Analysis of Protein Oxidative Modifications in Follicular Fluid from Fertile Women: Natural Versus Stimulated Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Ethical Approval
2.3. Sample Collection
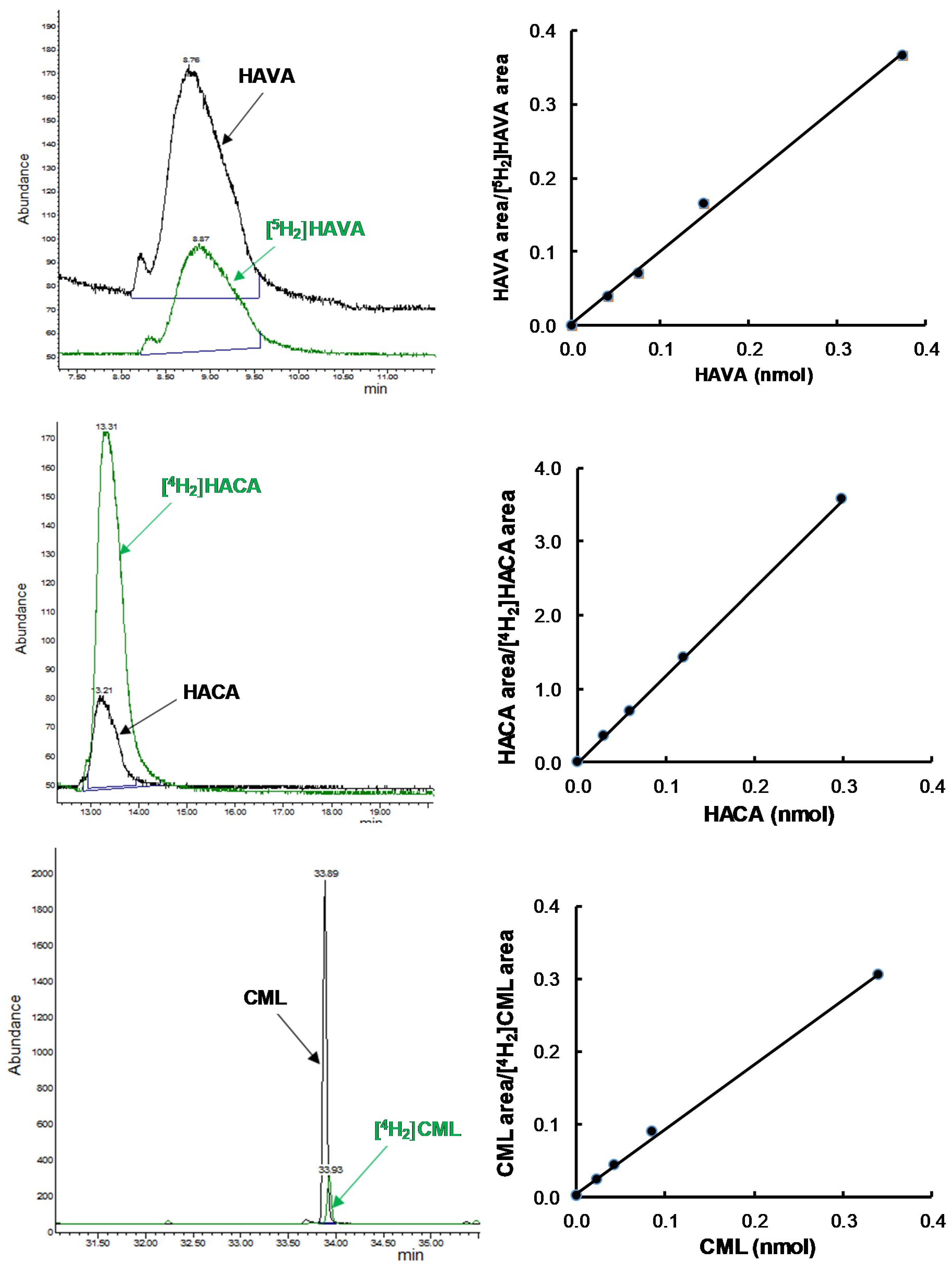
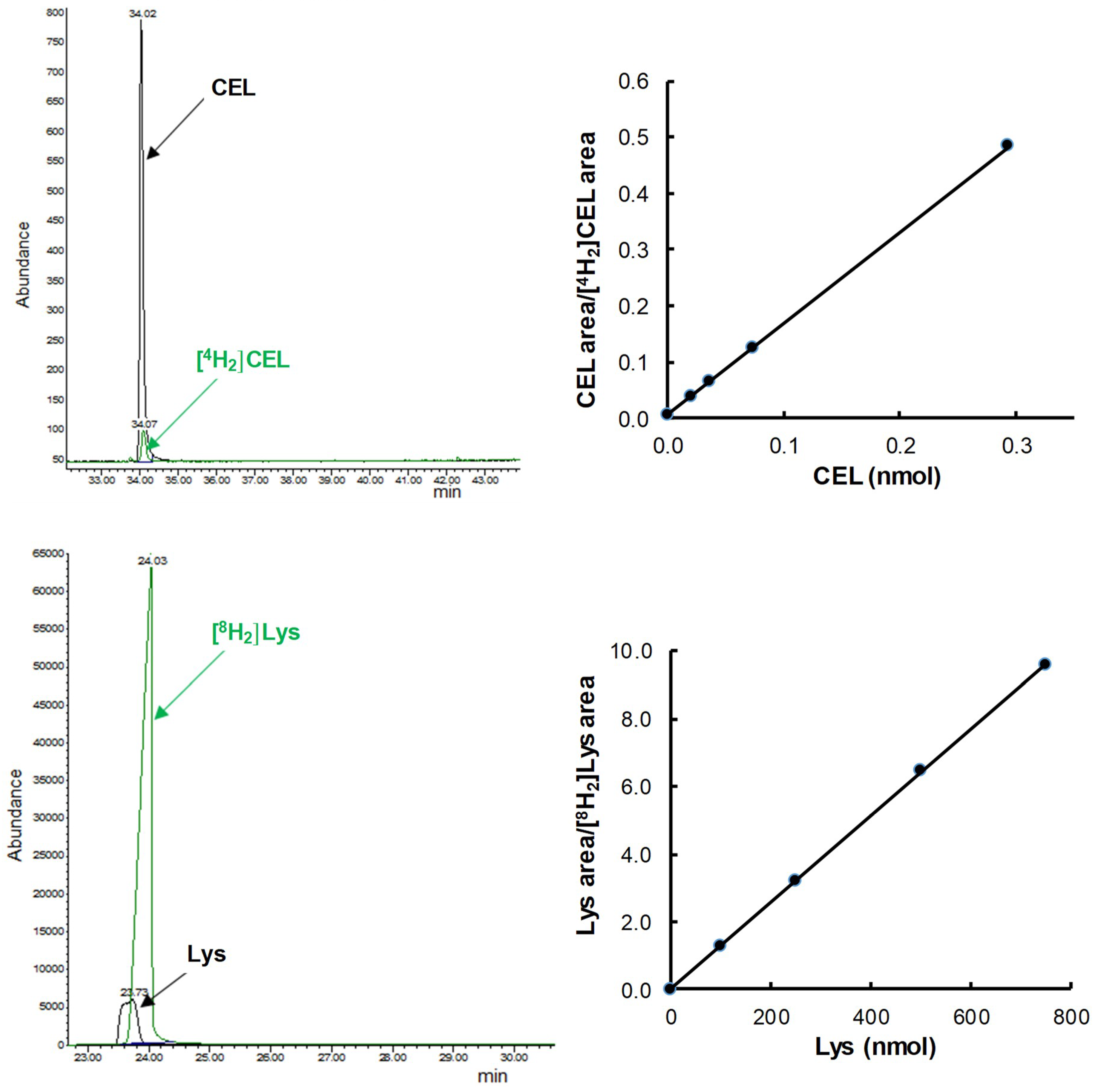
2.4. Measurement of GSA, AASA, CML, and CEL
2.5. Glucose Measurement
2.6. Statistical Analysis
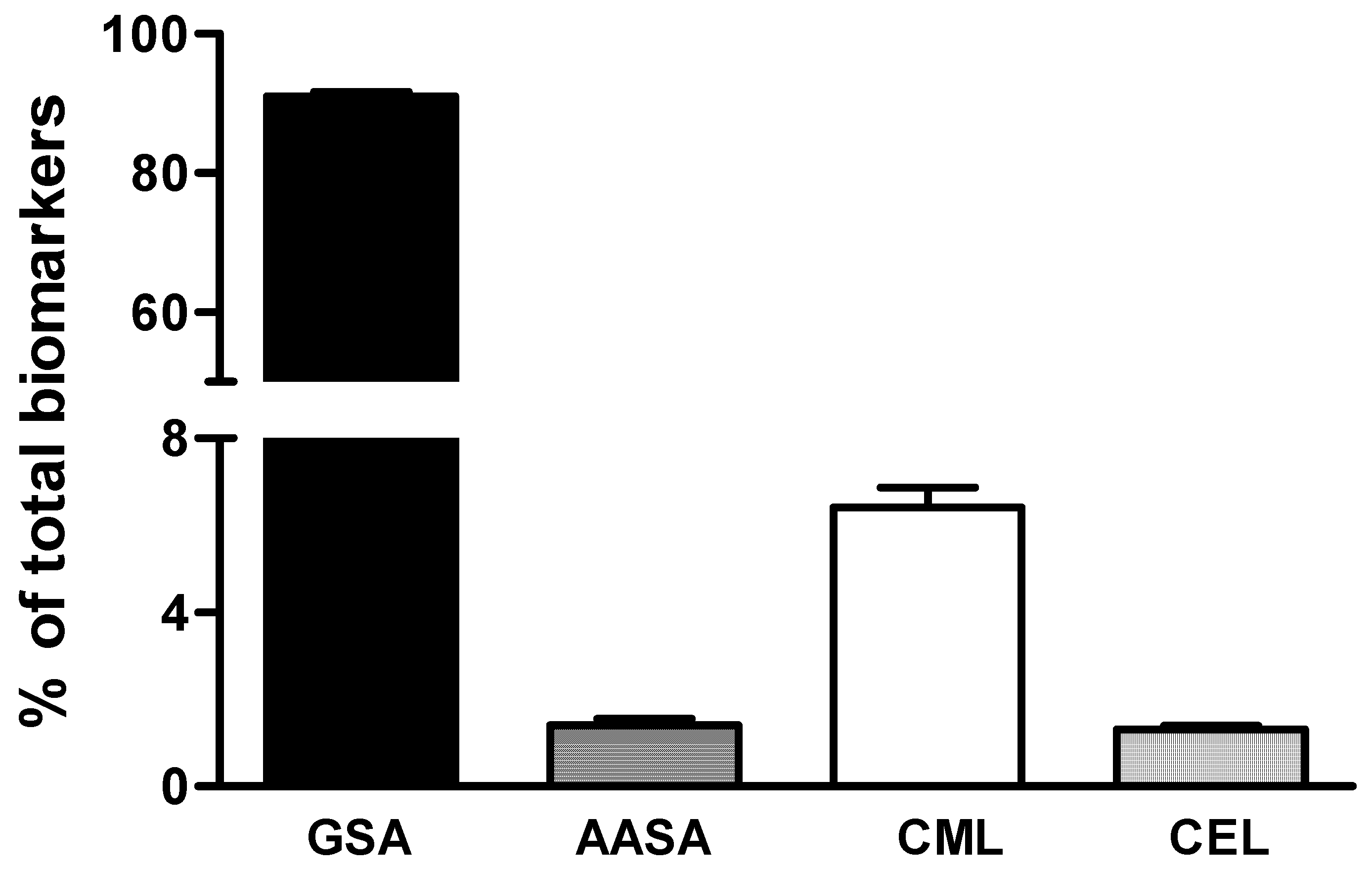
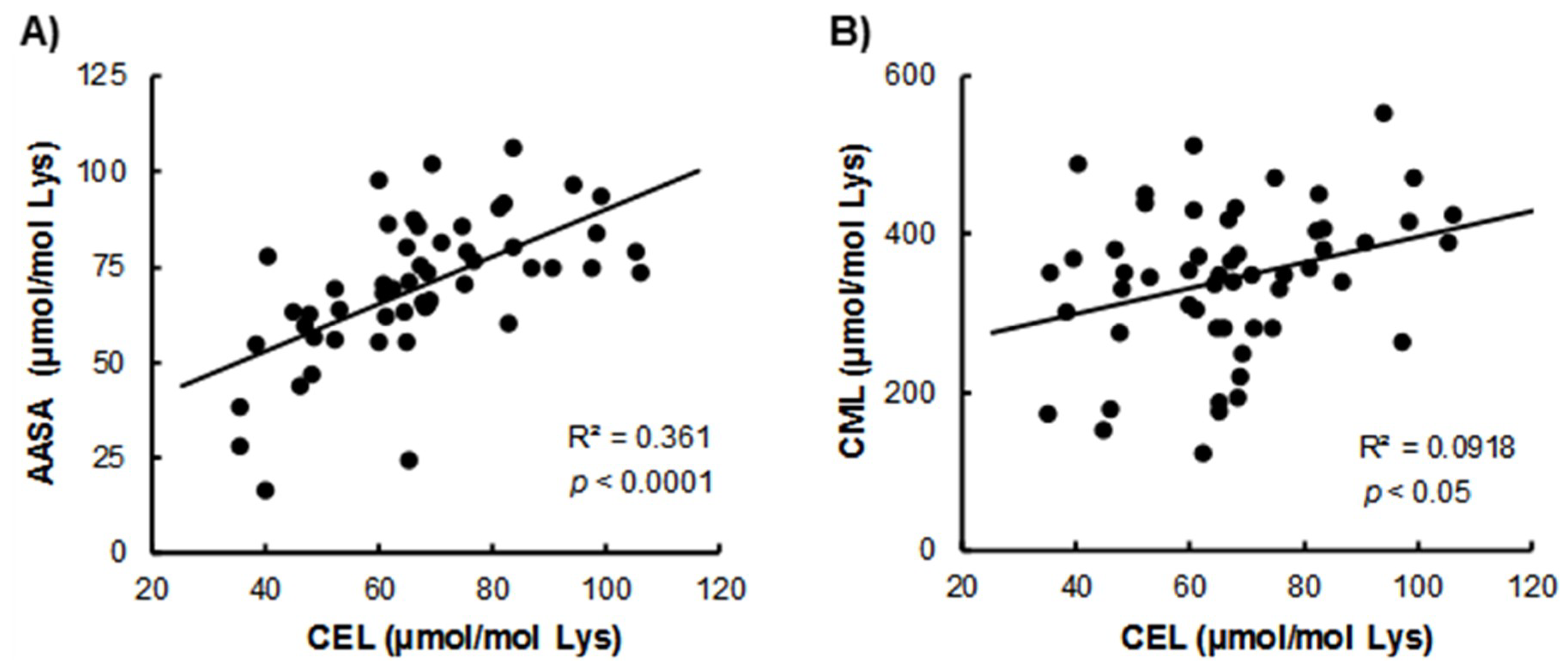
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amici, A.; Levine, R.L.; Tsai, L.; Stadtman, E.R. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J. Biol. Chem. 1989, 263, 3341–3346. [Google Scholar]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Tactacan, C.M.; Tan, S.X.; Lin, R.C.; Wouters, M.A.; Dawes, I.W. Cell cycle sensing of oxidative stress in Saccharomyces cerevisiae by oxidation of a specific cysteine residue in the transcription factor Swi6p. J. Biol. Chem. 2011, 286, 5204–5214. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R.; Moller, L.; Bartosz, G.; Bast, A.; Bertoni-Freddari, C.; Collins, A.; Cooke, M.; Coolen, G.; Haenen, A.M.; Hoberg, S.; et al. Biomarkers. Mol. Asp. Med. 2002, 23, 101–208. [Google Scholar] [CrossRef]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M.; Weber, D.; Grune, T.; Borsa, C.; et al. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Requena, J.R.; Chao, C.C.; Levine, R.L.; Stadtman, E.R. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M. Determisnation of Nε-(carboxymethyl)lysine in foods and related systems. Ann. N. Y. Acad. Sci. 2008, 1126, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Frye, E.B.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Kondapalli, L.A.; Celia, G.; Gordon, J.; DiMattina, M.; Payson, M. Natural cycle IVF reduces the risk of low birthweight infants compared with conventional stimulated IVF. Hum. Reprod. 2016, 31, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Liu, Y.; Zhang, R.; Wu, Y.; Huang, Y.; Liu, F.; Li, M.; Sun, S.; Xing, L.; et al. Maternal and Live-birth Outcomes of Pregnancies following Assisted Reproductive Technology: A Retrospective Cohort Study. Sci. Rep. 2016, 6, 35141. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Devroey, P.; Macklon, N.S. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet 2005, 365, 1807–1816. [Google Scholar] [CrossRef]
- Rizk, B.; Smitz, J. Ovarian hyperstimulation syndrome after superovulation using GnRH agonists for IVF and related procedures. Hum. Reprod. 1992, 7, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Aboulghar, M.A.; Mansour, R.T.; Serour, G.I.; Ramzy, A.M.; Amin, Y.M. Oocyte quality in patients with severe ovarian hyperstimulation syndrome. Fertil. Steril. 1997, 68, 1017–1021. [Google Scholar] [CrossRef]
- Jarkovska, K.; Martinkova, J.; Liskova, L.; Halada, P.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Proteome mining of human follicular fluid reveals a crucial role of complement cascade and key biological pathways in women undergoing in vitro fertilization. J. Proteome Res. 2010, 9, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Zamah, A.M.; Hassis, M.E.; Albertolle, M.E.; Williams, K.E. Proteomic analysis of human follicular fluid from fertile women. Clin. Proteom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, X.; Zhu, P.; Zhang, Y.; Wang, J.; Wang, Y.; Wang, W.; Liu, J.; Li, N.; Liu, F. Proteomic analysis of human follicular fluid associated with successful in vitro fertilization. Reprod. Biol. Endocrinol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, I.; Ruiz-Sanz, J.I.; del Agua, A.R.; Navarro, R.; Hernandez, M.L.; Matorras, R.; Prieto, B.; Ruiz-Larrea, M.B. Serum oxidizability and antioxidant status in patients undergoing in vitro fertilization. Fertil. Steril. 2010, 94, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Juncá, P.; Irarrázaval, I.; Rolle, A.J.; Gutiérrez, J.I.; Moreno, R.D.; Santos, M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015, 48, 68. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ahn, J.I.; Lee, A.R.; Ko, D.W.; Yang, W.S.; Lee, G.; Ahn, J.Y.; Lim, J.M. Adverse Effect of Superovulation Treatment on Maturation, Function and Ultrastructural Integrity of Murine Oocytes. Mol. Cells 2017, 40, 558–566. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Kollmann, Z.; Flück, C.E.; Stute, P.; Marti, U.; Weiss, B.; Bersinger, N.A. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: A comparative study between natural cycle IVF and conventional IVF. Hum. Reprod. 2014, 29, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, Z.; Schneider, S.; Fux, M.; Bersinger, N.A.; von Wolff, M. Gonadotrophin stimulation in IVF alters the immune cell profile in follicular fluid and the cytokine concentrations in follicular fluid and serum. Hum. Reprod. 2017, 32, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.R.; Troncoso, C.; Bosch, E.; Serra, V.; Simon, C.; Remohi, J.; Pellicer, A. Age and uterine receptiveness: Predicting the outcome of oocyte donation cycles. J. Clin. Endocrinol. Metab. 2005, 90, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Dalfo, E.; Ayala, V.; Bellmunt, M.J.; Prat, J.; Ferrer, I.; Portero-Otin, M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005, 280, 21522–21530. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Jinno, M.; Takeuchi, M.; Watanabe, A.; Teruya, K.; Hirohama, J.; Eguchi, N.; Miyazaki, A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum. Reprod. 2011, 26, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liang, Y.; Shao, Y.; Bian, W.; Fu, H.; Xu, J.; Sui, L.; Yao, B.; Li, M. Advanced glycation end product concentrations in follicular fluid of women undergoing IVF/ICSI with a GnRH agonist protocol. Reprod. Biomed. Online 2018, 36, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wiener-Megnazi, Z.; Vardi, L.; Lissak, A.; Shnizer, S.; Reznick, A.Z.; Ishai, D.; Lahav-Baratz, S.; Shiloh, H.; Koifman, M.; Dirnfeld, M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil. Steril. 2004, 82 (Suppl. 3), 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Attaran, M.; Pasqualotto, E.; Falcone, T.; Goldberg, J.M.; Miller, K.F.; Agarwal, A.; Sharma, R.K. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int. J. Fertil. Womens Med. 2000, 45, 314–320. [Google Scholar] [PubMed]
- Carbone, M.C.; Tatone, C.; Delle Monache, S.; Marci, R.; Caserta, D.; Colonna, R.; Amicarelli, F. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 2003, 9, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Temple, A.; Yen, T.Y.; Gronert, S. Identification of specific protein carbonylation sites in model oxidations of human serum albumin. J. Am. Soc. Mass Spectrom. 2006, 17, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.M.; Requena, J.R.; Crowley, J.R.; Thorpe, S.R.; Heinecke, J.W. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: A mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Investig. 1999, 104, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Naudi, A.; Jove, M.; Ayala, V.; Cabre, R.; Portero-Otin, M.; Pamplona, R. Non-enzymatic modification of aminophospholipids by carbonyl-amine reactions. Int. J. Mol. Sci. 2013, 14, 3285–3313. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Stadtman, E.R. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. USA 1992, 89, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Monroy, C.A.; Doorn, J.A.; Roman, D.L. Modification and functional inhibition of regulator of G-protein signaling 4 (RGS4) by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2013, 26, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Martin, J.H.; Aitken, R.J. Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis. Biol. Reprod. 2015, 92, 33. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Wu, Y.; Zhang, J.Y.; Hou, N.N.; Liu, A.X.; Pan, J.X.; Lu, J.Y.; Sheng, J.Z.; Huang, H.F. Preliminary proteomic analysis on the alterations in follicular fluid proteins from women undergoing natural cycles or controlled ovarian hyperstimulation. J. Assist. Reprod. Genet. 2015, 32, 417–427. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ruiz, I.; Meijide, S.; Hérnandez, M.-L.; Navarro, R.; Larreategui, Z.; Ferrando, M.; Ruiz-Larrea, M.-B.; Ruiz-Sanz, J.-I. Analysis of Protein Oxidative Modifications in Follicular Fluid from Fertile Women: Natural Versus Stimulated Cycles. Antioxidants 2018, 7, 176. https://doi.org/10.3390/antiox7120176
Pérez-Ruiz I, Meijide S, Hérnandez M-L, Navarro R, Larreategui Z, Ferrando M, Ruiz-Larrea M-B, Ruiz-Sanz J-I. Analysis of Protein Oxidative Modifications in Follicular Fluid from Fertile Women: Natural Versus Stimulated Cycles. Antioxidants. 2018; 7(12):176. https://doi.org/10.3390/antiox7120176
Chicago/Turabian StylePérez-Ruiz, Irantzu, Susana Meijide, María-Luisa Hérnandez, Rosaura Navarro, Zaloa Larreategui, Marcos Ferrando, María-Begoña Ruiz-Larrea, and José-Ignacio Ruiz-Sanz. 2018. "Analysis of Protein Oxidative Modifications in Follicular Fluid from Fertile Women: Natural Versus Stimulated Cycles" Antioxidants 7, no. 12: 176. https://doi.org/10.3390/antiox7120176
APA StylePérez-Ruiz, I., Meijide, S., Hérnandez, M.-L., Navarro, R., Larreategui, Z., Ferrando, M., Ruiz-Larrea, M.-B., & Ruiz-Sanz, J.-I. (2018). Analysis of Protein Oxidative Modifications in Follicular Fluid from Fertile Women: Natural Versus Stimulated Cycles. Antioxidants, 7(12), 176. https://doi.org/10.3390/antiox7120176