Abstract
The concentration of antioxidant compounds is constitutive and variable from species to species and is also variable considering the development of the plant tissue. In this review, we take into consideration the antioxidant changes and the physiological, biochemical and molecular factors that are able to modulate the accumulation of antioxidant compounds in ornamental flowers during the whole development process until the senescence. Many ornamental flowers are natural sources of very important bioactive compounds with benefit to the human health and their possible role as dietary components has been reported. The most part of antioxidants are flower pigments such as carotenoids and polyphenols, often present in higher concentration compared with the most common fruits and vegetables. The antioxidants content changes during development and during senescence many biochemical systems and molecular mechanisms are activated to counteract the increase of reactive oxygen species and free radicals. There is a tight correlation between antioxidants and senescence processes and this aspect is detailed and appropriately discussed.
1. Introduction
The source of nutraceutical compounds in human diet is almost exclusively provided by fruits and vegetables. However, flowers are becoming important sources of several bioactive compounds that can be added in the diet as food. In the ancient time flowers were mainly eaten for their medicinal properties rather than their nutritional value. Nowadays, several metabolomics studies revealed the chemical compositions of wild and ornamental flowers, showing the presence of important bioactive molecules. Often, wild flowers represent low cost sources of important natural antioxidants. However, edible flowers are also used by chefs to add color, fragrance and flavor to foods or drinks [1]. In the past, the relative low interest in the use of ornamental flowers as food sources was due to the limited analytical methods able to detect poison substances; ornamental flowers have been largely used as sources of natural poison and this explains why many common flowers used as garden decoration are poisonous.
The majority of edible flowers are vegetables such as pumpkins flowers or inflorescences (broccoli) and a lot of references on their chemical composition or nutritional values are available. Several species of ornamental flowers are also substantial sources of antioxidants able to remove the negative effect of free radicals. In humans, antioxidants exert important roles in preventing several degenerative diseases and stress related pathologies. The intake of antioxidant molecules can scavenge free radical species to defend the cells from damages. In flowers, important compounds with antioxidant activities and anti-inflammatory proprieties are represented by polyphenols, carotenoids and vitamin C (ascorbic acid) [2]. Polyphenols include more than 10,000 compounds and are among the most important natural antioxidant compounds [3]. Flowers with their pigments are rich in phenolics which include phenolic acids, flavonoids and anthocyanins. From an ecological point of view the flowers represent the most important organs for the plants, since they are responsible for the spatial and temporal species survival. The flower has to be protected during development in order to guarantee the fertilization. After pollination, the external parts of flowers such as petals or tepals, stamens and part of the pistil, such as style and stigma, senesce because they have completed their function.
Therefore, the variations in the levels of antioxidant compounds are related with floral developmental processes and especially with the last step of senescence. The plant defense mechanisms against external and internal senescence associated factors are extremely complex. Multidisciplinary studies using physiological, biochemical and molecular biology approaches should be able to give a clearer picture of this fascinating phenomena and its regulation.
This review focuses on the use of ornamental flowers as food and source of antioxidant compounds as well as on the molecular mechanisms of flower development and floral senescence in general. In particular, the role of antioxidant compounds, the function of senescence associated genes (SAGs), antioxidant genes and their regulatory networks from signal transduction to transcription and post-transcription are discussed. Flower development has been largely studied for understanding the biological processes linked with senescence. Since flowers constitute floriculture items, it is very important to study the function of factors involved in flower senescence to identify innovative strategies for preserving the ornamental quality during storage, prolong the flower life or vase life at the consumer stage and increase the antioxidant compound levels.
2. Antioxidants in Ornamental Flowers
Flowers are appreciated for their wide range of colors and aroma. These sensory attributes are valuable indicators of the external quality and the ornamental value as well as important properties used for culinary purpose. Many ornamental flowers contain high levels of antioxidant compounds, which are often even higher than common horticultural crops. One of the most important parameters used to estimate the antioxidant content is the determination of the total phenolics. Flowers with higher total phenolics content are Antigonon leptopus, Bougainvillea glabra, Tagetes erecta, Cosmos sulphureus, Prunus mume and Sophora viciifolia with values >100 mg/g DW (Table 1). Several analytical methods can be used to evaluate the antioxidant capacity of flower tissues such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Ferric-reducing antioxidant power (FRAP). The higher values of FRAP were found in Antigonon leptopus, Bougainvillea hybrid, Cosmos sulphureus, Nelumbo nucifera and Tagetes erecta (Table 1). With respect to fruit or vegetables such as apple and lettuce, all the species studied as edible flowers have higher antioxidant capacity expressed as FRAP and lower percentage of inhibition expressed as DPPH.

Table 1.
Total phenolic content (determined with Folin–Ciocalteu assay) and antioxidant capacity (FRAP and ORAC assay) in different edible flowers.
| Edible flower | Total phenolics | Antioxidant capacity (DPPH) | Antioxidant capacity (FRAP) | Ref. | ||
|---|---|---|---|---|---|---|
| mg/g DW | mg/g FW | g AsA equiv./kg FW | % inhibition | μmol Fe2+/g DW | ||
| Antigonon leptopus | 72.1–177.2 | 89.4 | 282.9–619.7 | [4] | ||
| Antirrhinum majus | 3.49 | 5.06 | - | [5] | ||
| Begonia boliviensis | 4.92 | 6.80 | - | [5] | ||
| Bougainvillea glabra | 138.2 | 307.1 | [4] | |||
| Bougainvillea hybrid | 50.0 | 91.4 | 588.0 | [4] | ||
| Centaurea cyanus | 4.76 | 6.81 | - | [5] | ||
| Chrysanthemum frutescens | 2.53 | 4.24 | - | [5] | ||
| Chrysanthemum parthenium | 2.72 | 4.21 | - | [5] | ||
| Clitoria ternatea | 59.0 | - | 73.0 | [4] | ||
| Cassia siamea | 88.5 | 97.6 | 163.7 | [6] | ||
| Cosmos sulphureus | 86.8–102.5 | 87.0 | 99.9–538.6 | [4] | ||
| Dianthus caryophyllus | 5.28 | 6.96 | [5] | |||
| Fuchsia x hybrid | 3.45 | 5.20 | [5] | |||
| Helianthus annuus L. | 47.1 | [7] | ||||
| Hemerocallis spp. | 69–160 | [8] | ||||
| Impatiens walleriana | 4.85 | 6.89 | [5] | |||
| Ixora chinensis | 82.4 | [4] | ||||
| Malvaviscus arboreus | 59.0 | 31.4 | 271.3 | [4] | ||
| Nelumbo nucifera | 60.0 | 96.9 | 585.4 | [4] | ||
| Plumeria obtusa | 37.0 | 69.6 | 260.3 | [4] | ||
| Prunus mume | 150 | [4] | ||||
| Rosa odorata | 5.02 | 6.85 | [5] | |||
| Rosa hydrida | 8.5 | [9] | ||||
| Sophora viciifolia | 143.8 | 20.7 | 3160 | [10] | ||
| Tagetes erecta | 98.0–212.9 | 94.3 | 329.4–609.2 | [4] | ||
| Tagetes patula | 4.58 | 6.70 | [5] | |||
| Telosma minor | 29.0 | 34.1 | 162.6 | [4] | ||
| Tropaeolum majus | 3.31 | 5.12 | [5] | |||
| Viola x wittrockiana | 5.11 | 6.65 | [5] | |||
| Malus domestica | 100–200 | 1.2–6.5 | 83 | 4.2–6.3 | [11] | |
| Lactuca sativa | 0.2–0.3 | 74–82 | 1.8–5.3 | [12] | ||
Among the flavonoids, quercetin was present in high concentrations. In Nelumbo nucifera and Plumeria obtuse this flavonoid reached values of 194–238 mg/100 g DW. In contrast, rutin was present at concentration over 50 mg/100 g DW in Bougainvillea hybrid, Ixora chinensis and Plumeria obtuse. In apple and lettuce this compound is usually not detectable. Quercetin and rutin are able to protect human health against diseases associated to oxidative stresses such as cancer and cardiovascular diseases [13]. The daily dose intake of quercitin should range from 10 to 20 mg. Another important flavonoid with beneficial effects on human health is kaempferol. In vitro assay showed the ability of kaempferol to affect positively several cancer-derived disorders such as apoptosis, angiogenesis, metastasis, and inflammation [14].
The flowers with higher content of kaempferol were Antigonon leptopus, Bougainvillea glabra and Tagetes erecta, ranging from 76 to 87 mg/100 g DW (Table 2).

Table 2.
Flavonoid compounds in different edible flowers expresses as mg/100 g DW, apple and lettuce were added for comparison. Data are means found in literature.
| Species | Apigenin | Catechin | Chlorogenic acid | Kaempferol | Myricetin | Quercetin | Rutin | Ref. |
|---|---|---|---|---|---|---|---|---|
| Antigonon leptopus | 0.83 | 75.9 | 47.5 | 5.7–21.9 | [15] | |||
| Bougainvillea glabra | 8.9 | 87.2 | 61.5 | 1.3 | [15] | |||
| Bougainvillea hybrida | - | 3.54 | 5.6 | 51.5 | [4] | |||
| Cassia siamea | - | - | - | 3.21 | 4.56 | 61.9 | 64 | [4] |
| Cosmos sulphureus | 7 | 25.6 | 60 | 19.7 | [15] | |||
| Hemerocallis spp. | - | 111.5 | 7.2 | 9 | 14.6 | [16] | ||
| Ixora chinensis | 0.64 | - | - | 3.77 | 5.18 | 102.4 | 139 | [4] |
| Leucaena leucocephalade | - | - | - | 4.23 | 5.72 | 67.1 | 16.2 | [4] |
| Malvaviscus arboreus | - | - | - | 3.18 | 5.05 | 33.6 | 27.7 | [4] |
| Nelumbo nucifera | 0.62 | - | - | 3.79 | 5 | 237.8 | 23.1 | [4] |
| Plumeria obtuse | - | - | - | 3.58 | 5.06 | 193.6 | 500.3 | [4] |
| Tagetes erecta | 8.4 | 83.4 | 54.8 | 5.1 | [15] | |||
| Malus x domestica | - | 38.8–99.3 | 75.1 | 3.1 | 30.9 | 7.7–13.20 | 82 | [17,18,19,20,21] |
| Lactuca sativa | <4 | nd | 47 | 2.9 | <1 | 42.9 | nd | [22,23] |
In apple kaempferol is under the detection limits, while in lettuce it is comparable with other flower.
The antioxidant compounds increase during early senescence and decline during advanced senescence. As a first reaction, antioxidant compound protect the cell from damaging substances such as hydrogen peroxide and other reactive oxygen species (ROS).
2.1. The Changes of Antioxidant Compounds Is Often Related to the Senescence Processes
The antioxidant compounds increase during early senescence and decline during advanced senescence. As a first reaction, antioxidant compound protect the cell from damaging substances such as hydrogen peroxide and other reactive oxygen species (ROS).
Senescence is a process of progressive oxidative deterioration that represents the last step in flower development and leads ultimately to the programmed cell death (PCD). ROS are a common feature in the life of aerobic organisms. They act as signal molecules and are involved in several physiological processes in both plants and animals. Senescence is a complex chain of phenomena and ROSs are responsible of several senescence related processes. In fact, a certain amount of ROS is always produced during the whole life of each aerobic organism and its levels are tightly controlled by antioxidant systems which involve the mutual action of enzymes and antioxidant compounds.
The onset of senescence requires the coordinated regulation of several genes which is triggered by internal and external factors. The plant hormones ethylene and abscisic acid play an important role as internal signals of flower senescence. Based on the response to ethylene, flowers are classified in ethylene sensitive or ethylene insensitive. In the first class, ethylene strongly regulates the senescence process. In some species such as Hibiscus (Hibiscus rosa-sinensis L.) both hormones are involved in the flower senescence [24].
Plants counteract the effects of oxidative stress by synthesizing new antioxidants, by utilizing pre-existing pools or activating specific routes to recycle and consume specific molecules. A challenge for future researches is to understand if senescence could stimulate the de novo synthesis of antioxidant compounds or if the antioxidant potential of flowers during senescence mainly depends on the pool of molecules already accumulated during flower development.
2.1.1. Ascorbic Acid (AsA)
The AsA represents a key molecule in plant metabolism. It has been recognized to play a central role in several physiological processes like photosynthesis, photo-protection, cell division, plant growth, stress responses, regeneration of other important molecule such as α-tocopherol. Moreover, it has been reported to act as a co-substrate for the biosynthesis of important plant hormones such as ethylene and gibberellic acid and to be the substrate for oxalate and tartrate biosynthesis [25,26,27].
The AsA has been reported to be involved in the process of cell wall metabolism [28] and cell wall oxidative scission [29]. Further studies are needed in order to clarify the complex net of correlations between the levels of AsA, cell wall polysaccharides, ROS and other antioxidant molecules.
The AsA was reported to be involved in regulation of some senescence associated genes (SAGs) during plant oxidative stress-induced senescence. In fact a study showed an up-regulation of specific SAG transcripts in the AsA-deficient Arabidopsis mutant vtc1 grown under a long-day photoperiod (16 h) [30], suggesting that AsA deficiency induces a senescent phenotype.
It has been suggested that the effects of AsA levels on flowering time could be related to alterations in phytohormone levels such as gibberellic acid (GA) ABA, SA and ethylene and that the redox status of AsA may play a role in signaling in this interconnected phytohormone network. [30]. In daylily (Hemerocallis hybrid) petals AsA levels decreased by one half after 12 h before flower opening and the levels remain low to 36 h after flower opening. A limiting amount of AsA has been thus mentioned by authors as an important factor in the ability of ascorbate peroxidase (APX) to reduce H2O2 [31]. The AsA was reported to rapidly decline during aging of Chrysanthemum petals and it was reported to interact synergistically with α-tocopherol in contrasting oxidative damage of lipid membranes [32].
2.1.2. Tocopherol
In plants, tocopherols together with tocotrienols are important antioxidants believed to act as scavenger of lipid peroxy radicals, which are responsible for lipid peroxidation [33,34,35]. The α-tocopherol represents the primary defense against lipid peroxidation, it has been reported to act in preventing radical-induced lipid peroxidation and its action is synergistically connected to AsA [32,36]. In petals from recently cut chrysanthemum flowers the ascorbic acid/α-tocopherol ratio declined during aging, suggesting that this antioxidant system is less efficient as the aging process started [32].
2.1.3. Phenolic Compounds
Phenolic secondary metabolites are widespread among plants and are involved in many plant functions, defense mechanisms and stress responses. Anthocyanins, a flavonoid subclass, are the main pigments in flowers acting as insect and animal attractants [37,38]. Anthocyanins are synthesized via the phenylpropanoid pathway. These compounds are known for their antioxidant properties and are among the most important plant’s antioxidants. They have been extensively studied and their pathway of accumulation has been well characterized [39,40,41].
The flavonols generally increase prior to anthocyanin accumulation during floral development and declined when anthocyanin began to accumulate [42,43]. This phenomenon is well tightly regulated and it was explained, hypothesizing a role for flavonols in protecting developing sexual organs from potentially harmful UV-B light [43,44].
The changes in concentration of various phenolic compounds and color parameters were measured for the first time at different developmental stages of miniature rose “KORcrisett” [9]. In their work, the authors referred to a previous research published by Takahama et al. in 1997 [45] in which the existence of a peroxidase/phenolics/ascorbate system active in the scavenging of hydrogen peroxide in plant cells was suggested and reported that a decline in phenolics and anthocyanins concentration at later stages of flower development may limit the role of this system, making the flower more vulnerable to oxidative stress. The authors thus suggested a role of phenolics in regulation of flower development [45]. During flower senescence in long life and ephemeral Hibiscus (Hibiscus rosa-sinensis L.) anthocyanins were very high in ephemeral flowers and were reported to be likely linked to senescence processes [24,46]. In petunias the flower longevity was inversely correlated to anthocyanins content [47]. On the contrary, other studies showed a negative correlation between high antocyanins content and flower longevity. In senescent Orchid flowers changes in the anthocyanin pattern determined color changes from white to pink or blue [48]. Analogously during flower senescence the anthocyanin accumulation increases and the application of senescence promoters (ethylene) or inhibitor (1-methylciclopropene) enhance or retard the anthocyanin accumulation [49]. These results indicate that phenolic compounds biosynthesis might be a response of flowers to cell disruption, but the accumulation of these phenolic compounds is inefficient to counteract the genetically programmed senescence.
2.1.4. Carotenoids
Carotenoids (in relation to abscissic acid ABA biosynthesis) levels generally decreased during senescence indicating a possible involvement as a substrate in biosynthesis of ABA through the indirect pathway. The highest carotenoids concentration was associated with shortest flower life, suggesting their potential source for ABA biosynthesis in petals. However, carotenoids degradation and ABA content should be monitored during flower development and senescence in all cultivars before to come to a clear conclusion [46].
3. Biochemical Pathways Involved in the Antioxidant Responses during Flower Senescence
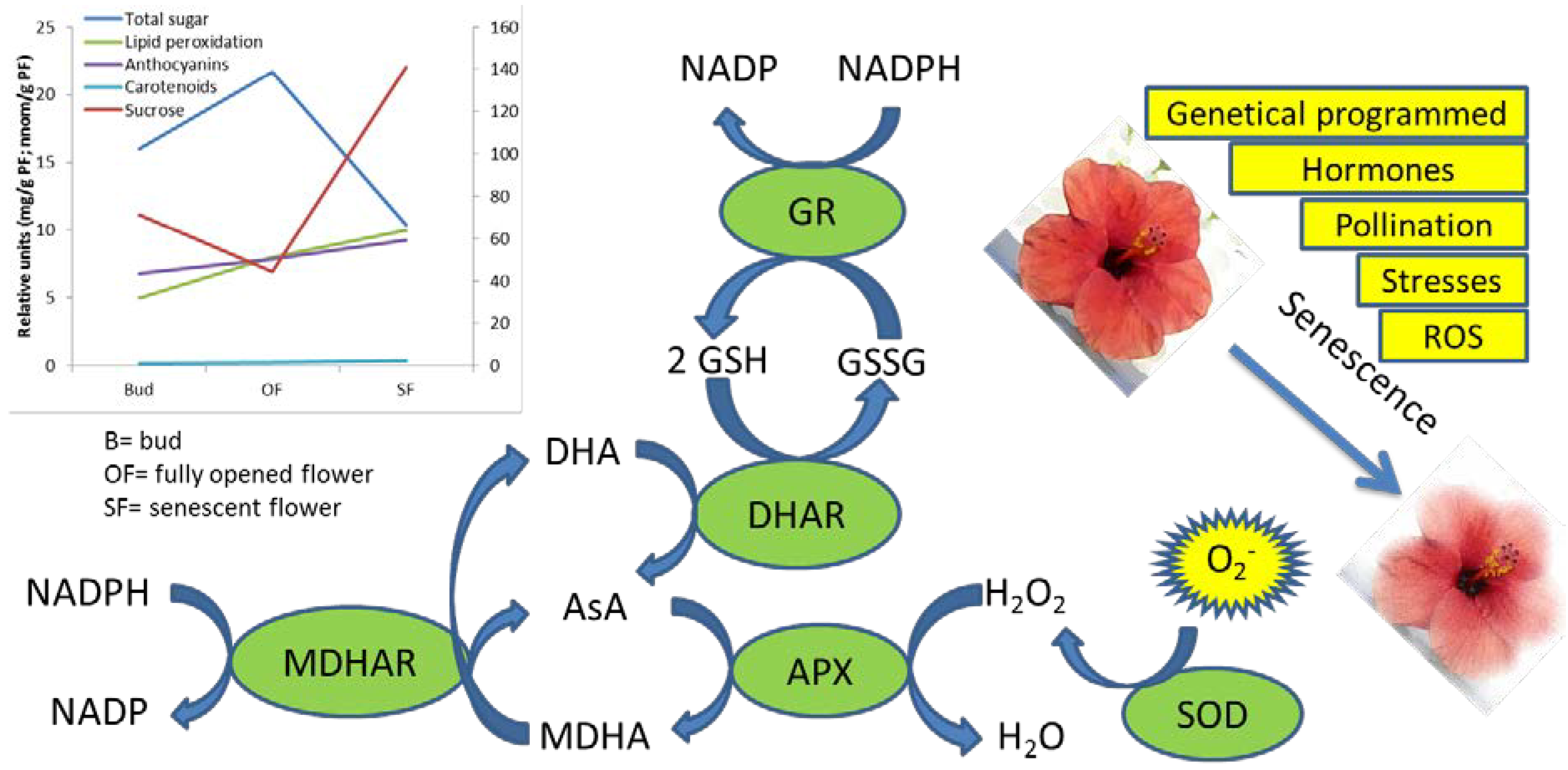
Senescence is tightly associated with a rise in ROS levels [50], whose production is accompanied by the activation of the enzymes involved in ROS scavenging such as superoxide dismutases (SODs), catalases (CATs) and ascorbate-glutathione cycle enzymes ascorbate peroxidase (APX), monodehydroascorbic reductase (MDHAR), dehydroascorbic reductase (DHAR) and glutathione reductase (GR) (Figure 1). These antioxidant enzymes show different pattern of activity among species in ethylene-dependent and independent manners. The levels of SODs were observed to decrease with the progression of senescence in petals from Chrysanthemum [32], Rose [51], day lily [52], Carnation [53], Iris [54], Gladiolus [55] and Freesia [56]. Class III plant peroxidase (POX), such as peroxiredoxins [57] and glutathione peroxidases [58] were recently identified as being involved in ROS scavenging by reaction with H2O2 or alkyl hydroperoxides [59]. During senescence CAT activity was shown to fall in Chrysanthemum [32], Gladiolus [55] and carnation [60], but to rise in day lily [52] and Iris [54]. Ascorbate peroxidase (APX) reduces H2O2 with the consequent oxidation of ascorbate to dehydroascorbate: H2O2 acts as a signaling molecule to induce PCD pathway and expression of defense-related genes (including glutathione S-transferase, glutathione peroxidase, superoxide dismutase) [61]. In Gladiolus [62], day lily [52] and Iris [54] the down regulation of APX over the senescence period has led to high accumulation levels of endogenous H2O2 that up-regulates SOD gene action. In contrast, in Chrysanthemum and carnation petals, APX activities increase during flower senescence accompanied by an increase in the number of peroxisomes [63,64].
More studies are needed in order to better understand the possible role of MDHAR and DHAR as their activity lead to AsA recycling and thus a better knowledge about the interaction between the activity of these enzyme with AsA levels it could represent an useful mean to reinforce flower’s own ability to counteract the progress of senescence, increasing their marketability and antioxidant quality. The GR activity showed diverse pattern of activity between species during floral senescence. In Gladiolus, GR levels fell at later stages of senescence [62,65]; in carnation, GR declined much earlier throughout senescence [60]; in Chrysanthemum, GR peaked twice, at late bud stage and again at the beginning of senescence [63]. All these studies clearly indicated that the activity of many ROS-scavenging enzymes follows a decline as senescence reaches the last phases, in concomitance with a reduction in the antioxidant content.
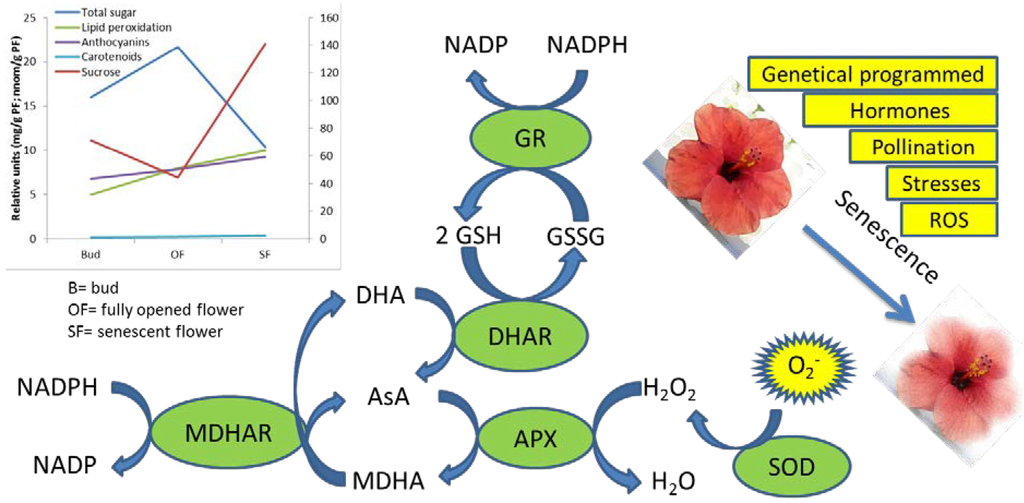
Figure 1.
Factors involved in the flower senescence and detoxification enzymes, which acts in scavenging the superoxide forms. In the graph, nutraceutical compounds and senescence markers such as lipid peroxidation during development and senescence are reported.
Figure 1.
Factors involved in the flower senescence and detoxification enzymes, which acts in scavenging the superoxide forms. In the graph, nutraceutical compounds and senescence markers such as lipid peroxidation during development and senescence are reported.
4. The Molecular Regulatory Networks of Flower Development and Senescence
4.1. Gene Regulation of Flower Development
Flower formation and development are controlled by many genes, mostly encoding for transcription factors. These genes are conventionally grouped into classes according to their role in the processes of flower initiation, meristem identity and organs determination [66]. In Arabidopsis, the class of Flowering Time genes control the flowering time and is regulated by environmental conditions such as cold temperature (vernalization) [67]. The key gene in the control of flowering time is the FLOWERING LOCUS C (FLC), a MADS-box transcription factor whose expression is regulated at the epigenetic level [68]. The repression of FLC target genes lead to a delay in the expression of the Floral Meristem identity genes. This latter class of genes includes: LEAFY (LFY) and APETALA1 (AP1), two transcription factors with partial overlapping roles in the determination of flower meristem identity; CAULIFLOWER (CAL) and UNUSUAL FLORAL ORGANS (UFO) both promoting the floral meristem fate, and TERMINAL FLOWER 1 (TFL1), which maintains the inflorescence meristem identity [69,70,71]. Other genes promoting meristem identity include SEPALLATA (SEP) and other uncharacterized genes that were shown to be target of LFY activity [72]. The class of Flower Organ Identity genes determine the floral-organ patterning of each whorl and include A, B, C, D, and E homeotic genes. The ABCDE model is found to be conserved among several plant species [73,74]. Class A specifies sepal identity in whorl 1 and along with class B determines petal identity in whorl 2; class B along with class C specifies stamen identity in whorl 3 and class C confers carpel identity in whorl 4; class D determines ovule identity and class E is required for petals, stamens and carpels specification [75].
4.2. Senescence Associated Genes (SAGs)
Few recent reviews on floral senescence are available [50,76]. Flower senescence is a programmed process characterized, in the terminal phase, by the degradation of nucleic acids, proteins, carbohydrates, lipids and membranes, all representing hallmarks of programmed cell death (PCD) [76]. These events are orchestrated at different levels, including control of metabolic changes, environmental and hormonal signaling in association with considerable modification of gene transcription and translation. The molecular and regulatory networks involved in flower senescence program have been largely investigated: a high number of senescence-associated genes (SAGs) were identified in flowers with different patterns of senescence such as Petunia, Dianthus caryophyllus and Hemerocallis [77,78,79]. Moreover, by means of PCR-based subtractive hybridization, microarray technology and EST analyses numerous genes with up- or down-regulation expression patterns were identified also in Narcissus [80], Alstroemeria [81], Iris [82], Rosa [83] and Mirabilis jalapa [84]. SAG-encoded proteins exert a variety of functions in macromolecule breakdown, ROS detoxification, ethylene biosynthesis, nutrient recycling and remobilization. SAGs include enzymes involved in lipid metabolism (glutathione-S-transferase, allene oxide synthase, acyl CoA oxidase, in-chain fatty acid hydroxylase and fatty acid elongase); hydrolase implicated in cell-wall degradation (β-glucosidase, β-galactosidase, pectin acetylesterase), a carboxy PEP mutase with putative role in membrane turnover and biosynthesis of phosphonates, and enzymes responsible for ethylene biosynthesis (S-adenosylmethionine synthetase, 1-aminocyclopropane-1-carboxylic acid synthase (ACC) and 1-aminocyclopropane-1-carboxylic acid oxidase) [85,86,87,88,89,90].
In Alstroemeria the down-regulation of a cytochrome P450 was associated with flower senescence [81] while in Petunia a tonoplast-localized cytochrome P450 highly similar to a tomato allene oxide synthase (AOS) was reported to be highly expressed in senescing petals [91].
In Rose, an homologous gene of a delta-9-desaturases was induced during senescence with putative role in degradation of saturated fatty acids of membrane lipids [92].
Work performed in Mirabilis jalapa identified down-regulated genes homologous to clock-associated (CCA1), far-red insensitive 219 (FIN219) and phytochrome A-related WD-40 repeat gene, suggesting that flower senescence might be under the control of the circadian clock through the phytochrome A pathway [93,94]. In addition, a RING zinc finger ankyrin repeat protein (MjXB3) was found highly expressed in senescing petals with putative roles in protein ubiquitination [76]. The involvement of an ubiquitin pathway for the degradation of petal proteins was also reported in Hemerocallis flowers [95]. Several cysteine and serine protease genes were shown to be up-regulated in Narcissus [96] and under exposure to ethylene in Petunia [97], Sandersonia [98] and Alstroemeria [99]. Thiol proteases (SEN11 and SEN102) were up-regulated in Hemerocallis sepals and found to be involved in protein hydrolysis at the late senescence stage [100]. Nine cysteine proteases were identified in senescent corollas of Dianthus caryophyllus and their expression was highly increased by ethylene [101] in parallel with the reduction of cysteine protease inhibitor [102].
It was shown that the gene expression levels of nuclease and DNA fragmentation increase until the advanced stages of flower senescence in Petunia: in particular, five single-stranded and double-stranded DNases were identified and induced by Ca2+ in senescent petals of pollinated flowers [103]. In Hemerocallis petals, the S1- and P-type endonucleases were identified with roles in fragmentation of single-stranded DNA and RNA [87]. Senescence-associated S-like RNases were also characterized in the petals and leaves of Arabidopsis and tomato [104,105].
All floral tissues undergo senescence. For example, the degeneration of anther tapetum was characterized by chromatin condensation and DNA degradation in Lobivia rauschii, Tillandsia albida and Hordeum vulgare [106,107]. In the cytoplasmic male sterility mutant (PET1-CMS) of Helianthus annuus the release of cytochrome c from the mitochondria into the cytoplasma caused premature PCD [108]. In papaver, the PCD deriving from self-incompatibility (SI) was induced by caspase-3-like activity to prevent aspecific pollen-tube growth and fertilization [109].
4.3. Transduction and Gene Regulation: Transcription Factors and Small Non Coding RNAs
The activation and expression pattern of SAGs are controlled at the levels of signal transduction, transcription and post-transcription [110].
G protein, secondary messenger calcium, polyamines and sugars signaling pathways were shown to induce floral senescence [76].
Before senescence starts, an increased level of G protein linked to the phospholipase a and c (PLA and PLC) was observed in Petunia petals: PLs hydrolyze the membrane component phosphatydylinositol diphosphate (PIP) to generate inositol triphosphate (IP3) and diacylglycerol (DAG), whose levels increase until the burst of ethylene [111].
As co-factor of protein kinases, the application of exogenous calcium in the orchid Phalaenopsis increased flower sensitivity to ethylene, thereby accelerating senescence [112].
In plants, polyamines (Pas) have roles in many physiological processes from the stimulation of cell division, differentiation and growth to stress response [113]. It has been shown that in Dianthus caryophyllus and gerbera petals the addition of different concentration of Pas, such as putrescine and spermine, caused a delay in senescence [114,115] probably inhibiting the ethylene synthesis. This is consistent with the results obtained by Lee et al. [116], where the application of a polyamine synthesis inhibitor on Dianthus caryophyllus petals led to increased levels of ACC synthase and aminocyclopropanecarboxylate oxidase (ACC oxidase) mRNAs as well as to increased ethylene production.
Finally, Hoeberichts et al., in 2007 [117] reported that in Dianthus caryophyllus petals the application of soluble sugar and sucrose inhibited ethylene signaling with subsequent repression of SAGs and delay in protein degradation.
By means of genetic and transcriptomic approaches several plant transcription factors were identified as regulators of plant senescence. Most of the isolated transcription factors were involved in the positive regulation of leaf senescence. However, some Arabidopsis mutants such as NAC transcription factor (ANAC092) [118], AUXIN RESPONSIVE FACTOR 2 (ARF2) [119] and TCP4 [120] showed a delay in floral senescence suggesting their role in promoting floral abscission. Furthermore, the promoter of TCP4 and ANAC092 genes contains W-box motifs indicating a possible regulation by WRKY transcription factors. In particular, WRKY53 was identified as leaf senescence promoting transcription factor that also accelerates flowering [121].
In Arabidopsis the NAC-domain, bHLH and MYB73 genes were shown to be up-regulated soon after anthesis and their expression was restricted to ovule senescence [122].
In the senescing petals of Dianthus caryophyllus the Aux/IAA3 transcription factors were up-regulated [117], while MYB-like and a MYC proteins were differentially regulated although their function is unclear [117]. Four Ethylene insensitive 3 EIN3 and EIN3-like (EIL) transcription factors were isolated from Dianthus caryophyllus petals and showed differential expression levels, with the up-regulated EIL3 as key regulator of ethylene-responsive genes [117].
An AP2 protein with very high homology to ETHYLENE RESPONSIVE FACTOR 2 (ERF2) showed an up-regulation at transcription level in petals of Narcissus [96].
The expression levels of genes homologous to bZIP and HD-Zip proteins were up-regulated in M. jalapa flowers [84].
Three MADS box genes belonging to the AP1/AGL9 subfamily were isolated from orchid flowers [123]. Members of the same family in Arabidopsis were reported to be up-regulated and closely associated with flower and silique senescence [124]; the constitutive overexpression of the MADS domain protein AGAMOUS like 15 (AGL15) caused a delay in floral abscission in Arabidopsis [125].
The regulation of flower senescence was also shown to occur through epigenetic mechanisms as evident in the histone deacetylase Arabidopsis (hda6) mutant where the mutation induced a delay in floral senescence [126].
The regulation of gene expression occurs at the post-transcriptional level through the action of small non coding RNAs. Among them, the 21-nt microRNAs (miRNAs) exert important regulatory roles in plant development by silencing complementary mRNA transcripts. The miRNAs have been identified as regulators of flowering time and floral organ identity by controlling the expression of specific transcription factors. For example, in Arabidopsis the miR164 family silence members of the NAC genes with function in organ boundary formation [127] and the miR172 family regulates APETALA2(AP2) and other AP2-like transcription factor genes acting as repressors of flowering [128].
In Arabidopsis miR164 and miR319 families were shown to be involved in the control of leaf senescence [129]; although it has not yet been investigated, it is tempting to speculate that the same miRNA families might control flower senescence by regulating NAC and TCP transcription factors. Apart from ethylene and jasmonic acid, whose function was demonstrated to induce organ senescence, auxin plays a role in floral organ abscission through a miR390-TAS3-ARF2 module: miR390 induces the cleavage of a TAS3 transcript for the generation of 21-nt trans acting short interfering RNAs (tasiRNAs) involved in the silencing of ARF2, a negative regulator of auxin responses [119].
A recent study in Rosa hybrid: “Vital”, “Maroussia”, and “Sympathy” and Rosa rugosa Thunb. reported the identification of certain miRNAs putatively involved in the regulation of genes related to coloring, like the flavonoid biosynthetic genes [130]. In Rosa petals miR166 and miR5139 appeared to target β-galactosidase and expansin genes involved in cell-wall modification in an ethylene-regulated manner [131].
In Arabidopsis it was shown that by repressing the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9) factor, miR156 regulates the FLOWERING LOCUS T (FT) expression during flowering and the accumulation of anthocyanin through the destabilization of a MYB-bHLH-WD40 transcriptional activation complex [132]: the high level of miR156 decreased the accumulation of anthocyanins and this result is consistent with the observed reduction of total anthocyanins concentration in petals [24,47] leading to a limited antioxidant defense and progress of senescence.
4.4. Antioxidant Genes
Antioxidant or ROS-scavenging genes have important roles in the control of ROS levels in different aspects of plant growth, development, response to stress and organ senescence. The increasing levels of reactive oxygen species (ROS) in senescing leaves and petals is accompanied by the differential regulation of genes involved in the protection against ROS. Transcriptome studies of leaves from Arabidopsis mutants, such as the immutans (im) variegation mutant [133], revealed that in response to oxidative stress the Cu-ZnSODs, FeSOD, CAT1, ferritin1, PODs, glutathione peroxidase (GPX2/GPX7), stromal APX, GR and alternative oxidase (AOX) genes were up-regulated whereas FeSOD1, CAT3, thylakoid APX and DHAR genes were down-regulated [134]. Interestingly, the work of Wagstaff et al. published in 2009 [135] showed that transcripts profiling of senescence in leaves, petals and siliques is similar, indicating that majority of SAGs are not tissue specific and supporting the hypothesis that petals are evolutionarily derived from leaves.
With respect to petal senescence large gene expression studies of CATs, APXs, lipoxygenases (LOX)s, SODs, GR and GSTs were performed in Arabidopsis [135], Erysimum linifolium [136], Iris [82] and Alstroemeria [137] and the expression patterns of some antioxidant genes were also identified through EST analysis in other species such as Petunia and Dianthus caryophyllus. Nevertheless, it is very likely that the expression pattern of antioxidant genes follows the same activity pattern of their encoded proteins, with a fall in the transcript abundances at the ethylene burst for ethylene sensitive species.
In Alstroemeria the expression levels of the CAT, APX, LOX and a Cu–Zn SOD genes were down-regulated and the DHAR gene was up-regulated, whereas in Arabidopsis these genes did not show variation in the mRNA abundance [50,137]. Members of the thioredoxin gene family were up-regulated in Erysimum and Alstroemeria [135,136,137]. Glutaredoxin genes and glutathione peroxidase were up-regulated in Arabidopsis and Erysimum, but down-regulated in Alstroemeria.
In Dianthus caryophyllus, two glutathione S-transferases (GST1, GST2) genes were up-regulated in concomitance with the increase of ethylene [138]. The functional role of most plant GSTs is still unclear, especially in petals: some act as glutathione peroxidises in Arabidopsis, while others might have roles in hormone metabolism [139] and anthocyanin biosynthesis [140].
An increased transcript level of metallothioneins (MTs) was reported in senescent leaves [141] petals [83], and ripening fruits [142]. MTs were highly represented in the transcriptome of Alstroemeria and in Erysimum senescent petals but not in Arabidopsis [50,81,137]. The function of such high levels of MTs might be related to the detoxification of metal ions (Cu2+ and Zn2+) released after enzyme and protein degradation.
During the initial stages of senescence the antioxidant response to oxidative stress is associated with an increment in the content of antioxidant compounds such as tocopherol, ascorbic acid (AsA) and glutathione, followed by a decrease as senescence reaches the ultimate phases.
The key genes in tocopherol biosynthesis were isolated such as the HPD encoding for 4-hydroxyphenylpyruvate dioxygenase and VTE2 encoding for homogentisate prenyltransferase. HPD expression analysis in rice and barley leaves showed that the mRNA levels were enhanced during leaf senescence [143] and tocopherols were found to be accumulated in the senescing leaves of several plants [144]. In particular, an increasing level of α- and γ-tocopherol were observed during senescence of Lilium cut flowers [145].
Ascorbic acid is synthetized via l-galactose [146], l-gulose [147], d-galacturonic and d-glucuronic pathways [148]. The genes encoding the biosynthetic enzymes were identified through the study of four AsA-deficient mutants (vtc1, vtc2, vtc3, and vtc4) [149]. As a result of the decreased enzyme activity, the vtc1 mutant had a reduced quantity of AsA respect to the wild-type [150]. Kotchoni et al. in 2009 [151] reported that low levels of AsA induced the up-regulation of circadian clock and photoperiodic pathway genes and the down-regulation of FLC, causing early flowering, while high levels of AsA delayed flowering and senescence irrespective of the photoperiod.
Mutants showing alterations in flower pigmentation were affected in the flavonoid biosynthesis and many of the involved regulatory genes were isolated from Arabidopsis, Zea mais, Antirrhinum majus, and Petunia [152]. The accumulation of anthocyanin depends on the expression level of chalcone synthase (CHS), chalcone isomerase (CHI), phenylalanine ammonia-lyase (PAL) and flavonoids 3′-hydroxylase (F3′H) biosynthetic genes. Their expression increases during the early stages of flower development and decreases at the later stages in Petunia, Malus domestica, Antirrhinum majus and Rose.
Interestingly, the overexpression of a GAST-like protein (GIP2) in Petunia promoted stem and corolla elongation by regulating the levels of ROS, acting as antioxidant [153].
5. Conclusions
With the advancement in sequencing technologies and transcriptome profiling tools, the global gene expression reprogramming of floral senescence has been elucidated in diverse species despite the function of many genes has yet to be validated. Among plant species, there are evident differences in the transcript levels of SAGs and antioxidant genes: these may be ascribed to the diversity of mechanisms established during senescence. Such differences could also be due to the variation of SAGs and antioxidant transcript levels among different cells that might undergo stages of senescence at different times within the same floral tissue.
Hence, still many aspects of floral senescence have to be deciphered. Firstly, a more comprehensive profiling of the small RNAs classes is necessary to better understand the gene regulatory mechanisms at the post-transcriptional level. Secondly, little information is available for abiotic stresses impacts on flower development and senescence. For example, a large variability exists in the senescence response to climate change depending on the climatic conditions such as temperature and on the plant species [154]. Both proteomic and metabolomics investigations are needed to determine the post-translational modifications and the metabolic changes. The combination of different “omics” platforms will surely provide a global and deeper view of all the networks implicated in flower development and senescence.
Despite the difficulty in transforming and regenerate certain species, the understanding of senescence regulatory networks will help to engineer flowers with an extended life. It was shown that Dianthus caryophyllus flowers expressing an antisense copy of ACC oxidase (ACO) or the expression of ethylene insensitive receptor etr1-1 in Petunia showing delay in floral abscission had an increased longevity respect to the wild type and to the application of any postharvest treatment [155,156]. Genetic engineering might be also addressed toward the manipulation of antioxidant gene expression and enzymes involved in antioxidants production pathway (i.e., ascorbic acid, polyphenols, etc.) both in ornamental flowers and edible flowers, the latter with direct positive implications for human nutrition and health. Senescence conditions generally determine an increase in antioxidant levels as they represent a key defense strategy against oxidative stress. As such these phases could be very interesting on both nutritional and ornamental point of view. Moreover, studies on transcription factors and genes responsible for antioxidant-related traits will be a valid support in the identification of varieties with a better attitude to storage and industrial processing as well as in the molecular assisted selection.
Further works should be addressed on the quality of edible flowers after harvest and the development of technologies that can be used for maintaining the nutritional value during storage. The marketability of edible flowers will be possible if appropriate food processing and storage methods will be studied and designed in order to reduce quality losses from the field to the consumers. Only if an organized food chain will be developed the ornamental flower will gain the global market. Postharvest treatments should be also optimized for these new foods considering that only safe and non-toxic compounds can be utilized for the storage of edible products.
Acknowledgments
This work was funded by the Italian Ministry of Agricultural, Food and Forestry Policies (MIPAAF) under the project TRACEFLOR.
Conflict of Interest
The authors declare no conflict of interest.
References
- Kelley, K.M.; Bridget, K.; Behe, B.K.; Biernbaum, J.A.; Poff, K.L. Consumer preference for edible flower color, container size, and price. HortScience 2001, 36, 801–804. [Google Scholar]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Haslam, E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Kaur, G.; Alamb, M.S.; Jabbar, Z.; Javed, K.; Athar, M. Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol. 2006, 108, 340–348. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Liang, Q.; Cui, J.; Li, H.; Liu, J.; Zhao, G. Florets of Sunflower (Helianthus annuus L.): Potential new sources of dietary fiber and phenolic acids. J. Agric. Food Chem. 2013, 61, 3435–3442. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential health enhancing properties of edible flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J. Am. Soc. Hortic.Sci. 2009, 134, 491–496. [Google Scholar]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef]
- Alía, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110–118. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. Review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH• radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Pospišil, J.; Levaj, B.; Delonga, K.; Đaković, S. Phenolic profiles of apricot, apple and pumpkin purees in evaluation of apricot nectars and jams authenticity. Food Chem. 2005, 91, 373–383. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hort. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Bilyk, A.; Sapers, G.M. Distribution of quercetin and kaempferol in lettuce, kale, chive, garlic chive, leek, horseradish, red radish, and red cabbage tissues. J. Agric. Food Chem. 1985, 33, 226–228. [Google Scholar] [CrossRef]
- Hertog, M.G.; Hollman, P.C.; Venema, D.P. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J. Agric. Food Chem. 1992, 40, 1591–1598. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Bot. 2011, 62, 5437–5452. [Google Scholar] [CrossRef]
- Davey, M.W.; van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Conklin, P.L. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001, 24, 383–394. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. Hopes, disillusions and more hopes from vitamin C. Cell. Mol. Life Sci. 2004, 61, 209–219. [Google Scholar] [CrossRef]
- Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef]
- Panavas, T.; Rubinstein, B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998, 133, 125–138. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.R.; Puntarulo, S. Oxidants and antioxidants during aging of Chrysanthemum petals. Plant Sci. 1997, 129, 157–165. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E: Application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Liebler, D.C. Antioxidant reactions of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 20–31. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Kunert, K.J.; Ederer, M. Leaf aging and lipid peroxidation: The role of the antioxidants vitamin C and E. Physiol. Plant. 1985, 65, 85–88. [Google Scholar] [CrossRef]
- Bohm, B.A. Introduction to Flavonoids; Hardwood Academic Publishers: Reading, UK, 1998. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant. 1999, 107, 142–149. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Noda, N.; Kanno, Y.; Kato, N.; Kazuma, K.; Suzuki, M. Regulation of gene expression involved in flavonol and anthocyanin biosynthesis during petal development in lisianthus (Eustoma grandiflorum). Physiol. Plant. 2004, 122, 305–313. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Gupta, A.P.; Kaul, V.K. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J. Sep. Sci. 2008, 31, 262–267. [Google Scholar] [CrossRef]
- Nielsen, K.; Deroles, S.C.; Markham, K.R.; Bradley, M.J.; Podivinsky, E.; Manson, D. Antisense flavonol synthase alters copigmentation and flower color in Lisianthus. Mol. Breed. 2002, 9, 217–229. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol. Plant. 1997, 101, 845–852. [Google Scholar] [CrossRef]
- Trivellini, A.; Vernieri, P.; Ferrante, A.; Serra, G. Physiological characterization of flower senescence in long life and ephemeral hibiscus (Hibiscus rosa-sinensis L.). Acta Hortic. 2007, 755, 457–464. [Google Scholar]
- Ferrante, A.; Vernieri, P.; Tognoni, F.; Serra, G. Changes in abscisic acid and flower pigments during flower senescence of Petunia. Biol. Plant. 2006, 50, 581–585. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Hogan, N.M.; Arditti, J. Postpollination phenomena in orchid flowers. IX. Induction and inhibition of ethylene evolution, anthocyanin synthesis, and perianth senescence. Bot. Gaz. 1980, 141, 422–427. [Google Scholar] [CrossRef]
- Macnish, A.J.; Jiang, C.Z.; Reid, M.S. Treatment with thidiazuron improves opening and vase life of iris flowers. Postharvest Biol. Technol. 2010, 56, 77–84. [Google Scholar] [CrossRef]
- Rogers, H.J. Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ. 2012, 35, 217–233. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.C.; Dixit, K. Flower bud opening and senescence in roses (Rosa hybrida L.). Plant Growth Regul. 2008, 55, 81–99. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Verma, A.K.; Datta, S.K. Oxidative stress and antioxidant activity as the basis of senescence in Hemerocallis (day lily) flowers. J. Agric. For. 2009, 1, 113–119. [Google Scholar]
- Droillard, M.J.; Paulin, A. Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from petals of carnation (Dianthus caryophyllus) during senescence. Plant Physiol. 1990, 94, 1187–1192. [Google Scholar] [CrossRef]
- Bailly, C.; Corbineau, F.; van Doorn, W.G. Free radical scavenging and senescence in Iris tepals. Plant Physiol. Biochem. 2001, 39, 649–656. [Google Scholar] [CrossRef]
- Yamane, K.; Kawabata, S.; Fujishige, N. Changes in activities of superoxide dismutase, catalase and peroxidase during senescence of Gladiolus florets. J. Jpn. Soc. Hortic. Sci. 1999, 68, 798–802. [Google Scholar] [CrossRef]
- Shu, Z.; Shi, Y.; Qian, H.; Tao, Y.; Tang, D. Distinct respiration and physiological changes during flower development and senescence in two Freesia cultivars. HortScience 2010, 45, 1088–1092. [Google Scholar]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; Nunes de Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Eshdat, Y.; Holland, D.; Faltin, Z.; Ben-Hayyim, G. Plant glutathione peroxidases. Physiol. Plant. 1997, 100, 234–240. [Google Scholar] [CrossRef]
- Welinder, K.G.; Justesen, A.F.; Kjærsga, I.V.H.; Jensen, R.B.; Rasmussen, S.K.; Jespersen, H.M.; Duroux, L. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 6063–6081. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Chen, S.; Han, L.; Li, Z. The role of N-lauroylethanolamine in the regulation of senescence of cut carnations (Dianthus caryophyllus). J. Plant Physiol. 2007, 164, 993–1001. [Google Scholar] [CrossRef]
- Desikan, R.; Reynolds, A.; Hancock, J.T.; Neill, S.J. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 1998, 330, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Decline in ascorbate peroxidase activity—A prerequisite factor for tepal senescence in Gladiolus. J. Plant Physiol. 2006, 163, 186–194. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Guiamet, J.; Montaldi, E.R.; Puntarulo, S. Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci. 1995, 104, 161–168. [Google Scholar] [CrossRef]
- Del Rio, L.A.; Palma, J.M.; Sandalio, L.M.; Corpas, F.J.; Pastori, G.M.; Bueno, P.; Lopez-Huertas, E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem. Soc. Trans. 1996, 24, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Ezhilmathi, K.; Singh, V.P.; Arora, A.; Sairam, R.K. Effect of 5-sulfosalicylic acid on antioxidant activity in relation to vase life of Gladiolus cut flowers. Plant Growth Regul. 2007, 51, 99–108. [Google Scholar] [CrossRef]
- Krizek, B.A.; Fletcher, J.C. Molecular mechanisms of flower development: An armchair guide. Nat. Rev. Genet. 2005, 6, 688–698. [Google Scholar] [CrossRef]
- Lohmann, J.U.; Weigel, D. Building beauty: The genetic control of floral patterning. Dev. Cell 2002, 2, 135–142. [Google Scholar] [CrossRef]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Alvarez, J.; Smyth, D.R.; Yanofsky, M.F.; Meyerowitz, E.M. LEAFY controls floral meristem identity in Arabidopsis. Cell 1992, 69, 843–859. [Google Scholar] [CrossRef]
- Parcy, F.; Nilsson, O.; Busch, M.A.; Lee, I.; Weigel, D. A genetic framework for floral patterning. Nature 1998, 395, 561–566. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Gustafson-Brown, C.; Pinyopich, A.; Ditta, G.S.; Yanofsky, M.F. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 1999, 11, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- William, D.A.; Su, Y.; Smith, M.R.; Lu, M.; Baldwin, D.A.; Wagner, D. Genomic identification of direct target genes of LEAFY. Proc. Natl. Acad. Sci. USA 2004, 101, 1775–1780. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Jack, T. Molecular and genetic mechanisms of floral control. Plant Cell 2004, 16, S1–S17. [Google Scholar] [CrossRef]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Tuteja, N. Integrated signaling in flower senescence: An overview. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef]
- Lawton, K.A.; Raghothama, K.G.; Goldsbrough, P.B.; Woodson, W.R. Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiol. 1990, 93, 1370–1375. [Google Scholar] [CrossRef]
- Valpuesta, V.; Lange, N.E.; Guerrero, C.; Reid, M.S. Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Mol. Biol. 1995, 28, 575–582. [Google Scholar] [CrossRef]
- Rubinstein, B. Regulation of cell death in flower petals. Plant Mol. Biol. 2000, 44, 303–318. [Google Scholar] [CrossRef]
- Hunter, D.A.; Ferrante, A.; Vernieri, P.; Reid, M.S. Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus “Dutch Master”). Physiol. Plant. 2004, 121, 313–321. [Google Scholar] [CrossRef]
- Breeze, E.; Wagstaff, C.; Harrison, E.; Bramke, I.; Rogers, H.; Stead, A.; Thomas, B.; Buchanan-Wollaston, V. Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnol. J. 2004, 2, 155–168. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Balk, P.A.; van Houwelingen, A.M.; Hoeberichts, F.A.; Hall, R.D.; Vorst, O.; van Wordragen, M.F. Gene expression during anthesis and senescence in Iris flowers. Plant Mol. Biol. 2003, 53, 845–863. [Google Scholar] [CrossRef]
- Channelière, S.; Rivière, S.; Scalliet, G.; Szecsi, J.; Jullien, F.; Dolle, C.; Vergnea, P.; Dumasa, C.; Bendahmanea, M.; Hugueneya, P.; et al. Analysis of gene expression in rose petals using expressed sequence tags. FEBS Lett. 2002, 515, 35–38. [Google Scholar] [CrossRef]
- Xu, X.; Gookin, T.; Jiang, C.Z.; Reid, M.S. Genes associated with opening and senescence of Mirabilis jalapa flowers. J. Exp. Bot. 2007, 58, 2193–2201. [Google Scholar] [CrossRef]
- Wang, H.; Brandt, A.S.; Woodson, W.R. A flower senescence-related mRNA from carnation encodes a novel protein related to enzymes involved in phosphonate biosynthesis. Plant Mol. Biol. 1993, 22, 719–724. [Google Scholar] [CrossRef]
- Park, K.Y.; Drory, A.; Woodson, W.R. Molecular cloning of an 1-aminocyclopropane-1-carboxylate synthase from senescing carnation flower petals. Plant Mol. Biol. 1992, 18, 377–386. [Google Scholar] [CrossRef]
- Panavas, T.; Pikula, A.; Reid, P.D.; Rubinstein, B.; Walker, E.L. Identification of senescence-associated genes from daylily petals. Plant Mol. Biol. 1999, 40, 237–248. [Google Scholar] [CrossRef]
- Woodson, W.R.; Park, K.Y.; Drory, A.; Larsen, P.B.; Wang, H. Expression of ethylene biosynthetic pathway transcripts in senescing carnation flowers. Plant Physiol. 1992, 99, 526–532. [Google Scholar] [CrossRef]
- Meyer, R.C.; Goldsbrough, P.B.; Woodson, W.R. An ethylene-responsive flower senescence-related gene from carnation encodes a protein homologous to glutathione S-transferases. Plant Mol. Biol. 1999, 17, 277–281. [Google Scholar]
- Michael, M.Z.; Savin, K.W.; Baudinette, S.C.; Graham, M.W.; Chandler, S.F.; Lu, C.Y.; Caesar, C.; Gautrais, I.; Young, R.; Nugent, C.D.; et al. Cloning of Ethylene Biosynthetic Genes Involved in Petal Senescence of Carnation and Petunia, and Their Antisense Expression in Transgenic Plants. In Cellular and Molecular Aspects of the Plant Hormone Ethylene; Pech, J.C., Latche, A., Balogue, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 298–303. [Google Scholar]
- Xu, Y.; Ishida, H.; Reisen, D.; Hanson, M.R. Upregulation of a tonoplast-localized cytochrome P450 during petal senescence in Petunia inflata. BMC Plant Biol. 2006, 6, 8. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Savin, K.; Cornish, E.; Tanaka, Y.; Ashikari, T.; Kusumi, T.; Murata, N. Senescence-induced expression of a homologue of Δ9 desaturase in rose petals. Plant Mol. Biol. 1995, 29, 627–635. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, C.Z.; Donnelly, L.; Reid, M.S. Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J. Exp. Bot. 2007, 58, 3623–3630. [Google Scholar] [CrossRef]
- McClung, C.R. Circadian rhythms in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 139–162. [Google Scholar] [CrossRef]
- Courtney, S.E.; Rider, C.C.; Stead, A.D. Changes in protein ubiquitination and the expression of ubiquitin-encoding transcripts in daylily petals during floral development and senescence. Physiol. Plant. 1994, 91, 196–204. [Google Scholar] [CrossRef]
- Hunter, D.A.; Steele, B.C.; Reid, M.S. Identification of genes associated with perianth senescence in Daffodil (Narcissus pseudonarcissus L. “Dutch Master”). Plant Sci. 2002, 163, 13–21. [Google Scholar] [CrossRef]
- Jones, M.L.; Chaffin, G.S.; Eason, J.R.; Clark, D.G. Ethylene-sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. J. Exp. Bot. 2005, 56, 2733–2774. [Google Scholar] [CrossRef]
- Eason, J.R.; Ryan, D.J.; Pinkney, T.T.; O’Donoghue, E.M. Programmed cell death during flower senescence: Isolation and characterization of cysteine proteases from Sandersonia aurantiaca. Funct. Plant Biol. 2002, 29, 1055–1064. [Google Scholar] [CrossRef]
- Wagstaff, C.; Leverentz, M.K.; Griffiths, G.; Thomas, B.; Chanasut, U.; Stead, A.D.; Rogers, H.J. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J. Exp. Bot. 2002, 53, 233–240. [Google Scholar] [CrossRef]
- Guerrero, C.; de la Calle, M.; Reid, M.S.; Valpuesta, V. Analysis of the expression of two thiolprotease genes from daylily (Hemerocallis spp.) during flower senescence. Plant Mol. Biol. 1998, 36, 565–571. [Google Scholar] [CrossRef]
- Jones, M.L.; Larsen, P.B.; Woodson, W.R. Ethylene-regulated expression of a carnation cysteine proteinase during flower petal senescence. Plant Mol. Biol. 1995, 28, 505–512. [Google Scholar] [CrossRef]
- Sugawara, H.; Shibuya, K.; Yoshioka, T.; Hashiba, T.; Satoh, S. Is a cysteine proteinase inhibitor involved in the regulation of petal wilting in senescing carnation (Dianthus caryophyllus L.) flowers? J. Exp. Bot. 2002, 53, 407–413. [Google Scholar] [CrossRef]
- Xu, Y.; Hanson, M.R. Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiol. 2000, 122, 1323–1233. [Google Scholar] [CrossRef]
- Taylor, C.B.; Bariola, P.A.; Raines, R.T.; Green, P.J. RNS2: A senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc. Natl. Acad. Sci. USA 1993, 90, 5118–5122. [Google Scholar] [CrossRef]
- Lers, A.; Khalchitski, A.; Lomaniec, E.; Burd, S.; Green, P.J. Senescence-induced RNases in tomato. Plant Mol. Biol. 1998, 36, 439–449. [Google Scholar] [CrossRef]
- Papini, A.; Mosti, S.; Brighigna, L. Programmed-cell death events during tapetum development of angiosperms. Protoplasma 1999, 207, 213–221. [Google Scholar] [CrossRef]
- Wang, M.; Hoekstra, S.; van Bergen, S.; Lamers, G.E.M.; Oppedijk, B.J.; van der Heijden, M.W. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol. Biol. 1999, 39, 489–501. [Google Scholar] [CrossRef]
- Balk, J.; Leaver, C.J. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 2010, 13, 1803–1818. [Google Scholar]
- Thomas, S.G.; Franklin-Tong, V.E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 2004, 429, 305–309. [Google Scholar] [CrossRef]
- Müller, R.; Stummann, B.M. Genetic regulation of ethylene perception and signal transduction related to flower senescence. J. Food Agric. Environ. 2003, 1, 87–94. [Google Scholar]
- Borochov, A.; Cho, M.H.; Boss, W.F. Plasma membrane lipid metabolism of Petunia petals during senescence. Physiol. Plant. 1994, 90, 279–284. [Google Scholar] [CrossRef]
- Porat, R.; Borochov, A.; Halevy, A.H. Pollination induced senescence in Phalaenopsis petals: Relationship of ethylene sensitivity to activity of GTP-binding proteins and protein phosphorylation. Physiol. Plant. 1994, 90, 679–684. [Google Scholar] [CrossRef]
- Kakkar, R.J.; Rai, V.K. Plant polyamines in flowering and fruit ripening. Phytochemistry 1993, 33, 1281–1288. [Google Scholar] [CrossRef]
- Bagni, N.; Tassoni, A. The Role of Polyamines in Relation to Flower Senescence. In Floriculture, Ornamental and Plant Biotechnology; Global Science Books, Ltd.: London, UK, Ikenobe, Japan, 2006; pp. 88–95. [Google Scholar]
- Upfold, S.J.; van Staden, J. Polyamines and carnation flower senescence: Endogenous levels and the effect of applied polyamines on senescence. Plant Growth Regul. 1991, 10, 355–362. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Park, K.Y. Effects of spermine on ethylene biosynthesis in cut carnation (Dianthus caryophyllus L.) flowers during senescence. J. Plant Physiol. 1997, 151, 68–73. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; van Doorn, W.G.; Vorst, O.; Hall, R.D.; van Wordragen, M.F. Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J. Exp. Bot. 2007, 58, 2873–2885. [Google Scholar] [CrossRef]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Nam, H.G. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef]
- Miao, Y.; Laun, T.; Zimmermann, P.; Zentgraf, U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol. Biol. 2004, 55, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Urbez, C.; Carbonell, J.; Granell, A.; Perez-Amador, M.A. A fertilization-independent developmental program triggers partial fruit development and senescence processes in pistils of Arabidopsis. Plant Physiol. 2010, 154, 163–172. [Google Scholar] [CrossRef]
- Fang, S.C.; Fernandez, D.E. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 2002, 130, 78–89. [Google Scholar] [CrossRef]
- Yu, H.; Goh, C.J. Identification and characterization of three orchid MADS-box genes of the AP1/AGL9 subfamily during floral transition. Plant Physiol. 2000, 123, 1325–1336. [Google Scholar] [CrossRef]
- Fernandez, D.E.; Heck, G.R.; Perry, S.E.; Patterson, S.E.; Bleecker, A.B.; Fang, S.C. The embryo MADS domain factor AGL15 acts postembryonically. Inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 2000, 12, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, L.; Zhou, C.; Yu, C.W.; Chaikam, V. HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J. Exp. Bot. 2008, 59, 225–234. [Google Scholar] [CrossRef]
- Laufs, P.; Peaucelle, A.; Morin, H.; Traas, J. MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 2004, 131, 4311–4322. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Rubio-Somoza, I.; Weigel, D. MicroRNA networks and developmental plasticity in plants. Trends Plant Sci. 2011, 16, 258–264. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.H.; Lim, C.J.; Lim, J.Y.; Ryu, J.Y.; Lee, B.W.; Choi, J.P.; Kim, W.B.; Lee, H.Y.; Choi, Y.; et al. Small RNA and transcriptome deep sequencing proffers insight into floral gene regulation in Rosa cultivars. BMC Genomics 2012, 13, 657. [Google Scholar] [CrossRef]
- Pei, H.; Ma, N.; Chen, J.; Zheng, Y.; Tian, J.; Li, J.; Zhang, S.; Fei, Z.; Gao, J. Integrative analysis of miRNA and mRNA profiles in response to ethylene in rose petals during flower opening. PLoS One 2013, 8, e64290. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Wetzel, C.M.; Jiang, C.Z.; Meehan, L.J.; Voytas, D.F.; Rodermel, S.R. Nuclear-organelle interactions: The immutans variegation mutant of Arabidopsis is plastid autonomous and impaired in carotenoid biosynthesis. Plant J. 1994, 6, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Mylona, P.V.; Polidoros, A.N. ROS Regulation of Antioxidant Genes. In Reactive Oxygen Species and Antioxidants in Higher Plants; Dutta Gupta, S., Ed.; Science Publishers: Enfield, New Hampshire, UK, 2010; pp. 101–127. [Google Scholar]
- Wagstaff, C.; Yang, T.J.; Stead, A.D.; Buchanan-Wollaston, V.; Roberts, J.A. A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J. 2009, 57, 690–705. [Google Scholar] [CrossRef]
- Price, A.M.; Aros Orellana, D.F.; Salleh, F.M.; Stevens, R.; Acock, R.; Buchanan-Wollaston, V.; Stead, A.D.; Rogers, H.J. A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol. 2008, 147, 1898–1912. [Google Scholar] [CrossRef]
- Wagstaff, C.; Bramke, I.; Breeze, E.; Thornber, S.; Harrison, E.; Thomas, B.; Rogers, H. A specific group of genes respond to cold dehydration stress in cut Alstroemeria flowers whereas ambient dehydration stress accelerates developmental senescence expression patterns. J. Exp. Bot. 2010, 61, 2905–2921. [Google Scholar] [CrossRef]
- Itzhaki, H.; Maxson, J.M.; Woodson, W.R. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc. Natl. Acad. Sci. USA 1994, 91, 8925–8929. [Google Scholar] [CrossRef]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002, 49, 515–532. [Google Scholar] [CrossRef]
- Larsen, E.S.; Alfenito, M.R.; Briggs, W.R.; Walbot, V. A carnation anthocyanin mutant is complemented by the glutathione S-transferases encoded by maize Bz2 and Petunia An9. Plant Cell Rep. 2003, 21, 900–904. [Google Scholar] [PubMed]
- Gibbings, J.G.; Cook, B.P.; Dufault, M.R.; Madden, S.L.; Khuri, S.; Turnbull, C.J.; Dunwell, J.M. Global transcript analysis of rice leaf and seed using SAGE technology. Plant Biotechnol. J. 2003, 1, 271–285. [Google Scholar] [CrossRef]
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Jan Blaas, J.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; van der Voetb, H.; et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 2000, 12, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Kleber-Janke, T.; Krupinska, K. Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta 1997, 203, 332–340. [Google Scholar] [CrossRef]
- Rise, M.; Cojocaru, M.; Gottlieb, H.E.; Goldschmidt, E.E. Accumulation of α-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol. 1989, 89, 1028–1030. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010, 179, 289–295. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Wolucka, B.A.; van Montagu, M. GDP-mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef]
- Nishikimi, M. Recent advance in the study of ascorbic acid biosynthesis. Seikagaku 1996, 68, 377–380. [Google Scholar] [PubMed]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar] [PubMed]
- Conklin, P.L.; Pallanca, J.E.; Last, R.L.; Smirnoff, N. l-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol. 1997, 115, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Kotchoni, S.O.; Larrimore, K.E.; Mukherjee, M.; Kempinski, C.F.; Barth, C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol. 2009, 149, 803–815. [Google Scholar] [PubMed]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Wigoda, N.; Ben-Nissan, G.; Granot, D.; Schwartz, A.; Weiss, D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J. 2006, 48, 796–805. [Google Scholar] [CrossRef]
- Wingler, A. Interactions between flowering and senescence regulation and the influence of low temperature in Arabidopsis and crop plants. Ann. Appl. Biol. 2011, 159, 320–338. [Google Scholar] [CrossRef]
- Savin, K.W.; Baudinette, S.C.; Graham, M.W.; Michael, M.Z.; Nugent, G.D.; Lu, C.Y.; Chandler, S.F.; Cornish, E.C. Antisense ACC Oxidase RNA delays carnation petal senescence. HortScience 1995, 30, 970–972. [Google Scholar]
- Wilkinson, J.Q.; Lanahan, M.B.; Clark, D.G.; Bleecker, A.B.; Chang, C.; Meyerowitz, E.M.; Klee, H.J. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat. Biotechnol. 1997, 15, 444–447. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
