Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
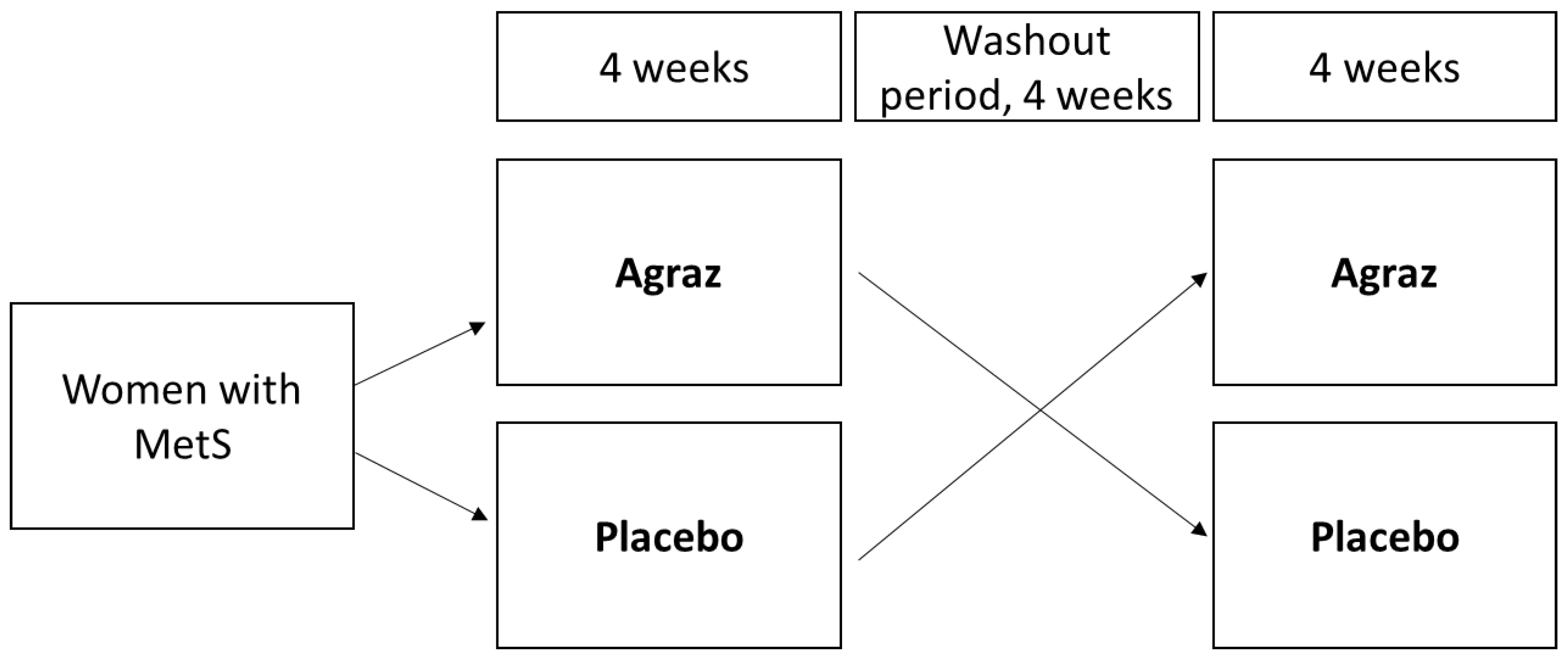
2.2. Experimental Design
2.3. Blood Collection and Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.4. Anthropometric and Blood Pressure Measurements
2.5. Biochemical Markers
2.6. PON1 Arylesterase Activity
2.7. PON1 Lactonase Activity
2.8. Myeloperoxidase (MPO)
2.9. ApoB Precipitation
2.10. Advanced Oxidation Protein Products (AOPP)
2.11. Cholesterol Efflux
2.12. Inflammatory Markers
2.13. Statistical Analysis
3. Results
3.1. Participant Characteristics and MetS Criteria
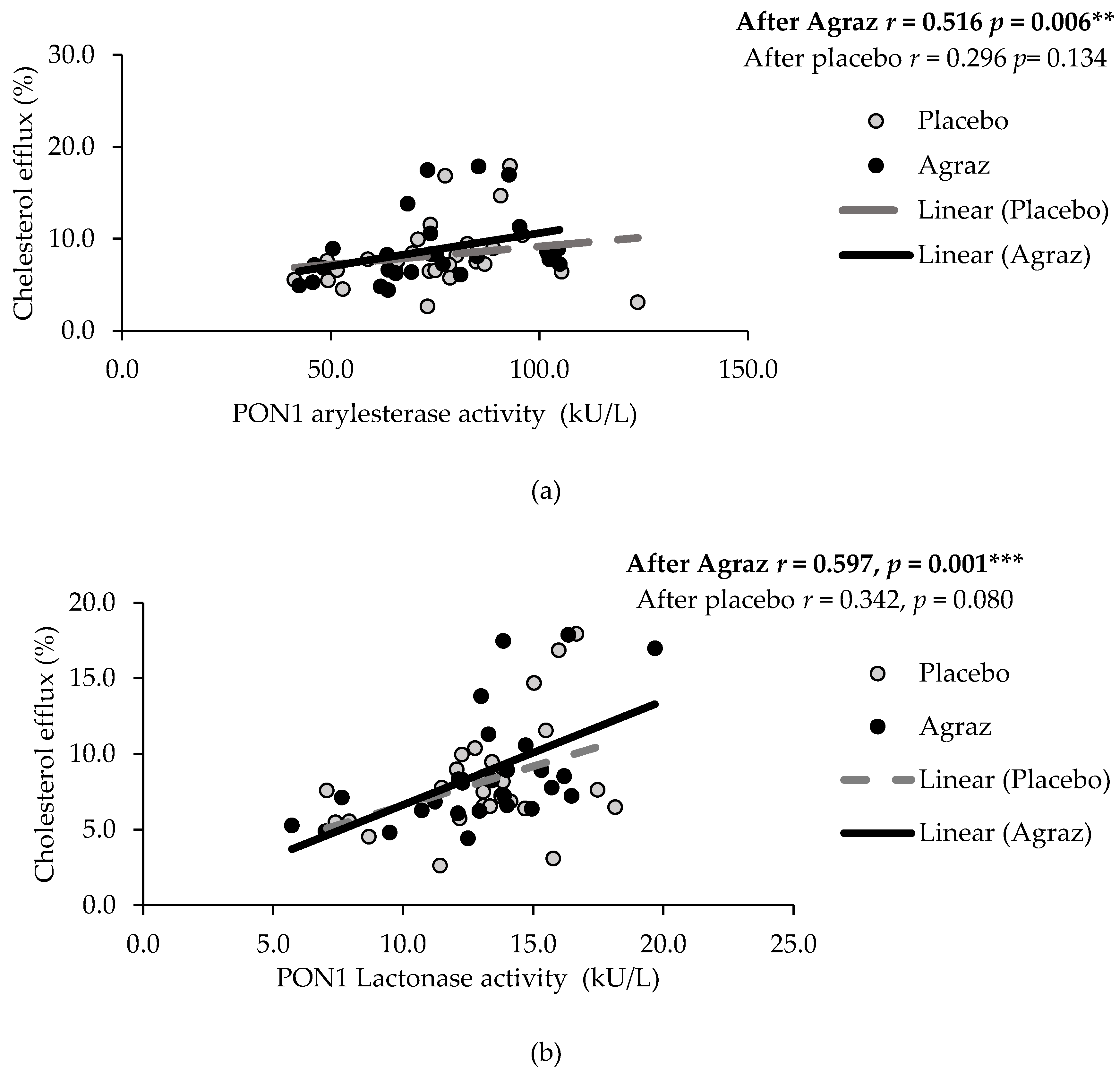
3.2. HDL Function and Related Oxidative Markers
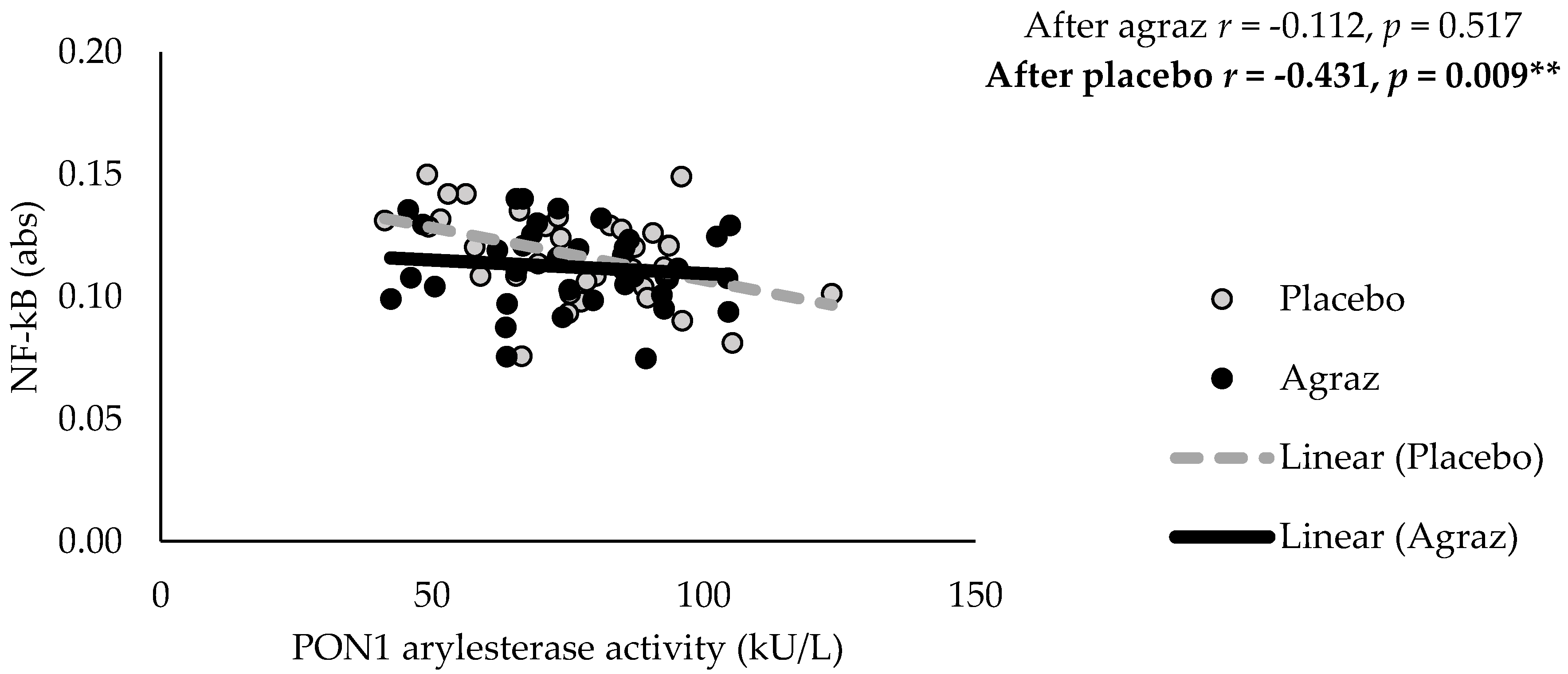
3.3. Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- WHO. Disease Burden and Mortality Estimates. Cause-Specific Mortality, 2000–2015. Global Health Estimates 2015: Estimated Deaths by Cause and Region, 2000 and 2015 (xls). Available online: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 8 May 2018).
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- Mohammadi, M.; Gozashti, M.H.; Aghadavood, M.; Mehdizadeh, M.R.; Hayatbakhsh, M.M. Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep. Biochem. Mol. Biol. 2017, 6, 74–79. [Google Scholar] [PubMed]
- Kim, S.-H.; Lee, J.-W.; Im, J.-A.; Hwang, H.-J. Monocyte chemoattractant protein-1 is related to metabolic syndrome and homocysteine in subjects without clinically significant atherosclerotic cardiovascular disease. Scand. J. Clin. Lab. Investig. 2011, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-J.; Lee, K.H.; Chung, J.H.; Park, Y.K.; Choi, M.K.; Oh, J.; Choi, J.W.; Lee, S.-H.; Chung, N.; Kang, S.-M. Circulating IL-8 levels in heart failure patients with and without metabolic syndrome. Clin. Chim. Acta 2009, 405, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.G.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High-Density Lipoprotein Cholesterol, High-Density Lipoprotein Particle Size, and Apolipoprotein A-I: Significance for Cardiovascular Risk. J. Am. Coll. Cardiol. 2008, 51, 634–642. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Gansevoort, R.T.; Sparks, C.E.; Dullaart, R.P.F. Inflammation reduces HDL protection against primary cardiac risk. Eur. J. Clin. Investig. 2010, 40, 483–489. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Hama, S.Y.; de Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic Syndrome Is Associated with Elevated Oxidative Stress and Dysfunctional Dense High-Density Lipoprotein Particles Displaying Impaired Antioxidative Activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; Dikkers, A.; de Boer, J.F.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; Schalkwijk, C.G.; Stehouwer, C.D.A.; Dullaart, R.P.F.; Tietge, U.J.F. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: The CODAM study. Sci. Rep. 2016, 6, 27367. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.A.; Vindis, C.; Hansel, B.; Nègre-Salvayre, A.; Therond, P.; Serrano, C.V.; Chantepie, S.; Salvayre, R.; Bruckert, E.; Chapman, M.J.; et al. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis 2008, 197, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Vaya, J.; Shih, D.; Aviram, M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis 2005, 179, 69–77. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Escher, G.; D’Souza, W.; Tchoua, U.; Grant, A.; Krozowski, Z.; Bukrinsky, M.; Sviridov, D. Enhancing apolipoprotein A-I-dependent cholesterol efflux elevates cholesterol export from macrophages in vivo. J. Lipid Res. 2008, 49, 2312–2322. [Google Scholar] [CrossRef]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef]
- Hine, D.; Mackness, B.; Mackness, M. Coincubation of PON1, APO A1, and LCAT increases the time HDL is able to prevent LDL oxidation. IUBMB Life 2012, 64, 157–161. [Google Scholar] [CrossRef]
- Aharoni, S.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013, 228, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K.; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Settle, M.; Brubaker, G.; Schmitt, D.; Hazen, S.L.; Smith, J.D.; Kinter, M. Localization of Nitration and Chlorination Sites on Apolipoprotein A-I Catalyzed by Myeloperoxidase in Human Atheroma and Associated Oxidative Impairment in ABCA1-dependent Cholesterol Efflux from Macrophages. J. Biol. Chem. 2005, 280, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L.; Heinecke, J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Investig. 1997, 99, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Velarde, G.P.; Sherazi, S.; Kraemer, D.F.; Bravo-Jaimes, K.; Butterfield, R.; Amico, T.; Steinmetz, S.D.; Guzman, M.; Martin, D.; Dodani, S.; et al. Clinical and Biochemical Markers of Cardiovascular Structure and Function in Women With the Metabolic Syndrome. Am. J. Cardiol. 2015, 116, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Capeillère-Blandin, C.; Gausson, V.; Descamps-Latscha, B.; Witko-Sarsat, V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2004, 1689, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Venturini, D.; Simão, A.N.C.; Dichi, I. Advanced oxidation protein products are more related to metabolic syndrome components than biomarkers of lipid peroxidation. Nutr. Res. 2015, 35, 759–765. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of Wonderful Variety Pomegranate Juice and Extract by Diabetic Patients Increases Paraoxonase 1 Association with High-Density Lipoprotein and Stimulates Its Catalytic Activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.-K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Lee, W.; Shin, S.; Kim, G.-Y.; Choi, B.; Choi, Y. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Lopera, Y.E.; Fantinelli, J.; González Arbeláez, L.F.; Rojano, B.; Ríos, J.L.; Schinella, G.; Mosca, S. Antioxidant Activity and Cardioprotective Effect of a Nonalcoholic Extract of Vaccinium meridionale Swartz during Ischemia-Reperfusion in Rats. Evid.-Based Complement. Altern. Med. ECAM 2013, 2013, 516727. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Espinosa-Moncada, J.; Marín-Echeverri, C.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Aristizábal-Rivera, J.C.; Blesso, C.N.; Fernandez, M.L.; Barona-Acevedo, J. Evaluation of agraz consumption on adipocytokines, inflammation and oxidative stress markers in women with metabolic syndrome. Nutrients 2018, 10, 1639. [Google Scholar] [CrossRef]
- Álvarez Monsalve, J.M.; González Zapata, L.I. Diseño de un cuestionario de frecuencia para evaluar ingesta alimentaria en la Universidad de Antioquia, Colombia. Nutr. Hosp. 2011, 26, 1333–1344. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Garcia, C.; Norris, G.H.; Lemos, B.S.; DiMarco, D.M.; Fernandez, M.L.; Blesso, C.N. Effects of Freeze-Dried Grape Powder on High-Density Lipoprotein Function in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Study. Metab. Syndr. Relat. Disord. 2018, 16, 464–469. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Attias, J.; Mahamid, R.; Aviram, M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010, 1, 99–109. [Google Scholar] [CrossRef]
- Gouedard, C.; Barouki, R.; Morel, Y. Dietary Polyphenols Increase Paraoxonase 1 Gene Expression by an Aryl Hydrocarbon Receptor-Dependent Mechanism. Mol. Cell. Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Toh, R.; Hasokawa, M.; Nakajima, H.; Honjo, T.; Otsui, K.; Mori, K.; Miyamoto-Sasaki, M.; Shinohara, M.; Nishimura, K.; et al. Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis 2014, 234, 288–294. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Abete, I.; Navas-Carretero, S.; Massis-Zaid, A.; Martinez, J.A.; Zulet, M.A. Oxidised LDL levels decreases after the consumption of ready-to-eat meals supplemented with cocoa extract within a hypocaloric diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 416–422. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Majewski, W.; Strzyżewski, K.W.; Kasprzak, M.P.; Iskra, M. Advanced Oxidation Protein Products and Carbonylated Proteins as Biomarkers of Oxidative Stress in Selected Atherosclerosis-Mediated Diseases. BioMed Res. Int. 2017, 2017, 4975264. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Rodriguez, L.C.; Hammond, L.; Disilvestro, R.; Hunter, J.M.; Pietrzkowski, Z. Acute reduction of serum 8-iso-PGF2-alpha and advanced oxidation protein products in vivo by a polyphenol-rich beverage; a pilot clinical study with phytochemical and in vitro antioxidant characterization. Nutr. J. 2011, 10, 67. [Google Scholar] [CrossRef]
- Simão, T.N.C.; Lozovoy, M.A.B.; Simão, A.N.C.; Oliveira, S.R.; Venturini, D.; Morimoto, H.K.; Miglioranza, L.H.S.; Dichi, I. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br. J. Nutr. 2013, 110, 1885–1894. [Google Scholar] [CrossRef]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Singh, P.; Anantharamaiah, G.M.; Chait, A.; Brunzell, J.; et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J.; et al. Flavonoids as Substrates and Inhibitors of Myeloperoxidase: Molecular Actions of Aglycone and Metabolites. Chem. Res. Toxicol. 2008, 21, 1600–1609. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Tian, R.; Peng, Y.-Y. Inhibitive Effects of Quercetin on Myeloperoxidase-Dependent Hypochlorous Acid Formation and Vascular Endothelial Injury. J. Agric. Food Chem. 2018, 66, 4933–4940. [Google Scholar] [CrossRef]
- Karlsen, A.; Paur, I.; Bøhn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Yilmaz, H.; Sayar, N.; Yilmaz, M.; Gurkan, U.; Sesal, C.; Tosu, R.; Cakmak, N.; Erer, B.; Oz, D.; Ciloglu, F.; et al. Serum paraoxonase 1 activity in women with metabolic syndrome. Kardiol. Pol. 2010, 68, 1219–1224. [Google Scholar]
- Han, C.Y.; Chiba, T.; Campbell, J.S.; Fausto, N.; Chaisson, M.; Orasanu, G.; Plutzky, J.; Chait, A. Reciprocal and Coordinate Regulation of Serum Amyloid A Versus Apolipoprotein A-I and Paraoxonase-1 by Inflammation in Murine Hepatocytes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1806–1813. [Google Scholar] [CrossRef]
- Du, C.; Shi, Y.; Ren, Y.; Wu, H.; Yao, F.; Wei, J.; Hou, Y.; Wu, M. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells. Drug Des. Dev. Ther. 2015, 9, 5099–5113. [Google Scholar]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef]
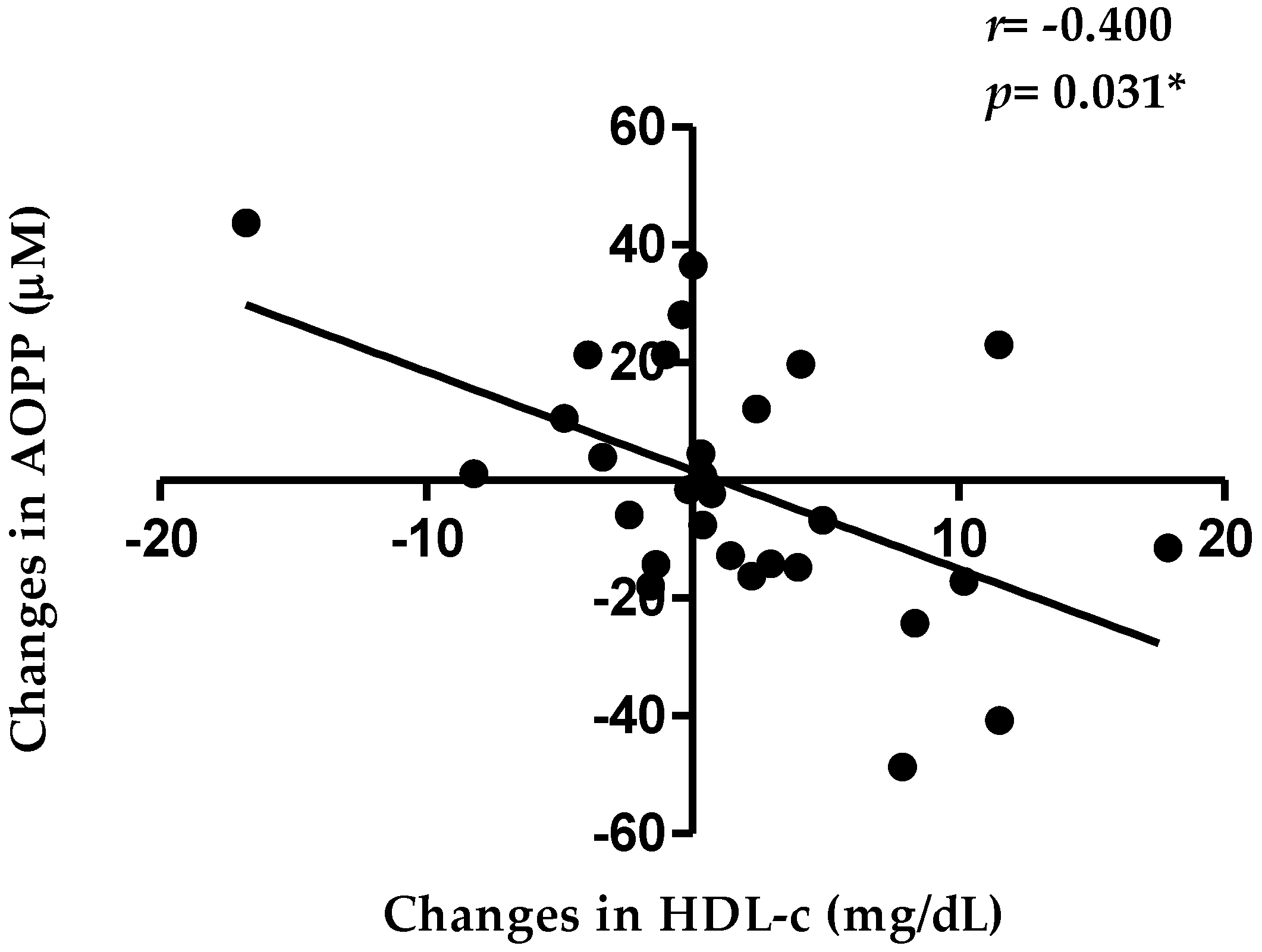
| Variables | Mean ± SD | ||
|---|---|---|---|
| Age (years) | 47.2 | ± | 9.4 |
| Waist circumference (cm) | 102 | ± | 9.2 |
| Systolic blood pressure (mm Hg) | 118.1 | ± | 12.5 |
| Diastolic blood pressure (mm Hg) | 76.1 | ± | 9.3 |
| Fasting glucose (mg/dL) | 94.2 | ± | 7.3 |
| HDL-c (mg/dL) | 42.2 | ± | 6.4 |
| Triglycerides (mg/dL) | 220.6 | ± | 88.9 |
| Variables | Placebo | Agraz | Δ Change (Agraz-Placebo) Mean ± SD | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||||||||
| HDL function markers | ||||||||||||
| Apo A1 (mg/dL) | 34 | 127.6 | ± | 43.1 | 29 | 132 | ± | 49 | 3.1 | ± | 40.8 | 0.597 |
| PON1 Arylesterase Activity (kU/L) | 38 | 77.1 | ± | 17.5 | 38 | 76.5 | ± | 17.5 | −0.7 | ± | 8.8 | 0.643 |
| PON1 Lactonase Activity (kU/L) | 38 | 12.6 | ± | 2.7 | 38 | 12.6 | ± | 2.8 | 0.2 | ± | 1.6 | 0.862 |
| Cholesterol efflux (%) | 27 | 8.2 | ± | 3.6 | 27 | 8.7 | ± | 3.8 | 0.5 | ± | 2.9 | 0.324 |
| HDL-related oxidative markers | ||||||||||||
| MPO (ng/mL) | 34 | 177.8 | ± | 74.6 | 34 | 175 | ± | 72.7 | −11.1 | ± | 72 | 0.795 |
| MPO/PON1 arylesterase ratio | 34 | 2.7 | ± | 1.6 | 34 | 2.6 | ± | 1.3 | −0.1 | ± | 1.2 | 0.770 |
| MPO/PON1 lactonase ratio | 34 | 15.5 | ± | 7.4 | 34 | 14.9 | ± | 6.9 | −0.7 | ± | 6.7 | 0.515 |
| AOPP (µM) | 29 | 99.5 | ± | 20.9 | 29 | 97.5 | ± | 17 | −2.0 | ± | 19.8 | 0.703 |
| Variables | Placebo | Agraz | Δ Change (Agraz-Placebo) Mean ± SD | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||||||||
| IL-1β (pg/mL) | 37 | 0.8 | ± | 0.4 | 37 | 0.8 | ± | 0.4 | 0.0 | ± | 0.2 | 0.748 |
| IL-6 (pg/mL) | 37 | 2.6 | ± | 2.1 | 37 | 2.1 | ± | 1.2 | −0.5 | ± | 1.5 | 0.271 |
| IL-8 (pg/mL) | 37 | 12.6 | ± | 5.6 | 37 | 12.1 | ± | 5.5 | −0.3 | ± | 2.6 | 0.322 |
| MCP-1 (pg/mL) | 38 | 251 | ± | 103 | 38 | 248.3 | ± | 106.6 | −2.6 | ± | 47.3 | 0.479 |
| TNF-α (pg/mL) | 38 | 4.7 | ± | 1.8 | 38 | 4.6 | ± | 1.5 | −0.1 | ± | 0.8 | 0.257 |
| NF-κB (abs) | 38 | 0.1 | ± | 0.02 | 38 | 0.1 | ± | 0.02 | 0.0 | ± | 0.02 | 0.290 |
| Changes in Variables | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | MCP-1 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|
| Apo A1 (mg/dL) | 0.151 | 0,022 | 0.056 | −0.087 | 0.030 |
| PON1 Arylesterase Activity (kU/L) | 0.215 | −0.273 | −0.106 | −0.060 | −0.012 |
| PON1 Lactonase Activity (kU/L) | 0.060 | −0.390* | −0.169 | 0.145 | −0.213 |
| MPO (ng/mL) | 0.102 | 0.707 *** | 0.338 | 0.413 * | 0.196 |
| MPO/PON1 arylesterase ratio | 0.097 | 0.682 *** | 0.349 | 0.393 * | 0.229 |
| MPO/PON1 lactonase ratio | 0.099 | 0.701 *** | 0.323 | 0.295 | 0.202 |
| AOPP (µM) | 0.098 | 0.080 | −0.228 | 0.170 | −0.087 |
| Cholesterol efflux (%) | −0.594 *** | −0.283 | −0.128 | −0.148 | −0.496 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Echeverri, C.; Blesso, C.N.; Fernández, M.L.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal, J.C.; Barona-Acevedo, J. Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome. Antioxidants 2018, 7, 185. https://doi.org/10.3390/antiox7120185
Marín-Echeverri C, Blesso CN, Fernández ML, Galvis-Pérez Y, Ciro-Gómez G, Núñez-Rangel V, Aristizábal JC, Barona-Acevedo J. Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome. Antioxidants. 2018; 7(12):185. https://doi.org/10.3390/antiox7120185
Chicago/Turabian StyleMarín-Echeverri, Catalina, Christopher N. Blesso, Maria Luz Fernández, Yeisson Galvis-Pérez, Gelmy Ciro-Gómez, Vitelbina Núñez-Rangel, Juan C. Aristizábal, and Jacqueline Barona-Acevedo. 2018. "Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome" Antioxidants 7, no. 12: 185. https://doi.org/10.3390/antiox7120185
APA StyleMarín-Echeverri, C., Blesso, C. N., Fernández, M. L., Galvis-Pérez, Y., Ciro-Gómez, G., Núñez-Rangel, V., Aristizábal, J. C., & Barona-Acevedo, J. (2018). Effect of Agraz (Vaccinium meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome. Antioxidants, 7(12), 185. https://doi.org/10.3390/antiox7120185







