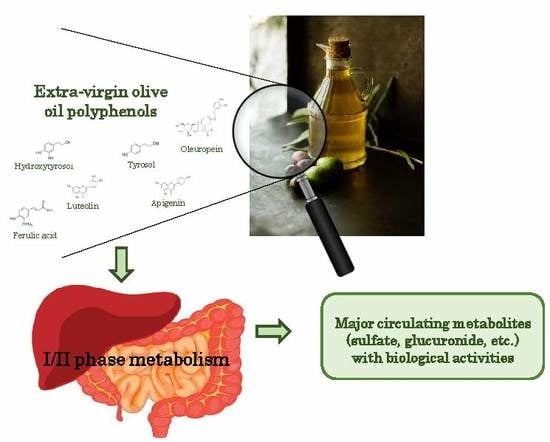
Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites
Abstract
1. Introduction
2. Olive Oil
2.1. Extra-Virgin Olive Oil Polyphenols
- -
- secoiridoids, where the most abundant are the dialdehydic form of decarboxymethyl elenolic acid linked to HT (3,4-DHPEA) or Tyr (p-HPEA), (3,4-DHPEA-EDA or p-HPEA-EDA), oleacein, oleuropein, an isomer of the oleuropein aglycon (HT linked to elenolic acid) (3,4-DHPEA-EA), and ligstroside aglycon (Tyr linked to elenolic acid) (p-HPEA-EA). p-HPEA-derivates and dialdehydic forms of oleuropein and ligstroside aglycon have also been detected as minor hydrophilic phenols of EVOO [19,24];
- -
- phenylethanoids, which possess a hydroxyl group attached to an aromatic hydrocarbon group, such as oleocanthal, HT (3,4-dihydroxyphenyl-ethanol or 3,4-DHPEA) and Tyr (p-hydroxyphenyl-ethanol or p-HPEA) [6]. Their concentration is usually low in fresh oils but increases during oil storage due to the hydrolysis of secoiridoids [25];
- -
- phenolic acids, which can be divided into two subgroups: hydroxybenzoic acid derivatives and hydroxycinnamic acid derivatives, such as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p- and o-coumaric acid, ferulic acid, and cinnamic acid [24];
- -
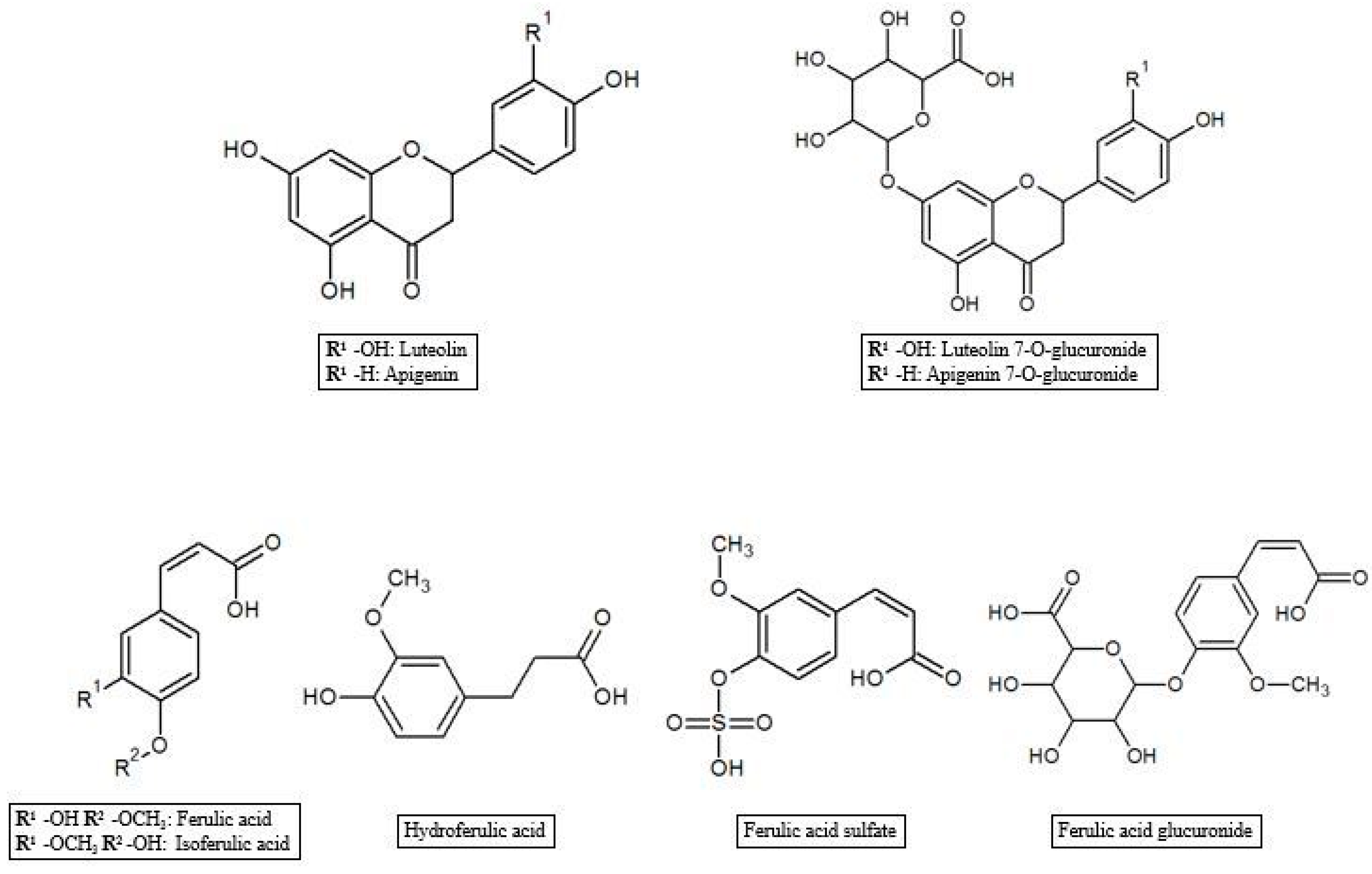
- flavonoids, which contain two benzene rings joined by a linear three carbon chain. Flavonoids are largely planar molecules and their structural variation comes in part from the pattern of modification by hydroxylation, methoxylation, prenylation, or glycosylation. Flavonoid aglycones are subdivided into flavones, flavonols, flavanones, and flavanols depending upon the presence of a carbonyl carbon at C-4, an OH group at C-3, a saturated single bond between C-2 and C-3, and a combination of carbonyl at C-4 with an OH group at C-3, respectively. Luteolin and apigenin are the most concentrated [24];
- -
- hydroxy-isocromans, a class which consists in only two compounds: 1-phenyl-6,7-dihydroxy-isochroman and 1-(39-methoxy-49-hydroxy) phenyl-6,7-dihydroxy- isochroman [26];
- -
- lignans, whose structure is not well understood, but it is based on the condensation of aromatic aldehydes. (+)-1-Acetoxypinoresinol and (+)-1-pinoresinol were characterized as the most concentrated lignans in EVOO [27].
2.2. Absorption and Distribution of Polyphenols
2.3. Metabolism of Polyphenols
3. Activity of Polyphenols Metabolites
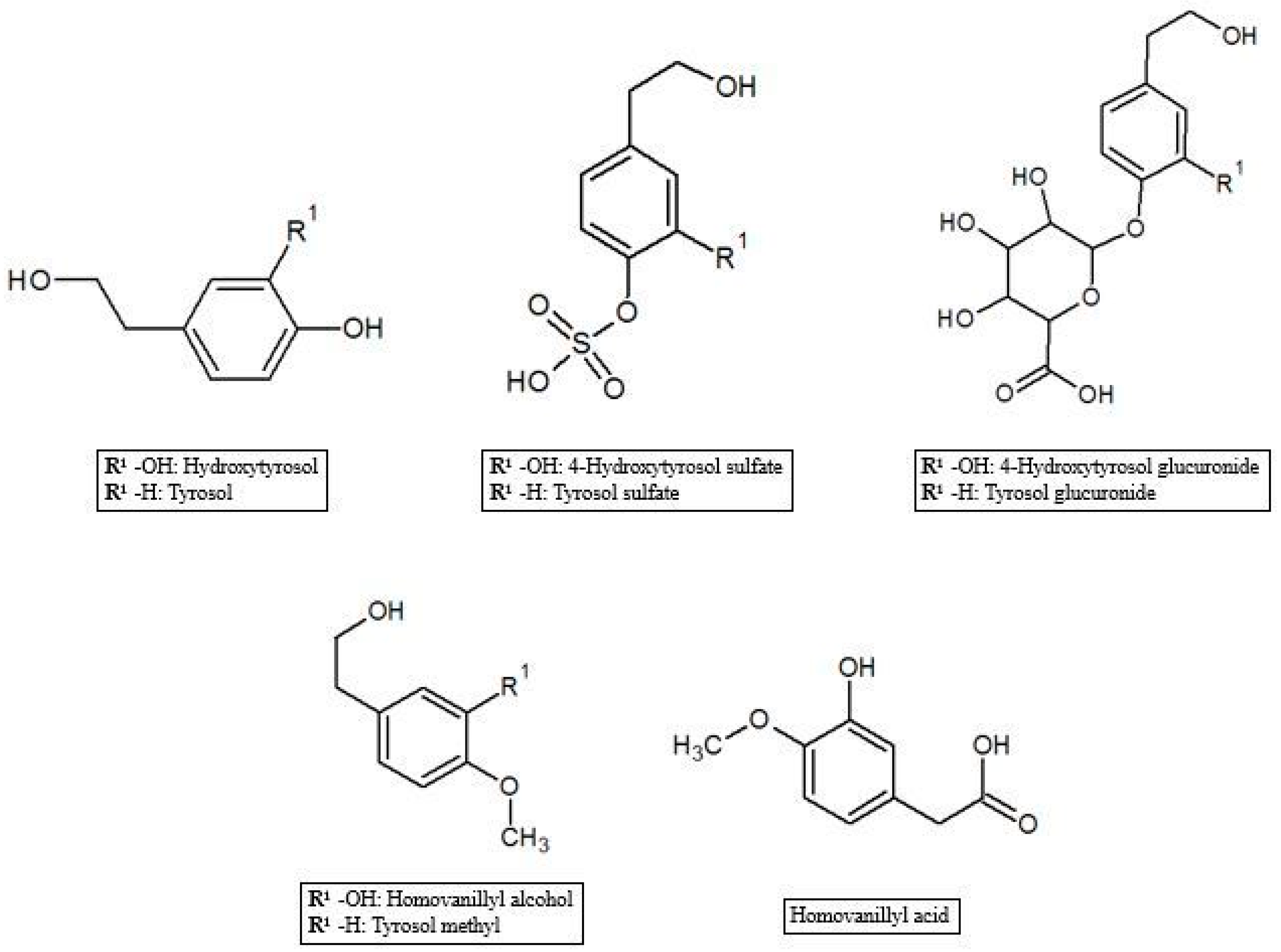
3.1. Hydroxytyrosol and Tyrosol Glucuronides and Sulfates
3.2. Homovanillic Acid and Homovanillyl Alcohol
3.3. Other Polyphenol Metabolites
4. Conclusions and Future Research
Funding
Acknowledgments
Conflicts of Interest
References
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean Diet and Nutritional Adequacy: A Review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The health benefiting mechanisms of virgin olive oil phenolic compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morato, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug. Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Incani, A.; Atzeri, A.; Angioni, A.; Campus, M.; Cauli, E.; Zurru, R.; Deiana, M. Antioxidant effect of natural table olives phenolic extract against oxidative stress and membrane damage in enterocyte-like cells. J. Food Sci. 2017, 82, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Burattini, S.; Battistelli, M.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Falcieri, E. Tyrosol prevents apoptosis in irradiated keratinocytes. J. Dermatol. Sci. 2015, 80, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Spencer, J.P.E.; Dessì, M.A. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Micioni Di Bonaventura, M.V.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB(1) tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. History and characteristics of the olive tree. In Olive Oil Chemistry and Technology; Boskou, D., Ed.; American Oil Chemists’ Society Press: Champaign, IL, USA, 1996. [Google Scholar]
- Huang, C.L.; Sumpio, E.B. Olive Oil, the Mediterranean Diet, and Cardiovascular Health. J. Am. Coll. Surg. 2008, 207, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.; Jerkovic, I.; Maldini, M.; Serreli, G. Phenolic Compounds, Antioxidant Activity, and Other Characteristics of Extra Virgin Olive Oils from Italian Autochthonous Varieties Tonda di Villacidro, Tonda di Cagliari, Semidana, and Bosana. J. Chem. 2016, 2016, 8462741. [Google Scholar] [CrossRef]
- Covas, M.I.; Ruiz-Gutierrez, V.; de la Torre, R.; Kafatos, A.; Lamuela-Raventos, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, 20–30. [Google Scholar] [CrossRef]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.G.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Uylaser, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Biological properties of olive oil phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Fito, M.; de la Torre, R.; Covas, M.I. Olive oil and oxidative stress. Mol. Nutr. Food Res. 2007, 51, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Savarese, M.; Paduano, A.; Scalfi, L.; Fogliano, V.; Sacchi, R. Healthy virgin olive oil: A matter of bitterness. Crit. Rev. Food Sci. Nutr. 2013, 55, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Coccioli, F.; Guiso, M.; Marra, C. The occurrence in olive oil of a new class of phenolic compounds: Hydroxy-isochromans. Food Chem. Toxicol. 2001, 77, 405–411. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignans and squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef]
- Soler, A.; Romero, M.P.; Macia, A.; Saha, S.; Furniss, C.S.M.; Kroon, P.A.; Motilva, M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco-2/TC7 cell line. Food Chem. 2001, 119, 703–714. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes dos Santos, G.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, X.-M.; Jiang, J.-G.; Zhu, W. Apigenin-7-O-b-d-glucuronide inhibits modified low-density lipoprotein uptake and foam cell formation in macrophages. J. Funct. Foods 2017, 35, 615–621. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.-G.; Zhang, X.-M.; Zhu, W. Identification of luteolin 7-O-β-d-glucuronide from Cirsium japonicum and its anti-inflammatory mechanism. J. Funct. Foods 2018, 46, 521–528. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Fitó, M.; Farré Albaladejo, M.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; De La Torre, R.; Covas, M.I. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp. Clin. Res. 2004, 30, 207–212. [Google Scholar] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Perez-Maňa, C.; Farre, M.; Rodrıguez-Morato, J.; Papaseit, E.; Pujadas, M.; Fitó, M.; Robledo, P.; Covas, M.I.; Cheynier, V.; Meudec, E.; et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Dessi, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Lopez de las Hazas, M.-C.; Piňol, C.; Macia, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubio, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Peyrol, J.; Meyer, G.; Obert, P.; Dangles, O.; Pechère, L.; Amiot, M.-J.; Riva, C. Involvement of bilitranslocase and beta-glucuronidase in the vascular protection by hydroxytyrosol and its glucuronide metabolites in oxidative stress conditions. J. Nutr. Biochem. 2018, 51, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Rho, S.; Kim, J.; Kim, M.Y.; Lee, D.H.; Kim, S.Y.; Choi, H.; Kim, H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007, 414, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J.; Stupans, I. Structural characterization of the metabolites of hydroxytyrosol, the principal phenolic component in olive oil, in rats. J. Agric. Food Chem. 2002, 50, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Farre, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I. Direct analysis of glucuronidated metabolites of main olive oil phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Rubió, L.; Serra, A.; Macià, A.; Piñol, C.; Romero, M.-P.; Motilva, M.J. In vivo distribution and deconjugation of hydroxytyrosol phase II metabolites in red blood cells: A potential new target for hydroxytyrosol. J. Funct. Foods 2014, 10, 139–143. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubió, L.; Hernaéz, A.; Tormos, C.; Motilva, M.J.; Fitó, M.; et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santiago, M.; Fonolla, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R.; Corella, D.; Castaňer, O.; Martınez-Gonzalez, M.A.; Salas-Salvador, J.; Vila, J.; Estruch, R.; Sorli, J.V.; Aros, F.; Fiol, M.; et al. Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: Virgin olive oil, wine, and catechol-methylathion. Am. J. Clin. Nutr. 2017, 105, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Valls, R.-M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.-P.; De la Torre, R.; Covas, M.I.; Solà, R.; Motilva, M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Garcia-Aloy, M.; Figueira, M.E.; Combet, E.; Mullen, W.; Bronze, M.R. High resolution mass spectrometric analysis of secoiridoids and metabolites as biomarkers of acute olive oil intake—An approach to study interindividual variability in humans. Mol. Nutr. Food Res. 2018, 62, 1700065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Fito, M.; Tourino, S.; Muñoz-Aguayo, D.; Pujadas, M.; Torres, J.L.; Joglar, J.; Farré, M.; Covas, M.I.; De la Torre, R. Antioxidant activities of hydroxytyrosol main metabolites do not contribute to beneficial health effects after olive oil ingestion. Drug Metab. Dispos. 2010, 38, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.Z.; Zhang, H.Y. Radical scavenging potential of phenolic compounds encountered in O. europaea products as indicated by calculation of bond dissociation enthalpy and ionization potential values. J. Agric. Food Chem. 2005, 53, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Incani, A.; Rosa, A.; Atzeri, A.; Loru, D.; Cabboi, B.; Melis, M.P.; Lucas, R.; Morales, J.C.; Dessì, M.A. Hydroxytyrosol glucuronides protect renal tubular epithelial cells against H2O2 induced oxidative damage. Chem. Biol. Interact. 2011, 193, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Silva, A.; Almeida, V.; Carvalheira, M.; Serra, C.; Rodrígues-Borges, J.E.; Fernandes, J.; Belo, L.; Santos-Silva, A. Protective activity of hydroxytyrosol metabolites on erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2013, 61, 6636–6642. [Google Scholar] [CrossRef] [PubMed]
- Muriana, F.J.G.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abia, R.; Lopez, S. Tyrosol and its metabolites as antioxidative and antiinflammatory molecules in human endothelial cells. Food Funct. 2017, 8, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dangles, O.; Rakotomanomana, N.; Baracchini, S.; Visioli, F. 3-O-hydroxytyrosol glucuronide and 4-O-hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar] [CrossRef] [PubMed]
- Catalan, U.; López de las Hazas, M.-C.; Pino, C.; Rubio, L.; Motilva, M.J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; Godinho-Pereirac,, J.; Macià, A.; Almeida, A.F.; Ventura, A.R.; Motilva, M.J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- Alberti, A.; Amorati, R.; Campredon, M.; Lucarini, M.; Macciantelli, D.; Pedulli, G.F. Antioxidant activity of some simple phenols present in olive oil. Acta Aliment. 2009, 38, 427–436. [Google Scholar] [CrossRef]
- Incani, A.; Deiana, M.; Corona, G.; Vafeiadou, K.; Vauzour, D.; Dessì, M.A.; Spencer, J.P.E. Involvement of ERK, Akt and JNK signalling in H2O2-induced cell injury and protection by hydroxytyrosol and its metabolite homovanillic alcohol. Mol. Nutr. Food Res. 2010, 54, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y.; Koike, T.; Matsuo, T.; Yoshida, Y. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J. Oleo Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Etienne, N.; Alonso, M.G.; de Pascual-Teresa, S.; Minihane, A.M.; Weinberg, P.D.; Rimbach, G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitam. Nutr. Res. 2005, 75, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Garcıa-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Menendez, J.A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Uptake and metabolism of olive oil polyphenols in human breast cancer cells using nano-liquid chromatography coupled to electrospray ionization-time of flight-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 898, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Corona, G.; Incani, A.; Loru, D.; Rosa, A.; Atzeri, A.; Melis, M.P.; Dessì, M.A. Protective effect of simple phenols from extravirgin olive oil against lipid peroxidation in intestinal Caco-2 cells. Food Chem. Toxicol. 2010, 48, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Colombo, E.; Fumagalli, M.; Abbiati, F.; Caruso, D.; Dell’Agli, M. Inhibition of NF-κB activity by minor polar components of extra-virgin olive oil at gastric level. Phytother. Res. 2012, 26, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E.; et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food. Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Tagliafierro, L.; Scala, I.; Granese, B.; Andria, G.; Zappia, V. The role of iron toxicity in oxidative stress-induced cellular degeneration in down syndrome: Protective effects of phenolic antioxidants. Curr. Nutr. Food Sci. 2012, 8, 206–212. [Google Scholar] [CrossRef]
- Serra, G.; Deiana, M.; Spencer, J.P.E.; Corona, G. Olive oil phenolics prevent oxysterol-induced proinflammatory cytokine secretion and reactive oxygen species production in human peripheral blood mononuclear cells, through modulation of p38 and JNK pathways. Mol. Nutr. Food Res. 2017, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid sugar esters are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid. J. Nutr. 2003, 133, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Van Rymenant, E.; Van Camp, J.; Pauwels, B.; Boydens, C.; Vanden Daele, L.; Beerens, K.; Brouckaert, P.; Smagghe, G.; Kerimi, A.; Williamson, G.; et al. Ferulic acid-4-O-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J. Nutr. Biochem. 2017, 44, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ochiai, H.; Mantani, N.; Kogure, T.; Shibahara, N.; Terasawa, K. Administration of isoferulic acid improved the survival rate of lethal influenza virus pneumonia in mice. Mediat. Inflamm. 2001, 10, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [PubMed]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2000, 129, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic acid, a new antiglycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef] [PubMed]
- Baeza, G.; Bachmair, E.M.; Wood, S.; Mateos, R.; Bravo, L.; de Roos, B. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 22, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Lee, J.-A.; Cho, E.J.; Choi, I. Effects of catechol O-methyl transferase inhibition on anti-inflammatory activity of luteolin metabolites. J. Food Sci. 2017, 82, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Saka, N.; Nozawa, R.; Sato, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001, 29, 1521–1524. [Google Scholar] [PubMed]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-d-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Kamalakararao, K.; Gopalakrishna, V.K.; Zenebe, H.; Govinda Rao, D.; Padal, S.B.; Krishna Chaithanya, K. Anti-inflammatory activity of bioactive flavonoid apigenin-7-O-β-d-glucuronide methyl ester from ethyl acetate leaf extract of Manilkara zapota on lipopolysaccharide induced pro-inflammatory mediators nitric oxide (NO), prostaglandin e2 (PGE2) in RAW 264.7 cells. Drug Invent. Today 2018, 10, 531–535. [Google Scholar]
| Compounds | Concentration Tested | In Vitro/In Vivo Model | Outcome | Reference |
|---|---|---|---|---|
| Mix of metabolites | 10–80 µM | In vitro red blood cells (RBC) | Protection of red blood cells from H2O2-induced oxidative injury | [64] |
| 10 mg/kg | ApoE−/− mice | Reduction of VCAM-1, ICAM-1, E-selectin and MCP-1 molecules secretion by inhibiting mRNA expression | [67] | |
| 1–5 μM | Human aortic endothelial cells (HAEC) | Reduction of p38δ, JNK1-3, CREB, AKT3, p53 and P70 S6 kinase phosphorylation, and of lymphocytes adhesion | [67] | |
| HT glucuronide | 100 μM | Rat aortic rings | Reduced endothelial dysfunction by blocking superoxide production | [43] |
| 2.3 µM | DPPH test | Good antioxidant and antiradical capacity | [48] | |
| 0.01–10 μM | In vitro Cu-induced oxidation of LDL | Loss of antioxidant activity with respect to HT | [61] | |
| 5–10 μM | Renal LLC-PK1 cells culture model | Protection of renal cells against H2O2-induced lipid peroxidation | [63] | |
| 10–25 µM | Human hepatocarcinoma HepG2 cells | Inhibition of tunicamycin-induced endoplasmic reticulum (ER) stress | [66] | |
| HT sulfate | 91 µM | DPPH test | Poor antioxidant and antiradical capacity | [48] |
| 2.5–10 µM | Caco-2 intestinal cells monolayers | Counteraction of the oxidizing action of oxidized cholesterol on intestinal cell membranes | [60] | |
| 10 µM | Neuroblastoma (SH-SY5Y) and dopaminergic (LUHMES) neuronal cells | Protective effects against oxidative stress | [68] | |
| Tyr glucuronide | 0.01–10 μM | In vitro Cu-induced oxidation of LDL | Loss of antioxidant activity with respect to Tyr | [61] |
| 100 µM | Endothelial HUVEC cells monolayers | Prevention of the phosphorylation of NF-κB signaling proteins and of the over-expression of adhesion molecules | [65] | |
| 0.1–0.5 mg/kg | Carrageenan-induced hind paw oedema in mice | Amelioration of plantar and ear edemas | [65] | |
| Tyr sulfate | 2.5–10 µM | Intestinal Caco-2 cells monolayers | Counteraction of the oxidizing action of oxidized cholesterol on intestinal cell membranes | [60] |
| 0.1–0.5 mg/kg | Carrageenan-induced hind paw oedema in mice | Amelioration of plantar and ear edemas | [65] | |
| 100 µM | Endothelial HUVEC cells monolayers | Prevention of the phosphorylation of NF-κB signaling proteins and of the over-expression of adhesion molecules | [65] | |
| Homovanillic acid | 14.8 µM | DPPH test | Good antioxidant and antiradical capacity | [48] |
| Homovanillyl alcohol | 11.4 µM | DPPH test | Good antioxidant and antiradical capacity | [48] |
| 5.4–146.5 mM | Human clinical study | Reduction of CVD and total mortality risk | [55] | |
| 0.3–1 µM | Renal LLC-PK1 cell culture model | Protection against H2O2-induced renal epithelial injury through interaction both MAP kinase and PI3 kinase pathways | [70] | |
| 2–20 µM | Endothelial HUVEC cells monolayers | Inhibition of ICAM-1, VCAM-1 and MCP-1 secretion | [73] | |
| 5–25 µM | Intestinal Caco-2 cells monolayers | Protection of cell membranes from oxidative damage induced by TBH | [75] | |
| 0.5–10 mM | Human gastric adenocarcinoma (AGS) cells | Inhibition of NF-kB driven transcription and nuclear translocation | [76] | |
| 0.5–25 µM | Endothelial HUVEC cells monolayers | Inhibition of cell surface expression of E-selectin, ICAM-1 and VCAM-1 adhesion molecules | [77] | |
| 1–7.5 µM | Human monocytic cells U937 | Reduced cell adhesion and ICAM-1 expression | [78] | |
| 10–50 µM | Erythrocytes by blood samples obtained from trisomic patients | Decreased oxidative stress-induced ROS generation | [79] | |
| 0.25–1 µM | Peripheral mononucleated blood cells (PBMCs) | Inhibition of oxysterols-induced production of proinflammatory cytokines, interleukin-1β, normal T-cell macrophage migration inhibitory factor. Decreased levels of reactive oxygen species (ROS) and phosphorylation of the p38 and JNK MAP kinases | [80] | |
| FA glucuronide | 11.42–114.2 µg/kg | Male Swiss mice | Elicitation of vasorelaxation of saphenous and femoral arteries and aortae. Decreased mean arterial pressure | [82] |
| IsoFA | 0.5–1 mg/day | Mice infected by intranasal inoculation of influenza virus | Inhibition of the progression of lethal influenza virus pneumonia | [83] |
| 1–13 μg/mL | In vitro lipid peroxidation, DPPH and ABTS tests | Good antioxidant activity | [84] | |
| 5.0 mg/kg | Streptozotocin-induced diabetic rats | Inhibition of hepatic gluconeogenesis and increase of the glucose utilization in peripheral tissue to lower plasma glucose | [85] | |
| 1.25–5 mM | In vitro Glycation of Bovine Serum Albumin (BSA) | Antiglycation properties against fructose and glucose-mediated glycation and oxidation of bovine serum albumin | [86] | |
| HydroFA | 0.01–100 μg/mL | In vitro platelet culture | Inhibitor of platelet activation by decreasing P-selectin expression | [87] |
| Luteolin sulfate | 40 μM | Macrophages Raw 264.7 cells | Anti-inflammatory activities as inhibition of LPS-stimulated iNOS expression and production of nitric oxide, TNF-α, IL-1β, and IL-6 | [88] |
| Luteolin glucuronide | 25–200 μg/mL | Macrophages Raw 264.7 cells | Anti-inflammatory activities as inhibition of LPS-stimulated iNOS expression and production of nitric oxide, TNF-α, IL-1β, and IL-6 | [33] |
| 40 μM | Macrophages Raw 264.7 cells | Anti-inflammatory activities as inhibition of LPS-stimulated iNOS expression and production of nitric oxide, TNF-α, IL-1β, and IL-6 | [88] | |
| 50 mM | Macrophages Raw 264.7 cells | Reduction of expression of LPS-stimulated inflammatory genes | [90] | |
| Apigenin glucuronide | 25–100 μg/mL | Macrophages Raw 264.7 cells | Inhibition of Ox-LDL uptake and the scavenger receptor CD36 mRNA and protein expression. Enhancement of the expression of scavenger receptor class B type 1, following inhibition of ERK1/2 phosphorylation | [32] |
| 12.5–100 µM | Macrophages Raw 264.7 cells | Counteraction of prostaglandin E2 (PGE2) production, LPS-induced mRNA expression of iNOS, COX-2 and TNF-α Decrease of the translocation of c-Jun into the nucleus inhibition p38 and ERK phosphorylation | [91] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. https://doi.org/10.3390/antiox7120170
Serreli G, Deiana M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants. 2018; 7(12):170. https://doi.org/10.3390/antiox7120170
Chicago/Turabian StyleSerreli, Gabriele, and Monica Deiana. 2018. "Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites" Antioxidants 7, no. 12: 170. https://doi.org/10.3390/antiox7120170
APA StyleSerreli, G., & Deiana, M. (2018). Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants, 7(12), 170. https://doi.org/10.3390/antiox7120170