Bioavailability of Plant-Derived Antioxidants
Abstract
:1. Introduction
2. Factors Affecting Oral Absorption of Xenobiotics

3. Evaluation of Oral Bioavailability

4. Bioactivity and Bioavailability: Are They Closely Matched?
5. Bioavailability Studies of the Major Classes of Natural Antioxidants (1998–2013)
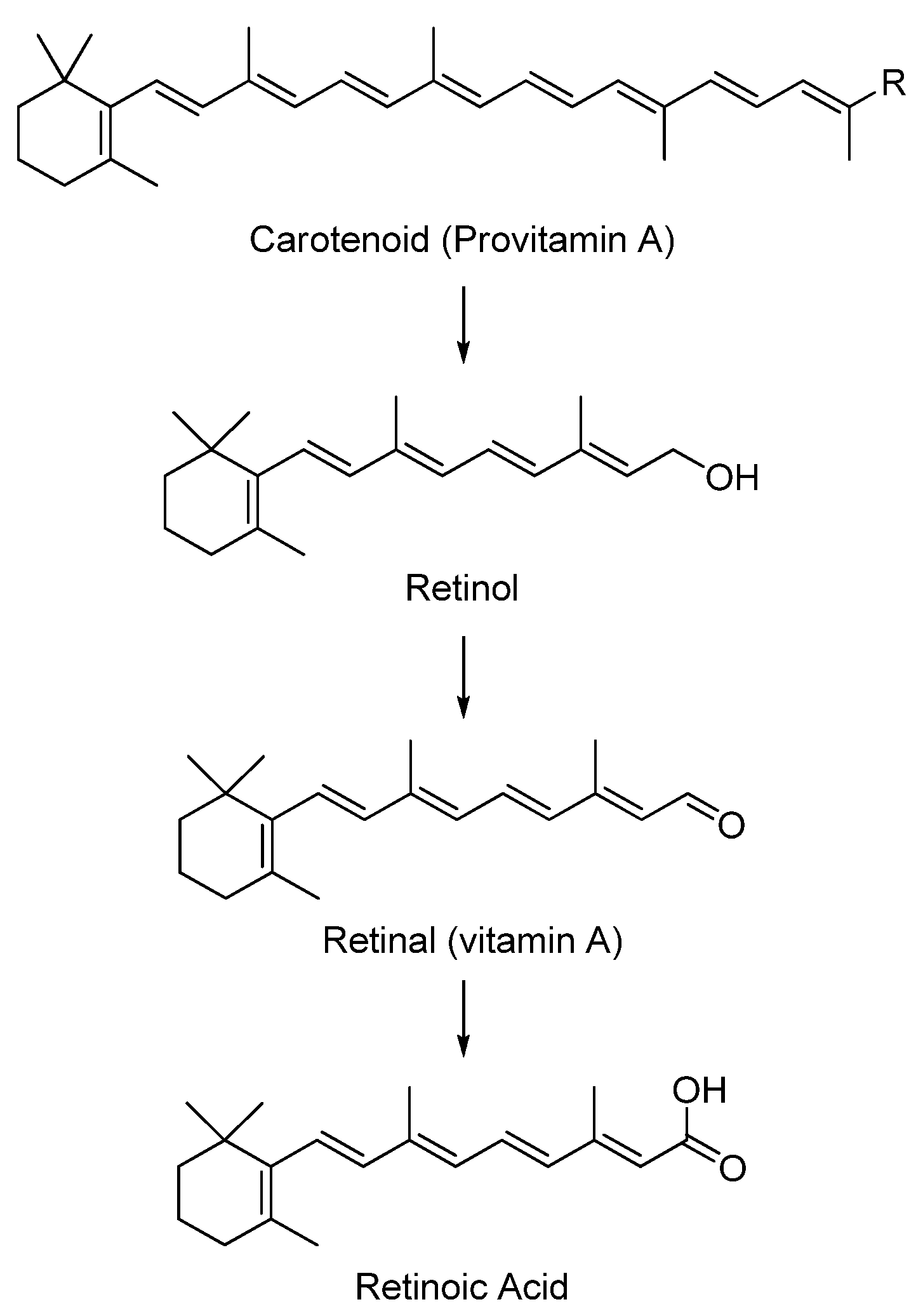
5.1. Carotenoids


5.2. Polyphenols
5.3. Organosulfur Compounds

6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Stoner, C.L.; Cleton, A.; Johnson, K.; Oh, D.-M.; Hallak, H.; Brodfuehrer, J.; Surendran, N.; Han, H.-K. Integrated oral bioavailability projection using in vitro screening data as a selection tool in drug discovery. Int. J. Pharm. 2004, 269, 241–249. [Google Scholar] [CrossRef]
- Jambhekar, S.S. Physicochemical and Biopharmaceutical Properties of Drug Substnaces and Pharmacokinetics. In Foye’s Principles of Medicinal Chemistry, 7th ed.; Lemke, T.L., Williams, D.A., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013; pp. 61–105. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Hen, Y.-H.; Liu, T.-T.; Li, C.; Cui, X.; White, R.E.; Cheng, K.-C. Evaluation of a novel in vitro Caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metab. Dispos. 2004, 32, 937–942. [Google Scholar] [PubMed]
- Fernandez-Garcia, E.; Carvajal-Lerida, I.; Jaren-Galan, M.; Garrido-Fernandez, J.; Perez-Galvez, A.; Hornero-Mendez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Niessen, W.M.A. Liquid Chromatography-Mass Spectrometry, 3rd ed.; CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hsieh, Y.; Korfmacher, W.A. Increasing speed and throughput when using hplc-ms/ms systems for drug metabolism and pharmacokinetic screening. Curr. Drug Metab. 2006, 7, 479–489. [Google Scholar] [CrossRef]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.-J.; Russell, R.M. Carotenoid bioavailability and bioconversion. Ann. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef]
- Biehler, E. Methods for assessing aspects of carotenoid bioavailability. Curr. Nutr. Food Sci. 2010, 6, 44–69. [Google Scholar] [CrossRef]
- Bohn, T. Bioavailability of non-provitamin A carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Faulks, R.M.; Southon, S. Challenges to understanding and measuring carotenoid bioavailability. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 95–100. [Google Scholar] [CrossRef]
- Johnson, E.J. Human Studies on Bioavailability and Serum Response of Carotenoids. In Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 265–277. [Google Scholar]
- Olson, J.A. Bioavailability of carotenoids. Arch. Latinoam. Nutr. 1999, 49, 21–25. [Google Scholar]
- Parker, R.S.; Swanson, J.E.; You, C.-S.; Edwards, A.J.; Huang, T. Bioavailability of carotenoids in human subjects. Proc. Nutr. Soc. 1999, 58, 155–162. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids—A review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Schwartz, S.J. Food Matrix and Processing Modulates Carotenoid Bioavailability. In Proceedings of Pacifichem 2010, International Chemical Congress of Pacific Basin Societies, Honolulu, HI, USA, 15–20 December 2010.
- Southon, S.; Faulks, R.M. Carotenoids in Food: Bioavailability and Functional Benefits. In Phytochemical Functional Foods; Johnson, I., Williamson, G., Eds.; Woodhead Publishing in Food Science and Technology: Cambridge, England, 2003; pp. 107–127. [Google Scholar]
- Tanumihardjo, S.A.; Palacios, N.; Pixley, K.V. Provitamin A carotenoid bioavailability: What really matters? Int. J. Vitam. Nutr. Res. 2010, 80, 336–350. [Google Scholar] [CrossRef]
- Van het Hof, K.H.; Gaertner, C.; West, C.E.; Tijburg, L.B.M. Potential of vegetable processing to increase the delivery of carotenoids to man. Int. J. Vitam. Nutr. Res. 1998, 68, 366–370. [Google Scholar] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability of carotenoids. Pure Appl. Chem. 1997, 69, 2145–2150. [Google Scholar] [CrossRef]
- West, C.E.; Castenmiller, J.J.M. Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int. J. Vitam. Nutr. Res. 1998, 68, 371–377. [Google Scholar] [PubMed]
- Bresnahan, K.A.; Arscott, S.A.; Khanna, H.; Arinaitwe, G.; Dale, J.; Tushemereirwe, W.; Mondloch, S.; Tanumihardjo, J.P.; de Moura, F.F.; Tanumihardjo, S.A. Cooking enhances but the degree of ripeness does not affect provitamin A carotenoid bioavailability from bananas in Mongolian gerbils. J. Nutr. 2012, 142, 2097–2104. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Estimation of carotenoid bioavailability from fresh stir-fried vegetables using an in vitro digestion/Caco-2 cell culture model. J. Nutr. Biochem. 2000, 11, 574–580. [Google Scholar] [CrossRef]
- Graebner, I.T.; Siqueira, E.M.A.; Arruda, S.F.; de Souza, E.M.T. Carotenoids from native Brazilian dark-green vegetables are bioavailable: A study in rats. Nutr. Res. 2004, 24, 671–679. [Google Scholar] [CrossRef]
- Liu, C.-S.; Glahn, R.P.; Liu, R.H. Assessment of carotenoid bioavailability of whole foods using a Caco-2 cell culture model coupled with an in vitro digestion. J. Agric. Food Chem. 2004, 52, 4330–4337. [Google Scholar] [CrossRef]
- O’Connell, O.; Ryan, L.; O’Sullivan, L.; Aherne-Bruce, S.A.; O’Brien, N.M. Carotenoid micellarization varies greatly between individual and mixed vegetables with or without the addition of fat or fiber. Int. J. Vitam. Nutr. Res. 2008, 78, 238–246. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.D.J.; Gardea, A.A.; Yahia, E.M.; Failla, M.L. Carotenoid composition in “Ataulfo” mango and their bioavailability and bioconversion to vitamin A. Acta Hortic. 2010, 877, 1245–1252. [Google Scholar]
- Ramos, M.I.L.; Siqueira, E.M.A.; Isomura, C.C.; Barbosa, A.M.J.; Arruda, S.F. Bocaiuva (Acrocomia aculeata (Jacq.) Lodd) improved vitamin A status in rats. J. Agric. Food Chem. 2007, 55, 3186–3190. [Google Scholar] [CrossRef]
- Yahia, E.M.; Ramirez-Padilla, G.K.; Carrillo-Lopez, A. Carotenoid content of five fruits and vegetables and their bioconversion to vitamin A measured by retinol accumulation in rat livers. Acta Hortic. 2009, 841, 619–623. [Google Scholar]
- Zakaria-Rungkat, F.; Djaelani, M.; Setiana, M.; Rumondang, E.; Nurrochmah, E. Carotenoid bioavailability of vegetables and carbohydrate-containing foods measured by retinol accumulation in rat livers. J. Food Compos. Anal. 2000, 13, 297–310. [Google Scholar] [CrossRef]
- Perez-Galvez, A.; Martin, H.D.; Sies, H.; Stahl, W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br. J. Nutr. 2003, 89, 787–793. [Google Scholar] [CrossRef]
- Hageman, S.H.; She, L.; Furr, H.C.; Clark, R.M. Excess vitamin E decreases canthaxanthin absorption in the rat. Lipids 1999, 34, 627–631. [Google Scholar] [CrossRef]
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Eldridge, A.L.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403. [Google Scholar] [PubMed]
- Goltz, S.R.; campbell, W.W.; Chitchumroonchockchai, C.; Failla, M.L.; Ferruzzi, M.G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol. Nutr. Food Res. 2012, 56, 866–877. [Google Scholar] [CrossRef]
- Hornero-Mendez, D.; Minquez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [PubMed]
- Blas, J.; Perez-Rodriguez, L.; Bortolotti, G.R.; Vinuela, J.; Marchant, T.A. Testosterone increases bioavailability of carotenoids: Insights into the honesty of sexual signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 18633–18637. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Erdman, J.W., Jr. Combined consumption of soy germ and tomato powders results in altered isoflavone and carotenoid bioavailability in rats. J. Agric. Food Chem. 2011, 59, 5335–5341. [Google Scholar] [CrossRef]
- Biehler, E.; Kaulmann, A.; Hoffmann, L.; Krause, E.; Bohn, T. Dietary and host-related factors influencing carotenoid bioaccessibility from spinach (Spinacia oleracea). Food Chem. 2011, 125, 1328–1334. [Google Scholar] [CrossRef]
- Alminger, M.; Svelander, C.; Wellner, A.; Martinez-Tomas, R.; Bialek, L.; Larque, E.; Perez-Llamas, F. Applicability of in vitro models in predicting the in vivo bioavailability of lycopene and α-carotene from differently processed soups. Food Nutr. Sci. 2012, 3, 477–489. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E.; Linssen, J.P.H.; van het Hof, K.H.; Voragen, A.G.J. The food matrix of spinach is a limiting factor in determining the bioavailability of α-carotene and to a lesser extent of lutein in humans. J. Nutr. 1999, 129, 349–356. [Google Scholar] [PubMed]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P.; Mikail, C.; Abou, L.; Charbonnier, M.; Caris-Veyrat, C.; Goupy, P.; Portugal, H.; Lairon, D.; Amiot, M.-J. Enrichment of tomato paste with 6% tomato peel increases lycopene and α-carotene bioavailability in men. J. Nutr. 2005, 135, 790–794. [Google Scholar] [PubMed]
- Ryan, L.; O’Connell, O.; O’Sullivan, L.; Aherne, S.A.; O’Brien, N.M. Micellarisation of carotenoids from raw and cooked vegetables. Plant Food Hum. Nutr. 2008, 63, 127–133. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Francis, D.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-β-carotene varieties of tomatoes. J. Agric. Food Chem. 2007, 55, 1597–1603. [Google Scholar] [CrossRef]
- Van het Hof, K.H.; de Boer, B.C.J.; Tijburg, L.B.M.; Lucius, B.R.H.M.; Zijp, I.; West, C.E.; Hautvast, J.G.A.J.; Weststrate, J.A. Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride-rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J. Nutr. 2000, 130, 1189–1196. [Google Scholar] [PubMed]
- Chitchumroonchockchai, C.; Failla, M.L. Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by Caco-2 human intestinal cells. J. Nutr. 2006, 136, 588–594. [Google Scholar] [PubMed]
- Odeberg, J.M.; Lignell, A.; Pettersson, A.; Hoglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Aherne, S.A.; O’Brien, N.M. Investigation of β-carotene and lutein transport in Caco-2 cells: Carotenoid-carotenoid interactions and transport inhibition by ezetimibe. Int. J. Vitam. Nutr. Res. 2009, 79, 337–347. [Google Scholar] [CrossRef]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.-F.; Veyrat, C.C.; Borel, P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef]
- Cardinault, N.; Tyssandier, V.; Grolier, P.; Winklhofer-Roob, B.M.; Ribalta, J.; Bouteloup-Demenage, C.; Rock, E.; Borel, P. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur. J. Nutr. 2003, 42, 315–323. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, H.-J.; Park, S.J.; Choi, H.-M. Factors effecting the bioavailability of carotenoid in elderly Korean women. Taehan Chiyok Sahoe Yongyang Hakhoechi 2003, 8, 822–830. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Khan, N.; Monagas, M.; Liorach, R.; Urpi-Sarda, M.; Rabassa, M.; Estuch, R.; Andres-Lacueva, C. Targeted and metabolomic study of biomarkers of cocoa powder consumption: Effects on inflammatory biomarkers in patients at high risk of cardiovascular disease. Agro Food Ind. Hi Tech 2010, 21, 51–54. [Google Scholar]
- Scalbert, A.; Rios, L.Y.; Gonthier, M.-P.; Manach, C.; Morand, C.; Remesy, C. The specificity of cocoa polyphenols recent advances in their bioavailability. Polyphen. Actual. 2002, 22, 14–18. [Google Scholar]
- Wakame, K.; Kitadate, K. Function of oligonol, low-molecular weight polyphenol of new generation. Aromatopia 2011, 107, 87–91. [Google Scholar]
- Lambert, J.D.; Yang, C.S. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. 2003, 523–524, 201–208. [Google Scholar] [CrossRef]
- Lambert, J.D.; Hong, J.; Lee, M.-J.; Sang, S.; Meng, X.; Lu, H.; Yang, C.S. Biotransformation and bioavailability of tea polyphenols: Implications for cancer prevention research. ACS Symp. Ser. 2005, 909, 212–224. [Google Scholar] [CrossRef]
- Lu, H.; Meng, X.; Lee, M.-J.; Li, C.; Maliakal, P.; Yang, C.S. Bioavailability and biological activity of tea polyphenols. ACS Symp. Ser. 2003, 851, 9–15. [Google Scholar] [CrossRef]
- Belles, V.V.; Franch, P.C.; San-Jose, L.G.; Rodriguez, P.M. Bioavailability of flavonoids in beer. In vivo antioxidant effects. Part I. Cerveza Malta 2009, 46, 65–71. [Google Scholar]
- Urquiaga, I.; Leighton, F. Wine and health: Evidence and mechanisms. World Rev. Nutr. Diet. 2005, 95, 122–139. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; di benedetto, R.; Garguilo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Konishi, T.; Rahman, M.M. Improving the Bioavailability of Polyphenols. In Biotechnology in Functional Foods and Nutraceuticals; Bagchi, D., Lau, F.C., Ghosh, D.K., Eds.; CRC: Boca Raton, FL, USA, 2010; pp. 81–90. [Google Scholar]
- Manach, C.; Scalbert, A.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Salucci, M.; Bugianesi, R.; Maiani, G. Dietary flavonoids: Intake and bioavailability. Recent Res. Dev. Nutr. 2001, 4, 65–79. [Google Scholar]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Carle, E.; Strass, G.; Kesenheimer, B.; Herbst, M.; Bitsch, I. Bioavailability of antioxidative compounds from Brettacher apple juice in humans. Innov. Food Sci. Emerg. Technol. 2000, 1, 245–249. [Google Scholar] [CrossRef]
- Cherubini, A.; Beal, M.F.; Frei, B. Black tea increases the resistance of human plasma to lipid peroxidation in vitro, but not ex vivo. Free Radic. Biol. Med. 1999, 27, 381–387. [Google Scholar] [CrossRef]
- Nifli, A.-P.; Kampa, M.; Alexaki, V.-I.; Notas, G.; Castanas, E. Polyphenol interaction with the T47D human breast cancer cell line. J. Dairy Res. 2005, 72, 44–50. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Pusztai, A. Evaluation of polyphenol bioavailability in rat small intestine. Eur. J. Nutr. 2001, 40, 84–90. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.-Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar]
- Redeuil, K.; Smarrito-Menozzi, C.; Guy, P.; Rezzi, S.; Dionisi, F.; Williamson, G.; Nagy, K.; Renouf, M. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 4678–4688. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Ema, K.; Tokuda, Y.; Monobe, M.; Tachibana, H.; Sameshima, Y.; Kuriyama, S. Effect of green tea powder (Camellia sinensis L. cv. Benifuuki) particle size on O-methylated EGCG absorption in rats; The Kakegawa Study. Cytotechnology 2011, 63, 171–179. [Google Scholar] [CrossRef]
- Renouf, M.; Guy, P.; Marmet, C.; Longet, K.; Fraering, A.-L.; Moulin, J.; Barron, D.; Dionisi, F.; Cavin, C.; Steiling, H. Plasma appearance and correlation between coffee and green tea metabolites in human subjects. Br. J. Nutr. 2010, 104, 1635–1640. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Gonzalez-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Green, R.J.; Peters, C.M.; Neilson, A.P.; Janle, E.M. The influence of food formulation on digestive behavior and bioavailability of catechin polyphenols. Acta Hortic. 2009, 841, 121–127. [Google Scholar]
- Keogh, J.B.; McInerney, J.; Clifton, P.M. The effect of milk protein on the bioavailability of cocoa polyphenols. J. Food Sci. 2007, 72, S230–S233. [Google Scholar] [CrossRef]
- Dupas, C.J.; Marsset-Bagllieri, A.C.; Ordonaud, C.S.; Ducept, F.M.G.; Maillard, M.-N. Coffee antioxidant properties: Effects of milk addition and processing conditions. J. Food Sci. 2006, 71, S253–S258. [Google Scholar] [CrossRef]
- Biasutto, L.; Marotta, E.; de Marchi, U.; Zoratti, M.; Paradisi, C. Ester-based precursors to increase the bioavailability of quercetin. J. Med. Chem. 2007, 50, 241–253. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Dora, C.L.; Andrade, E.L.; Chaves, J.S.; Silva, L.F.C.; Lemos-Senna, E.; Calixto, J.B. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res. 2010, 61, 288–297. [Google Scholar] [CrossRef]
- Mazzarino, L.; Silva, L.F.C.; Curta, J.C.; Licinio, M.A.; Costa, A.; Pacheco, L.K.; Siqueira, J.M.; Montanari, J.; Romero, E.; Assreuy, J.; et al. Curcumin-loaded lipid and polymeric nanocapsules stabilized by nonionic surfactants: An in vitro and in vivo antitumor activity on B16-F10 melanoma and macrophage uptake comparative study. Biomed. Nanotechnol. 2011, 7, 406–414. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [PubMed]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in mesocarp/epicarp and endocarp of fresh acai (Euterpe oleracea Mart.) and their antioxidant activities and bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velaco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Johnson, I.T. Glucosinolates in the human diet. Bioavailability and implications for health. Phytochem. Rev. 2003, 1, 183–188. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil-Izquierdo, A.; Moreno, D.A. Bioavailability and Metabolism of Phenolic Compounds and Glucosinolates. In Designing Functional Foods. Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; McClements, D.A., Decker, E.A., Eds.; CRC Press: Woodhead Publishing Limited, Cambridge, UK, 2009; pp. 194–229. [Google Scholar]
- Kensler, T.W.; Chen, J.-G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.-B.; Wu, Y. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2605–2613. [Google Scholar] [CrossRef]
- Shrivastava, S. S-allyl-cysteines reduce amelioration of aluminum induced toxicity in rats. Am. J. Biochem. Biotechnol. 2011, 7, 74–83. [Google Scholar] [CrossRef]
- Singh, Y.P.; Singh, R.A. Insilico studies of organosulfur-functional active compounds in garlic. BioFactors 2010, 36, 297–311. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309-325. https://doi.org/10.3390/antiox2040309
Abourashed EA. Bioavailability of Plant-Derived Antioxidants. Antioxidants. 2013; 2(4):309-325. https://doi.org/10.3390/antiox2040309
Chicago/Turabian StyleAbourashed, Ehab A. 2013. "Bioavailability of Plant-Derived Antioxidants" Antioxidants 2, no. 4: 309-325. https://doi.org/10.3390/antiox2040309
APA StyleAbourashed, E. A. (2013). Bioavailability of Plant-Derived Antioxidants. Antioxidants, 2(4), 309-325. https://doi.org/10.3390/antiox2040309



