Melatonin Enhances Muscle Development and Suppresses Fat Deposition in Cashmere Goats by Implicating Gut Microbiota and Ameliorating Systemic Antioxidant Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Carcass Trait Analysis
2.3. Assessment of Meat Quality
2.4. Measurement of the Chemical Profile of Musclesn
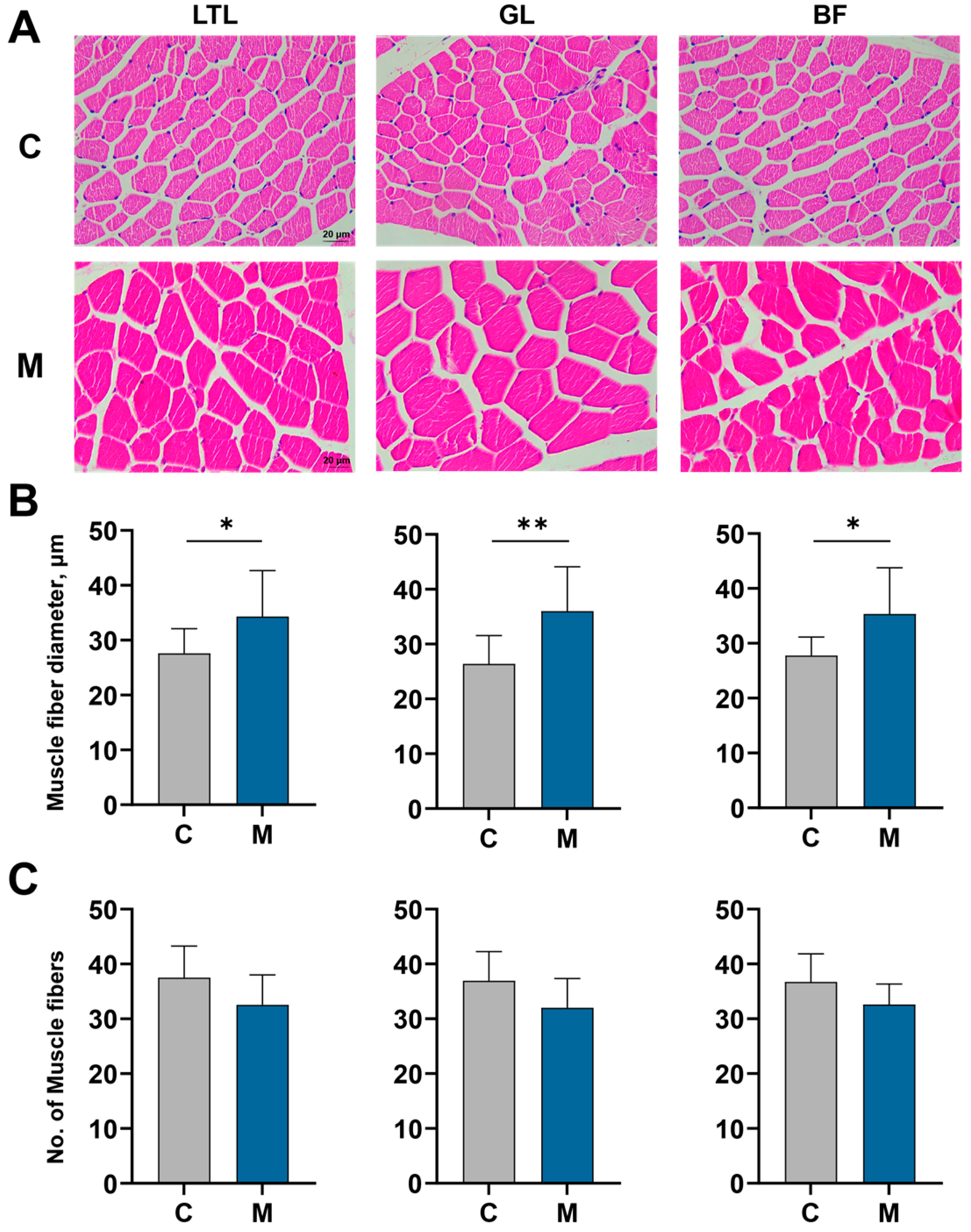
2.5. Tissue Histology Examination
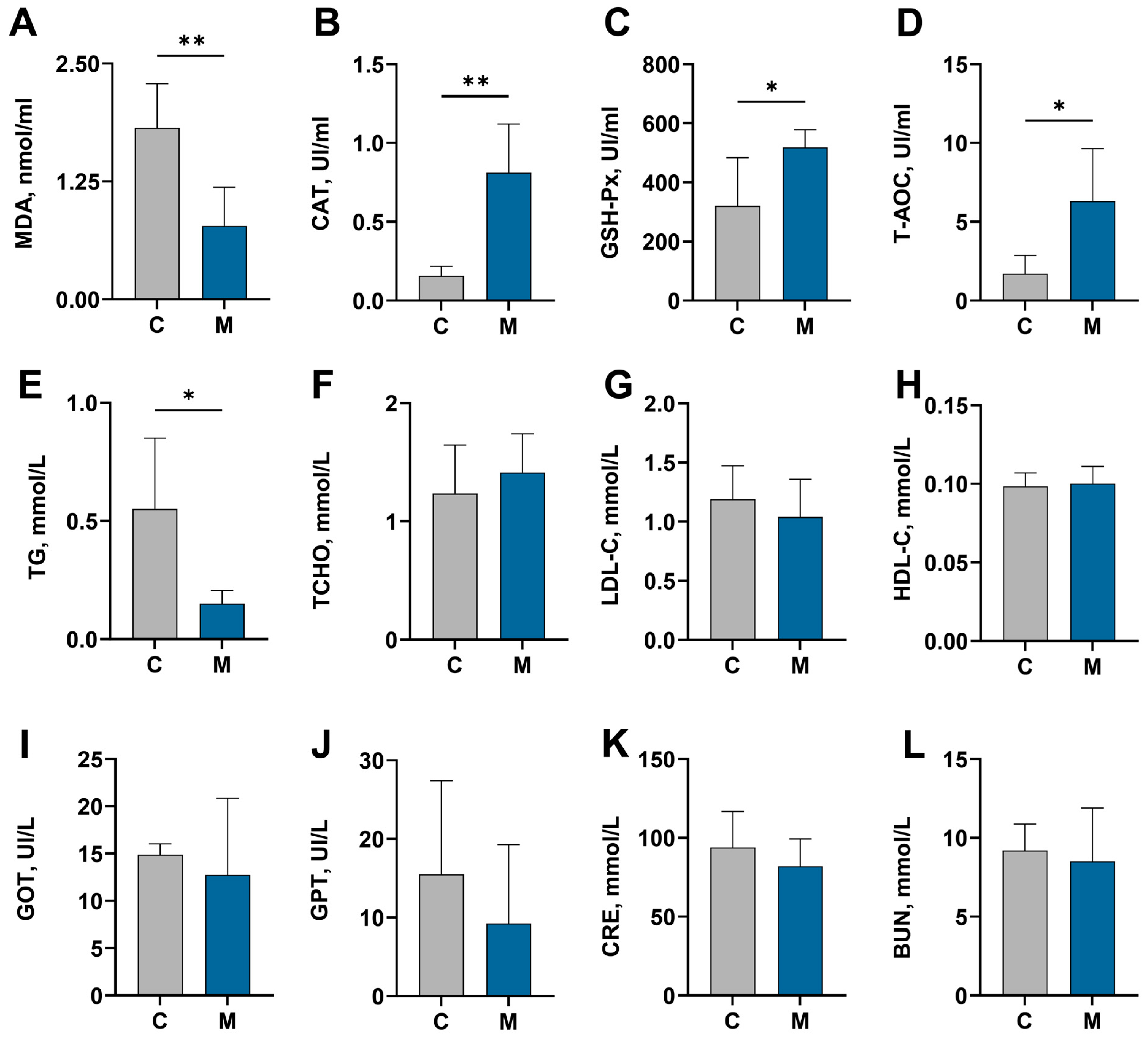
2.6. Serum Sample Collection and Biochemical Assessment
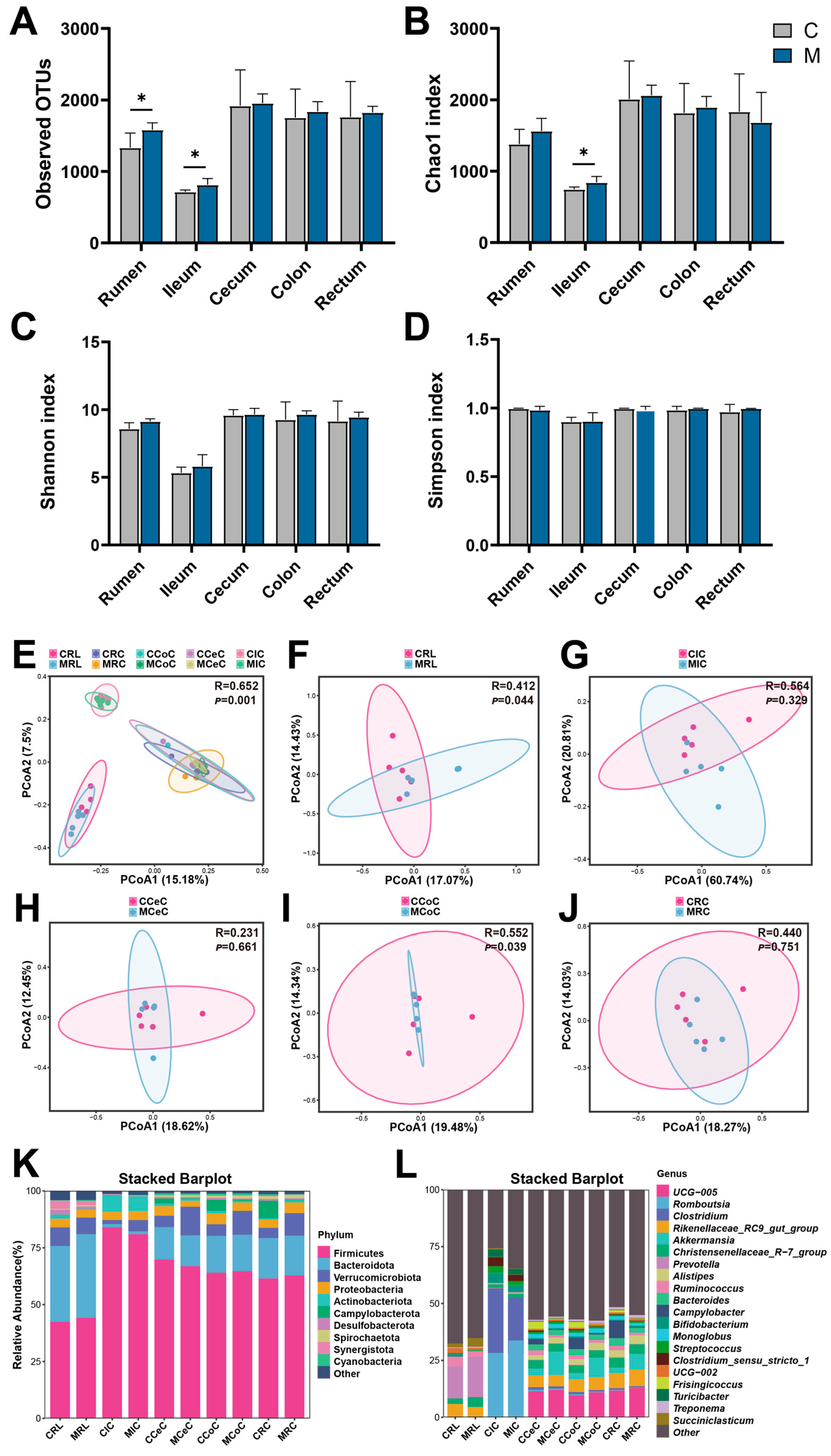
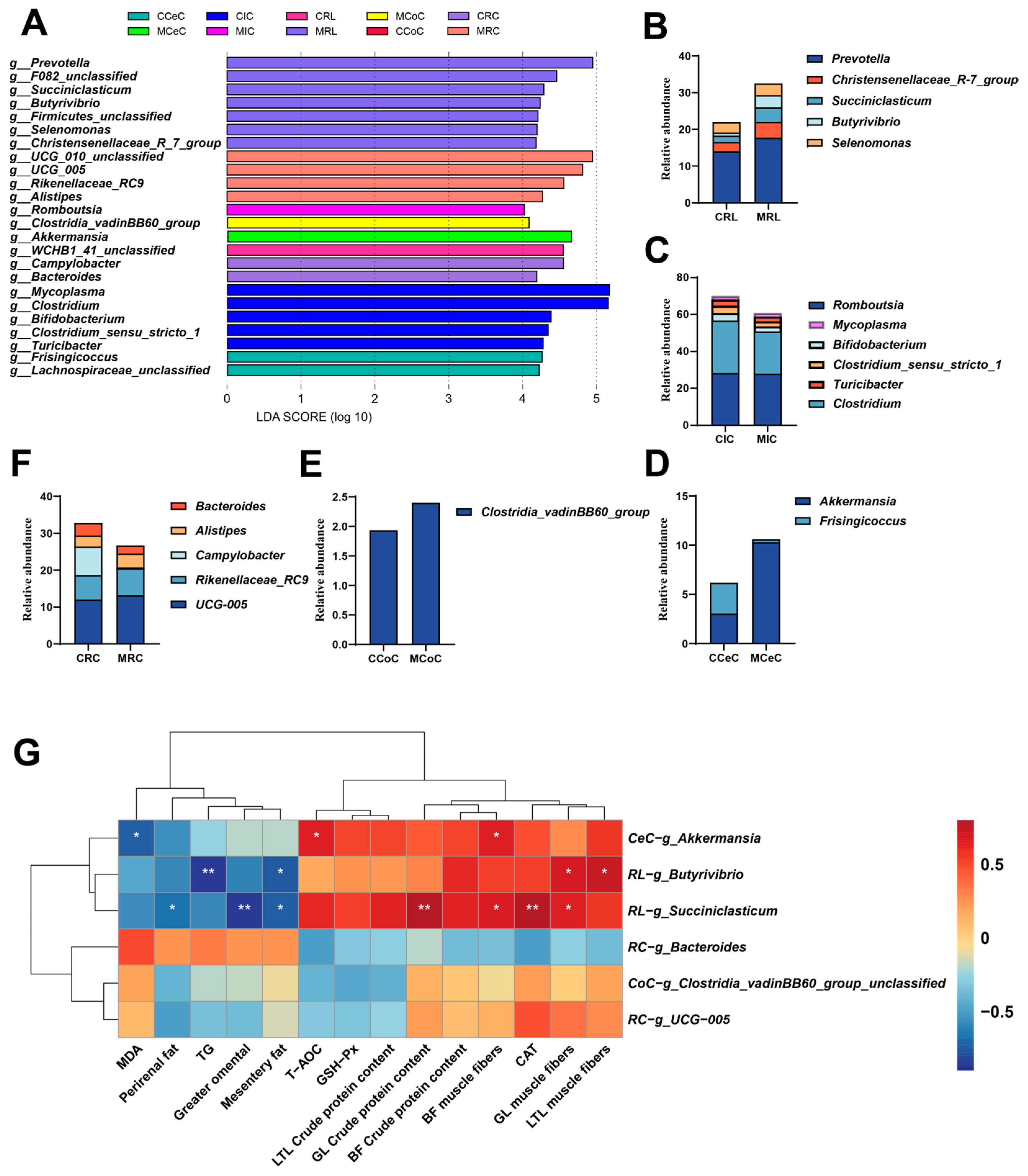
2.7. Gastrointestinal Microbiota Analysis
2.8. Statistical Analyses
3. Results
3.1. Slaughter Performance
3.2. Muscle Characteristics and Meat Quality
3.3. Blood Biochemical Parameters
3.4. Histological Structure of the Digestive Tract and Composition of the Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.; Sun, X.; Zhao, S.; Hu, M.; Li, D.; Qi, S.; Jiao, X.; Sun, Y.; Wang, C.; Zhu, X.; et al. Dietary alfalfa powder supplementation improves growth and development, body health, and meat quality of tibetan sheep. Food Chem. 2022, 396, 133709. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Li, H.; Xiang, H.; Zhang, C.; Du, Z.; Huang, L.; Zhu, J. MiR-196a promotes lipid deposition in goat intramuscular preadipocytes by targeting MAP3k1 and activating PI3k-akt pathway. Cells 2024, 13, 1459. [Google Scholar] [CrossRef]
- Duan, C. The Research on Patterns and Mechanisms of Cashmere Growth Induced by Melatonin; China Agricultural University: Beijing, China, 2016. [Google Scholar]
- Duan, T.; Wu, Z.; Zhang, H.; Liu, Y.; Li, Y.; Zhang, W. Effects of melatonin implantation on carcass characteristics, meat quality and tissue levels of melatonin and prolactin in inner mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, Z.; Zhang, W. Melatonin’s role in hair follicle growth and development: A cashmere goat perspective. Int. J. Mol. Sci. 2025, 26, 2844. [Google Scholar] [CrossRef]
- Han, D.; Zheng, Z.; Su, Z.; Wang, X.; Ding, S.; Wang, C.; He, L.; Zhang, W. Melatonin promotes muscle growth and redirects fat deposition in cashmere goats via gut microbiota modulation and enhanced antioxidant capacity. Antioxidants 2025, 14, 645. [Google Scholar] [CrossRef]
- Xing, T.; Gao, F.; Tume, R.K.; Zhou, G.; Xu, X. Stress effects on meat quality: A mechanistic perspective. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, Y.; Wang, L.; Gao, K.; Jiang, Z. A high-fat diet increases body fat mass and up-regulates expression of genes related to adipogenesis and inflammation in a genetically lean pig. J. Zhejiang Univ. B Sci. 2018, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, X.; Zhao, X.; Liu, T.; Jin, Y.; Duan, Y. Effects of l-arginine supplementation on fat deposition and meat quality in growing lambs: Interactions with gut microbiota and metabolic signalling pathways. Food Chem. 2025, 479, 143677. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, Q.; Zhou, X.; Duan, Y.; Yang, Y.; Gong, S.; Han, M.; Liu, Y.; Yang, Z.; Chen, Q.; et al. Role of brain-gut-muscle axis in human health and energy homeostasis. Front. Nutr. 2022, 9, 947033. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, X.; Lu, T.; An, W.; Chen, S.; Li, S.; Miao, H.; Han, X. Co-cultivation of lactobacillus acidophilus and bacillus subtilis mediates the gut-muscle axis affecting pork quality and flavor. J. Anim. Sci. Biotechnol. 2025, 16, 93. [Google Scholar] [CrossRef]
- Dou, L.; Liu, C.; Chen, X.; Yang, Z.; Hu, G.; Zhang, M.; Sun, L.; Su, L.; Zhao, L.; Jin, Y. Supplemental clostridium butyricum modulates skeletal muscle development and meat quality by shaping the gut microbiota of lambs. Meat Sci. 2023, 204, 109235. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Chen, Y.; Song, G.; Zhao, C.; Qi, W.; Wang, Y. Interactions between anthocyanins and gut microbiota in promoting healthy aging. J. Future Foods 2025, 5, 229–238. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, M.; Wei, S.; Zhu, L.; Yan, R.; Jin, M.; Wang, Y. Variation of meat quality and relationship to gut microbiota among different pig breeds. Microb. Biotechnol. 2025, 18, e70139. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, H.; Wei, Y.; Niu, J.; Hao, S.; Sun, H.; Tang, G.; Qi, C.; Ge, J. Protective effect of melatonin against metabolic disorders and neuropsychiatric injuries in type 2 diabetes mellitus mice. Phytomedicine 2024, 131, 155805. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Farjallah, M.A.; Hammouda, O.; Zouch, M.; Ghattassi, K.; Graja, A.; Driss, T.; Chamari, K.; Souissi, N. Effect of melatonin ingestion on physical performance, metabolic responses, and recovery after an intermittent training session. Physiol. Int. 2018, 105, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of melatonin on skeletal muscle and exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, N.; Jiao, H.; Zhang, J.; Li, C.; Ren, W.; Reiter, R.J.; Su, S. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 2021, 71, e12754. [Google Scholar] [CrossRef]
- Xia, S.; Gao, W.; Li, Y.; Ma, J.; Gong, S.; Gao, Z.; Tang, W.; Tian, W.; Tang, S. Effects of melatonin on intestinal function and bacterial compositions in sucking piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1139–1148. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, T.; He, M.; Yang, B.; Wang, Z.; Liu, Y. Melatonin protects against colistin-induced intestinal inflammation and microbiota dysbiosis. J. Pineal Res. 2024, 76, e12989. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, W.; Xiao, B.; Wang, Y.; Li, Y.; Wu, A.; Zhang, Q.; Liu, X.; Liu, S.; Yuan, Z.; et al. Melatonin alleviates t-2 toxin-induced intestinal injury by enhancing gut barrier function and modulating microbiota in weaned piglets. J. Agric. Food Chem. 2025, 73, 6903–6916. [Google Scholar] [CrossRef]
- Yang, C.; Xu, J.; Ren, Q.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef]
- Tan, B.; Yin, Y.; Liu, Z.; Li, X.; Xu, H.; Kong, X.; Huang, R.; Tang, W.; Shinzato, I.; Smith, S.B.; et al. Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 2009, 37, 169–175. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiao, Y.; Ma, L.; Lyu, W.; Peng, H.; Wang, X.; Ren, Y.; Li, J. Low dose of sucralose alter gut microbiome in mice. Front. Nutr. 2022, 9, 848392. [Google Scholar] [CrossRef]
- Zheng, Z.; Han, D.; Su, Z.; He, L.; Zhang, W. Effect of melatonin on the production performance, blood biochemical parameters, nutrient digestibility, and gastrointestinal microbiome of liaoning cashmere goats. Agriculture 2024, 14, 1983. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Su, Z.; Li, S.; Qin, J.; Ren, N.; He, L.; Zhang, W. Melatonin alleviates intestinal damage in neonate mice following salmonella typhimurium and LPS challenges involving gut microbiota remodeling. Int. Immunopharmacol. 2026, 168, 115761. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of floccularia luteovirens regulate intestinal immune response, and oxidative stress activity through MAPK/nrf2/keap1 signaling pathway in immunosuppressive mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.; Leite, A.; Vasconcelos, L.; Plaza, J.; Abecia, J.; Revilla, I.; Palacios, C.; Teixeira, A. Effect of melatonin implants on carcass characteristics and meat quality of slow-growing chickens. Poult. Sci. 2025, 104, 104913. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, L.; Huang, Y.; Wang, Y.; Song, W.; Zhen, L. Effect of dietary supplementation with melatonin on meat quality, fatty acid profiles and metabolome differences in broiler breast. J. Food Compost. Anal. 2023, 121, 105410. [Google Scholar] [CrossRef]
- Chen, W.; Tu, Y.; Cai, P.; Wang, L.; Zhou, Y.; Liu, S.; Huang, Y.; Zhang, S.; Gu, X.; Yi, W.; et al. Melatonin supplementation promotes muscle fiber hypertrophy and regulates lipid metabolism of skeletal muscle in weaned piglets. J. Anim. Sci. 2023, 101, skad256. [Google Scholar] [CrossRef]
- Reid, D.S.; Geary, T.W.; Zezeski, A.L.; Waterman, R.C.; Van Emon, M.L.; Messman, R.D.; Burnett, D.D.; Lemley, C.O. Effects of prenatal and postnatal melatonin supplementation on overall performance, male reproductive performance, and testicular hemodynamics in beef cattle. J. Anim. Sci. 2023, 101, skad111. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wu, X.; Chen, X.; Paengkoum, P.; Han, Y.; Yang, Y.; Wang, X.; Zhao, J.; Lu, S.; Chen, H.; et al. Effects of dietary allicin supplementation on meat quality, antioxidant enzymes, fiber characteristics, and flavor composition of guizhou black goats. Meat Sci. 2025, 231, 109962. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hou, P.; Liu, L.; Zhao, L.; Zhang, X.; Yang, C.; Huang, X.; Ge, T.; Zheng, J.; Wen, Y.; et al. Effects of the dietary protein level on growth performance, nitrogen metabolism, serum biochemical index, and meat quality of suffolk×hu f1 lambs. J. Agric. Food Res. 2025, 21, 101808. [Google Scholar] [CrossRef]
- Afzal, A. Melatonin as a multifunctional modulator: Emerging insights into its role in health, reproductive efficiency, and productive performance in livestock. Front. Physiol. 2024, 15, 1501334. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- Yang, W.; Tang, K.; Wang, Y.; Zhang, Y.; Zan, L. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 2017, 7, 15080. [Google Scholar] [CrossRef]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Grabowska, M.G.; Wawrzyniak, D.; Rolle, K.; Chomczy Ski, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 1, 39–312. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Luo, X.; Ge, C.; Lv, Y.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Itaconic acid alleviates perfluorooctanoic acid-induced oxidative stress and intestinal damage by regulating the keap1/nrf2/ho-1 pathway and reshaping the gut microbiota. Int. J. Mol. Sci. 2024, 25, 9826. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef]
- Diao, X.; Duan, C.; Yao, L.; Qin, J.; He, L.; Zhang, W. Melatonin promotes the development of secondary hair follicles in adult cashmere goats by activating the keap1-nrf2 signaling pathway and inhibiting the inflammatory transcription factors NFkappaB and AP-1. Int. J. Mol. Sci. 2023, 24, 3403. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lu, W.; Zhou, X.; Li, X.; Zeng, S.; Li, S.; Yan, C.; Zhu, R.; Cai, G.; Zheng, W.; et al. Ameliorative effects of e. Cristatum fermented albino tea at the regreening stage on fat deposition of youth chicken. Poult. Sci. 2025, 104, 105240. [Google Scholar] [CrossRef]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef]
- Song, C.; Pantopoulos, K.; Chen, G.; Zhong, C.; Zhao, T.; Zhang, D.; Luo, Z. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1alpha-PPARgamma pathway. Cell. Mol. Life Sci. 2022, 79, 394. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, C.; Tian, H.; Weng, X.; Lin, C.; Zhang, D.; Zhao, Y.; Li, X.; Cheng, J.; et al. Rumen microbiome and fat deposition in sheep: Insights from a bidirectional mendelian randomization study. NPJ Biofilms Microbiomes 2024, 10, 129. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, D.; Zhang, D.; Huang, K.; Zhang, Y.; Li, X.; Zhao, Y.; Zhao, L.; Xu, Q.; Yang, X.; et al. Exploring the cecal microbial community associated with fat deposition in sheep and its possible pathways of action. Microbiol. Spectr. 2025, 13, e01424–e01488. [Google Scholar] [CrossRef]
- Guo, X.; Xu, J.; Zhao, Y.; Wang, J.; Fu, T.; Richard, M.L.; Sokol, H.; Wang, M.; Li, Y.; Liu, Y.; et al. Melatonin alleviates heat stress-induced spermatogenesis dysfunction in male dairy goats by regulating arachidonic acid metabolism mediated by remodeling the gut microbiota. Microbiome 2024, 12, 233. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, Y.; Wang, Y.; Liu, M.; Zhu, P.; Cui, K.; Yang, C.; Xu, C.; Feng, T.; Liu, Q. Host-driven remodeling of rumen microbiota supports lactation metabolism in buffalo. Front. Microbiol. 2025, 16, 1617388. [Google Scholar] [CrossRef] [PubMed]
- Ilina, L.A.; Filippova, V.A.; Brazhnik, E.A.; Dubrovin, A.V.; Yildirim, E.A.; Dunyashev, T.P.; Laptev, G.Y.; Novikova, N.I.; Sobolev, D.V.; Yuzhakov, A.A.; et al. The comparative analysis of the ruminal bacterial population in reindeer (Rangifer tarandus L.) From the russian arctic zone: Regional and seasonal effects. Animals 2021, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Sun, J.; Qiu, X.; Zhang, Y.; Wang, J.; Wang, Q.; Huang, J.; Ge, L.; Liu, Z. The intestinal microbiota contributes to the growth and physiological state of muscle tissue in piglets. Sci. Rep. 2021, 11, 11237. [Google Scholar] [CrossRef]
- Li, Q.; Huo, J.; Ni, G.; Zhang, F.; Zhang, S.; Zhang, X.; Wang, R.; Jiao, J.; Yu, Z.; Pu, X.; et al. Reductive acetogenesis is a dominant process in the ruminant hindgut. Microbiome 2025, 13, 28. [Google Scholar] [CrossRef]
- Shi, M.; Li, Z.; Hu, S.; Zhang, P.; Meng, S.; Huang, L.; Miao, Z.; Zhang, J. Microbiome-proteome analysis of gastrointestinal microbiota and longissimus thoracis muscle proteins in cattle with high and low grades of marbling. BMC Vet. Res. 2024, 20, 563. [Google Scholar] [CrossRef]
- Pi, Y.; Mu, C.; Gao, K.; Liu, Z.; Peng, Y.; Yu, K.; Su, Y.; Zhu, W. Increasing carbohydrates or nitrogenous compounds by cecal infusion leads to an opposite influence on colonic microbiota and host metabolism in a pig model. Anim. Nutr. 2025, 23, 62–77. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Hu, L.; Zhang, G.; Lu, H.; Luo, H.; Zhao, S.; Zhu, H.; Wang, Y. Characterization of the microbial communities along the gastrointestinal tract in crossbred cattle. Animals 2022, 12, 825. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | (%) | Nutrient Levels 2 | |
|---|---|---|---|
| Alfalfa | 35.00 | ME, (MJ/Kg) | 9.69 |
| Peanut straw | 35.00 | DM, (%) | 89.93 |
| Corn | 13.08 | EE, (%) | 3.27 |
| Extruded Corn | 4.62 | CP, (%) | 15.24 |
| Soybean Meal | 6.40 | NDF, (%) | 50.30 |
| Corn Gluten Meal | 1.50 | ADF, (%) | 36.23 |
| Extruded Full-fat Soybean | 2.00 | Ca, (%) | 1.24 |
| Limestone | 0.45 | P, (%) | 0.46 |
| Salt | 0.21 | ||
| Premix 1 | 0.45 | ||
| Molasses | 0.90 | ||
| Monocalcium Phosphate | 0.15 | ||
| Ammonium Chloride | 0.15 | ||
| Choline Chloride | 0.03 | ||
| Tannic Acid | 0.06 | ||
| Total | 100 |
| Item | CON | MT | p-Value |
|---|---|---|---|
| Slaughter weight (kg) | 33.44 ± 0.45 | 32.05 ± 1.50 | 0.448 |
| Carcass weight (kg) | 14.70 ± 1.24 | 14.19 ± 0.84 | 0.771 |
| Carcass yield (%) | 43.83 ± 3.32 | 44.20 ± 1.21 | 0.926 |
| Height at withers (cm) | 54.05 ± 1.02 | 54.82 ± 0.96 | 0.894 |
| Length of body (cm) | 54.78 ± 1.22 | 57.90 ± 1.58 | 0.225 |
| Circumference of chest (cm) | 9.60 ± 0.35 | 9.58 ± 0.15 | 0.961 |
| Head weight (kg) | 2.36 ± 0.03 b | 2.60 ± 0.07 a | 0.023 |
| Hoof weight (kg) | 0.71 ± 0.09 | 0.94 ± 0.03 | 0.071 |
| GR (cm) | 3.00 ± 0.21 | 2.95 ± 0.70 | 0.975 |
| Rib-eye area (cm2) | 16.11 ± 1.57 | 15.40 ± 1.35 | 0.763 |
| Perirenal fat (g) | 274.88 ± 18.91 b | 170.42 ± 23.87 a | 0.015 |
| Greater omental (g) | 655.94 ± 32.52 b | 323.48 ± 32.42 a | 0.001 |
| Mesentery fat (g) | 346.40 ± 24.96 b | 196.87 ± 8.71 a | 0.013 |
| Item | CON | MT | p-Value | |
|---|---|---|---|---|
| Weight (g) | Heart | 84.64 ± 8.97 | 95.56 ± 5.83 | 0.388 |
| Liver | 469.98 ± 39.16 | 536.24 ± 19.57 | 0.213 | |
| Spleen | 28.42 ± 3.11 | 35.44 ± 2.64 | 0.162 | |
| Lung | 271.50 ± 19.94 | 335.58 ± 26.55 | 0.123 | |
| Kidney | 79.36 ± 6.15 | 88.80 ± 9.74 | 0.363 | |
| Index (%) | Heart | 0.25 ± 0.03 | 0.30 ± 0.01 | 0.255 |
| Liver | 1.41 ± 0.13 | 1.68 ± 0.07 | 0.099 | |
| Spleen | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.650 | |
| Lung | 0.81 ± 0.08 b | 1.04 ± 0.06 a | 0.041 | |
| Kidney | 0.23 ± 0.02 | 0.28 ± 0.03 | 0.260 | |
| Testes (g) | 183.54 ± 17.25 | 183.14 ± 2.66 | 0.984 | |
| Rumen weight (kg) | 3.16 ± 0.75 | 4.20 ± 0.91 | 0.454 | |
| Net rumen (kg) | 0.66 ± 0.05 | 0.91 ± 0.17 | 0.222 | |
| Rumen pH | 6.60 ± 0.39 | 6.68 ± 0.14 | 0.870 | |
| Item | CON | MT | p-Value | |
|---|---|---|---|---|
| LTL | pH45min | 6.42 ± 0.26 | 6.54 ± 0.27 | 0.529 |
| pH24h | 5.46 ± 0.07 | 6.16 ± 0.39 | 0.017 | |
| L* | 36.50 ± 0.73 | 37.12 ± 2.08 | 0.586 | |
| a* | 17.13 ± 1.60 | 17.27 ± 0.62 | 0.826 | |
| b* | 4.01 ± 0.36 | 4.13 ± 0.46 | 0.670 | |
| Drip loss (%) | 7.95 ± 3.72 | 7.26 ± 3.15 | 0.786 | |
| Cooking loss (%) | 50.54 ± 4.85 | 43.53 ± 6.68 | 0.251 | |
| Shear force (kgf) | 11.26 ± 0.87 | 13.15 ± 1.98 | 0.118 | |
| Moisture content (%) | 71.02 ± 0.01 | 71.15 ± 0.01 | 0.790 | |
| Crude protein content (%) | 26.19 ± 0.34 b | 27.18 ± 0.38 a | 0.032 | |
| Ether extract content (%) | 2.03 ± 0.19 | 1.73 ± 0.25 | 0.357 | |
| GL | pH45min | 6.40 ± 0.26 | 6.73 ± 0.23 | 0.094 |
| pH24h | 5.73 ± 0.22 | 5.78 ± 0.23 | 0.750 | |
| L* | 37.08 ± 1.60 | 38.17 ± 2.23 | 0.452 | |
| a* | 19.05 ± 1.72 | 18.50 ± 0.99 | 0.595 | |
| b* | 4.72 ± 0.34 | 4.89 ± 0.54 | 0.596 | |
| Drip loss (%) | 8.31 ± 3.26 b | 3.41 ± 1.01 a | 0.021 | |
| Cooking loss (%) | 49.61 ± 4.16 | 50.23 ± 3.67 | 0.827 | |
| Shear force (kgf) | 9.72 ± 3.34 | 12.88 ± 2.00 | 0.144 | |
| Moisture content (%) | 70.68 ± 0.86 | 67.62 ± 2.06 | 0.235 | |
| Crude protein content (%) | 24.29 ± 0.66 b | 28.39 ± 1.52 a | 0.038 | |
| Ether extract content (%) | 2.03 ± 0.19 | 1.73 ± 0.25 | 0.658 | |
| BF | pH 45 min | 6.39 ± 0.15 | 6.76 ± 0.42 | 0.137 |
| pH 24 h | 5.79 ± 0.20 | 5.70 ± 0.15 | 0.483 | |
| L* | 37.60 ± 2.06 | 37.00 ± 1.36 | 0.643 | |
| a* | 17.40 ± 1.70 | 16.77 ± 0.73 | 0.514 | |
| b* | 4.39 ± 0.35 | 4.26 ± 0.37 | 0.604 | |
| Drip loss (%) | 9.61 ± 2.72 b | 5.44 ± 2.09 a | 0.041 | |
| Cooking loss (%) | 50.41 ± 4.22 | 50.88 ± 4.68 | 0.884 | |
| Shear force (kgf) | 12.15 ± 1.86 | 12.72 ± 0.77 | 0.583 | |
| Moisture content (%) | 70.97 ± 0.30 | 70.06 ± 0.42 | 0.113 | |
| Crude protein content (%) | 25.56 ± 0.13 b | 27.22 ± 0.21 a | 0.004 | |
| Ether extract content (%) | 2.13 ± 0.20 | 2.49 ± 0.30 | 0.335 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Su, Z.; Zheng, Z.; Lu, M.; Han, D.; Qin, J.; Yin, T.; Quan, Z.; Ding, S.; He, L.; Zhang, W. Melatonin Enhances Muscle Development and Suppresses Fat Deposition in Cashmere Goats by Implicating Gut Microbiota and Ameliorating Systemic Antioxidant Status. Antioxidants 2026, 15, 11. https://doi.org/10.3390/antiox15010011
Su Z, Zheng Z, Lu M, Han D, Qin J, Yin T, Quan Z, Ding S, He L, Zhang W. Melatonin Enhances Muscle Development and Suppresses Fat Deposition in Cashmere Goats by Implicating Gut Microbiota and Ameliorating Systemic Antioxidant Status. Antioxidants. 2026; 15(1):11. https://doi.org/10.3390/antiox15010011
Chicago/Turabian StyleSu, Zhenyu, Zibin Zheng, Mulong Lu, Di Han, Jiaxin Qin, Tianzhu Yin, Zhiguo Quan, Shiwei Ding, Liwen He, and Wei Zhang. 2026. "Melatonin Enhances Muscle Development and Suppresses Fat Deposition in Cashmere Goats by Implicating Gut Microbiota and Ameliorating Systemic Antioxidant Status" Antioxidants 15, no. 1: 11. https://doi.org/10.3390/antiox15010011
APA StyleSu, Z., Zheng, Z., Lu, M., Han, D., Qin, J., Yin, T., Quan, Z., Ding, S., He, L., & Zhang, W. (2026). Melatonin Enhances Muscle Development and Suppresses Fat Deposition in Cashmere Goats by Implicating Gut Microbiota and Ameliorating Systemic Antioxidant Status. Antioxidants, 15(1), 11. https://doi.org/10.3390/antiox15010011






