The Role of Magnesium in the Secondary Phase After Traumatic Spinal Cord Injury. A Prospective Clinical Observer Study
Abstract
1. Introduction
2. Patients, Material, and Methods
2.1. Patient Demographics
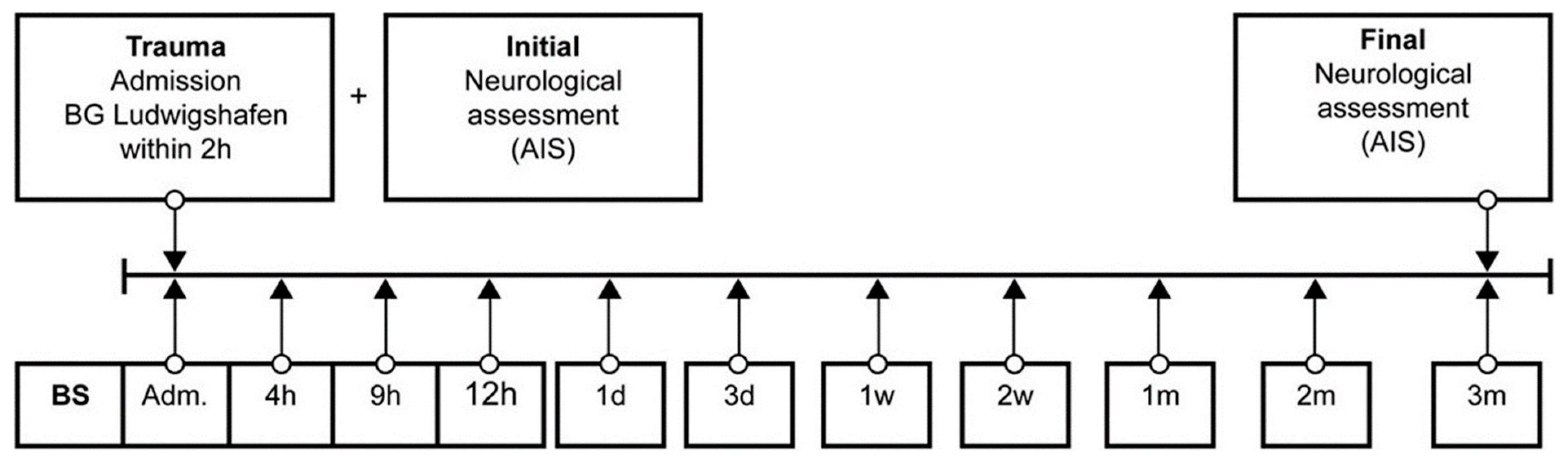
2.2. Material and Methods
3. Statistical Analysis
4. Results
4.1. Mg-Analysis
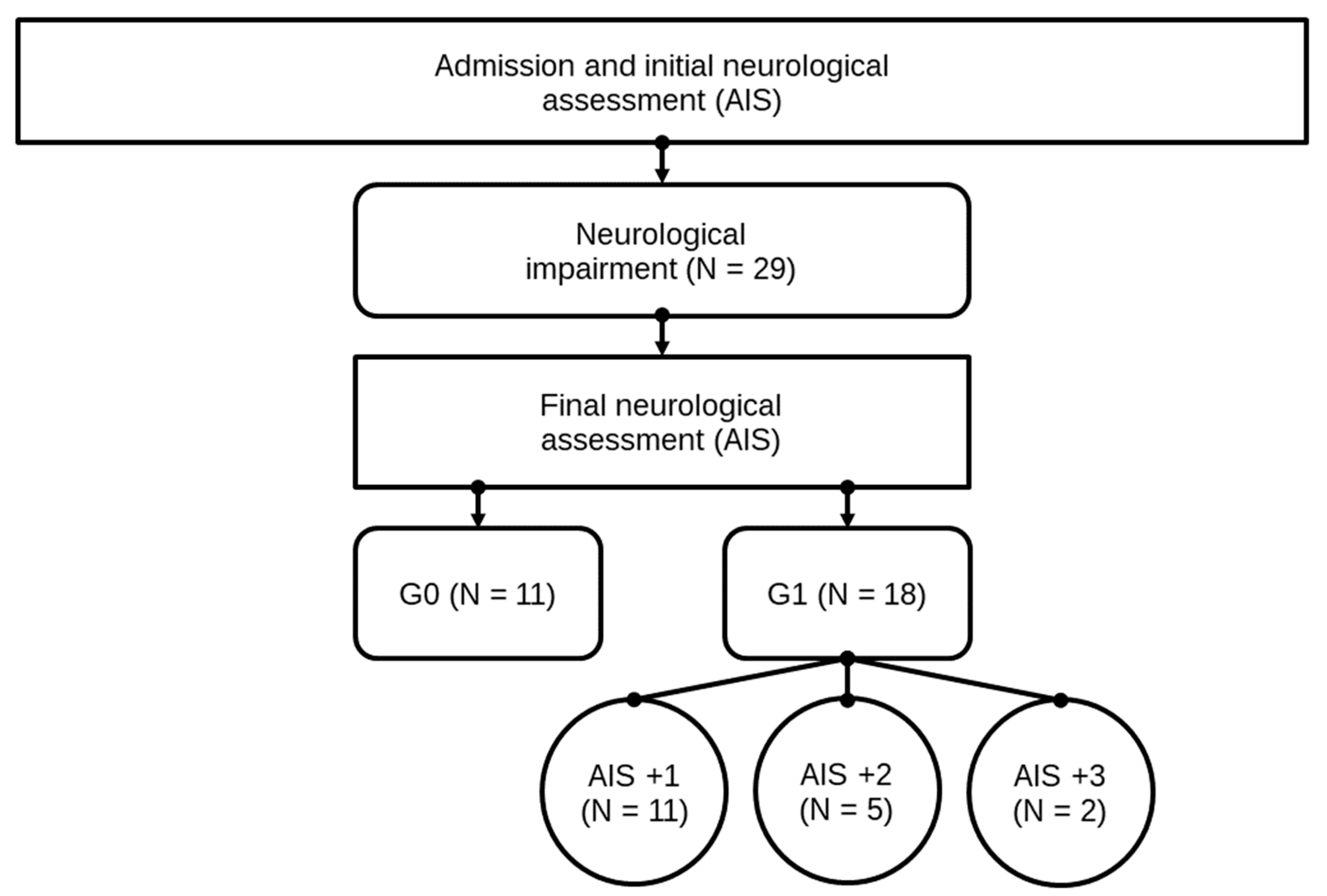
4.1.1. Entire Patient Collective
4.1.2. Comparison of Group G1 and G0
4.1.3. Comparison Within Group G1: AIS imp. = +1 and AIS imp. > +1
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. Off. J. N. Am. Spine Soc. 2004, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Child, C.; Bruckner, T.; Gerner, H.J.; Daniel, V.; Biglari, B. Posttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int. J. Mol. Sci. 2015, 16, 7900–7916. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Little, J.W.; Tessler, A.; Burns, A.S. Spinal shock revisited: A four-phase model. Spinal Cord 2004, 42, 383–395. [Google Scholar] [CrossRef]
- McKinley, W.; Meade, M.A.; Kirshblum, S.; Barnard, B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch. Phys. Med. Rehabil. 2004, 85, 1818–1825. [Google Scholar] [CrossRef]
- Burns, A.S.; Ditunno, J.F. Establishing prognosis and maximizing functional outcomes after spinal cord injury: A review of current and future directions in rehabilitation management. Spine 2001, 26, S137–S145. [Google Scholar] [CrossRef]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Romani, A.M.P. Intracellular magnesium homeostasis. In Magnesium in the Central Nervous System; Robert Vink, M.N., Ed.; University of Adelaide Press, Barr Smith Library, The University of Adelaide: Adelaide, Australia, 2011; pp. 13–58. [Google Scholar]
- Maguire, M.E. Hormone-sensitive magnesium transport and magnesium regulation of adenylate cyclase. Trends Pharmacol. Sci. 1984, 5, 73–77. [Google Scholar] [CrossRef]
- Romani, A.M.; Scarpa, A. Regulation of cellular magnesium. Front. Biosci. A J. Virtual Libr. 2000, 5, D720–D734. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.; Maguire, M.E. Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2002, 15, 271–283. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genetics 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. Ltrpc7 is a Mg. Atp-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Gunther, T.; Vormann, J. Mg2+ efflux is accomplished by an amiloride-sensitive Na+/Mg2+ antiport. Biochem. Biophys. Res. Commun. 1985, 130, 540–545. [Google Scholar] [CrossRef]
- Guther, T.; Vormann, J.; Forster, R. Regulation of intracellular magnesium by Mg2+ efflux. Biochem. Biophys. Res. Commun. 1984, 119, 124–131. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Noronha, J.L.; Matuschak, G.M. Magnesium in critical illness: Metabolism, assessment, and treatment. Intensive Care Med. 2002, 28, 667–679. [Google Scholar] [CrossRef]
- Ryan, M.F.; Barbour, H. Magnesium measurement in routine clinical practice. Ann. Clin. Biochem. 1998, 35, 449–459. [Google Scholar] [CrossRef]
- Lemke, M.; Demediuk, P.; McIntosh, T.K.; Vink, R.; Faden, A.I. Alterations in tissue Mg+, Na+ and spinal cord EDEMA following impact trauma in rats. Biochem. Biophys. Res. Commun. 1987, 147, 1170–1175. [Google Scholar] [CrossRef]
- Cernak, I.; Savic, V.J.; Kotur, J.; Prokic, V.; Veljovic, M.; Grbovic, D. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J. Neurotrauma 2000, 17, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Kaptanoglu, E.; Beskonakli, E.; Solaroglu, I.; Kilinc, A.; Taskin, Y. Magnesium sulfate treatment in experimental spinal cord injury: Emphasis on vascular changes and early clinical results. Neurosurg. Rev. 2003, 26, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Magerl, F.; Aebi, M.; Gertzbein, S.D.; Harms, J.; Nazarian, S. A comprehensive classification of thoracic and lumbar injuries. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 1994, 3, 184–201. [Google Scholar] [CrossRef]
- Heller, R.; Daniel, V.; Swing, T.; Akbar, M.; Gerner, H.J.; Biglari, B.; Moghaddam-Alvandi, A. P29 MMP-8 and MMP-9 serum levels as possible early markers for remission after traumatic spinal cord injury. Injury 2016, 47, S35. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.; Daniel, V.; Swing, T.; Akbar, M.; Gerner, H.J.; Biglari, B. Exploratory study to suggest the possibility of MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury. Spinal Cord 2017, 55, 8–15. [Google Scholar] [CrossRef]
- Moghaddam, A.; Sperl, A.; Heller, R.; Gerner, H.J.; Biglari, B. sCD95l in serum after spinal cord injury. Spinal Cord 2016, 54, 957–960. [Google Scholar] [CrossRef]
- Moghaddam, A.; Sperl, A.; Heller, R.; Kunzmann, K.; Graeser, V.; Akbar, M.; Gerner, H.J.; Biglari, B. Elevated serum insulin-like growth factor 1 levels in patients with neurological remission after traumatic spinal cord injury. PLoS ONE 2016, 11, e0159764. [Google Scholar] [CrossRef]
- Ferbert, T.; Child, C.; Graeser, V.; Swing, T.; Akbar, M.; Heller, R.; Biglari, B.; Moghaddam, A. Tracking spinal cord injury: Differences in cytokine expression of IGF-1, TGF- B1, and sCD95l can be measured in blood samples and correspond to neurological remission in a 12-week follow-up. J. Neurotrauma 2017, 34, 607–614. [Google Scholar] [CrossRef]
- Heller, R.A.; Raven, T.F.; Swing, T.; Kunzmann, K.; Daniel, V.; Haubruck, P.; Akbar, M.; Grutzner, P.A.; Schmidmaier, G.; Biglari, B.; et al. CCL-2 as a possible early marker for remission after traumatic spinal cord injury. Spinal Cord 2017, 55, 1002–1009. [Google Scholar] [CrossRef]
- Biglari, B.; Heller, R.A.; Hörner, M.; Sperl, A.; Bock, T.; Reible, B.; Haubruck, P.; Grützner, P.A.; Moghaddam, A. Novel approach to an early assessment of a patient’s potential for neurological remission after acute spinal cord injury: Analysis of hemoglobin concentration dynamics. J. Spinal Cord Med. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.A.; Seelig, J.; Bock, T.; Haubruck, P.; Grutzner, P.A.; Schomburg, L.; Moghaddam, A.; Biglari, B. Relation of selenium status to neuro-regeneration after traumatic spinal cord injury. J. Trace Elem. Med. Biol. 2019, 51, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; Buchler, A.; Swing, T.; Child, C.; Biehl, E.; Reitzel, T.; Bruckner, T.; Ferbert, T.; Korff, S.; Rief, H.; et al. Serum sCD95l concentration in patients with spinal cord injury. J. Int. Med. Res. 2015, 43, 250–256. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Chanimov, M.; Berman, S.; Gofman, V.; Weissgarten, Y.; Averbukh, Z.; Cohen, M.L.; Vitin, A.; Bahar, M. Total cell associated electrolyte homeostasis in rat spinal cord cells following apparently irreversible injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2006, 12, Br63–Br67. [Google Scholar]
- Naomi, L.; Cook, F.C. Corinna Van den Heuvel The role of magnesium in cns injury. In Magnesium in the Central Nervous System; Robert Vink, M.N., Ed.; University of Adelaide Press, Barr Smith Library, The University of Adelaide: Adelaid, Australia, 2011; pp. 167–179. [Google Scholar]
- Lemke, M.; Faden, A.I. Edema development and ion changes in rat spinal cord after impact trauma: Injury dose-response studies. J. Neurotrauma 1990, 7, 41–54. [Google Scholar] [CrossRef]
- Blache, D.; Devaux, S.; Joubert, O.; Loreau, N.; Schneider, M.; Durand, P.; Prost, M.; Gaume, V.; Adrian, M.; Laurant, P.; et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic. Biol. Med. 2006, 41, 277–284. [Google Scholar] [CrossRef]
- Regan, R.F.; Jasper, E.; Guo, Y.; Panter, S.S. The effect of magnesium on oxidative neuronal injury in vitro. J. Neurochem. 1998, 70, 77–85. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Anderson, D.K.; Panter, S.S.; Hallaway, P.E.; Eaton, J.W. Hemoglobin potentiates central nervous system damage. J. Clin. Investig. 1987, 79, 662–664. [Google Scholar] [CrossRef]
- Pazdernik, T.L.; Layton, M.; Nelson, S.R.; Samson, F.E. The osmotic/calcium stress theory of brain damage: Are free radicals involved? Neurochem. Res. 1992, 17, 11–21. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Nutrition; Trauma; the Brain. Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel; Erdman, J., Oria, M., Pillsbury, L., Eds.; National Academies Press (US) Copyright 2011 by the National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- Sen, A.P.; Gulati, A. Use of magnesium in traumatic brain injury. Neurotherapeutics 2010, 7, 91–99. [Google Scholar] [CrossRef]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Demediuk, P.; Panter, S.S.; Vink, R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugere, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Et Biophys. Acta 2000, 1501, 91–98. [Google Scholar] [CrossRef]
- Gok, B.; Okutan, O.; Beskonakli, E.; Kilinc, K. Effects of magnesium sulphate following spinal cord injury in rats. Chin. J. Physiol. 2007, 50, 93–97. [Google Scholar] [PubMed]
- Van den Heuvel, C.; Vink, R. The role of magnesium in traumatic brain injury. Clin. Calcium 2004, 14, 9–14. [Google Scholar]
- Feldman, Z.; Gurevitch, B.; Artru, A.A.; Oppenheim, A.; Shohami, E.; Reichenthal, E.; Shapira, Y. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J. Neurosurg. 1996, 85, 131–137. [Google Scholar] [CrossRef]
- Hoane, M.R. Magnesium therapy and recovery of function in experimental models of brain injury and neurodegenerative disease. Clin. Calcium 2004, 14, 65–70. [Google Scholar]
- Hoane, M.R.; Knotts, A.A.; Akstulewicz, S.L.; Aquilano, M.; Means, L.W. The behavioral effects of magnesium therapy on recovery of function following bilateral anterior medial cortex lesions in the rat. Brain Res. Bull. 2003, 60, 105–114. [Google Scholar] [CrossRef]
- Heath, D.L.; Vink, R. Optimization of magnesium therapy after severe diffuse axonal brain injury in rats. J. Pharmacol. Exp. Ther. 1999, 288, 1311–1316. [Google Scholar]
- Kwon, B.K.; Roy, J.; Lee, J.H.; Okon, E.; Zhang, H.; Marx, J.C.; Kindy, M.S. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: Preclinical refinement and optimization. J. Neurotrauma 2009, 26, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Ditor, D.S.; John, S.M.; Roy, J.; Marx, J.C.; Kittmer, C.; Weaver, L.C. Effects of polyethylene glycol and magnesium sulfate administration on clinically relevant neurological outcomes after spinal cord injury in the rat. J. Neurosci. Res. 2007, 85, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, D.B.; Dailey, A.T.; Lundin, D.; Zhou, J.; Lipson, A.; Falicov, A.; Shaffrey, C.I. Magnesium efficacy in a rat spinal cord injury model. J. Neurosurg. Spine 2009, 10, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Temkin, N.R.; Anderson, G.D.; Winn, H.R.; Ellenbogen, R.G.; Britz, G.W.; Schuster, J.; Lucas, T.; Newell, D.W.; Mansfield, P.N.; Machamer, J.E.; et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet. Neurol. 2007, 6, 29–38. [Google Scholar] [CrossRef]
- Dhandapani, S.S.; Gupta, A.; Vivekanandhan, S.; Sharma, B.S.; Mahapatra, A.K. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. Indian J. Neurotrauma 2008, 5, 27–33. [Google Scholar] [CrossRef]
- Vink, R.; Cook, N.L.; van den Heuvel, C. Magnesium in acute and chronic brain injury: An update. Magnes. Res. 2009, 22, 158S–162S. [Google Scholar] [CrossRef]



| All (n = 29) | G0 (n = 11) | G1 (n = 18) | p-Value | |
|---|---|---|---|---|
| Sex | 0.65 | |||
| female | 8 (28) | 2 (18) | 6 (33) | |
| male | 21 (72) | 9 (82) | 12 (67) | |
| Age | 0.62 | |||
| min | 15 | 22 | 15 | |
| max | 75 | 65 | 75 | |
| median (IQR) | 43 (23.00, 54.00) | 44 (27.00, 49.00) | 38.50 (21.00, 56.25) | |
| mean (95% CI) | 40.69 (34.07, 47.31) | 41.73 (32.72, 50.74) | 40.06 (30.73, 49.38) | |
| Etiology | 0.40 | |||
| fall | 19 (66) | 8 (73) | 11 (61) | |
| traffic | 8 (28) | 2 (18) | 6 (33) | |
| other | 2 (6) | 1 (9) | 1 (6) | |
| AO | 0.23 | |||
| A | 18 (62) | 5 (45) | 13 (72) | |
| B | 6 (21) | 4 (36) | 2 (11) | |
| C | 5 (17) | 2 (18) | 3 (17) | |
| NLI | 0.21 | |||
| cervical | 11 (38) | 5 (45) | 6 (33) | |
| thoracic | 10 (34) | 5 (45) | 5 (28) | |
| lumbar | 8 (28) | 1 (9) | 7 (39) | |
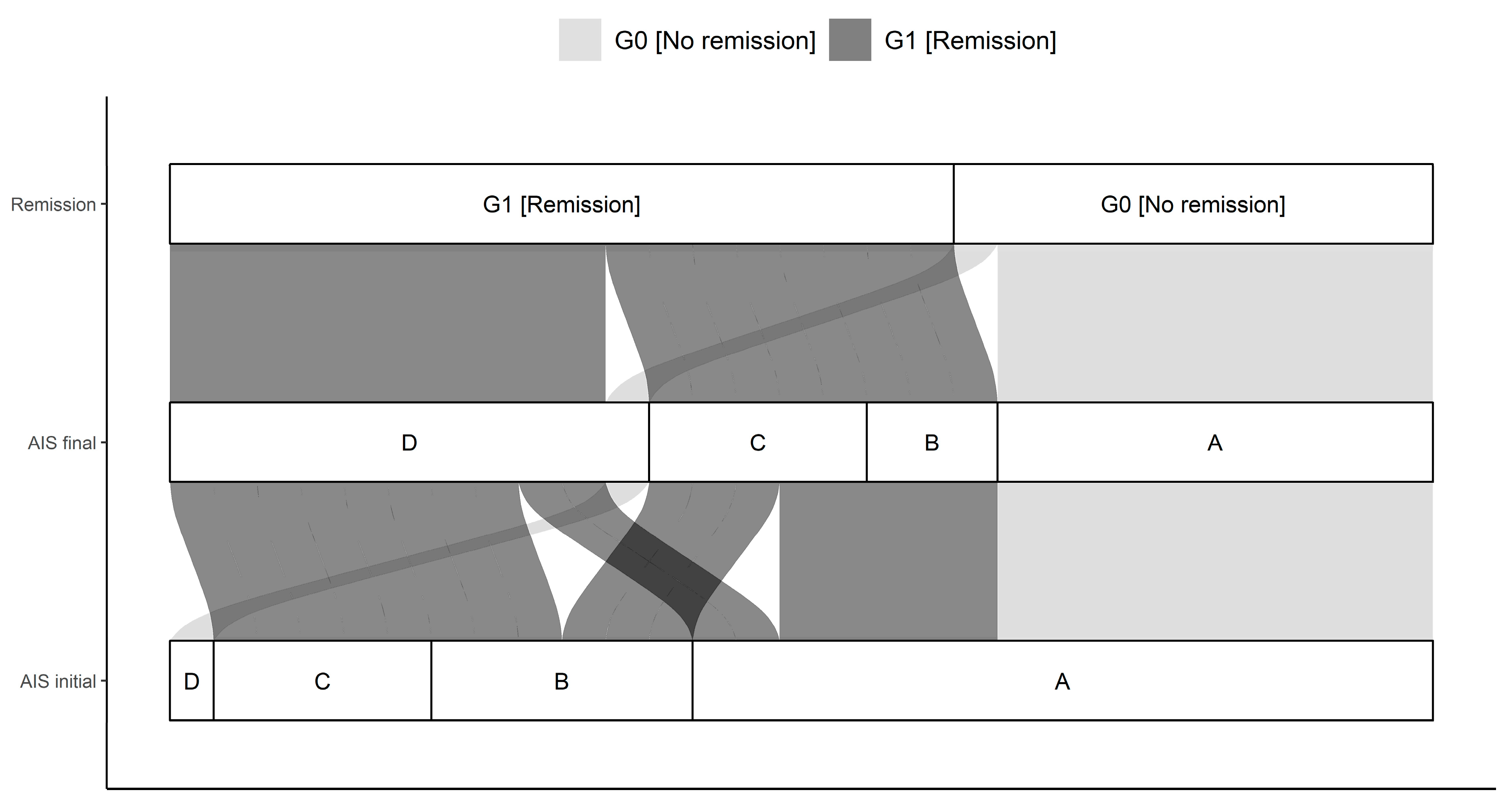
| AIS initial | <0.01 | |||
| A | 17 (59) | 10 (91) | 7 (39) | |
| B | 6 (21) | 0 (0) | 6 (33) | |
| C | 5 (17) | 0 (0) | 5 (28) | |
| D | 1 (3) | 1 (9) | 0 (0) | |
| AIS final | <0.01 | |||
| A | 10 (34) | 10 (91) | 0 (0) | |
| B | 3 (10) | 0 (0) | 3 (17) | |
| C | 5 (17) | 0 (0) | 5 (28) | |
| D | 11 (38) | 1 (9) | 10 (56) |
| AIS Grade | Clinical State |
|---|---|
| A | Complete—No motor or sensory function is preserved in the sacral segments S4–S5 |
| B | Incomplete—Sensory but not motor function is preserved below the NLI and includes the sacral segments S4–S5 |
| C | Incomplete—Motor function is preserved below the NLI, and more than half of the key muscles below the NLI have a muscle grade less than 3 |
| D | Incomplete—Motor function is preserved below the NLI, and at least half of the key muscles below the NLI have a muscle grade of 3 or more |
| E | Normal—Motor and sensory function is normal |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperl, A.; Heller, R.A.; Biglari, B.; Haubruck, P.; Seelig, J.; Schomburg, L.; Bock, T.; Moghaddam, A. The Role of Magnesium in the Secondary Phase After Traumatic Spinal Cord Injury. A Prospective Clinical Observer Study. Antioxidants 2019, 8, 509. https://doi.org/10.3390/antiox8110509
Sperl A, Heller RA, Biglari B, Haubruck P, Seelig J, Schomburg L, Bock T, Moghaddam A. The Role of Magnesium in the Secondary Phase After Traumatic Spinal Cord Injury. A Prospective Clinical Observer Study. Antioxidants. 2019; 8(11):509. https://doi.org/10.3390/antiox8110509
Chicago/Turabian StyleSperl, André, Raban Arved Heller, Bahram Biglari, Patrick Haubruck, Julian Seelig, Lutz Schomburg, Tobias Bock, and Arash Moghaddam. 2019. "The Role of Magnesium in the Secondary Phase After Traumatic Spinal Cord Injury. A Prospective Clinical Observer Study" Antioxidants 8, no. 11: 509. https://doi.org/10.3390/antiox8110509
APA StyleSperl, A., Heller, R. A., Biglari, B., Haubruck, P., Seelig, J., Schomburg, L., Bock, T., & Moghaddam, A. (2019). The Role of Magnesium in the Secondary Phase After Traumatic Spinal Cord Injury. A Prospective Clinical Observer Study. Antioxidants, 8(11), 509. https://doi.org/10.3390/antiox8110509







