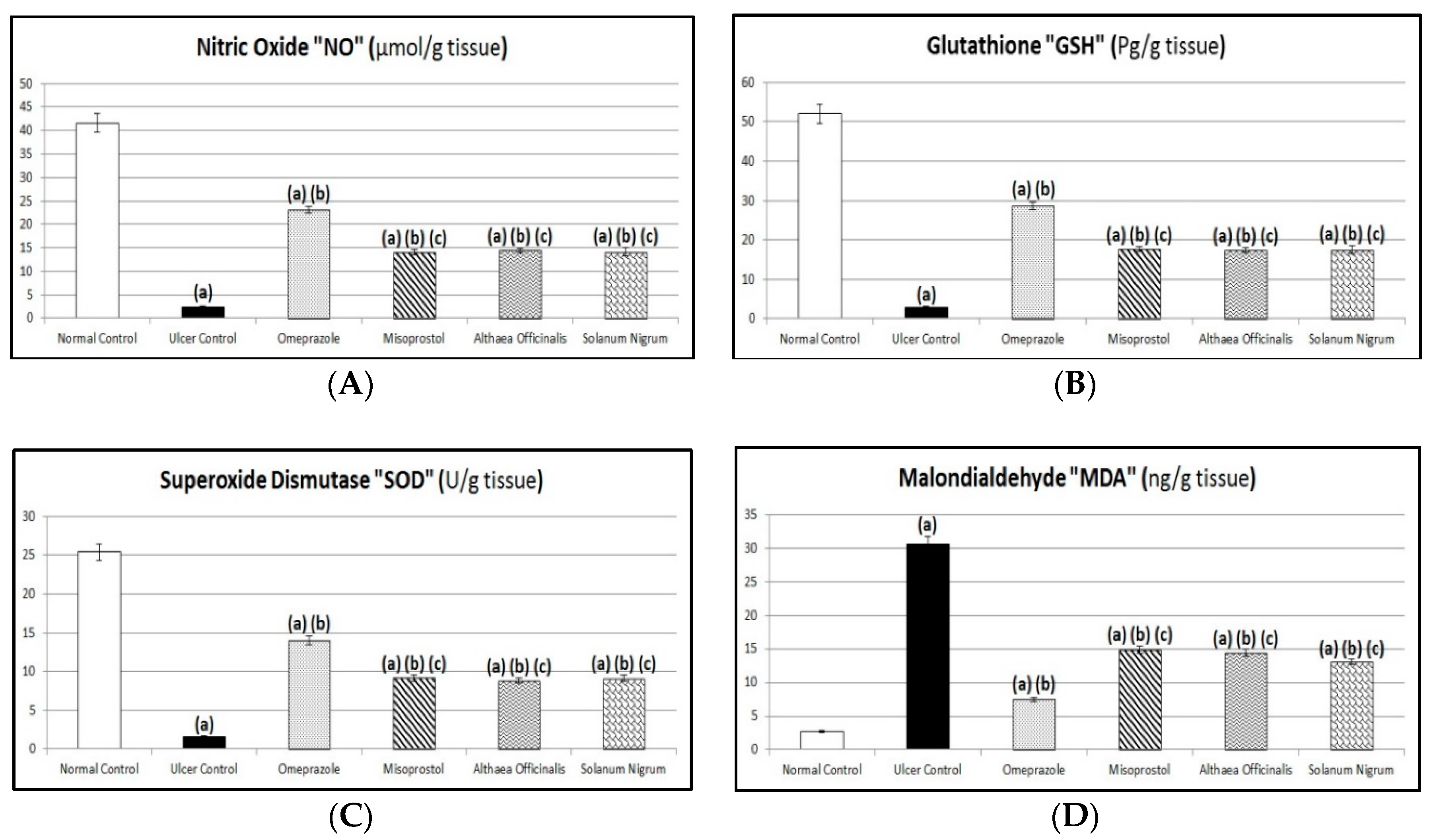
3.7. Oxidative Stress Biomarkers (Gastric Mucosal NO, GSH, and MDA Contents and SOD Activity)
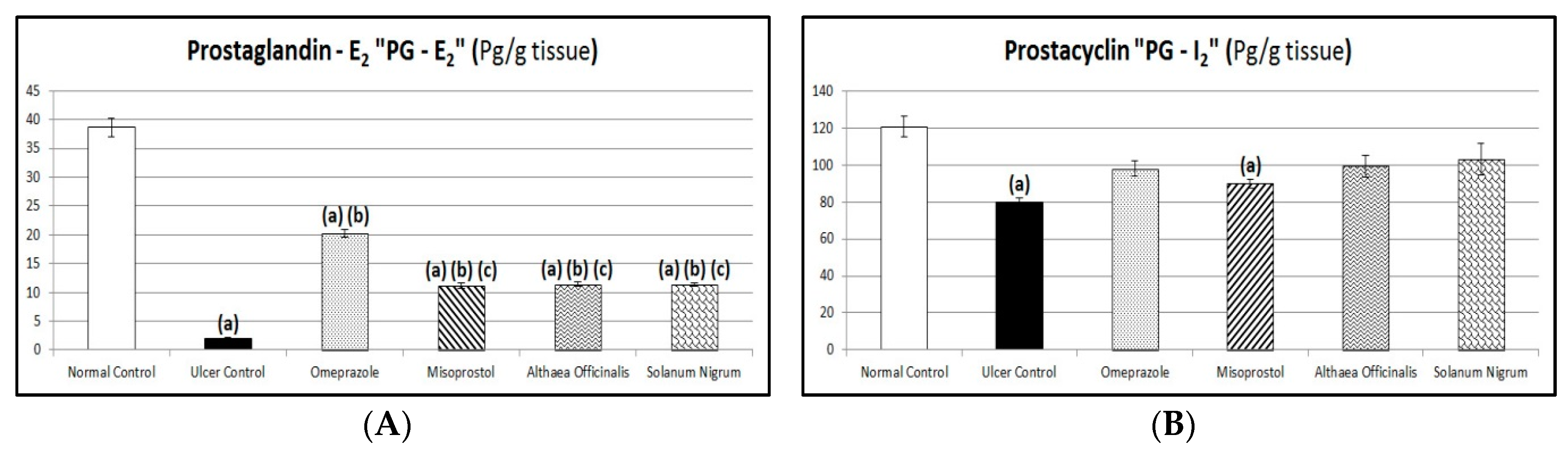
3.8. Protective Factors (Gastric Mucosal PG-E2 and PG-I2 Contents)
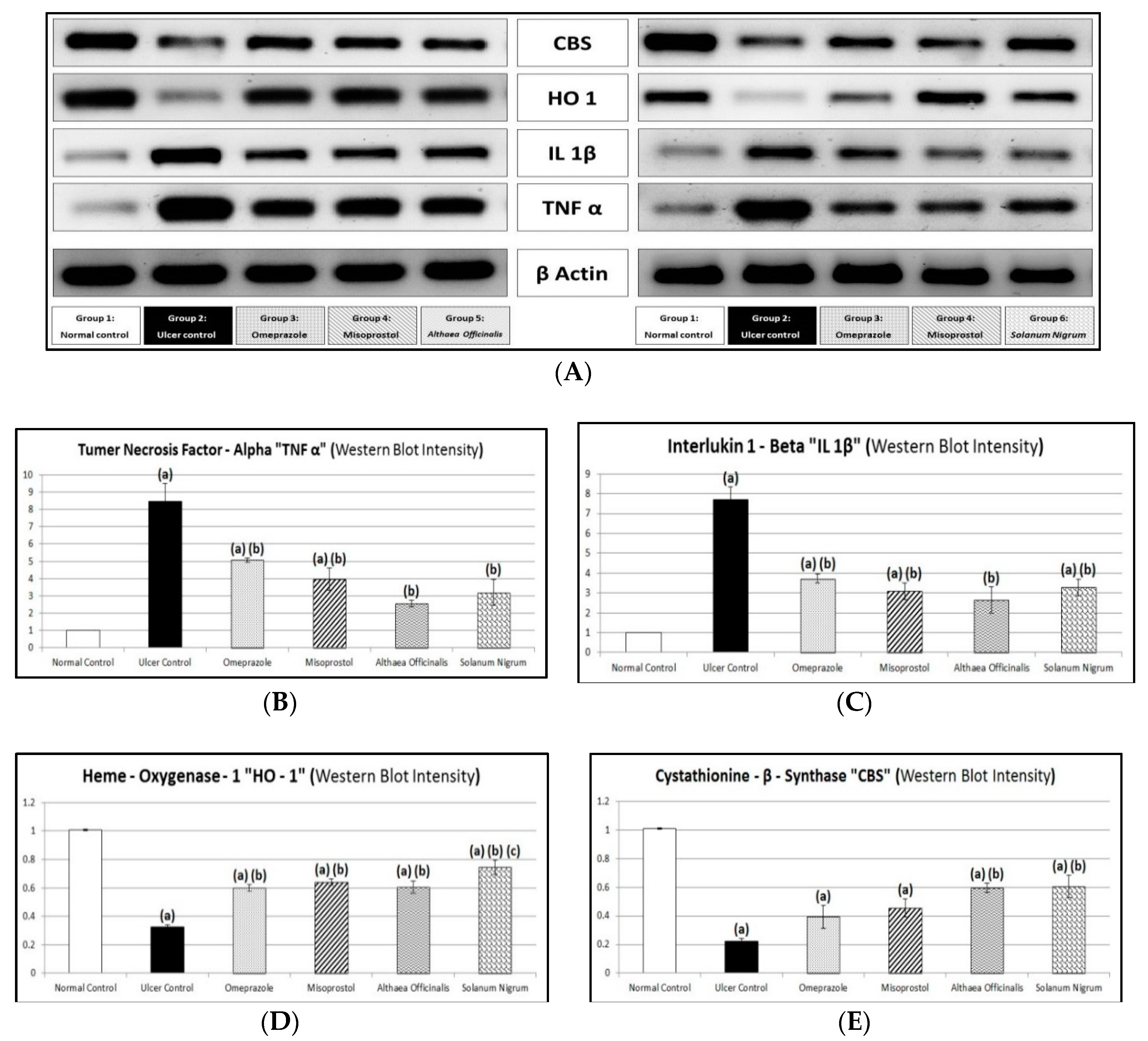
3.9. Immunological Markers (Gastric Mucosal TNF-α and IL-1β Contents and CBS and HO-1 Activities) Estimated by Western Blot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jainu, M.; Devi, C.S.S. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. J. Ethnopharmacol. 2006, 104, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Eamlamnam, K.; Patumraj, S.; Visedopas, N.; Thong-Ngam, D. Effects of Aloe vera and sucralfate on gastric microcirculator changes, cytokine levels and gastric ulcer healing in rats. World J. Gastroenterol. 2006, 12, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.S. Use of scavenging oxygen-derived free radicals to protect the rat against aspirin-and ethanol-induced erosive gastritis. J. Pharm. Sci. 1992, 81, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.I.; McQuaid, K.R.; Laine, L.; Walsh, J.H. Acid peptic disorders. In Textbook of Gastroenterolgy, 2nd ed.; Yamada, T., Ed.; Lippincott, J.B.: Philadelphia, PA, USA, 1995; pp. 1347–1430. [Google Scholar]
- Jainu, M.; Devi, C.S.S. Antioxidant effect of methanolic extract of Solanum nigrum berries on aspirin-induced gastric mucosal injury. Indian J. Clin. Biochem. 2004, 19, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, B.W.; Kwon, H.J.; Nam, S.W. Curative effect of selenium against indomethacin-induced gastric ulcers in rats. J. Microbiol. Biotechnol. 2011, 21, 400–404. [Google Scholar] [PubMed]
- Bhalke, R.D.; Giri, M.A.; Anarthe, S.J.; Pal, S.C. Antiulcer activity of the ethanol extract of leaves of Sesbania Grandiflora (Linn.). Int. J. Pharm. Sci. 2010, 2, 206–208. [Google Scholar]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Biswas, K.; Bandyopadhyay, U.; Chattopadhyay, I.; Varadaraj, A.; Ali, E.; Banerjee, R.K. A Novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem. 2003, 278, 10993–11001. [Google Scholar] [CrossRef]
- Yeomans, N.D.; Tulassay, Z.; Juhász, L.; Rácz, I.; Howard, J.M.; Van Rensburg, C.J.; Swannell, A.J.; Hawkey, C.J. A Comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal anti-inflammatory drugs. N. Engl. J. Med. 1998, 338, 719–726. [Google Scholar] [CrossRef]
- Penney, A.G.; Andrews, F.J.; O’Brien, P.E. Effects of misoprostol on delayed ulcer healing induced by aspirin. Dig. Dis. Sci. 1994, 39, 934–939. [Google Scholar] [CrossRef]
- De Sales, I.R.P.; Formiga, R.D.O.; Machado, F.D.F.; Nascimento, R.F.; Pessoa, M.M.B.; Barros, M.E.F.X.; Vieira, G.C.; Gadelha, F.A.A.F.; Marinho, A.F.; Barbosa Filho, J.M.; et al. Cytoprotective, antioxidant and anti-inflammatory mechanism related to antiulcer activity of Cissampelos sympodialis Eichl. in animal models. J. Ethnopharmacol. 2018, 222, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Herve, E.E.; Bernard, G.N.; Leandre, K.K.; Paul, Y.A.; Etienne, E.E. Acute toxicity and gastric anti-ulcer activity of an aqueous extract of the leaves of Macaranga barteri Mll. Arg (Euphorbiaceae) on rat models. J. Med. Plant Res. 2018, 12, 96–105. [Google Scholar]
- Kumar, M.R.; Niyas, M.K.; Mani, T.T.; Rahiman, O.M.F.; Kumar, S.B. A review on medicinal plants for peptic ulcer. Pharm. Lett. 2011, 3, 414–420. [Google Scholar]
- Zakaria, Z.A.; Hisamb, E.E.A.; Rofieeb, M.S.; Norhafizahc, M.; Somchita, M.N.; Tehd, L.K.; Salleh, M.Z. In vivo antiulcer activity of the aqueous extract of Bauhinia purpurea leaf. J. Ethnopharmacol. 2011, 137, 1047–1054. [Google Scholar] [CrossRef]
- Ahmad, A.; Kumar, V.; Maurya, S.K. Natural antiulcer agents: A pharmacological review. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 535–541. [Google Scholar]
- Gohar, A.A.; Zaki, A.A. Assessment of some herbal drugs for prophylaxis of peptic ulcer. Iran. J. Pharm. Res. 2014, 13, 1081–1086. [Google Scholar]
- Inas, Z.A.; Hala, A.K.; Gehan, H.H. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci. J. 2011, 8, 433–445. [Google Scholar]
- Zheng, Y.F.; Xie, J.H.; Xu, Y.F.; Liang, Y.Z.; Mo, Z.Z.; Jiang, W.W.; Chen, X.Y.; Liu, Y.H.; Yu, X.D.; Huang, P.; et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem.-Biol. Interact. 2014, 222, 27–36. [Google Scholar] [CrossRef]
- Saiah, W.; Halzoune, H.; Djaziri, R.; Tabani, K.; Koceir, E.A.; Omari, N. Antioxidant and gastroprotective actions of butanol fraction of Zingiber officinale against diclofenac sodium-induced gastric damage in rats. J. Food Biochem. 2018, 42, 1–12. [Google Scholar] [CrossRef]
- Al Mofleh, I.A.; Al Rashed, R.S. Nonsteroidal antiinflammatory drug-induced gastrointestinal injuries and related adverse reactions: Epidemiology, pathogenesis, and management. Saudi J. Gastroenterol. 2007, 13, 107–113. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013, 2013, 796405. [Google Scholar] [CrossRef]
- Yang, H.; Lu, Y.; Zeng, X.F.; Li, L.; Zhang, R.P.; Ren, Z.K.; Liu, X. Antichronic gastric ulcer effect of zinc-baicalin complex on the acetic acid-induced chronic gastric ulcer rat model. Gastroenterol. Res. Pract. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Awaad, A.S.; El-Meligy, R.M.; Soliman, G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013, 17, 101–124. [Google Scholar] [CrossRef]
- Hage-Sleiman, R.; Mroueh, M.; Daher, C.F. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm. Biol. 2011, 49, 327–333. [Google Scholar] [CrossRef]
- Benchikh, F. Pharmacological Effects of Myrtus Communis L. on the Gastrointestinal Tract of Rats and Mice. Ph.D. Thesis, Department of Biology and Animal Physiology, Faculté des Sciences de la Nature et de la Vie, Université Ferhat Abbas Sétif 1, Setif, Algeria, 2018. [Google Scholar]
- Ahmed, O.E.I.; Hashim, N.M.; Ibrahim, M.Y.; Abdulrahman, A.A.; Adam, H.; Arbab, I.A. Gastroprotective effects of (+)-catechin hydrate on ethanol-induced gastric ulcer in rats Cienc. Tec. Vitivinic. 2016, 31, 1–30. [Google Scholar]
- Rao, C.V.; Venkataramana, K. A pharmacological review on natural antiulcer agents. J. Glob. Trends Pharm. Sci. 2013, 4, 1118–1131. [Google Scholar]
- Dwivedi, S.K.; Verma, C. Potential medicinal plants used as antiulcer agents. Int. J. Pharm. Res. Sci. 2013, 2, 885–891. [Google Scholar]
- Pillai, S.I.; Kandaswamy, M.; Subramanian, S. Antiulcerogenic and ulcer healing effects of Indian propolis in experimental rat ulcer models. J. Api. Prod. Api.Med. Sci. 2010, 2, 21–28. [Google Scholar] [CrossRef]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Sadighara, P.; Gharibi, S.; Jafari, A.M.; Khaniki, G.J.; Salari, S. The antioxidant and flavonoids contents of Althaea officinalis L. flowers based on their color. Avicenna J. Phytomed. 2012, 2, 113–117. [Google Scholar]
- Palle, S.; Kanakalatha, A.; Kavitha, C.N. Gastroprotective and antiulcer effects of Celastrus paniculatus seed oil against several gastric ulcer models in rats. J. Diet. Suppl. 2018, 15, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Karimi, A.; Ouguerram, K.; Vahidi-Ataabadi, N.; Eshraghi-Jazi, F.; Mansouri, A.; Nematbakhsh, M. Lack of nephroprotective efficacy of Althaea officinalis flower extract against gentamicin renal toxicity in male rats. Int. J. Prev. Med. 2014, 5, 1360–1363. [Google Scholar] [PubMed]
- Fallahpour, F.; Banaee, M.; Javadzade1, N. Effects of dietary marshmallow (Althaea officinalis L.) extract on growth performance and body composition of common carp (Cyprinus Carpio). Int. J. Adv. Biol. Biom. Res. 2014, 2, 2453–2460. [Google Scholar]
- Perez, R.M.; Perez, J.A.; Garcia, L.M.; Sossa, H. Neuropharmacological activity of Solanum nigrum fruit. J. Ethnopharmacol. 1998, 62, 43–48. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mohammed, R.; Abouzid, S.F.; Rateb, M.E.; Sayed, A.M. Cytotoxicity of Solanum nigrum L. green fruits on breast (MCF-7) and liver (HepG-2) cancer cell lines. Pharm. Innov. J. 2015, 3, 87–89. [Google Scholar]
- Harborne, J.B. Phytochemical Methods, A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 2007; pp. 1–34. ISBN 81-8128-410-4. [Google Scholar]
- Singh, A.P.; Shukla, V.; Khare, P. Effects of Plumeria obtusa Linn. in peptic ulcer induced by pylorus ligation & indomethacin. J. Pharm. Sci. Innov. 2012, 1, 26–32. [Google Scholar]
- Takao, T.; Watanabe, N.; Yagi, I.; Sakata, K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Delazar, A.; Byres, M.; Gibbons, S.; Kumarasamy, Y.; Modarresi, M.; Nahar, L.; Shoeb, M.; Sarker, S.D. Iridoid glycosides from Eremostachys Glabra. J. Nat. Prod. 2004, 67, 1584–1587. [Google Scholar] [CrossRef]
- Levi, S.; Goodlad, R.A.; Lee, C.Y.; Stamp, G.; Walport, M.J.; Wright, N.A.; Hodgson, H.J.F. Inhibitory effect of non-steroidal anti-inflammatory drugs on mucosal cell proliferation associated with gastric ulcer healing. Lancet 1990, 336, 840–843. [Google Scholar] [CrossRef]
- Shay, H.; Komarow, S.A.; Fels, S.S.; Meranze, D.; Gruenstein, M.; Siplet, H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 1945, 5, 43–61. [Google Scholar]
- Ajeigbe, K.O.; Oladejo, E.O.; Emikpe, B.O.; Asuk, A.A.; Olaleye, S.B. The dual modulatory effect of folic acid supplementation on indomethacin induced gastropathy in the rat. Eur. J. Biol. Sci. 2011, 3, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.S.; Banik, A.; Singh, A.; Singh, M. Evaluation of anti-ulcer activity of aqueous and ethanolic extract of whole plant of Clitoria ternatea in albino Wistar rats. Int. J. Pharm. Sci. Drug Res. 2015, 7, 33–39. [Google Scholar]
- Pendley, C.E.; Fitzpatrick, L.R.; Ewing, R.W.; Molino, B.F.; Martin, G.E. The gastrin/cholecystokinin-B receptor antagonist L-365,260 reduces basal acid secretion and prevents gastrointestinal damage induced by aspirin, ethanol, and cysteamine in the rat. J. Pharmacol. Exp. Ther. 1993, 265, 1348–1354. [Google Scholar] [PubMed]
- Khayyal, M.T.; El-Ghazaly, M.A.; Kenawy, S.A.; Seif-El-Nasr, M.; Mahran, L.G.; Kafafi, Y.A.; Okpanyi, S.N. Antiulcenogenic effect of some gastro intestinally acting plant extracts and their combination. Arzneimittel-Forschung 2001, 51, 545–553. [Google Scholar] [PubMed]
- Cho, C.H.; Olge, C.W. Cholinergic mediated gastric mast cells degranulation with subsequent histamine H1 and H2 receptors activation in stress ulceration in rats. Eur. J. Pharmacol. 1979, 55, 23–33. [Google Scholar] [CrossRef]
- Hano, J.; Bugajski, J.; Danek, L.; Wantuch, C. The effect of neuroleptics on the development of gastric ulcers in rats exposed to restraint cold stress. Pol. J. Pharmacol. Pharm. 1976, 28, 37–47. [Google Scholar] [PubMed]
- Drury, R.A.; Wallington, E.A. Carleton’s Histological Techniques, 6th ed.; Oxford University Press: London, UK, 1980; p. 183. [Google Scholar]
- Corne, S.J.; Morrisey, S.M.; Woods, R.J. A method for the quantitative estimation of gastric barrier mucus. J. Physiol. 1974, 242, 116–117. [Google Scholar]
- Al Asmari, A.; Al Shahrani, H.; Al Masri, N.; Al Faraidi, A.; Elfaki, I.; Arshaduddin, M. Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2016, 3, 105–113. [Google Scholar] [CrossRef]
- Shay, H.; Sun, D.C.H.; Gruenstein, M. A quantitative method for measuring spontaneous gastric secretion in the rat. Gastroenterology 1954, 26, 906–913. [Google Scholar] [CrossRef]
- Grossman, M.I. Physiology for physician. Mon. Publ. Am. Physiol. Soc. 1963, 1, 1–5. [Google Scholar]
- Brodie, D.A.; Hooke, K.F. The effect of vasoactive agents on stress-induced gastric hemorrhage in the rat. Digestion 1971, 4, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.B. A modified method for the determination of pepsinogen in urine (uropepsin). Scand. J. Clin. Investig. 1954, 6, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.M.; Guevara-Guzman, R.; Zorrilla, J.; Hinton, M.R.; Broad, K.D.; Mimmack, M.; Ohkura, S. Formation of olfactory memories mediated by nitric oxide. Nature 1997, 388, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Nat. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. Western blotting: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int. J. PharmTech Res. 2013, 5, 1378–1385. [Google Scholar]
- Rani, Y.S.; Reddy, V.J.; Basha, S.J.; Koshma, M.; Hanumanthu, G.; Swaroopa, P. A review on Solanum nigrum. World J. Pharm. Pharm. Sci. 2017, 6, 293–303. [Google Scholar]
- Erkin, B.; Dokmeci, D.; Altaner, S.; Turan, F.N. Gastroprotective effect of L-carnitine on indomethacin-induced gastric mucosal injury in rats: A preliminary study. Folia Med. 2006, 48, 86–89. [Google Scholar]
- Ezzat, S.M.; Choucry, M.A.; Kandil, Z.A. Antibacterial, antioxidant, and topical anti-inflammatory activities of Bergia ammannioides: A wound-healing plant. Pharm. Biol. 2016, 54, 215–224. [Google Scholar] [CrossRef]
- AlRashdi, A.S.; Salama, S.M.; Alkiyumi, S.S.; Abdulla, M.A.; Hadi, A.H.A.; Abdelwahab, S.I.; Taha, M.M.; Hussiani, J.; Asykin, N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/Ethanol-induced gastric mucosal injury in rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Kahraman, A.; Erkasap, N.; Koken, T.; Serteser, M.; Aktepe, F.; Erkasap, S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 2003, 183, 133–142. [Google Scholar] [CrossRef]
- Son, Y.O.; Kim, J.; Lim, J.C.; Chung, Y.; Chung, G.H.; Lee, J.C. Ripe fruits of Solanum nigrum L. inhibits cell growth and induces apoptosis in MCF-7 cells. Food Chem. Toxicol. 2003, 41, 1421–1428. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, A.; Gupta, S.; Sarethy, I.P.; Gabrani, R. Solanum nigrum: Current perspectives on therapeutic properties. Altern. Med. Rev. 2011, 16, 78–85. [Google Scholar] [PubMed]
- Shah, S.A.; Akhtar, N.; Akram, M.; Shah, P.A.; Saeed, T.; Ahmed, K.; Asif, H.M. Pharmacological activity of Althaea officinalis L. J. Med. Plant Res. 2011, 5, 5662–5666. [Google Scholar]
- Wang, T.; Zhao, S.; Wang, Y.; Yang, Y.; Yao, L.; Chu, L.; Du, H.; Fu, F. Protective effects of escin against indomethacin-induced gastric ulcer in mice. Toxicol. Mech. Method 2014, 24, 560–566. [Google Scholar] [CrossRef]
- Hoshino, T.; Tsutsumi, S.; Tomisato, W.; Hwang, H.J.; Tsuchiya, T.; Mizushima, T. Prostaglandin-E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J. Biol. Chem. 2003, 278, 12752–12758. [Google Scholar] [CrossRef]
- Redlak, M.J.; Power, J.J.; Miller, T.A. Role of mitochondria in aspirin-induced apoptosis in human gastric epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 731–738. [Google Scholar] [CrossRef]
- Jiang, G.L.; Im, W.B.; Donde, Y.; Wheeler, L.A. EP4 agonist alleviates indomethacin-induced gastric lesions and promotes chronic gastric ulcer healing. World J. Gastroenterol. 2009, 15, 5149–5156. [Google Scholar] [CrossRef]
- Koizumi, S.; Odashima, M.; Otaka, M.; Jin, M.; Linden, J.; Watanabe, S.; Ohnishi, H. Attenuation of gastric mucosal inflammation induced by indomethacin through activation of the A2A adenosine receptor in rats. J. Gastroenterol. 2009, 44, 419–425. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Oxidative stress involvement and gene expression in indomethacin-induced gastropathy. Redox Rep. 2006, 11, 243–253. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T. Gastroprotective effect of rutin against indomethacin-induced ulcers in rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 742–750. [Google Scholar] [CrossRef]
- Jaarin, K.; Gapor, M.T.; Nafeeza, M.I.; Ffauzee, A.M. Effect of various doses of palm vitamin E and tocopherol on aspirin-induced gastric lesions in rats. Int. J. Exp. Pathol. 2002, 83, 295–301. [Google Scholar] [CrossRef]
- Dengiz, G.O.; Gürsan, N. Effects of Momordica charantia L. (Cucurbitaceae) on indomethacin-induced ulcer model in rats. Turk. J. Gastroenterol. 2005, 16, 85–88. [Google Scholar]
- Sairam, K.; Rao, C.V.; Dora Babu, M.; Agrawal, V.K.; Goel, R.K. Antiulcerogenic activity of methanolic extract of Emblica officinalis. J. Ethnopharmacol. 2002, 82, 1–9. [Google Scholar] [CrossRef]
- Balekar, N.; Jain, D.K.; Jawanjal, H. Evaluation of antiulcer activity of bark extract of Albizzia lebbeck linn. South Pac. J. Pharma Bio Sci. 2013, 1, 43–50. [Google Scholar]
- Sahoo, S.K.; Sahoo, H.B.; Priyadarshini, D.; Soundarya, G.; Kumar, C.K.; Rani, K.U. Antiulcer activity of ethanolic extract of Salvadora indica (W.) leaves on albino rats. J. Clin. Diagn. Res. 2016, 10, 7–10. [Google Scholar] [CrossRef]
- Gwee, K.A.; Goh, V.; Lima, G.; Setia, S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: Risks versus benefits. J. Pain Res. 2018, 11, 361–374. [Google Scholar] [CrossRef]
- Guha, P.; Dey, A.; Chatterjee, A.; Chattopadhyay, S.; Bandyopadhyay, S.K. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by l-arginine. Br. J. Pharmacol. 2010, 159, 726–734. [Google Scholar] [CrossRef]
- Jamian, S.S.; Mehrani, S.; Asilan, K.S.; Tabrizi, A.T.; Goharian, A. Responses of seedling growth and germination parameters in three medicinal plants under drought stress. Int. J. Agric. Crop. Sci. 2014, 7, 191–195. [Google Scholar]
- Deters, A.; Zippel, J.; Hellenbrand, N.; Pappai, D.; Possemeyer, C.; Hensel, A. Aqueous extracts and polysaccharides from Marshmallow roots (Althea officinalis L.): Cellular internalisation and stimulation of cell physiology of human epithelial cells in vitro. J. Ethnopharmacol. 2010, 127, 62–69. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Abbasabadi, Z.; Abdollahi, M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int. J. Pharmacol. 2013, 9, 108–124. [Google Scholar] [CrossRef]
- Zaghlool, S.S.; Shehata, B.A.; Abo-Seif, A.A.; El-Latif, H.A.A. Comparison between the protective effects of famotidine, ginger and marshmallow on pyloric ligation-induced peptic ulcer in rats. J. Bioequiv. Bioavailab. 2015, 7, 170–178. [Google Scholar]
- Aburjai, T.A.; Oun, I.M.; Auzi, A.A.; Hudaib, M.M. Volatile oil constituents of fruits and leaves of Solanum nigrum L. growing in Libya. J. Essent. Oil Bear. Plant 2014, 17, 397–404. [Google Scholar] [CrossRef]
- Atanu, F.O.; Ebiloma, U.G.; Ajayi, E.I. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol. Mol. Biol. Rev. 2011, 6, 1–8. [Google Scholar]
- Heeba, G.H.; Hassan, M.K.A.; Amin, R.S. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: Role of nitric oxide and prostaglandins. Eur. J. Pharmacol. 2009, 607, 188–193. [Google Scholar] [CrossRef]
- Huligol, S.V.; Kumar, V.H.; Narendar, K. Evaluation of gastroprotective role of alpha-tocopherol in indomethacin induced peptic ulcer in albino rats. Int. J. Pharmacol. Clin. Sci. 2012, 1, 39–44. [Google Scholar]
- Allam, M.M.; El-Gohary, O.A. Gastroprotective effect of ghrelin against indomethacin-induced gastric injury in rats: Possible role of heme oxygenase-1 pathway. Gen. Physiol. Biophys. 2017, 36, 321–330. [Google Scholar] [CrossRef]
- Sabina, E.P.; Rasool, M. Therapeutic efficacy of Indian ayurvedic herbal formulation triphala on lipid peroxidation, antioxidant status and inflammatory mediator TNF-α in adjuvant-induced arthritic mice. Int. J. Biol. Chem. 2007, 1, 149–155. [Google Scholar]
- Adhikary, B.; Yadav, S.K.; Roy, K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Black tea and Theaflavins assist healing of indomethacin-induced gastric ulceration in mice by antioxidative action. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Mishra, V.; Agrawal, M.; Onasanwo, S.A.; Madhur, G.; Rastogi, P.; Pandey, H.P.; Palit, G.; Narender, T. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine 2013, 20, 506–511. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Jala’a, A.; Al-Masoodi, E.A. Evaluation of the gastroprotective effect of misoprostol, chitosan and their combination on indomethacin induced gastric ulcer in rats. Med. J. Basrah Univ. 2011, 29, 1–8. [Google Scholar] [CrossRef]
- Hage-Sleiman, R. Effect of Water Extract of Althaea Officinalis Flowers on Inflammation, Gastric Ulcer, Bacterial Activity, Platelet Aggregation, Glycemia, and Lipidemia. Master’s Thesis, Science, Molecular Biology, Lebanese American University, Beirut, Lebanon, 2011. [Google Scholar]
- Shree, G.K.; Parvathi, S.; Ramkumar, P.S.S.; Priya, S.S. Pharmacological and phytochemical evaluation of anti-ulcerogenic potential of Solanum nigrum. Int. J. Pharm. Sci. Res. 2012, 3, 2837–2840. [Google Scholar]
- Malash, A.M.; Abdallah, D.M.; Agha, A.M.; Kenawy, S.A. Gastroprotective efficacy of coenzyme Q10 in indomethacin-induced gastropathy: Other potential mechanisms. Ulcers 2012, 2012, 957898. [Google Scholar] [CrossRef]
- Adewoye, E.O.; Salami, A.T. Anti-ulcerogenic mechanism of magnesium in indomethacin induced gastric ulcer in rats. Niger. J. Physiol. Sci. 2013, 28, 193–199. [Google Scholar] [PubMed]
- Nascimento, A.M.; Maria-Ferreira, D.; De Souza, E.F.J.; De Souza, L.M.; Sassaki, G.L.; Iacomini, M.; Werner, M.F.D.P.; Cipriani, T.R. Gastroprotective effect and chemical characterization of a polysaccharide fraction from leaves of Croton cajucara. Int. J. Biol. Macromol. 2017, 95, 153–159. [Google Scholar] [CrossRef]
- Ravichandran, M.; Mubarak, H.; Eswari, N. Gastroprotective activity of a siddha drug kadukkai chooranam against indomethacin and pylorus ligation induced gastric ulcer in rats. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 195–209. [Google Scholar]
- Matsuzaki, J.; Suzuki, H.; Minegishi, Y.; Sugai, E.; Tsugawa, H.; Yasui, M.; Hibi, T. Acid suppression by proton pump inhibitors enhances aquaporin-4 and KCNQ1 expression in gastric fundic parietal cells in mouse. Dig. Dis. Sci. 2010, 55, 3339–3348. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Banerjee, D.; Bauri, A.K.; Chattopadhyay, S.; Bandyopadhyay, S.K. Healing property of the Piper betel phenol, allylpyrocatechol against indometacin-induced stomach ulceration and mechanism of action. World J. Gastroenterol. 2007, 13, 3705–3713. [Google Scholar] [CrossRef]
- Kumar, B.G.; Kumari, D.; Rajeshwar, G.; Umadevi, V.; Kotla, N.G. Antiulcer activity of ethanolic extract of Terminalia catappa leaves against gastric ulcers by pyloric ligation induced model in rats. Int. J. Pharm. Sci. Drug Res. 2014, 6, 38–40. [Google Scholar]
- Megala, J.; Geetha, A. Antiulcerogenic activity of hydroalcoholic fruit extract of Pithecellobium dulce in different experimental ulcer models in rats. J. Ethnopharmacol. 2012, 142, 415–421. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Yoshida, N.; Kondo, M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig. Dis. Sci. 1998, 43, 30–34. [Google Scholar]
- Antonisamy, P.; Arasu, M.V.; Dhanasekaran, M.; Choi, K.C.; Aravinthan, A.; Kim, N.S.; Kang, C.W.; Kim, J.H. Protective effects of trigonelline against indomethacin-induced gastric ulcer in rats and potential underlying mechanisms. Food Funct. 2016, 7, 398–408. [Google Scholar] [CrossRef]
- Bafna, P.A.; Balaraman, R. Effect of activit, a herbomineral formulation, on experimentally-induced gastric lesions in rats. J. Appl. Pharm. Sci. 2011, 1, 134–139. [Google Scholar]
- Polat, B.; Albayrak, Y.; Suleyman, B.; Dursun, H.; Odabasoglu, F.; Yigiter, M.; Suleyman, H. Antiulcerative effect of dexmedetomidine on indomethacin-induced gastric ulcer in rats. Pharmacol. Rep. 2011, 63, 518–526. [Google Scholar] [CrossRef]
- Lanas, A.; Bajador, E.; Serrano, P.; Fuentes, J.; Carreño, S.; Guardia, J.; Sanz, M.; Montoro, M.; Sáinz, R. Nitrovasodilators, low-dose aspirin, other nonsteroidal anti-inflammatory drugs, and the risk of upper gastrointestinal bleeding. N. Engl. J. Med. 2000, 343, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Tsukimi, Y.; Okabe, S. Recent advances in gastrointestinal pathophysiology: Role of heat shock proteins in mucosal defense and ulcer healing. Biol. Pharm. Bull. 2001, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.L.R.; Rodrigues, O.R.L.; Da Silva, D.M.; Galdino, P.M.; De Paula, J.R.; Romão, W.; Da Costa, H.B.; Vaz, B.G.; Ghedini, P.C.; Costa, E.A. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. J. Ethnopharmacol. 2014, 155, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Hatware, K.V.; Sharma, S.; Patil, K.; Shete, M.; Karri, S.; Gupta, G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in wistar rats. Biomed. Pharmacother. 2018, 103, 317–325. [Google Scholar] [CrossRef]
- Buttgereit, F.; Burmester, G.R.; Simon, L.S. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2-specific inhibitors. Am. J. Med. 2001, 110, 13–19. [Google Scholar] [CrossRef]
- Sousa, G.A.; Oliveira, I.S.; Silva-Freitas, F.V.; Viana, A.F.S.; Neto, B.P.; Cunha, F.V.M.; Gonçalves, R.L.; Lima Filho, A.C.M.; Amaral, M.P.; Rita De Cássia, M.O.; et al. Gastroprotective effect of ethanol extracts of cladodes and roots of Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. Ex Rowl (Cactaceae) on experimental ulcer models. J. Ethnopharmacol. 2018, 218, 100–108. [Google Scholar] [CrossRef]
- Antonisamy, P.; Kannan, P.; Aravinthan, A.; Duraipandiyan, V.; Valan Arasu, M.; Ignacimuthu, S.; Abdullah Al-Dhabi, N.; Kim, J.H. Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: Investigation of potential mechanisms of action. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Joshi, S.V.; Kedar, K.A.; Markana, U.V.; Lodha, S.R.; Shah, P.D.; Vyas, H.G.; Vyas, R.B.; Vyas, B.A.; Kalyankar, G.G. Alteration of gastric mucus secretion in rats treated with Abelmoschus esculentus seed mucilage. Pharm. Lett. 2011, 3, 183–188. [Google Scholar]
- He, J.; Liang, J.; Zhu, S.; Zhao, W.; Zhang, Y.; Sun, W. Protective effect of taurohyodeoxycholic acid from Pulvis Fellis Suis on trinitrobenzene sulfonic acid-induced ulcerative colitis in mice. Eur. J. Pharmacol. 2011, 670, 229–235. [Google Scholar] [CrossRef]
- Uc, A.; Zhu, X.; Wagner, B.A.; Buettner, G.R.; Berg, D.J. Heme oxygenase-1 is protective against nonsteroidal anti-inflammatory drug–induced gastric ulcers. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 471–476. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef]
- Shen, F.; Zhao, C.S.; Shen, M.F.; Wang, Z.; Chen, G. The role of hydrogen sulfide in gastric mucosal damage. Med. Gas Res. 2019, 9, 88–92. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide–releasing diclofenac derivative in the rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Mard, S.A.; Ashabi, A.; Badavi, M.; Dianat, M. Protective effects of vitamin B6 alone and in combination with L-cysteine and NaHS on ethanol and indomethacin-induced gastric lesions in mice. Iran. J. Basic Med. Sci. 2015, 18, 253–258. [Google Scholar]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Rizzo, G.; Mencarelli, A.; Orlandi, S.; Zanardo, R.; Renga, B.; Di Sante, M.; Morelli, A.; et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 2005, 129, 1210–1224. [Google Scholar] [CrossRef]
- Medeiros, J.V.R.; Bezerra, V.H.; Gomes, A.S.; Barbosa, A.L.R.; Lima-Junior, R.C.P.; Soares, P.M.G.; Brito, G.A.C.; Ribeiro, R.A.; Cunha, F.Q.; Souza, M.H. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: Role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 2009, 330, 764–770. [Google Scholar] [CrossRef]
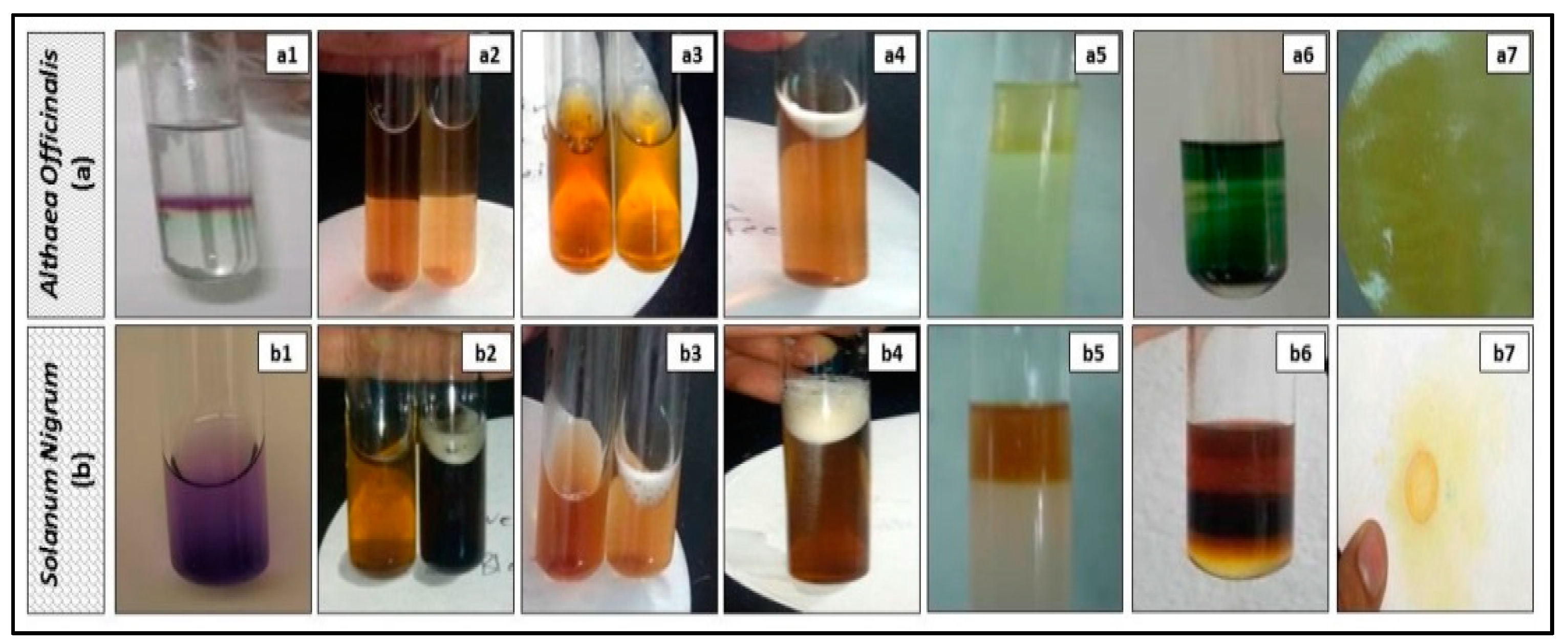

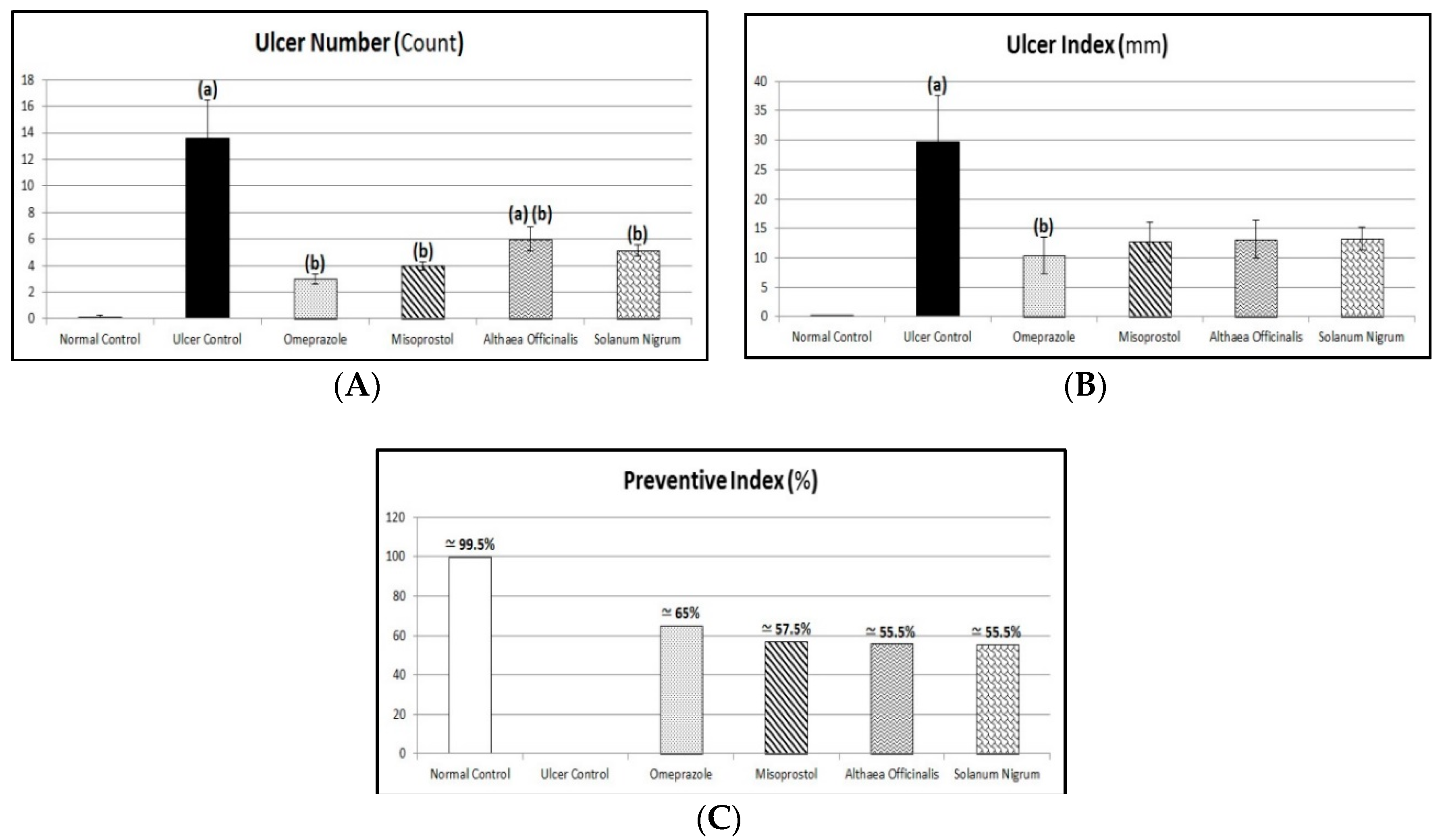
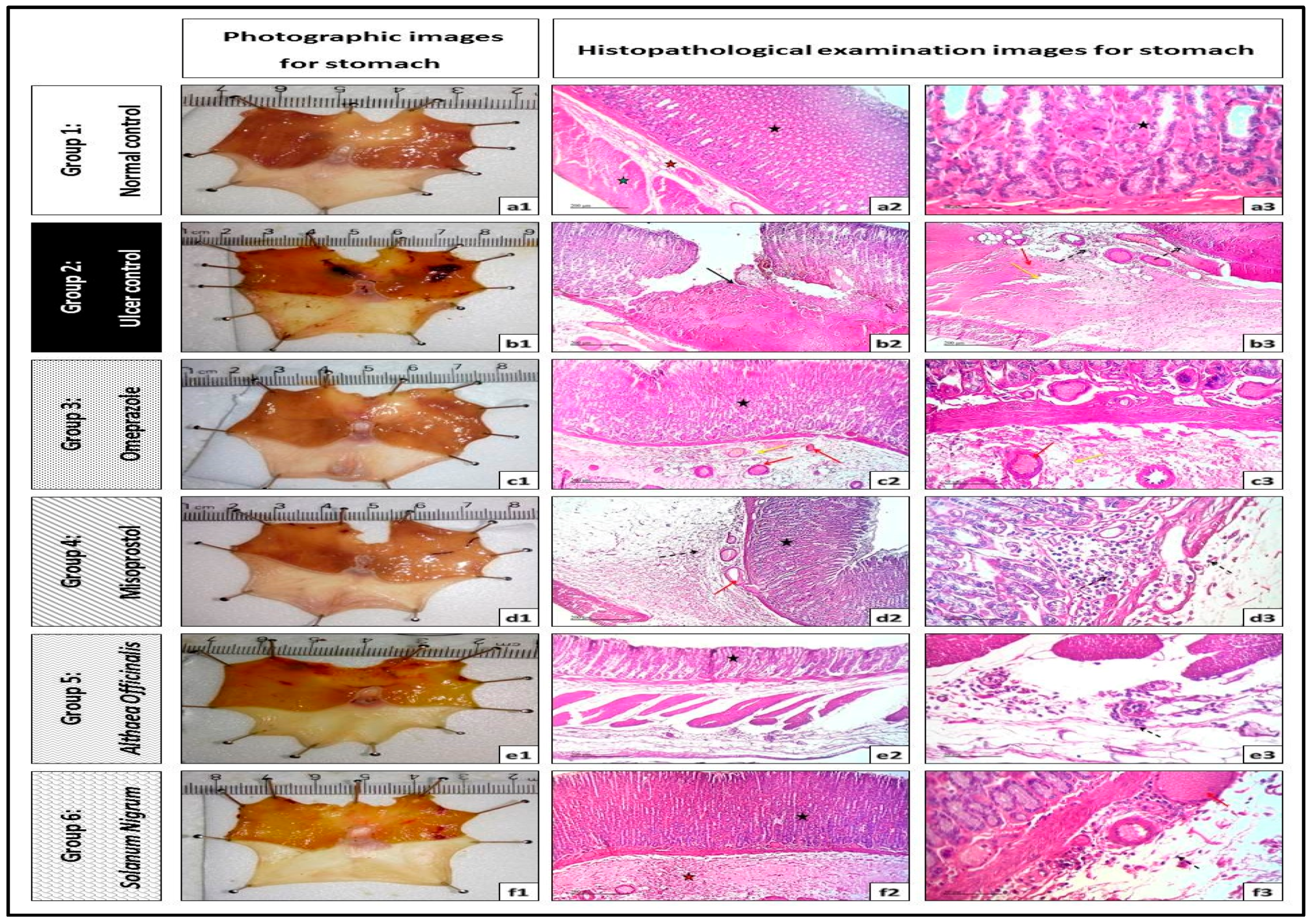
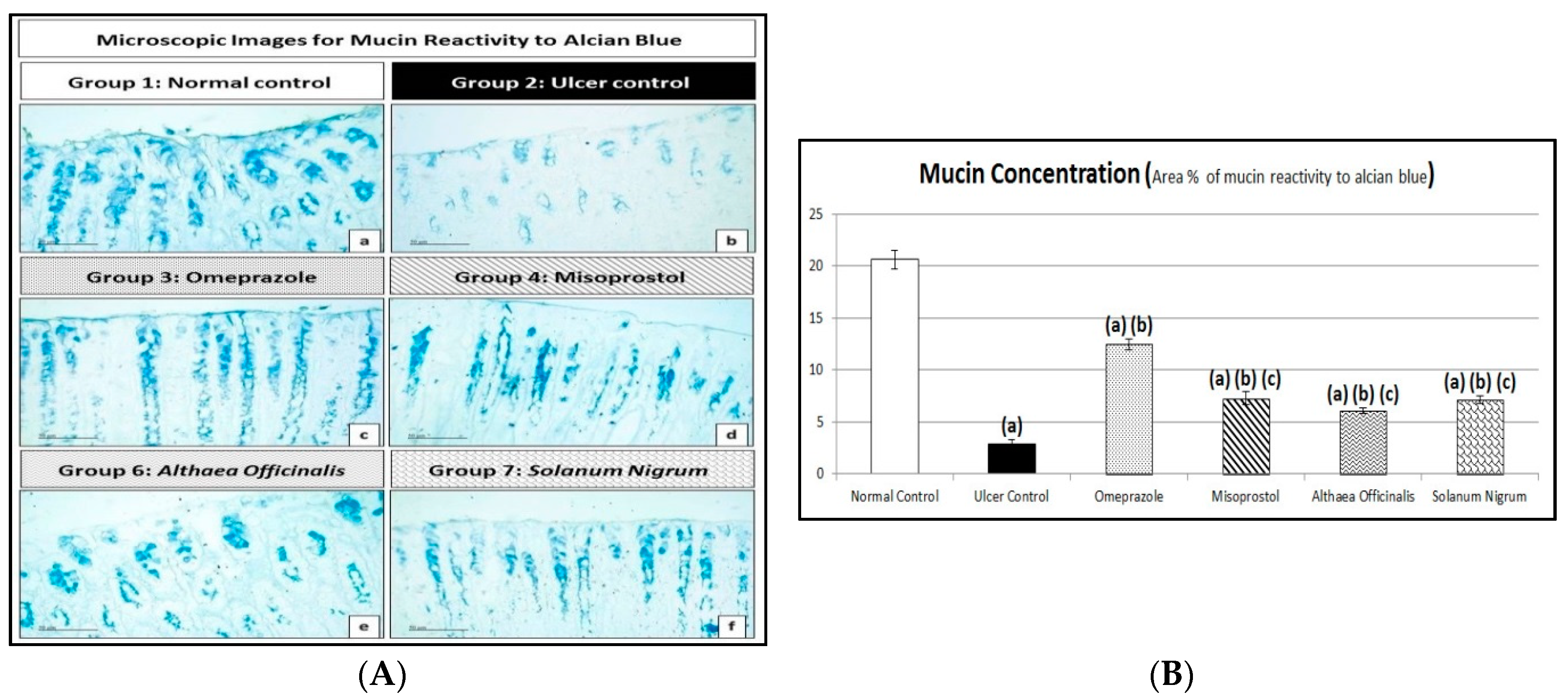
| No. | Phytochemical Tests | Results | |
|---|---|---|---|
| Althaea officinalis | Solanum nigrum | ||
| 1- | Test for Carbohydrates (Molish test) | ++ ve | ++ ve |
| 2- | Test for Tannins (FeCl3 test) | + ve | ++ ve |
| 3- | Test for Flavonoids (NaOH/HCL test) | ++ ve | ++ ve |
| 4- | Test for Saponins (Froth test) | + ve | ++ ve |
| 5- | Test for Anthraquinon Glycosides (Born-trager test) | – ve | – ve |
| 6- | Test for Sterols and Triterpenoids (Salkowski test) | – ve | ++ ve |
| 7- | Test for Alkaloids (Dragendorff test) | – ve | ++ ve |
| 8- | Test for Volatile Oils (Sudan III test) | + ve | – ve |
| 9- | Test for Cardiac Glycosides (Keller–Killiani test) | – ve | – ve |
| 10- | Test for Cyanogenic Glycosides (Sodium picrate paper test) | – ve | – ve |
| Group No. | Pre Treatment | Mucosal Ulcer/Necrosis | Edema | Inflammatory Cells Infiltration |
|---|---|---|---|---|
| Group 1 | Normal control | – ve | – ve | – ve |
| Group 2 | Gastric ulcer control | +++ ve | +++ ve | ++ ve |
| Group 3 | Omeprazole (20 mg/kg, p.o.) | – ve | ++ ve | – ve |
| Group 4 | Misoprostol (300 µg/kg, p.o.) | – ve | ++ ve | + ve |
| Group 5 | Althaea officinalis (100 mg/kg, p.o.) | – ve | + ve | + ve |
| Group 6 | Solanum nigrum (200 mg/kg, p.o.) | – ve | ++ ve | + ve |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaghlool, S.S.; Abo-Seif, A.A.; Rabeh, M.A.; Abdelmohsen, U.R.; Messiha, B.A.S. Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats. Antioxidants 2019, 8, 512. https://doi.org/10.3390/antiox8110512
Zaghlool SS, Abo-Seif AA, Rabeh MA, Abdelmohsen UR, Messiha BAS. Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats. Antioxidants. 2019; 8(11):512. https://doi.org/10.3390/antiox8110512
Chicago/Turabian StyleZaghlool, Sameh S., Ali A. Abo-Seif, Mohamed A. Rabeh, Usama Ramadan Abdelmohsen, and Basim A. S. Messiha. 2019. "Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats" Antioxidants 8, no. 11: 512. https://doi.org/10.3390/antiox8110512
APA StyleZaghlool, S. S., Abo-Seif, A. A., Rabeh, M. A., Abdelmohsen, U. R., & Messiha, B. A. S. (2019). Gastro-Protective and Anti-Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin-Induced Ulceration in Rats. Antioxidants, 8(11), 512. https://doi.org/10.3390/antiox8110512






