Molecular Regulation of FOXO1 and Its Pathophysiological Significance in Endometriosis: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
Search Strategy and Selection Criteria
3. Results
3.1. Roles of FOXO in Endometrial Function and Its Involvement in Endometriosis
3.1.1. Functions of FOXO and Its Role in the Endometrium
3.1.2. Aberrant FOXO1 Regulation and Pathogenesis in Endometriosis
3.2. Signaling Pathways Associated with FOXO
3.2.1. The PI3K Pathway
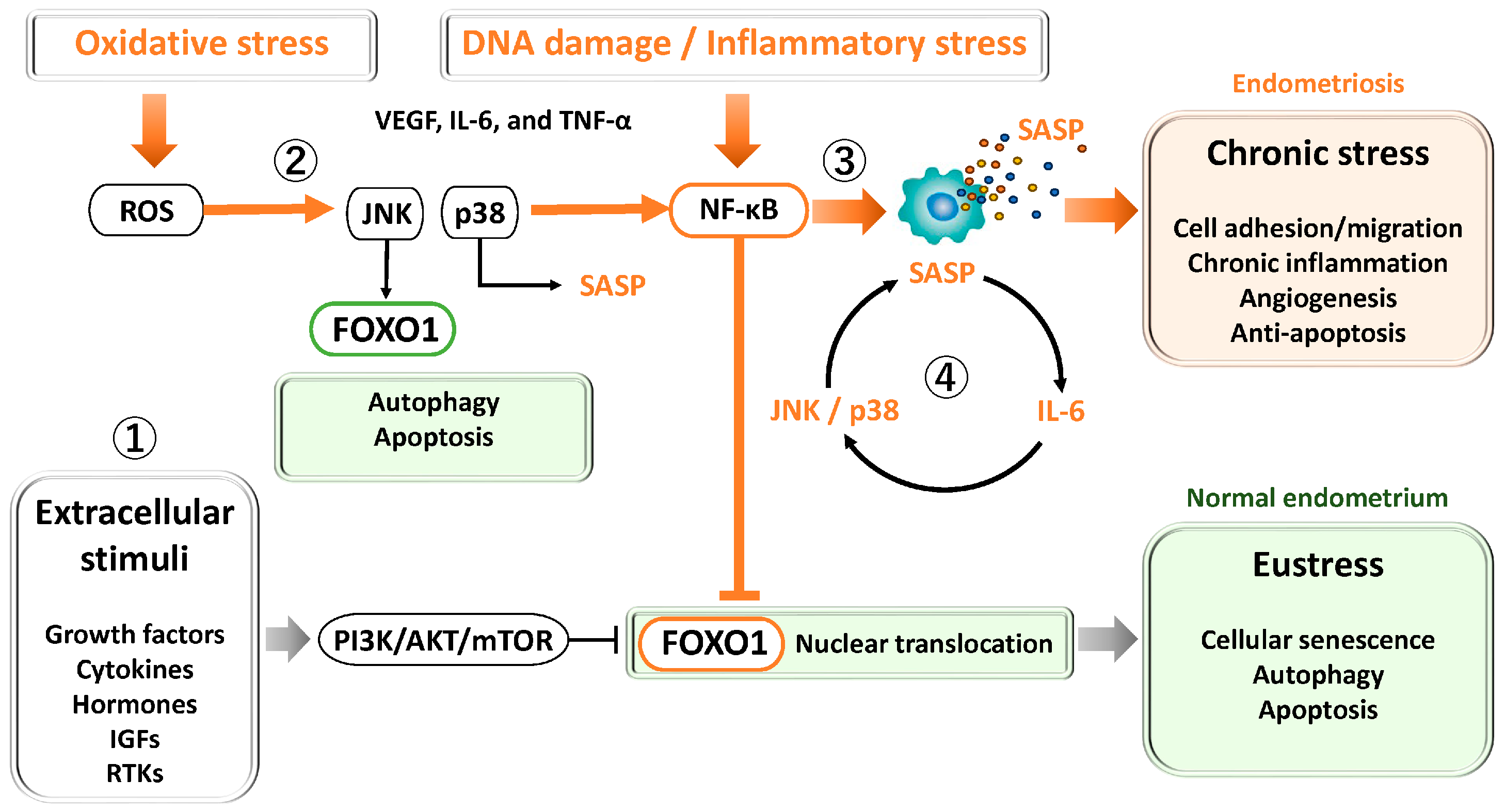
3.2.2. JNK and p38
3.2.3. NF-κB and STAT3
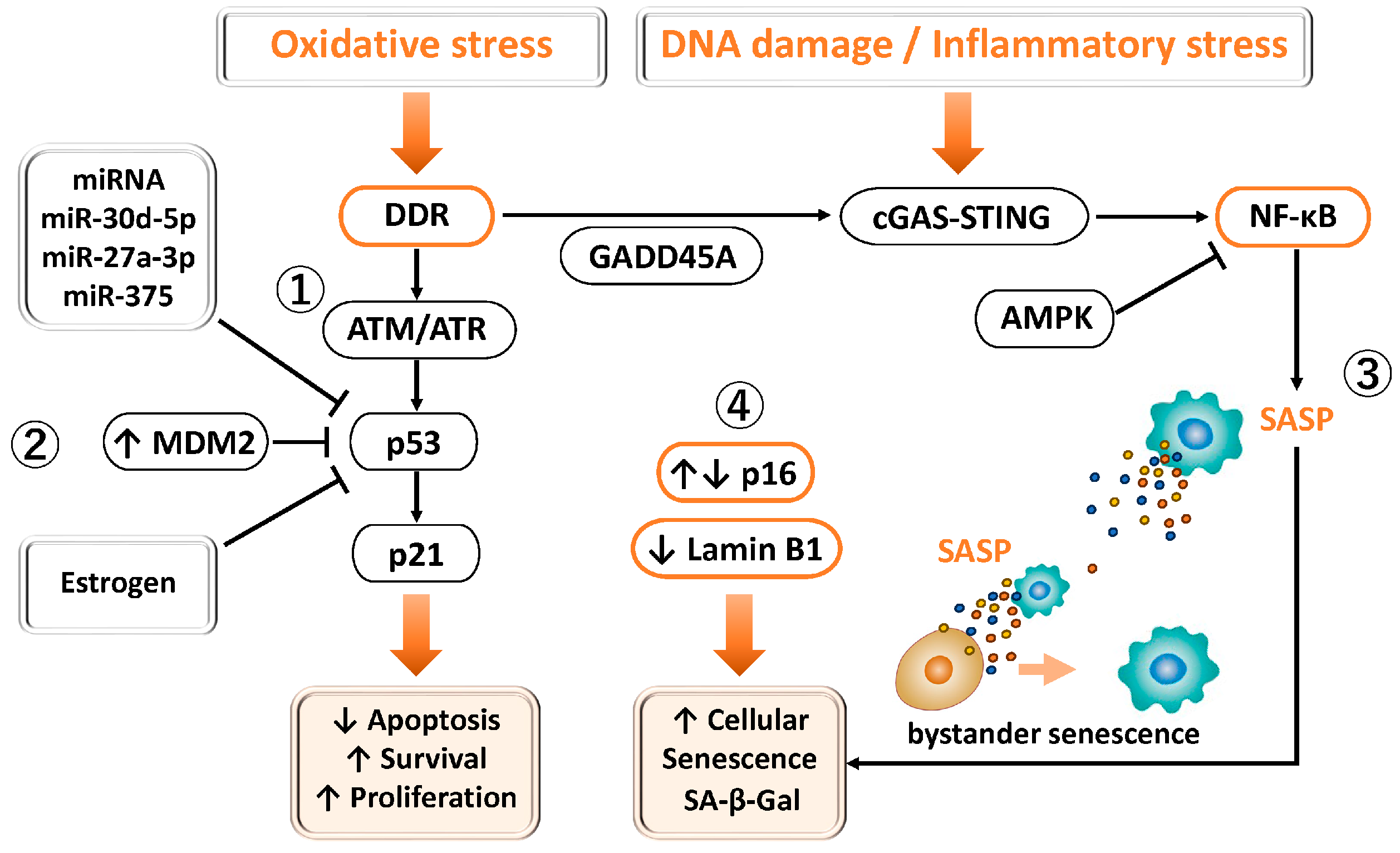
3.2.4. P53
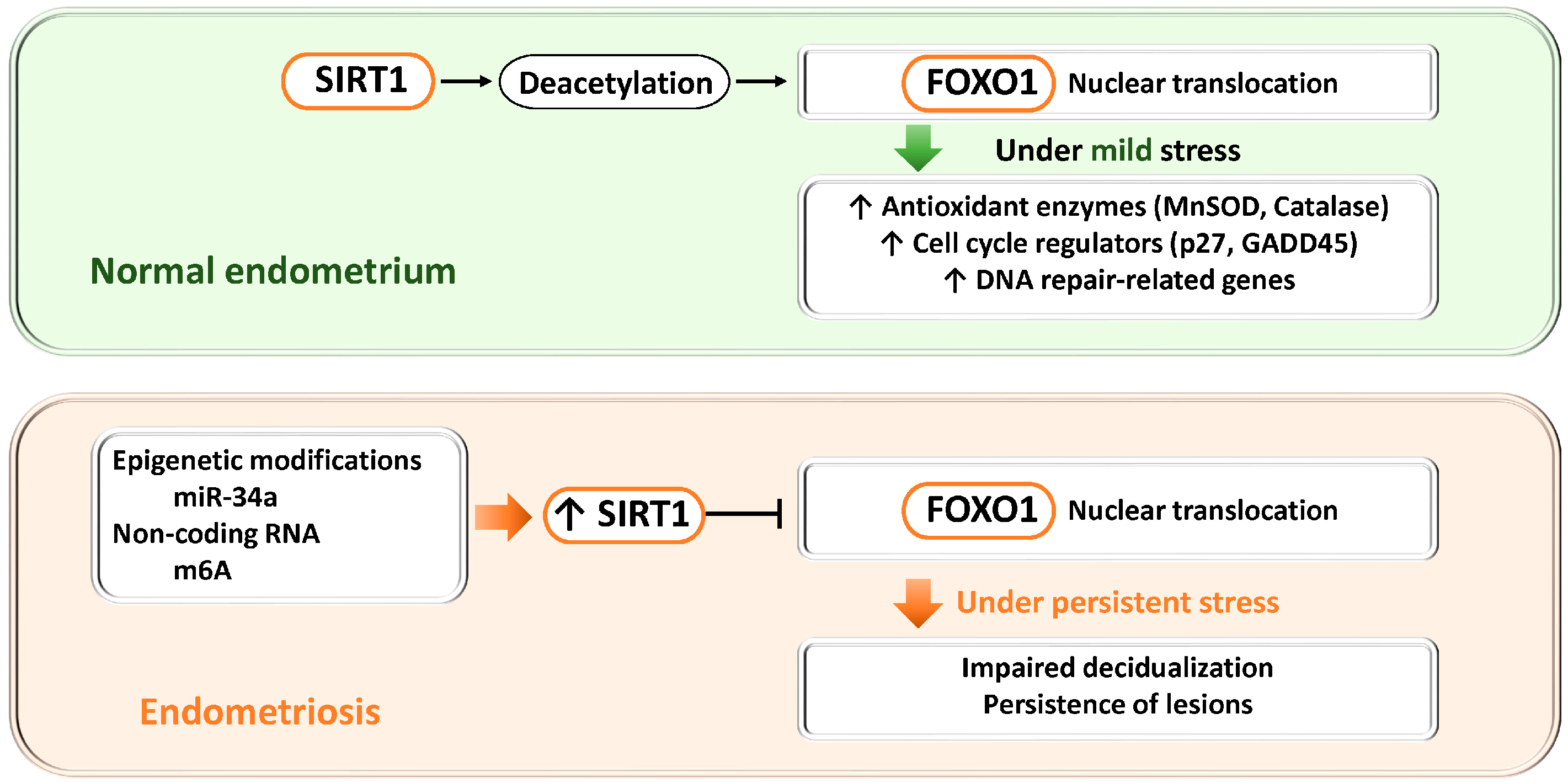
3.2.5. SIRT1
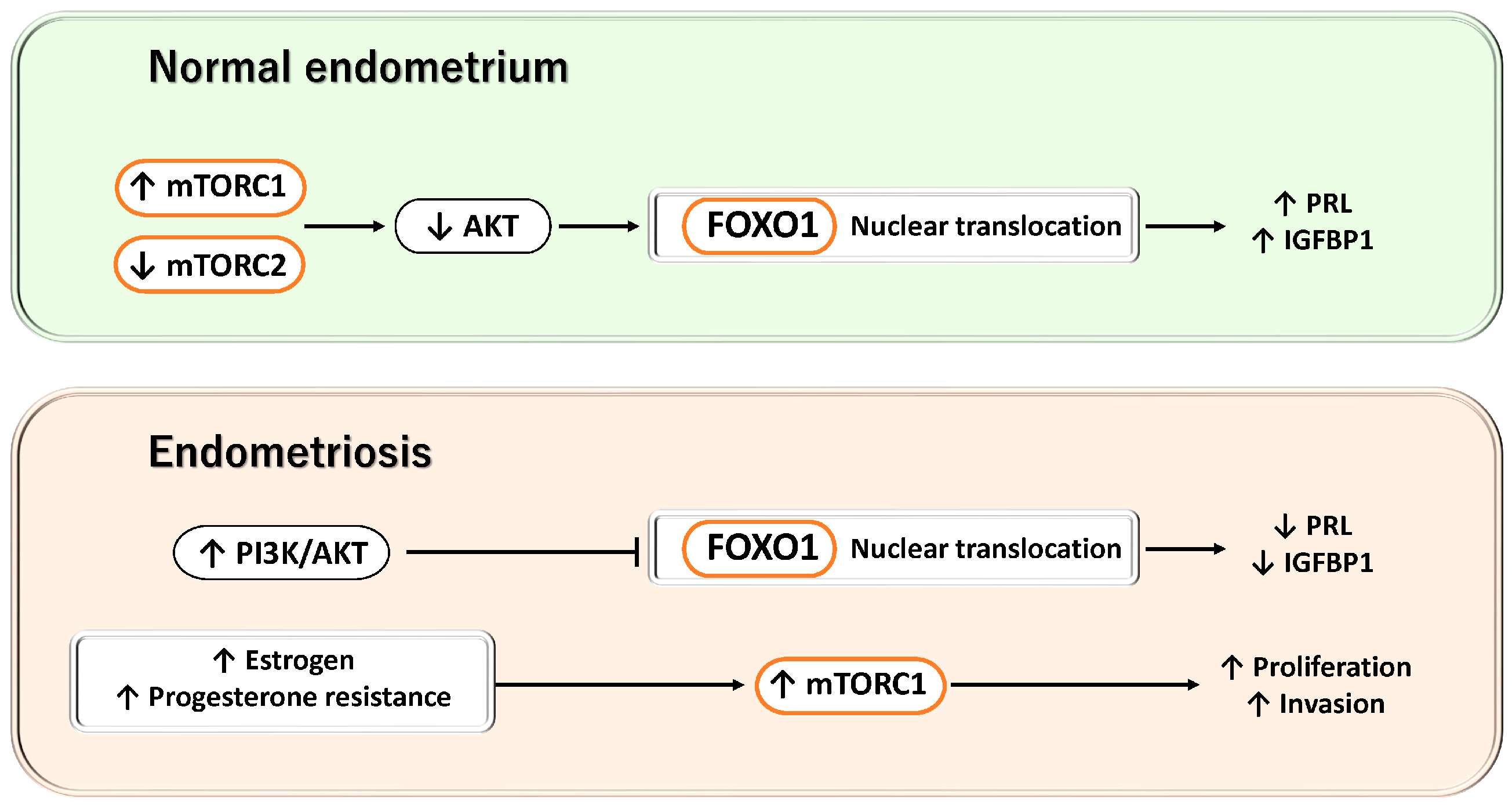
3.2.6. mTOR
3.3. Regulatory Mechanisms of FOXO1 in the Pathophysiology of Endometriosis
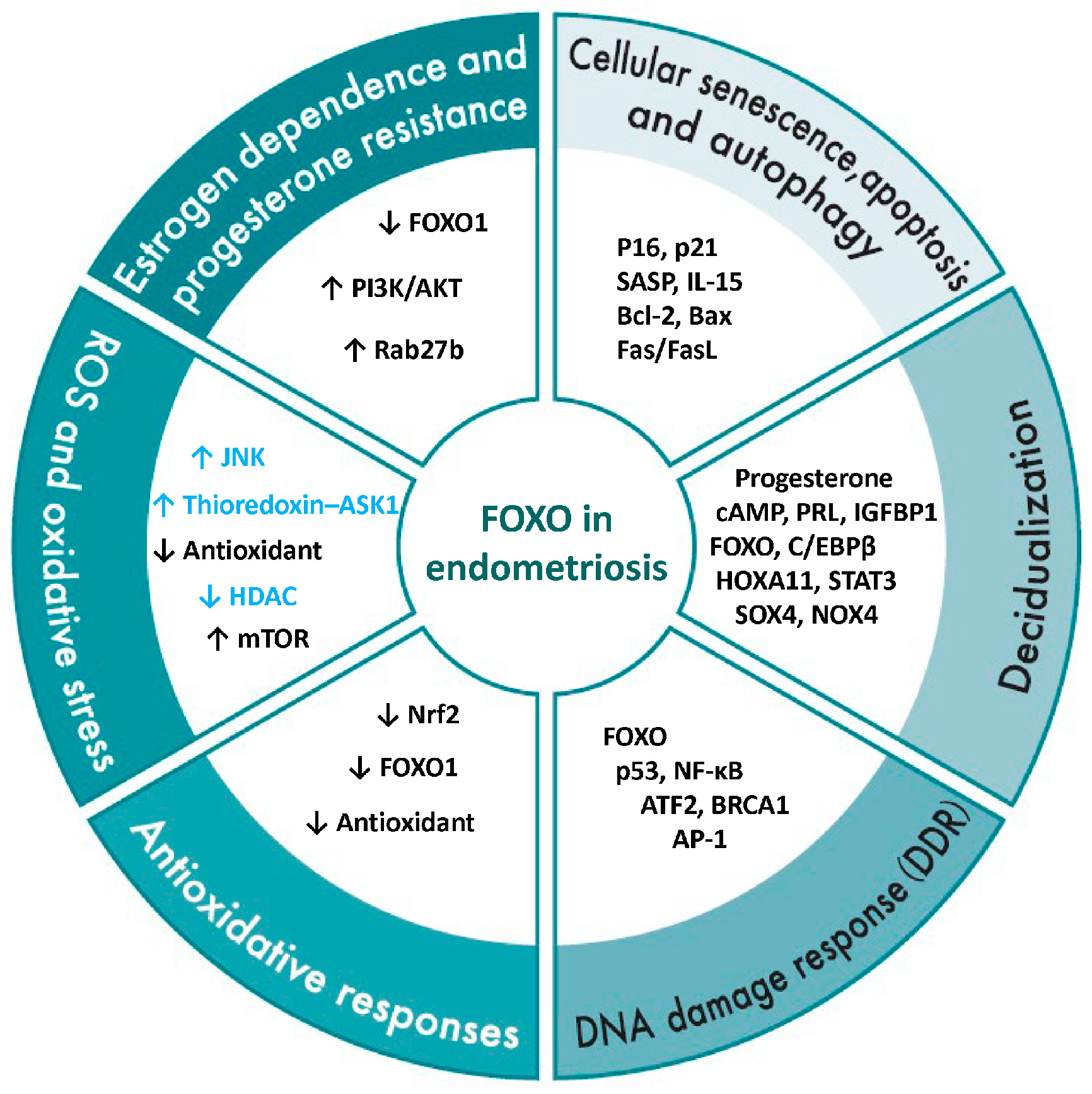
3.3.1. Estrogen Dependence and Progesterone Resistance
3.3.2. ROS and Oxidative Stress
3.3.3. Antioxidant Response
3.3.4. DNA Damage Response (DDR)
3.3.5. Decidualization
3.3.6. Cellular Senescence, Apoptosis, and Autophagy
4. Discussion
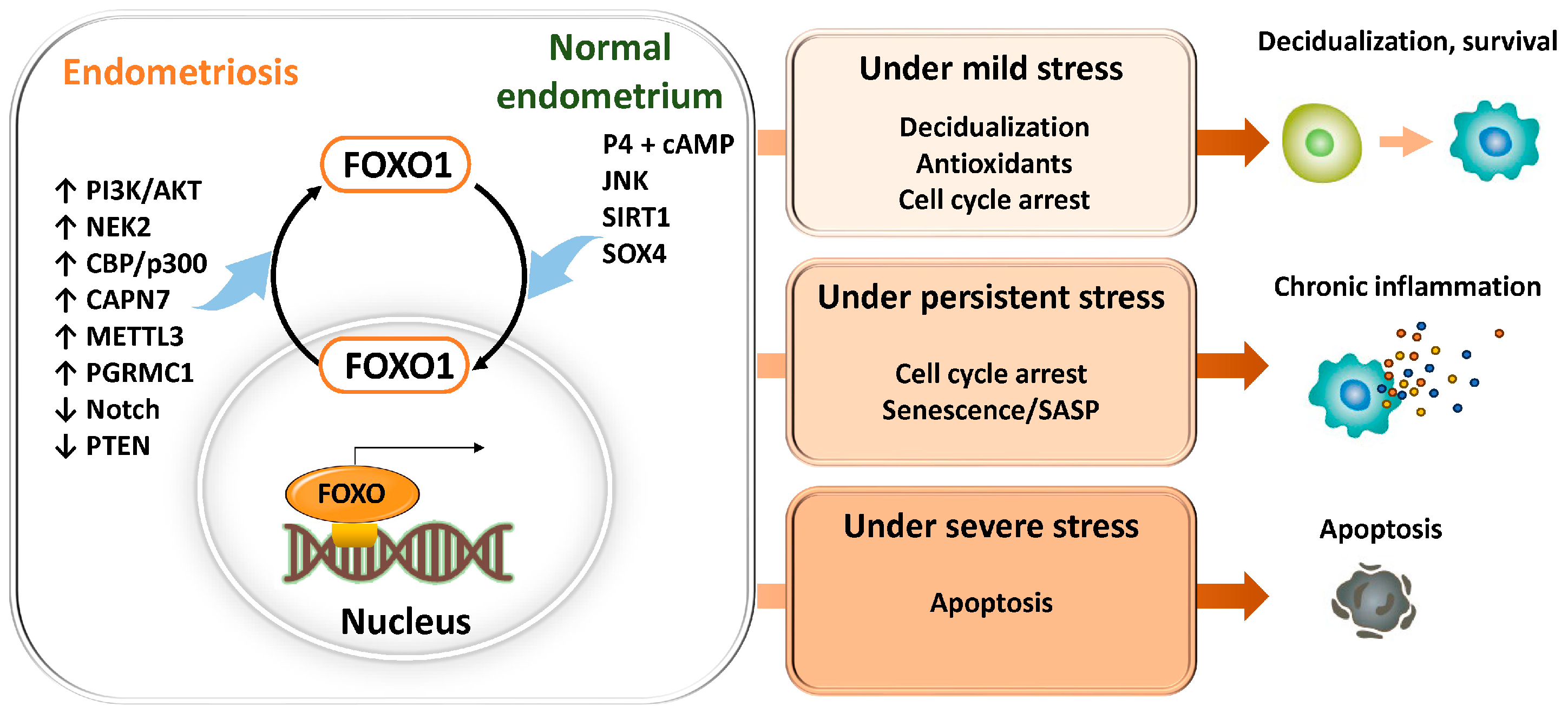
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 14-3-3 | 14-3-3 Proteins |
| 4EBP1 | Eukaryotic Translation Initiation Factor 4E-Binding Protein 1 |
| AKT | Protein Kinase B (PKB) |
| AMPK | AMP-Activated Protein Kinase |
| AP-1 | Activator Protein 1 |
| ASL | Fas Ligand (CD95L) |
| ATF2 | Activating Transcription Factor 2 |
| ATGs | Autophagy-related Genes |
| AREs | Antioxidant response elements |
| ASK1 | Apoptosis Signal-Regulating Kinase 1 |
| ATM | Ataxia-telangiectasia mutated |
| ATR | ATM and Rad3-related |
| BAD | Bcl-2-Associated Death Promoter |
| Bak | Bcl-2 Homologous Antagonist/Killer |
| BAX | Bcl-2-Associated X Protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| BH3 | Bcl-2 Homology 3 |
| BIM | Bcl-2 Interacting Mediator of Cell Death (BCL2L11) |
| BMP | Bone Morphogenetic Protein |
| BNIP3 | BCL2/adenovirus E1B 19 kDa Protein-Interacting Protein 3 |
| BRCA1 | Breast Cancer Type 1 Susceptibility Protein |
| cAMP | Cyclic Adenosine Monophosphate |
| CAPN7 | Calpain 7 |
| CAT | Catalase |
| CBP/p300 | CREB-Binding Protein/E1A Binding Protein p300 |
| CD44 | Cluster of Differentiation 44 |
| CDK4 | Cyclin-Dependent Kinase 4 |
| C/EBPβ | CCAAT/Enhancer-Binding Protein Beta |
| Chk1 | Checkpoint Kinase 1 |
| Chk2 | Checkpoint Kinase 2 |
| COX2 | Cyclooxygenase-2 |
| CTNNB1 | Catenin Beta 1 |
| DDR | DNA damage response |
| EMT | epithelial–mesenchymal transition |
| ERβ | Estrogen Receptor Beta |
| ERK | Extracellular Signal-Regulated Kinase |
| FHL2 | Four and a half LIM domain protein 2 |
| FOXO | Forkhead box O |
| GADD45 | Growth Arrest and DNA Damage-inducible 45 |
| GADD45A | Growth Arrest and DNA Damage-Inducible Alpha |
| GCL | Glutamate–Cysteine Ligase |
| GSH | Glutathione |
| GPX1 | Glutathione Peroxidase 1 |
| HDAC | Histone deacetylase |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| HO-1 | Heme oxygenase-1 |
| HOXA11 | Homeobox A11 |
| HSPs | Heat Shock Proteins |
| hTERT | Human Telomerase Reverse Transcriptase |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN-γ | Interferon Gamma |
| IGFs | Insulin-like growth factors |
| IGFBP1 | Insulin-Like Growth Factor Binding Protein 1 |
| IL17RB | Interleukin 17 Receptor B |
| IRF3 | Interferon Regulatory Factor 3 |
| JAK | Janus Kinase |
| JNK | c-Jun N-terminal Kinase |
| Keap1 | Kelch-Like ECH-Associated Protein 1 |
| LC3 | Microtubule-associated protein 1 light chain 3 (MAP1LC3) |
| MAPKAPK2 | Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 |
| METTL3 | Methyltransferase-Like 3 (N6-methyladenosine RNA methyltransferase) |
| MDM2 | Mouse Double Minute 2 homolog |
| miRNAs | MicroRNAs |
| MMPs | Matrix Metalloproteinases |
| MRE11 | Meiotic Recombination 11 Homolog |
| mtDNA | mitochondrial DNA |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| mTORC2 | Mechanistic Target of Rapamycin Complex 2 |
| NAD | Nicotinamide Adenine Dinucleotide |
| NEK2 | NIMA (Never in Mitosis Gene A)-Related Kinase 2 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NOXA | Phorbol-12-Myristate-13-Acetate-Induced Protein 1 (PMAIP1) |
| NOX4 | NADPH Oxidase 4 |
| NQO1 | NADPH quinone oxidoreductase 1 |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| OXPHOS | Mitochondrial oxidative phosphorylation |
| p21 | Cyclin-Dependent Kinase Inhibitor 1A (CDKN1A) |
| p27 | Cyclin-Dependent Kinase Inhibitor 1B (CDKN1B) |
| PGRMC1 | Progesterone Receptor Membrane Component 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PKA | Protein Kinase A |
| γH2AX | Phosphorylated H2A Histone Family Member X |
| PRDX3 | Peroxiredoxin 3 |
| PRMT5 | Protein Arginine Methyltransferase 5 |
| PRL | Prolactin |
| PTEN | Phosphatase and Tensin Homolog |
| PUMA | p53 Upregulated Modulator of Apoptosis (BBC3) |
| Rab27b | Ras-Related Protein Rab-27b |
| RAD50 | RAD50 Double Strand Break Repair Protein |
| RAD51 | RAD51 Recombinase |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted (CCL5) |
| RTKs | Receptor tyrosine kinases |
| S6K | Ribosomal Protein S6 Kinase |
| SA-β-gal | Senescence-Associated Beta-Galactosidase |
| SASP | Senescence-Associated Secretory Phenotype |
| SESN | Sestrins |
| SIRT1 | Sirtuin 1 (NAD+-dependent deacetylase) |
| SOD2 | Superoxide Dismutase 2 (MnSOD) |
| SOX4 | SRY-Box Transcription Factor 4 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TBK1 | TANK-Binding Kinase 1 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TSC1 | Tuberous Sclerosis Complex 1 |
| TSC2 | Tuberous Sclerosis Complex 2 |
| ULK1 | Unc-51-Like Autophagy Activating Kinase 1 |
| uNK | Uterine Natural Killer (Cells) |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular Endothelial Growth Factor |
| WIP1 | Wild-Type p53-Induced Phosphatase 1 |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Adilbayeva, A.; Kunz, J. Pathogenesis of Endometriosis and Endometriosis-Associated Cancers. Int. J. Mol. Sci. 2024, 25, 7624. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Harada, M. Cellular senescence in the pathogenesis of ovarian dysfunction. J. Obstet. Gynaecol. Res. 2024, 50, 800–808. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Rethinking the pathogenesis of endometriosis: Complex interactions of genomic, epigenetic, and environmental factors. J. Obstet. Gynaecol. Res. 2024, 50, 1771–1784. [Google Scholar] [CrossRef]
- Dai, W.; Guo, R.; Na, X.; Jiang, S.; Liang, J.; Guo, C.; Fang, Y.; Na, Z.; Li, D. Hypoxia and the endometrium: An indispensable role for HIF-1alpha as therapeutic strategies. Redox Biol. 2024, 73, 103205. [Google Scholar] [CrossRef] [PubMed]
- Bedrick, B.S.; Courtright, L.; Zhang, J.; Snow, M.; Amendola, I.L.S.; Nylander, E.; Cayton-Vaught, K.; Segars, J.; Singh, B. A Systematic Review of Epigenetics of Endometriosis. F S Rev. 2024, 5, 100070. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, H.; Nishio, M.; Umetani, M.; Imanaka, S.; Hashimoto, H.; Kobayashi, H. Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology. Int. J. Mol. Sci. 2025, 26, 9125. [Google Scholar] [CrossRef]
- Tamura, K.; Yoshie, M.; Kusama, K.; Tsuru, A. Mechanisms of Decidual Dysfunction and Infertility in Endometriosis: Roles of Prostaglandins and SASP. Reprod. Med. Biol. 2025, 24, e12663. [Google Scholar] [CrossRef]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef]
- Schmidt, M.; Fernandez de Mattos, S.; van der Horst, A.; Klompmaker, R.; Kops, G.J.; Lam, E.W.; Burgering, B.M.; Medema, R.H. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin, D. Mol. Cell Biol. 2002, 22, 7842–7852. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.F.; Grenho, I.; Martel, P.J.; Ferreira, B.I.; Link, W. FOXO family isoforms. Cell Death Dis. 2023, 14, 702, Erratum in Cell Death Dis. 2023, 14, 797. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Pavone, M.E.; Lu, Z.; Wei, J.; Kim, J.J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, E35–E43. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, D.; Jakson, I.; Babayeva, S.; Salamon, D.; Rethi, B.; Gidlöf, S.; Hirschberg, A.L. Dysregulation of In Vitro Decidualization of Human Endometrial Stromal Cells by Insulin via Transcriptional Inhibition of Forkhead Box Protein O1. PLoS ONE 2017, 12, e0171004. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Jin, Z.; Liu, H.; Xu, C. Scribble downregulation in adenomyosis compromises endometrial stromal decidualization by decreasing FOXO1 expression. Hum. Reprod. 2021, 37, 93–108. [Google Scholar] [CrossRef]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 2011, 1813, 1938–1945. [Google Scholar] [CrossRef]
- Li, B.; Yan, Y.P.; Liang, C.; He, Y.Y.; Wang, Y.; Li, M.Y.; Chen, S.T.; Li, Y.; Liu, A.X.; Yan, G.J.; et al. Primary Cilia Restrain PI3K-AKT Signaling to Orchestrate Human Decidualization. Int. J. Mol. Sci. 2022, 23, 15573. [Google Scholar] [CrossRef]
- Wang, M.; Sun, F.; Zhang, S.; Zhang, X.; Sun, Y.; Yu, T.; Li, Y.; Jiang, A.; Qiao, P.; Ren, C.; et al. NEK2 promotes the development of ovarian endometriosis and impairs decidualization by phosphorylating FOXO1. Cell. Mol. Life Sci. 2024, 81, 237. [Google Scholar] [CrossRef] [PubMed]
- 22van der Heide, L.P.; Smidt, M.P. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem. Sci. 2005, 30, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Salazar, G.; Patrushev, N.; Alexander, R.W. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J. Biol. Chem. 2011, 286, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Q.; Li, A.; Yang, Y.; Li, X.X.; Zhang, L.N.; Guo, H.C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018, 193, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; Weijzen, S.; de Vries-Smits, A.M.; Saarloos, I.; de Ruiter, N.D.; Bos, J.L.; Burgering, B.M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta 2010, 1804, 1684–1689. [Google Scholar] [CrossRef]
- Kajihara, T.; Jones, M.; Fusi, L.; Takano, M.; Feroze-Zaidi, F.; Pirianov, G.; Mehmet, H.; Ishihara, O.; Higham, J.M.; Lam, E.W.; et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol. Endocrinol. 2006, 20, 2444–2455. [Google Scholar] [CrossRef]
- Fan, W.; Li, S.W.; Li, W.H.; Wang, Y.; Gong, Y.; Ma, Q.H.; Luo, S. FOXO1 expression and regulation in endometrial tissue during the menstrual cycle and in early pregnancy decidua. Gynecol. Obstet. Invest. 2012, 74, 56–63. [Google Scholar] [CrossRef]
- Labied, S.; Kajihara, T.; Madureira, P.A.; Fusi, L.; Jones, M.C.; Higham, J.M.; Varshochi, R.; Francis, J.M.; Zoumpoulidou, G.; Essafi, A.; et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol. Endocrinol. 2006, 20, 35–44. [Google Scholar] [CrossRef]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef]
- Adiguzel, D.; Celik-Ozenci, C. FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy. Hum. Reprod. Update 2021, 27, 570–583. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Kojima, J.; Yonekawa, R.; Azumi, M.; Kusama, K.; Nishi, H.; Tamura, K. PGRMC1 Regulates Cellular Senescence via Modulating FOXO1 Expression in Decidualizing Endometrial Stromal Cells. Biomolecules 2022, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, T.; Brosens, J.J.; Ishihara, O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med. Mol. Morphol. 2013, 46, 61–68, Erratum in Med. Mol. Morphol. 2013, 46, 69. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Lee, S.K.; Gye, M.C. Cyclic changes in the expression of p57(kip2) in human endometrium and its regulation by steroid hormones in endometrial stromal cells in vitro. Reprod. Sci. 2012, 19, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.L.; Lee, S.I.; Park, H.W.; Lee, S.K.; Kim, T.H.; Kang, J.; Park, S.R. SIRT1 suppresses in vitro decidualization of human endometrial stromal cells through the downregulation of forkhead box O1 expression. Reprod Biol. 2022, 22, 100672. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Long, X.; Weng, R.; Xiong, W.; Liang, J.; Liu, J.; Sun, J.; Cai, X.; Zhang, L.; et al. METTL3-regulated m6A modification impairs the decidualization of endometrial stromal cells by regulating YTHDF2-mediated degradation of FOXO1 mRNA in endometriosis-related infertility. Reprod. Biol. Endocrinol. 2023, 21, 99. [Google Scholar] [CrossRef]
- Huang, P.; Deng, W.; Bao, H.; Lin, Z.; Liu, M.; Wu, J.; Zhou, X.; Qiao, M.; Yang, Y.; Cai, H.; et al. SOX4 facilitates PGR protein stability and FOXO1 expression conducive for human endometrial decidualization. eLife 2022, 11, e72073. [Google Scholar] [CrossRef]
- Su, R.W.; Strug, M.R.; Joshi, N.R.; Jeong, J.W.; Miele, L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J. Clin. Endocrinol. Metab. 2015, 100, E433–E442. [Google Scholar] [CrossRef]
- Kang, N.; Shan, H.; Wang, J.; Mei, J.; Jiang, Y.; Zhou, J.; Huang, C.; Zhang, H.; Zhang, M.; Zhen, X.; et al. Calpain7 negatively regulates human endometrial stromal cell decidualization in EMs by promoting FoxO1 nuclear exclusion via hydrolyzing AKT1. Biol. Reprod. 2022, 106, 1112–1125. [Google Scholar] [CrossRef]
- Bunch, K.; Tinnemore, D.; Huff, S.; Hoffer, Z.S.; Burney, R.O.; Stallings, J.D. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod. Sci. 2014, 21, 190–197. [Google Scholar] [CrossRef]
- Kim, T.H.; Young, S.L.; Sasaki, T.; Deaton, J.L.; Schammel, D.P.; Palomino, W.A.; Jeong, J.W.; Lessey, B.A. Role of SIRT1 and Progesterone Resistance in Normal and Abnormal Endometrium. J. Clin. Endocrinol. Metab. 2022, 107, 788–800, Erratum in J. Clin. Endocrinol. Metab. 2022, 107, e2217. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; He, Y.; Liu, J.; Chen, Y.; Huang, J.; Qi, G.; Li, P. SIRT1 upregulation promotes epithelial-mesenchymal transition by inducing senescence escape in endometriosis. Sci. Rep. 2022, 12, 12302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhao, X.; Wu, H.; Li, J.; Cheng, Y.; Guo, Q.; Cao, X.; Liang, T.; Sun, L.; et al. METTL3-mediated m6A modification of SIRT1 mRNA inhibits progression of endometriosis by cellular senescence enhancing. J. Transl. Med. 2023, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef]
- Jin, X.; Feng, J.; Cheng, X. LncRNA IGF2-AS promotes endometriosis progression through targeting miR-370-3p/IGF2 axis and activating PI3K/AKT/mTOR signaling pathway. J. Assist. Reprod. Genet. 2022, 39, 2699–2710. [Google Scholar] [CrossRef]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum. Reprod. Update 2016, 22, 382–403. [Google Scholar] [CrossRef]
- Gentilini, D.; Busacca, M.; Di Francesco, S.; Vignali, M.; Viganò, P.; Di Blasio, A.M. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol. Hum. Reprod. 2007, 13, 317–322. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Liu, S. Pharmacological insights into targeting PI3K/Akt and NF-kappaB pathways for treating endometriosis-associated infertility. Pak. J. Pharm. Sci. 2025, 38, 449–455. [Google Scholar]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tsuzuki-Nakao, T.; Kida, N.; Matsuo, Y.; Maruyama, T.; Okada, H.; Hirota, K. Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Biomedicines 2023, 11, 210. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Tong, D.; Li, S.; Yu, X.; Liu, B.; Jiang, L.; Liu, K. Research advances in endometriosis-related signaling pathways: A review. Biomed. Pharmacother. 2023, 164, 114909. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Asada, S.; Daitoku, H.; Matsuzaki, H.; Saito, T.; Sudo, T.; Mukai, H.; Iwashita, S.; Kako, K.; Kishi, T.; Kasuya, Y.; et al. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell. Signal. 2007, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, H.; Han, J.; Ishikawa, F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 2003, 8, 131–144. [Google Scholar] [CrossRef]
- Bora, G.; Yaba, A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1610–1623. [Google Scholar] [CrossRef]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16(ink4a) and beta-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef]
- Shazand, K.; Baban, S.; Privé, C.; Malette, B.; Croteau, P.; Lagacé, M.; Racine, J.B.; Hugo, P. FOXO1 and c-jun transcription factors mRNA are modulated in endometriosis. Mol. Hum. Reprod. 2004, 10, 871–877. [Google Scholar] [CrossRef]
- Ouyang, Z.; Deng, J.; Zhang, L.; Shen, F. How transcription factors regulate apoptosis in endometriosis (Review). Mol. Med. Rep. 2025, 32, 289. [Google Scholar] [CrossRef]
- Cakmak, H.; Seval-Celik, Y.; Arlier, S.; Guzeloglu-Kayisli, O.; Schatz, F.; Arici, A.; Kayisli, U.A. p38 Mitogen-Activated Protein Kinase is Involved in the Pathogenesis of Endometriosis by Modulating Inflammation, but not Cell Survival. Reprod. Sci. 2018, 25, 587–597. [Google Scholar] [CrossRef]
- Kobayashi, H.; Umetani, M.; Nishio, M.; Shigetomi, H.; Imanaka, S.; Hashimoto, H. Molecular Mechanisms of Cellular Senescence in Age-Related Endometrial Dysfunction. Cells 2025, 14, 858. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef]
- Kawamura, K.; Matsumura, Y.; Kawamura, T.; Araki, H.; Hamada, N.; Kuramoto, K.; Yagi, H.; Onoyama, I.; Asanoma, K.; Kato, K. Endometrial senescence is mediated by interleukin 17 receptor B signaling. Cell Commun. Signal. 2024, 22, 363, Erratum in Cell Commun. Signal. 2024, 22, 370. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int. J. Biol. Sci. 2022, 18, 4400–4413. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Zdrojkowski, Ł.; Jasiński, T.; Ferreira-Dias, G.; Pawliński, B.; Domino, M. The Role of NF-kappaB in Endometrial Diseases in Humans and Animals: A Review. Int. J. Mol. Sci. 2023, 24, 2901. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Wu, X.; Peng, W.; Zhong, Y.; Cai, Y.; Chen, W.; Liu, L.; Tan, B.; Chen, T. Gut Microbiota-Derived Acetate Ameliorates Endometriosis via JAK1/STAT3-Mediated M1 Macrophage Polarisation. Microb. Biotechnol. 2025, 18, e70202. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, J.E.; Cho, Y.J.; Park, M.J.; O’Malley, B.W. Genomic Function of Estrogen Receptor beta in Endometriosis. Endocrinology 2019, 160, 2495–2516. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Mamillapalli, R.; Flores, V.A.; Taylor, H.S. Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol. Hum. Reprod. 2021, 27, gaab016. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, R.; Defrère, S.; Devoto, L. Nuclear factor-kappaB: A main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil. Steril. 2012, 98, 520–528. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. Persistent activation of signal transducer and activator of transcription 3 via interleukin-6 trans-signaling is involved in fibrosis of endometriosis. Hum. Reprod. 2022, 37, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Q.; Sun, J.; Du, W.; Chen, Z.; Yu, M.; Tao, J.; Zhou, Y.; Zhao, Y.; Zhang, Q. The cGAS-STING pathway promotes endometriosis by up-regulating autophagy. Int. Immunopharmacol. 2023, 117, 109644. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Lv, L.; Liu, C.; Wang, L.; Sun, Y.N.; Zhao, Z.; Shi, B.; Li, Q.; Hao, G.M. Molecular regulation of DNA damage and repair in female infertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 103. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell. 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Bane, K.; Desouza, J.; Shetty, D.; Choudhary, P.; Kadam, S.; Katkam, R.R.; Fernandes, G.; Sawant, R.; Dudhedia, U.; Warty, N.; et al. Endometrial DNA damage response is modulated in endometriosis. Hum. Reprod. 2021, 36, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Fang, Q.J.; Zhao, X.B. A research on the protein expression of p53, p16, and MDM2 in endometriosis. Medicine 2019, 98, e14776. [Google Scholar] [CrossRef] [PubMed]
- Allavena, G.; Carrarelli, P.; Del Bello, B.; Luisi, S.; Petraglia, F.; Maellaro, E. Autophagy is upregulated in ovarian endometriosis: A possible interplay with p53 and heme oxygenase-1. Fertil. Steril. 2015, 103, 1244–1251.e1. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Wang, Y.; Lin, A.; Lian, L.; Cao, H.; Gu, W.; Li, T.; Sun, Q. Expression of nm23-H1, p53, and integrin beta1 in endometriosis and their clinical significance. Int. J. Clin. Exp. Pathol. 2020, 13, 1024–1029. [Google Scholar]
- Béliard, A.; Noël, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]
- Goumenou, A.; Panayiotides, I.; Mahutte, N.G.; Matalliotakis, I.; Fragouli, Y.; Arici, A. Immunohistochemical expression of p53, MDM2, and p21Waf1 oncoproteins in endometriomas but not adenomyosis. J. Soc. Gynecol. Investig. 2005, 12, 263–266. [Google Scholar] [CrossRef]
- Khalaj, K.; Miller, J.E.; Lingegowda, H.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019, 4, e128846. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Habara, M.; Hanaki, S.; Sato, Y.; Miki, Y.; Shimada, M. FOXO1 promotes cancer cell growth through MDM2-mediated p53 degradation. J. Biol. Chem. 2024, 300, 107209. [Google Scholar] [CrossRef]
- Malvezzi, H.; Dobo, C.; Filippi, R.Z.; Mendes do Nascimento, H.; Palmieri da Silva E Sousa, L.; Meola, J.; Piccinato, C.A.; Podgaec, S. Altered p16(Ink4a), IL-1beta, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. Int. J. Mol. Sci. 2022, 23, 2476. [Google Scholar] [CrossRef]
- Goumenou, A.G.; Matalliotakis, I.M.; Tzardi, M.; Fragouli, I.G.; Mahutte, N.G.; Arici, A. p16, retinoblastoma (pRb), and cyclin D1 protein expression in human endometriotic and adenomyotic lesions. Fertil. Steril. 2006, 85, 1204–1207. [Google Scholar] [CrossRef]
- Malvezzi, H.; Viana, B.G.; Dobo, C.; Filippi, R.Z.; Podgaec, S.; Piccinato, C.A. Depleted lamin B1: A possible marker of the involvement of senescence in endometriosis? Arch. Gynecol. Obstet. 2018, 297, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Liu, F.; Cheng, Z.; Wang, W. Cell fate decision mediated by p53 pulses. Proc. Natl. Acad. Sci. USA 2009, 106, 12245–12250. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert. Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar] [CrossRef]
- Daitoku, H.; Sakamaki, J.; Fukamizu, A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim. Biophys. Acta 2011, 1813, 1954–1960. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef]
- Rezk, N.A.; Lashin, M.B.; Sabbah, N.A. MiRNA 34-a regulate SIRT-1 and Foxo-1 expression in endometriosis. Noncoding RNA Res. 2021, 6, 35–41. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Salotti, J.; Johnson, P.F. Regulation of senescence and the SASP by the transcription factor C/EBPbeta. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef] [PubMed]
- Payea, M.J.; Anerillas, C.; Tharakan, R.; Gorospe, M. Translational Control during Cellular Senescence. Mol. Cell. Biol. 2021, 41, e00512-20. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061, Erratum in Nat. Cell Biol. 2021, 23, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015, 17, 1205–1217, Erratum in Nat. Cell Biol. 2015, 17, 1370. [Google Scholar] [CrossRef]
- Chen, C.C.; Jeon, S.M.; Bhaskar, P.T.; Nogueira, V.; Sundararajan, D.; Tonic, I.; Park, Y.; Hay, N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev. Cell. 2010, 18, 592–604. [Google Scholar] [CrossRef]
- Zhang, X.; Evans, T.D.; Chen, S.; Sergin, I.; Stitham, J.; Jeong, S.J.; Rodriguez-Velez, A.; Yeh, Y.S.; Park, A.; Jung, I.H.; et al. Loss of Macrophage mTORC2 Drives Atherosclerosis via FoxO1 and IL-1beta Signaling. Circ. Res. 2023, 133, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Aobulikasimu, A.; Sun, W.; Liu, S.; Xie, W.; Sun, W. Targeted regulation of FoxO1 in chondrocytes prevents age-related osteoarthritis via autophagy mechanism. J. Cell. Mol. Med. 2022, 26, 3075–3082. [Google Scholar] [CrossRef]
- Baek, M.O.; Song, H.I.; Han, J.S.; Yoon, M.S. Differential regulation of mTORC1 and mTORC2 is critical for 8-Br-cAMP-induced decidualization. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Driva, T.S.; Schatz, C.; Sobočan, M.; Haybaeck, J. The Role of mTOR and eIF Signaling in Benign Endometrial Diseases. Int. J. Mol. Sci. 2022, 23, 3416. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Lengyel, F.; Vértes, Z.; Kovács, K.A.; Környei, J.L.; Sümegi, B.; Vértes, M. Effect of estrogen and inhibition of phosphatidylinositol-3 kinase on Akt and FOXO1 in rat uterus. Steroids 2007, 72, 422–428. [Google Scholar] [CrossRef]
- Guzeloglu Kayisli, O.; Kayisli, U.A.; Luleci, G.; Arici, A. In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol. Reprod. 2004, 71, 714–721. [Google Scholar] [CrossRef]
- Shan, J.; Chang, L.Y.; Li, D.J.; Wang, X.Q. Rab27b promotes endometriosis by enhancing invasiveness of ESCs and promoting angiogenesis. Am. J. Reprod. Immunol. 2023, 90, e13762. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Papaconstantinou, J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006, 20, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.L.; Shavlakadze, T.; Grounds, M.D.; Arthur, P.G. Differential thiol oxidation of the signaling proteins Akt, PTEN or PP2A determines whether Akt phosphorylation is enhanced or inhibited by oxidative stress in C2C12 myotubes derived from skeletal muscle. Int. J. Biochem. Cell Biol. 2015, 62, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ng, S.; Wang, J.; Zhou, J.; Tan, S.H.; Yang, N.; Lin, Q.; Xia, D.; Shen, H.M. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy 2015, 11, 629–642. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Fusi, L.; Higham, J.; Lee, Y.; Lei, K.; Hanyaloglu, A.C.; Lam, E.W.; Christian, M.; Brosens, J.J. NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling. Endocrinology 2011, 152, 730–740. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Dalla Torre, M.; Pittari, D.; Boletta, A.; Cassina, L.; Sitia, R.; Anelli, T. Mitochondria remodeling during endometrial stromal cell decidualization. Life Sci. Alliance 2024, 7, e202402627. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Wilson, M.S.; Lam, E.W. FOXO transcription factors: From cell fate decisions to regulation of human female reproduction. Adv. Exp. Med. Biol. 2009, 665, 227–241. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhong, Z.; Wei, C.; Liu, Y.; Zhu, X. Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Front. Immunol. 2023, 14, 1134663. [Google Scholar] [CrossRef]
- Capobianco, A.; Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shi, L.; Li, M.; Yin, X.; Ji, X. Oxidative stress in endometriosis: Sources, mechanisms and therapeutic potential of antioxidants (Review). Int. J. Mol. Med. 2025, 55, 72. [Google Scholar] [CrossRef]
- Ni, C.; Li, D. Ferroptosis and oxidative stress in endometriosis: A systematic review of the literature. Medicine 2024, 103, e37421. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef]
- Ragu, S.; Droin, N.; Matos-Rodrigues, G.; Barascu, A.; Caillat, S.; Zarkovic, G.; Siberchicot, C.; Dardillac, E.; Gelot, C.; Guirouilh-Barbat, J.; et al. A noncanonical response to replication stress protects genome stability through ROS production, in an adaptive manner. Cell Death Differ. 2023, 30, 1349–1365. [Google Scholar] [CrossRef]
- Wang, Q.; Sztukowska, M.; Ojo, A.; Scott, D.A.; Wang, H.; Lamont, R.J. FOXO responses to Porphyromonas gingivalis in epithelial cells. Cell Microbiol. 2015, 17, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Xu, T.; Zhang, H.; Yu, A. FOXO1-dependent DNA damage repair is regulated by JNK in lung cancer cells. Int. J. Oncol. 2014, 44, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, M.; Fu, X.; Meng, X.; Ma, Y.; Liu, H.; Li, Q.; Xu, H.; Chen, J. Bushen Wenyang Huayu Decoction inhibits autophagy by regulating the SIRT1-FoXO-1 pathway in endometriosis rats. J. Ethnopharmacol. 2023, 308, 116277. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N.; Kashida, S.; Takiguchi, S.; Nakamura, Y.; Kato, H. Induction of superoxide dismutase by decidualization in human endometrial stromal cells. Mol. Hum. Reprod. 2000, 6, 178–184. [Google Scholar] [CrossRef]
- Marcellin, L.; Santulli, P.; Chouzenoux, S.; Cerles, O.; Nicco, C.; Dousset, B.; Pallardy, M.; Kerdine-Römer, S.; Just, P.A.; Chapron, C.; et al. Alteration of Nrf2 and Glutamate Cysteine Ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic. Biol. Med. 2017, 110, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Mani, A.M.; Wu, Z.H. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J. Cancer Metastasis Treat. 2017, 3, 45–59. [Google Scholar] [CrossRef]
- Christmann, M.; Kaina, B. Transcriptional regulation of human DNA repair genes following genotoxic stress: Trigger mechanisms, inducible responses and genotoxic adaptation. Nucleic Acids Res. 2013, 41, 8403–8420. [Google Scholar] [CrossRef]
- Bhoumik, A.; Lopez-Bergami, P.; Ronai, Z. ATF2 on the double—Activating transcription factor and DNA damage response protein. Pigment Cell Res. 2007, 20, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Brunet, A.; Grenier, J.M.; Datta, S.R.; Fornace, A.J., Jr.; DiStefano, P.S.; Chiang, L.W.; Greenberg, M.E. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 2002, 296, 530–534. [Google Scholar] [CrossRef]
- Inci, G.; Warkad, M.S.; Kang, B.G.; Lee, N.K.; Suh, H.W.; Lim, S.S.; Kim, J.; Kim, S.C.; Lee, J.Y. FOXO3a Mediates Homologous Recombination Repair (HRR) via Transcriptional Activation of MRE11, BRCA1, BRIP1, and RAD50. Molecules 2022, 27, 8623. [Google Scholar] [CrossRef]
- Miki, Y.; Tanji, K.; Mori, F.; Utsumi, J.; Sasaki, H.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Autophagy mediators (FOXO1, SESN3 and TSC2) in Lewy body disease and aging. Neurosci. Lett. 2018, 684, 35–41. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Jeong, S.A.; Kim, K.; Lee, J.H.; Cha, J.S.; Khadka, P.; Cho, H.S.; Chung, I.K. Akt-mediated phosphorylation increases the binding affinity of hTERT for importin alpha to promote nuclear translocation. J. Cell Sci. 2015, 128, 2287–2301. [Google Scholar] [CrossRef]
- Kao, S.H.; Huang, H.C.; Hsieh, R.H.; Chen, S.C.; Tsai, M.C.; Tzeng, C.R. Oxidative damage and mitochondrial DNA mutations with endometriosis. Ann. N. Y. Acad. Sci. 2005, 1042, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, J.H.; Lee, J.H.; Yoon, J.K.; Yun, B.H.; Park, J.H.; Seo, S.K.; Sung, H.J.; Kim, H.S.; Cho, S.; et al. Association Between Impairment of DNA Double Strand Break Repair and Decreased Ovarian Reserve in Patients with Endometriosis. Front. Endocrinol. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Lu, Z.; Goto, T.; Fusi, L.; Higham, J.; Francis, J.; Withey, A.; Hardt, J.; Cloke, B.; Stavropoulou, A.V.; et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol. Endocrinol. 2007, 21, 2334–2349. [Google Scholar] [CrossRef]
- Tamura, I.; Jozaki, K.; Sato, S.; Shirafuta, Y.; Shinagawa, M.; Maekawa, R.; Taketani, T.; Asada, H.; Tamura, H.; Sugino, N. The distal upstream region of insulin-like growth factor-binding protein-1 enhances its expression in endometrial stromal cells during decidualization. J. Biol. Chem. 2018, 293, 5270–5280. [Google Scholar] [CrossRef]
- Lynch, V.J.; Brayer, K.; Gellersen, B.; Wagner, G.P. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: Towards inferring the core transcriptional regulators of decidual genes. PLoS ONE 2009, 4, e6845. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, Y.; He, H.; Xin, Q.; Tu, Z.; Kong, S.; Cui, T.; Wang, B.; Quan, S.; Li, B.; et al. FoxM1 Directs STAT3 Expression Essential for Human Endometrial Stromal Decidualization. Sci. Rep. 2015, 5, 13735. [Google Scholar] [CrossRef]
- Thieffry, C.; Van Wynendaele, M.; Samain, L.; Tyteca, D.; Pierreux, C.; Marbaix, E.; Henriet, P. Spatiotemporal expression pattern of Progesterone Receptor Component (PGRMC) 1 in endometrium from patients with or without endometriosis or adenomyosis. J. Steroid Biochem. Mol. Biol. 2022, 223, 106153. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Negishi, R.; Mukoyama, T.; Yonekawa, R.; Kojima, J.; Azumi, M.; Kusama, K.; Nishi, H.; Tamura, K. Regulatory action of PGRMC1 on cyclic AMP-mediated COX2 expression in human endometrial cells. J. Pharmacol. Sci. 2023, 153, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, Y.M.; Wang, X.; Wetendorf, M.; Franco, H.L.; Mo, Q.; Wang, T.; Lanz, R.B.; Young, S.L.; Lessey, B.A.; Spencer, T.E.; et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018, 14, e1007787. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tang, S.; Jiang, P.; Geng, T.; Cope, D.I.; Dunn, T.N.; Guner, J.; Radilla, L.A.; Guan, X.; Monsivais, D. Impaired bone morphogenetic protein (BMP) signaling pathways disrupt decidualization in endometriosis. Commun. Biol. 2024, 7, 227. [Google Scholar] [CrossRef]
- Cai, X.; Xu, M.; Zhang, H.; Zhang, M.; Wang, J.; Mei, J.; Zhang, Y.; Zhou, J.; Zhen, X.; Kang, N.; et al. Endometrial stromal PRMT5 plays a crucial role in decidualization by regulating NF-kappaB signaling in endometriosis. Cell Death Discov. 2022, 8, 408. [Google Scholar] [CrossRef]
- Sengupta, A.; Molkentin, J.D.; Yutzey, K.E. FoxO transcription factors promote autophagy in cardiomyocytes. J. Biol. Chem. 2009, 284, 28319–28331. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, W.; Yang, J.; Ma, K.; Li, X.; Wang, Y.; Wang, D.; Wang, L.; Zhang, Y.; Yin, Y.; et al. FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy 2012, 8, 1712–1723. [Google Scholar] [CrossRef]
- Lu, J.; Huang, J.; Zhao, S.; Xu, W.; Chen, Y.; Li, Y.; Wang, Z.; Dong, Y.; You, R.; Cao, J.; et al. FOXO1 Is a Critical Switch Molecule for Autophagy and Apoptosis of Sow Endometrial Epithelial Cells Caused by Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 1172273. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; Berga, S.L.; Zou, E.; Washington, J.; Song, S.; Marzullo, B.J.; Bagchi, I.C.; Bagchi, M.K.; Yu, J. Interleukin-1beta induces and accelerates human endometrial stromal cell senescence and impairs decidualization via the c-Jun N-terminal kinase pathway. Cell Death Discov. 2024, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Yao, T. Role for autophagy-related markers Beclin-1 and LC3 in endometriosis. BMC Womens Health 2022, 22, 264. [Google Scholar] [CrossRef]
- Pizzimenti, C.; Fiorentino, V.; Ruggeri, C.; Franchina, M.; Ercoli, A.; Tuccari, G.; Ieni, A. Autophagy Involvement in Non-Neoplastic and Neoplastic Endometrial Pathology: The State of the Art with a Focus on Carcinoma. Int. J. Mol. Sci. 2024, 25, 12118. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Molecular mechanism of autophagy and apoptosis in endometriosis: Current understanding and future research directions. Reprod. Med. Biol. 2024, 23, e12577. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J.; Wei, B. Autophagy in endometriosis: Friend or foe? Biochem. Biophys. Res. Commun. 2018, 495, 60–63. [Google Scholar] [CrossRef]
- Gebel, H.M.; Braun, D.P.; Tambur, A.; Frame, D.; Rana, N.; Dmowski, W.P. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil. Steril. 1998, 69, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Arici, A. Apoptosis and the pathogenesis of endometriosis. Semin. Reprod. Med. 2003, 21, 165–172. [Google Scholar] [CrossRef]
- Sturlese, E.; Salmeri, F.M.; Retto, G.; Pizzo, A.; De Dominici, R.; Ardita, F.V.; Borrielli, I.; Licata, N.; Laganà, A.S.; Sofo, V. Dysregulation of the Fas/FasL system in mononuclear cells recovered from peritoneal fluid of women with endometriosis. J. Reprod. Immunol. 2011, 92, 74–81. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen Receptor beta Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Deryabin, P.; Griukova, A.; Nikolsky, N.; Borodkina, A. The link between endometrial stromal cell senescence and decidualization in female fertility: The art of balance. Cell. Mol. Life Sci. 2020, 77, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horné, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial-Mesenchymal Transition in Endometriosis-When Does It Happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Endometriosis: From iron and macrophages to exosomes. Is the sky clearing? Hum. Reprod. Open. 2024, 2024, hoae034. [Google Scholar] [CrossRef] [PubMed]
- Buzzio, O.L.; Lu, Z.; Miller, C.D.; Unterman, T.G.; Kim, J.J. FOXO1A differentially regulates genes of decidualization. Endocrinology 2006, 147, 3870–3876. [Google Scholar] [CrossRef] [PubMed]
- Lasick, K.A.; Jose, E.; Samayoa, A.M.; Shanks, L.; Pond, K.W.; Thorne, C.A.; Paek, A.L. FOXO nuclear shuttling dynamics are stimulus-dependent and correspond with cell fate. Mol. Biol. Cell. 2023, 34, ar21. [Google Scholar] [CrossRef]
- Weng, Q.; Liu, Z.; Li, B.; Liu, K.; Wu, W.; Liu, H. Oxidative Stress Induces Mouse Follicular Granulosa Cells Apoptosis via JNK/FoxO1 Pathway. PLoS ONE 2016, 11, e0167869. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Rizvi, F.; Raisuddin, S.; Kakkar, P. FoxO proteins’ nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic. Biol. Med. 2014, 76, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Vlahopoulos, S.A.; Granot, Z. Regulation of Bim in Health and Disease. Oncotarget 2015, 6, 23058–23134. [Google Scholar] [CrossRef]
- Qiang, L.; Banks, A.S.; Accili, D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J. Biol. Chem. 2010, 285, 27396–27401. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tian, M.; Huang, Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, R239–R253. [Google Scholar] [CrossRef]
- Guo, X.; Peng, K.; He, Y.; Xue, L. Mechanistic regulation of FOXO transcription factors in the nucleus. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189083. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.K.; Liu, H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kobayashi, H.; Shigetomi, H.; Nishio, M.; Umetani, M.; Imanaka, S.; Hashimoto, H. Molecular Regulation of FOXO1 and Its Pathophysiological Significance in Endometriosis: A Narrative Review. Antioxidants 2026, 15, 3. https://doi.org/10.3390/antiox15010003
Kobayashi H, Shigetomi H, Nishio M, Umetani M, Imanaka S, Hashimoto H. Molecular Regulation of FOXO1 and Its Pathophysiological Significance in Endometriosis: A Narrative Review. Antioxidants. 2026; 15(1):3. https://doi.org/10.3390/antiox15010003
Chicago/Turabian StyleKobayashi, Hiroshi, Hiroshi Shigetomi, Miki Nishio, Mai Umetani, Shogo Imanaka, and Hiratsugu Hashimoto. 2026. "Molecular Regulation of FOXO1 and Its Pathophysiological Significance in Endometriosis: A Narrative Review" Antioxidants 15, no. 1: 3. https://doi.org/10.3390/antiox15010003
APA StyleKobayashi, H., Shigetomi, H., Nishio, M., Umetani, M., Imanaka, S., & Hashimoto, H. (2026). Molecular Regulation of FOXO1 and Its Pathophysiological Significance in Endometriosis: A Narrative Review. Antioxidants, 15(1), 3. https://doi.org/10.3390/antiox15010003





