Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity
Abstract
1. Introduction
2. Materials and Methods
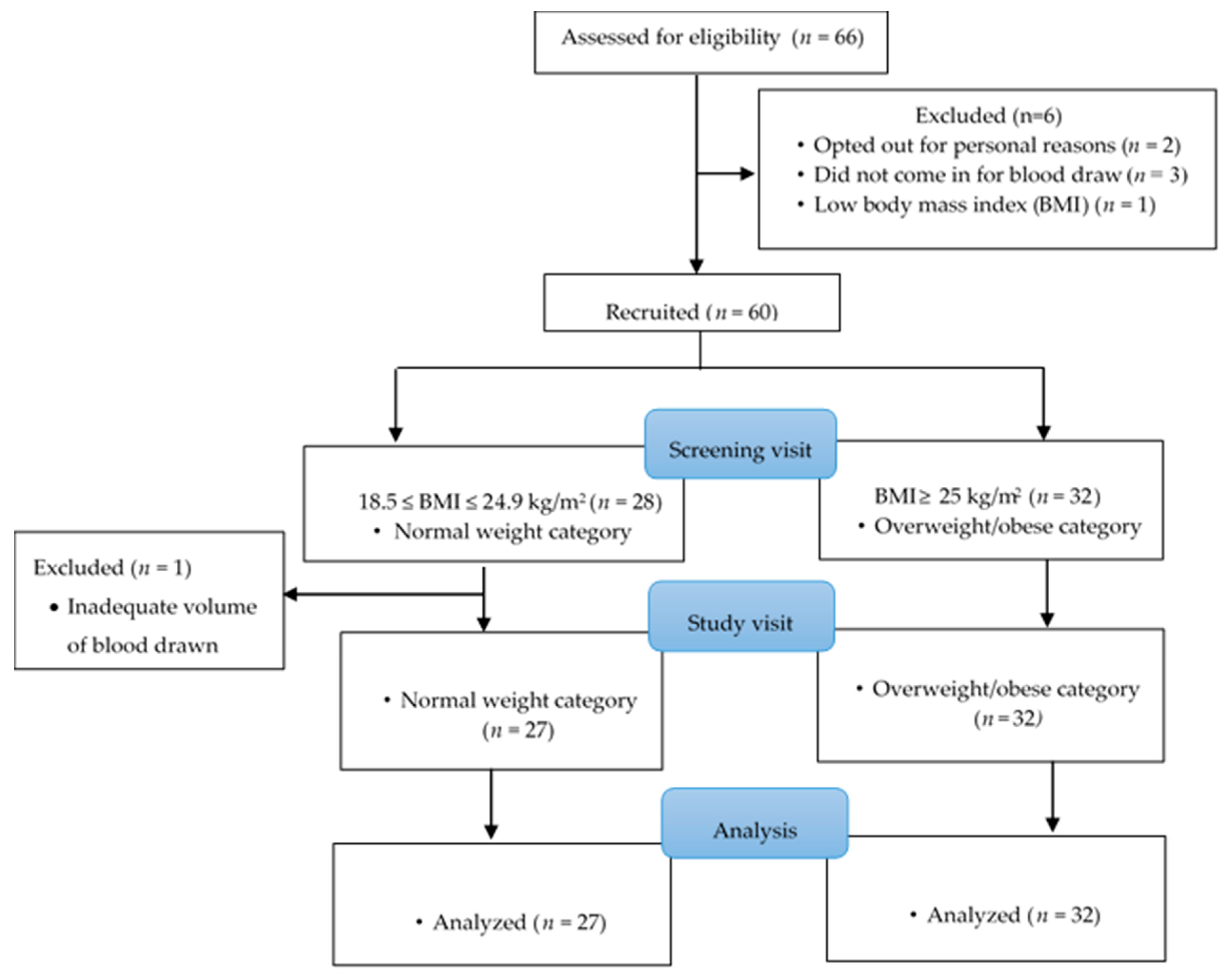
2.1. Study Participants and Recruitment
2.2. Study Design
2.3. Anthropometry
2.4. Dietary and Nutrient Intake
2.5. Sample Analyses
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, K.V. Epidemiology of Anemia in Older Adults. Semin. Hematol. 2008, 45, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Le, C.H.H. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012). PLoS ONE 2016, 11, e0166635. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; Petocz, P.; Samman, S. Micronutrient status in female university students: Iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients 2014, 6, 5103–5116. [Google Scholar] [CrossRef] [PubMed]
- Anemia of Inflammation & Chronic Disease | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases n.d. Available online: https://www.niddk.nih.gov/health-information/blood-diseases/anemia-inflammation-chronic-disease (accessed on 6 September 2018).
- Hunter, P. The inflammation theory of disease. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, L.T.; Kroot, J.J.; Tromp, M.; van der Hoeven, J.G.; Swinkels, D.W.; Pickkers, P. Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: An observational study. Crit. Care 2011, 15, R9. [Google Scholar] [CrossRef]
- Roy, C.N. Anemia of inflammation. Hematology 2010, 2010, 276–280. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Nemeth, E. Targeting the Hepcidin-Ferroportin Axis in the Diagnosis and Treatment of Anemias. Adv. Hematol. 2010, 2010, 750643. [Google Scholar] [CrossRef]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Méd. Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Madu, A.J.; Ughasoro, M.D. Anaemia of Chronic Disease: An In-Depth Review. Med. Princ. Pract. 2017, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Hegde, V.; Dubuisson, O.; Gao, Z.; Dhurandhar, N.V.; Ye, J. Interplay of pro- and anti-inflammatory cytokines to determine lipid accretion in adipocytes. Int. J. Obes. (Lond.) 2013, 37, 1490–14908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karatela, S.; Ward, N.I. Trace elements and human obesity: An overview. Manipal J. Nurs. Health Sci. 2016, 2, 10. [Google Scholar]
- Petkova-Marinova, T.V.; Ruseva, B.K.; Atanasova, B.D. Selenium deficiency as a risk factor for development of anemia. J. Biomed. Clin. Res. 2017, 10, 9–17. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Volp, A.C.P.; Marques-Rocha, J.L.; Ribeiro, S.M.R.; Navarro-Blasco, I.; Zulet, M.Á.; Martínez, J.A.; Bressan, J. Modulators of erythrocyte glutathione peroxidase activity in healthy adults: An observational study. Redox Rep. 2014, 19, 251–258. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.; Wang, T.; Jiang, H.; Guo, M.; Zhou, E.; Sun, Y.; Yang, Z.; Xu, S.; Cao, Y.; et al. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 2014, 37, 478–485. [Google Scholar] [CrossRef]
- Gürgöze, M.K.; Denizmen Aygün, A.; Ölçücü, A.; Doğan, Y.; Yılmaz, E. Plasma selenium status in children with iron deficiency anemia. J. Trace Elem. Med. Biol. 2004, 18, 193–196. [Google Scholar] [CrossRef]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.; Guralnik, J.; Fried, L.P. Serum antioxidants, inflammation, and total mortality in older women. Am. J. Epidemiol. 2006, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Van Nhien, N.; Yabutani, T.; Khan, N.C.; Khanh, L.N.B.; Ninh, N.X.; Chung, L.T.K.; Thuy, L.T.X.; Motonaka, J.; Nakaya, Y. Association of low serum selenium with anemia among adolescent girls living in rural Vietnam. Nutrition 2009, 25, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hardison, R.C.; Kennett, M.J.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. Selenoproteins regulate stress erythroid progenitors and spleen microenvironment during stress erythropoiesis. Blood 2018, 131, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Tron, K.; Novosyadlyy, R.; Dudas, J.; Samoylenko, A.; Kietzmann, T.; Ramadori, G. Upregulation of heme oxygenase-1 gene by turpentine oil-induced localized inflammation: Involvement of interleukin-6. Lab. Investig. 2005, 85, 376–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Houghton, L.A.; Parnell, W.R.; Thomson, C.D.; Green, T.J.; Gibson, R.S. Serum zinc Is a major predictor of anemia and mediates the effect of selenium on hemoglobin in school-aged children in a nationally representative survey in New Zealand. J. Nutr. 2016, 146, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Health and Nutrition Health Survey. Anthropometry Procedures Manual n.d. Version Current January 2007. Available online: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/anthro.pdf (accessed on 7 September 2018).
- Armah, S.M.; Boy, E.; Chen, D.; Candal, P.; Reddy, M.B. Regular Consumption of a High-Phytate Diet Reduces the Inhibitory Effect of Phytate on Nonheme-Iron Absorption in Women with Suboptimal Iron Stores. J. Nutr. 2015, 145, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.; Cook, R.; O’Connor, H.; Rooney, K.; Steinbeck, K. The relationship between obesity and hypoferraemia in adults: A systematic review. Obes. Rev. 2012, 13, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.T.; Hamed, A.I.; Kharshoum, R.R. Iron homeostasis and serum hepcidin-25 levels in obese children and adolescents: Relation to body mass index. Horm. Res. Paediatr. 2013, 80, 11–17. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef]
- Nikonorov, A.A.; Skalnaya, M.G.; Tinkov, A.A.; Skalny, A.V. Mutual interaction between iron homeostasis and obesity pathogenesis. J. Trace Elem. Med. Biol. 2015, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Vaja, V.; Babitt, J.L.; Lin, H.Y. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am. J. Hematol. 2012, 87, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.E.; Rooney, K.B.; Steinbeck, K.S.; Griffin, H.J.; Petocz, P.; O’Connor, H.T. Iron, hepcidin and inflammatory status of young healthy overweight and obese women in Australia. PLoS ONE 2013, 8, e68675. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Matoušková, P.; Hanousková, B.; Skálová, L. MicroRNAs as potential regulators of glutathione peroxidases expression and their role in obesity and related pathologies. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Allman-Farinelli, M. Association between antioxidant intake/status and obesity: A systematic review of observational studies. Biol. Trace Elem. Res. 2017, 175, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. (Eds.) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Combs, G.F. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Petrova, J.; Manolov, V.; Hadjidekova, S.; Yonova, D.; Bogov, B.; Vasilev, V.; Tzatchev, K.; Petrova, M.; Vazelov, E.; Georgieva, I. Quantification of hepcidin, Se, Zn, SOD and GPX in chronic kidney disease patients with ischemic stroke. Austin J. Cardiovasc. Dis. Atheroscler. 2017, 4, 1032. [Google Scholar]
- Manolov, V.; Hadjidekova, S.; Petrova, J.; Vasilev, V.; Petrova, M.; Traykov, L.; Tzatchev, K.; Borislav, M.; Grozdanova, R.E.; Bogov, I. The role of iron homeostasis in alzheimer’s disease. J. Alzheimers Neurodegener. Dis. 2017, 3, 1–4. [Google Scholar] [CrossRef]
| Nutrient/Calories | Normal (n = 27) | Overweight/Obese (n = 32) | Total | p-Value 2 |
|---|---|---|---|---|
| Caloric intake, kcal | 1844 (1423, 2611) | 1878 (1347, 2234) | 1875 (1357, 2304) | 0.667 |
| Total fat, g | 66.28 (56, 107) | 80.67 (53, 98) | 72.01 (54, 104) | 0.982 |
| Protein, g | 70.52 (50, 97) | 73.1 (52, 96) | 73.16 (51, 97) | 0.994 |
| Selenium, mcg | 99.42 (70, 151) | 102 (83, 153) | 101.82 (79, 152) | 0.645 |
| Iron, mg | 17.24 (10, 20) | 12.41 (10, 16) | 13.33 (10, 19) | 0.272 |
| Zinc, mg | 8.97 (6, 16) | 9.28 (7, 12) | 9.19 (6, 14) | 0.886 |
| Vitamin A, mcg | 352.51 (214, 442) | 318.71 (180, 427) | 352.51 (190, 430) | 0.667 |
| Vitamin C, mg | 88 (52, 124) | 43.37 (19, 95) | 57.44 (34, 103) | 0.028 * |
| Vitamin E, mg | 11.22 (7, 21) | 9.39 (7, 12) | 10.53 (7, 13) | 0.19 |
| BMI/Biochemical Markers | Normal (n = 27) | Overweight/Obese (n = 32) | Total | p-Value 2 |
|---|---|---|---|---|
| Mean | Mean | Mean | ||
| BMI, kg/m2 | 22.43 ± 0.34 | 29.28 ± 0.74 | 26.15 ± 0.62 | <0.001 *** |
| Erythrocyte GPX activity, nmol/min/mL | 1001 ± 76 | 1077 ± 75 | 1042 ± 523 | 0.766 |
| Plasma GPX activity, nmol/min/mL | 84.29 ± 3.59 | 79.48 ± 3.31 | 81.75 ± 2.41 | 0.169 |
| Hemoglobin 3, g/dL | 13.48 ± 0.30 | 13.25 ± 0.35 | 13.35 ± 0.23 | 0.616 |
| Transferrin saturation, % | 29.33 ± 2.98 | 26.59 ± 2.21 | 27.85 ± 1.79 | 0.456 |
| Serum iron, µg/dL | 98.37 ± 8.96 | 91.06 ± 7.55 | 94.41 ± 5.71 | 0.528 |
| Ferritin 4, ng/mL | 36.2 (13, 101) | 41.07 (13,136) | 38.76 (13, 118) | 0.677 |
| TIBC 4, µg/dL | 350.37 (284, 432) | 347.56 (296, 407) | 348.84 (287, 420) | 0.874 |
| Plasma selenium 4, ng/mL | 114.38 (90, 145) | 112.06 (92, 137) | 113.1 (91, 141) | 0.363 |
| SEPP1 4, ng/mL | 360.77 (290, 450) | 352.13 (276, 446) | 356.05 (284, 450) | 0.347 |
| Hepcidin 4, ng/mL | 9.95 (3, 29) | 15.09 (3, 67) | 12.47 (3, 47) | 0.218 |
| Demographic and Biochemical Indicators | Hepcidin ng/mL (n = 55) 1 | Serum Iron, ug/dL (n = 55) | Transferrin Saturation, % (n = 55) | Hemoglobin, g/dL (n = 54) | ||||
|---|---|---|---|---|---|---|---|---|
| Predictors | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p |
| Plasma GPX activity, nmol/min/mL | −0.02 ± 0.01 | 0.009 | −0.24 ± 0.33 | 0.474 | −0.03 ± 0.09 | 0.705 | −0.01 ± 0.01 | 0.208 |
| Ferritin, ng/mL 1 | 1.01 ± 0.12 | <0.001 | 13.26 ± 6.07 | 0.035 | 6.58 ± 1.59 | <0.001 | 0.51 ± 0.16 | 0.002 |
| C-reactive protein, mg/L 1 | 0.02 ± 0.09 | 0.849 | −6.47 ± 4.53 | 0.161 | −2.23 ± 1.18 | 0.067 | −0.08 ± 0.12 | 0.483 |
| Erythrocyte GPX activity, nmol/min/mL | −0.00 ± 0.00 | 0.776 | −0.00 ± 0.01 | 1.000 | 0.00 ± 0.00 | 0.839 | 0.00 ± 0.00 | 0.808 |
| Selenoprotein P, ng/mL 1 | −1.23 ± 0.54 | 0.028 | −26.15 ± 26.74 | 0.334 | −4.26 ± 6.99 | 0.545 | −0.93 ± 0.70 | 0.192 |
| Gender | ||||||||
| Male | −0.38 ± 0.32 | 0.245 | 20.92 ± 15.80 | 0.193 | 6.07 ± 4.13 | 0.149 | 1.94 ± 0.41 | <0.001 |
| Age, years | 0.01 ± 0.04 | 0.887 | 1.65 ± 1.85 | 0.376 | 0.37 ± 0.48 | 0.454 | 0.05 ± 0.05 | 0.343 |
| Weight status, kg/m 2 | ||||||||
| Overweight/obese | 0.23 ± 0.26 | 0.386 | 0.14 ± 12.77 | 0.991 | −0.07 ± 3.34 | 0.984 | 0.30 ± 0.34 | 0.383 |
| Ethnicity | ||||||||
| Black | −0.12 ± 0.31 | 0.699 | −10.00 ± 15.17 | 0.513 | −3.92 ± 3.96 | 0.328 | −1.27 ± 0.41 | 0.003 |
| Hispanic | −0.46 ± 0.40 | 0.262 | −25.83 ± 19.88 | 0.201 | −9.34 ± 5.19 | 0.079 | −0.73 ± 0.53 | 0.173 |
| Other Race 2 | 0.33 ± 0.37 | 0.376 | −4.22 ± 18.22 | 0.818 | −4.93 ± 4.76 | 0.307 | −0.54 ± 0.49 | 0.273 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larvie, D.Y.; Doherty, J.L.; Donati, G.L.; Armah, S.M. Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity. Antioxidants 2019, 8, 463. https://doi.org/10.3390/antiox8100463
Larvie DY, Doherty JL, Donati GL, Armah SM. Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity. Antioxidants. 2019; 8(10):463. https://doi.org/10.3390/antiox8100463
Chicago/Turabian StyleLarvie, Doreen Yvonne, Jeanne Lynn Doherty, George L. Donati, and Seth Mensah Armah. 2019. "Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity" Antioxidants 8, no. 10: 463. https://doi.org/10.3390/antiox8100463
APA StyleLarvie, D. Y., Doherty, J. L., Donati, G. L., & Armah, S. M. (2019). Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity. Antioxidants, 8(10), 463. https://doi.org/10.3390/antiox8100463





