PTEN/PKM2/ERα-Driven Glyoxalase 1 Overexpression Sustains PC3 Prostate Cancer Cell Growth Through MG-H1/RAGE Pathway Desensitization Leading to H2O2-Dependent KRIT1 Downregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. IHC of Prostate Tissues
2.3. Cell Cultures
2.4. Cell Lysates
2.5. Western Blot
2.6. Glo1, PTEN, p-AKT, and p-mTOR Evaluation
2.7. Glo1 Enzymatic Activity Assay
2.8. RNA Isolation, Reverse Transcription, and qRT-PCR
2.9. Measurement of H2O2 Levels
2.10. siRNA Transfection
2.11. MG-H1 Detection
2.12. Ectopic Expression of Glo1 and PTEN
2.13. Cell Proliferation
2.14. Colony Formation
2.15. Apoptosis Detection
2.16. Statistical Analysis
3. Results
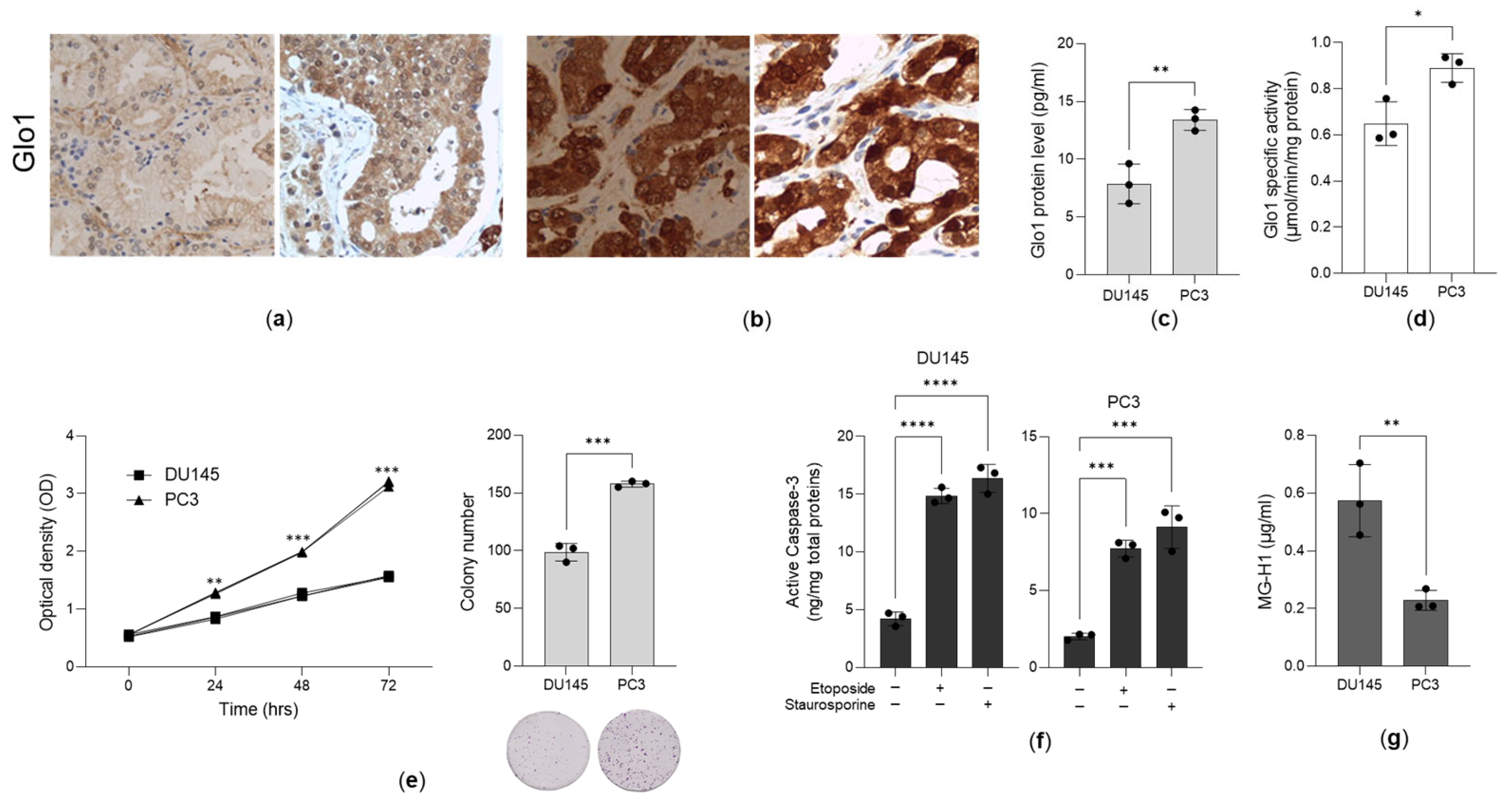
3.1. Glo1 Is Overexpressed in Aggressive PCa Tissues and Cell Lines
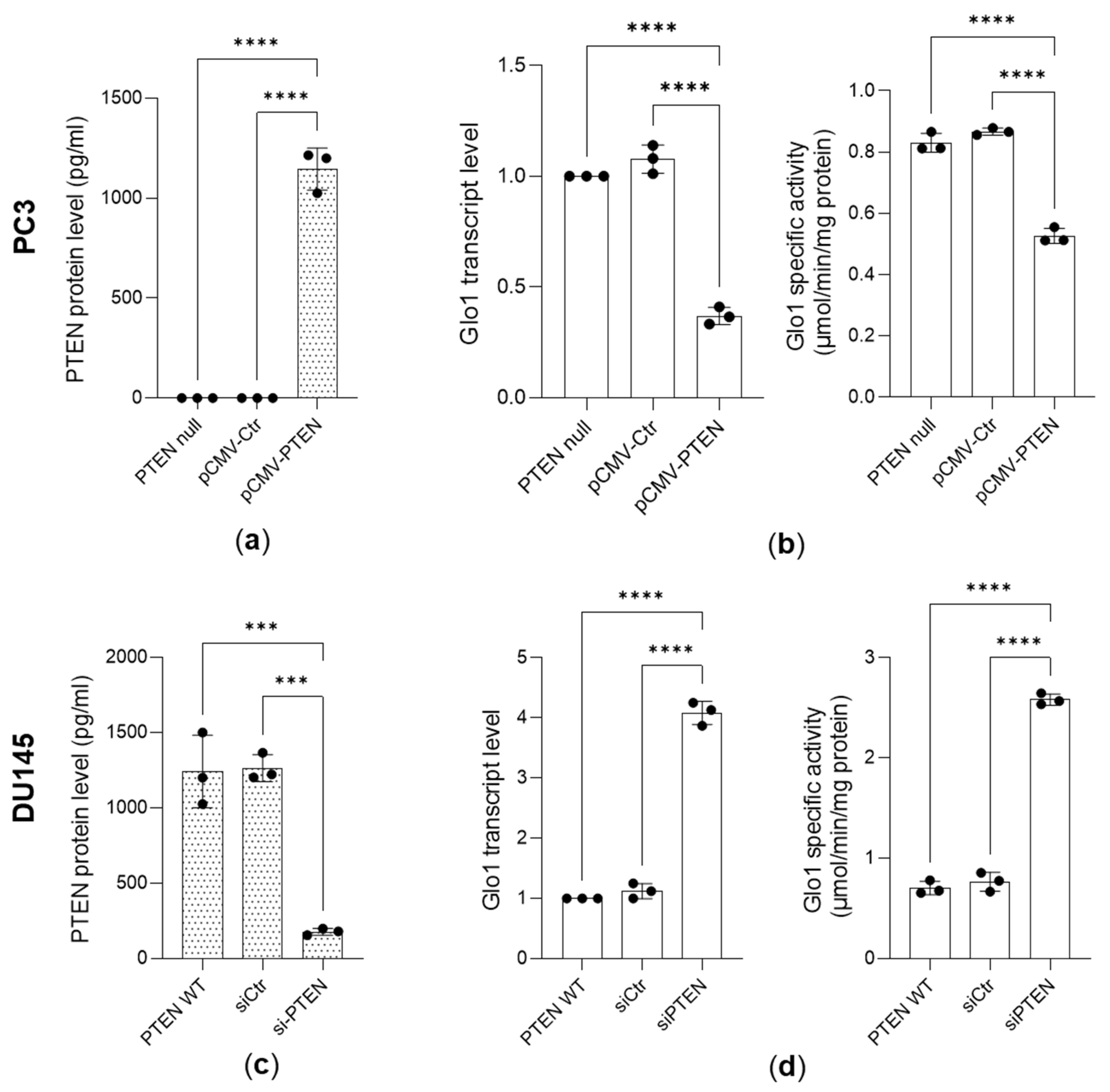
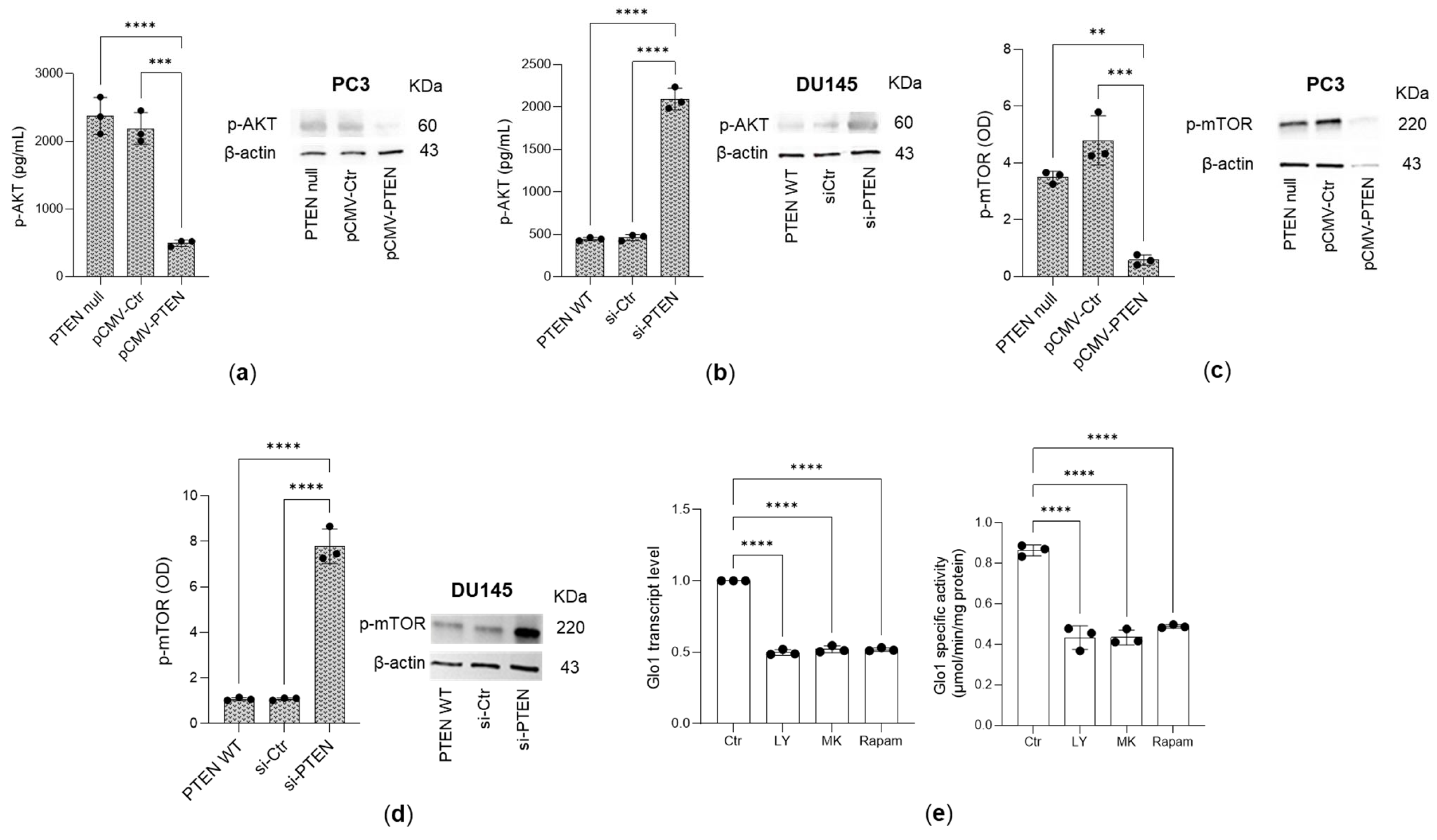
3.2. Glo 1 Upregulation Is Driven by the PTEN/PI3K/AKT/mTOR Pathway
3.3. Pyruvate Kinase (PK)M2 and Estrogen Receptor Alpha (ERα) Are Involved in PI3/AKT/mTOR Pathway-Triggered Glyoxalase 1 (Glo1) Upregulation
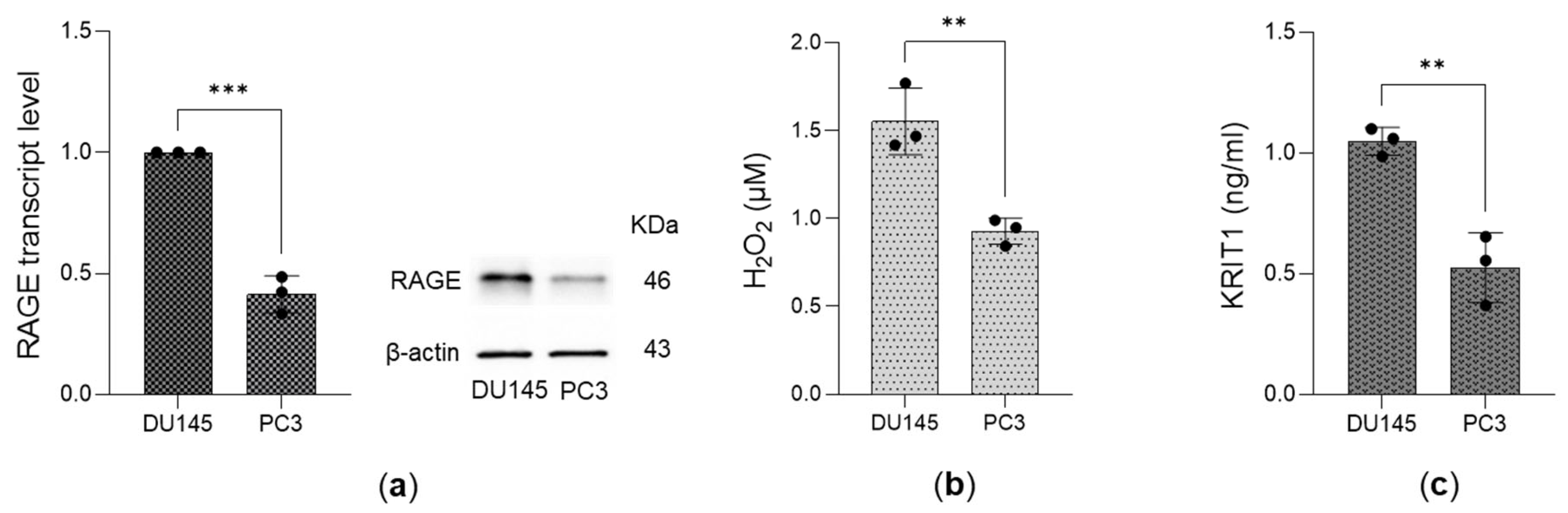
3.4. Expression of the Receptor for AGEs (RAGE), Levels of Hydrogen Peroxide (H2O2), and KRIT1 Expression in DU145 and PC3 Cells
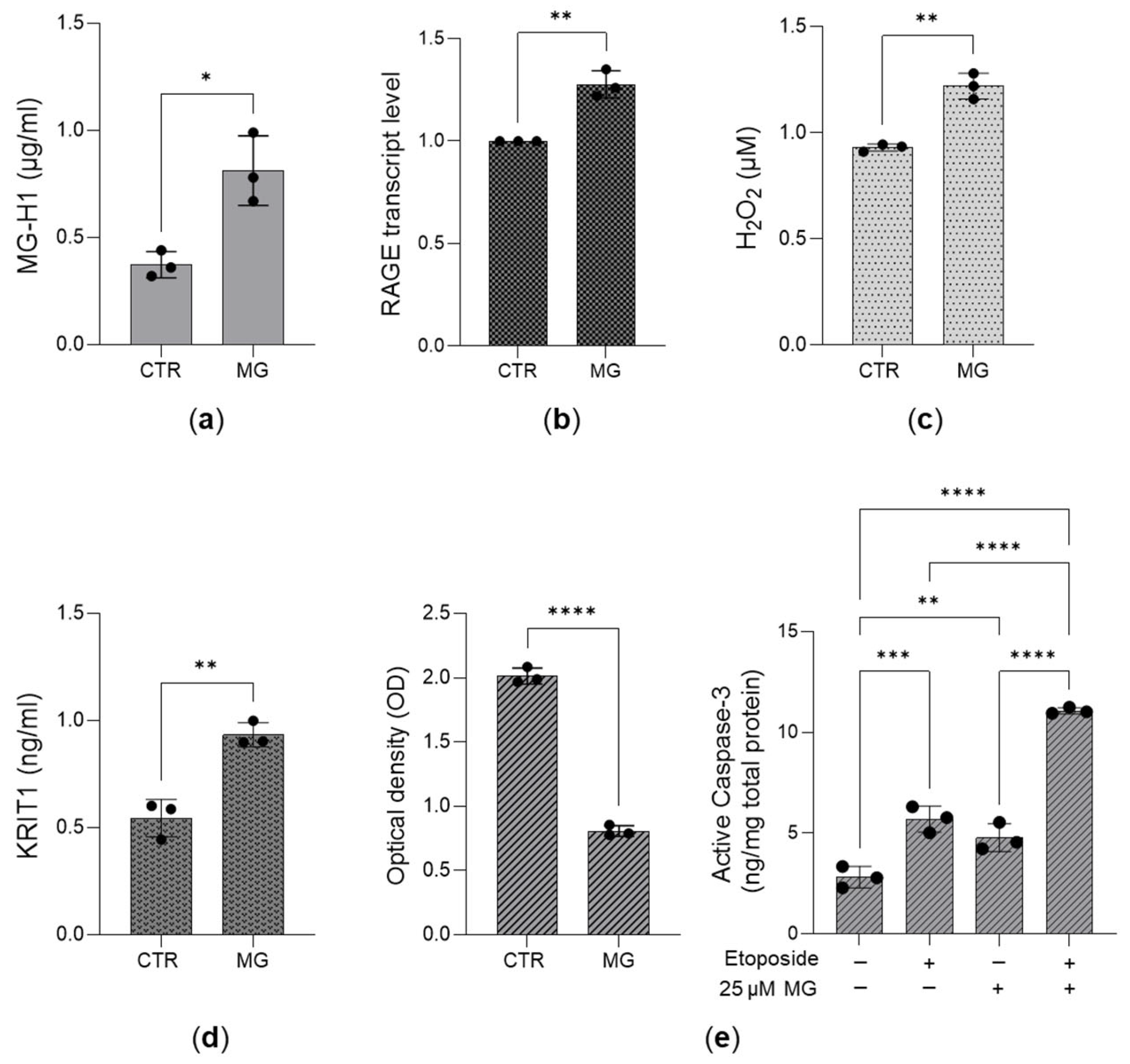
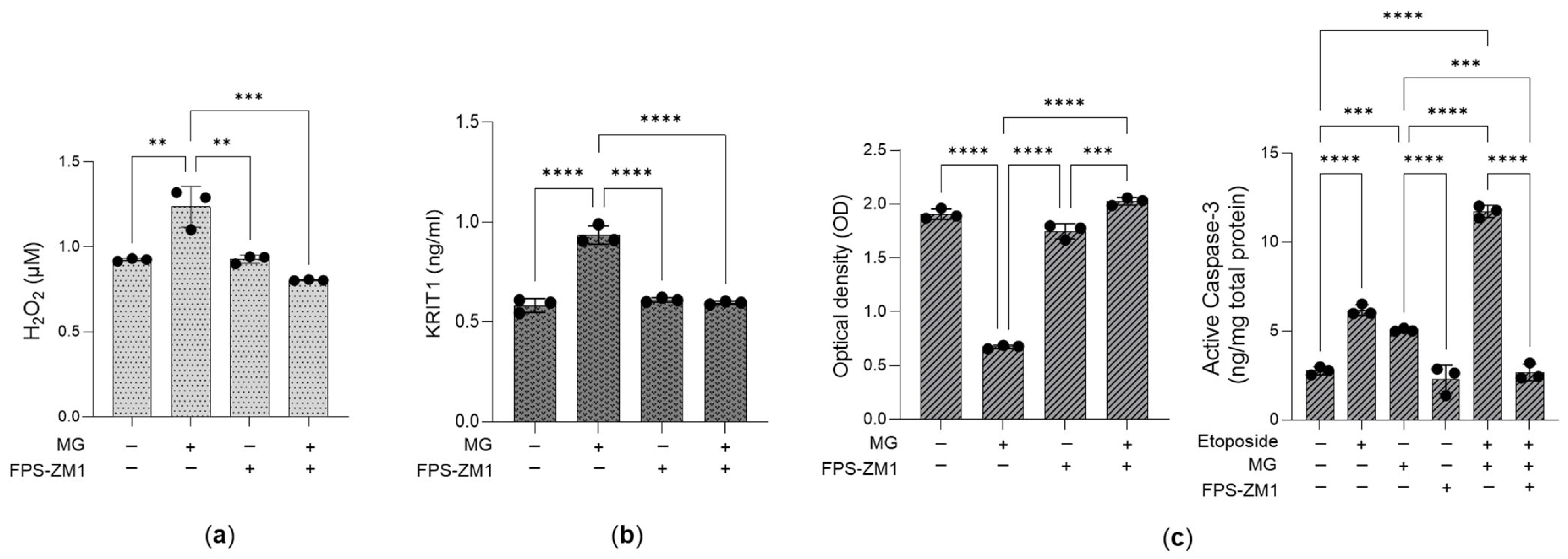
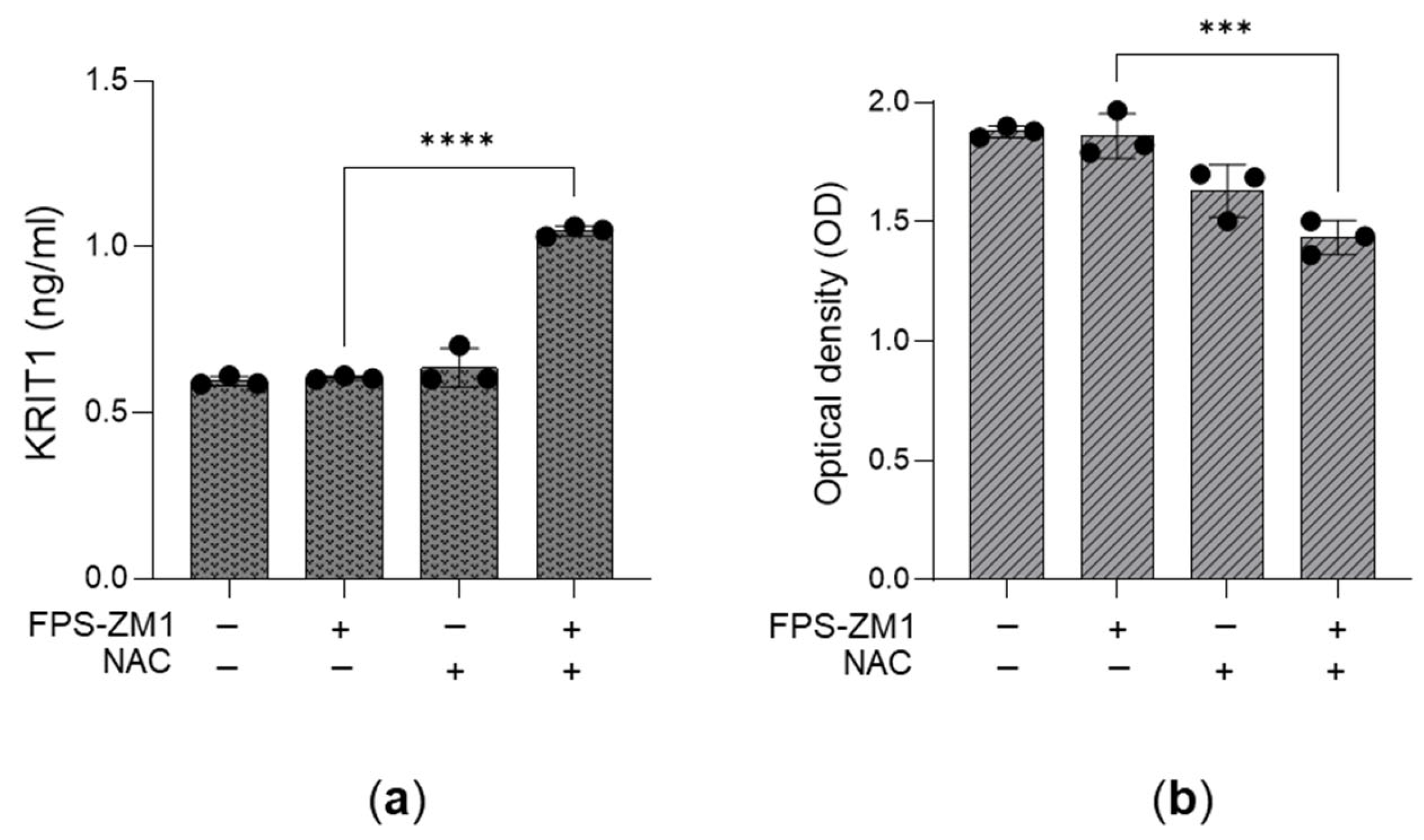
3.5. The Desensitization of the MG-H1/RAGE/H2O2/KRIT1 Axis Sustains the Growth of Aggressive PC3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Glo1 | Glyoxalase 1 |
| MG | Methylglyoxal |
| MG-H1 | MG-derived hydroimidazolone 1 |
| H2O2 | Hydrogen peroxide |
| KRIT1 | Krev interaction trapped 1 |
| PCa | Prostate cancer |
| AGEs | Advanced glycation end products |
| PTEN | Phosphatase and tensin homolog |
| PKM2 | Pyruvate kinase M2 |
| PEP | Phosphoenolpyruvate |
References
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.N.; Ponces Freire, A.; Cordeiro, C. The Glyoxalase Pathway: The First Hundred Years… and Beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef]
- Antognelli, C.; Talesa, V. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef]
- Nokin, M.-J.; Durieux, F.; Bellier, J.; Peulen, O.; Uchida, K.; Spiegel, D.A.; Cochrane, J.R.; Hutton, C.A.; Castronovo, V.; Bellahcène, A. Hormetic Potential of Methylglyoxal, a Side-Product of Glycolysis, in Switching Tumours from Growth to Death. Sci. Rep. 2017, 7, 11722. [Google Scholar] [CrossRef]
- Semchyshyn, H. Reactive Carbonyls Induce TOR- and Carbohydrate-Dependent Hormetic Response in Yeast. Sci. World J. 2020, 2020, 4275194. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Nigro, C.; Nicolò, A.; Prevenzano, I.; Formisano, P.; Beguinot, F.; Miele, C. The Dual-Role of Methylglyoxal in Tumor Progression—Novel Therapeutic Approaches. Front. Oncol. 2021, 11, 645686. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Zhang, M.; Liu, M. Dual Roles of Methylglyoxal in Cancer. Front. Oncol. 2025, 15, 1557162. [Google Scholar] [CrossRef]
- Anjaly, K.; Tiku, A.B. Exogenous Treatment of Caffeic Acid and Methylglyoxal Synergistically Enhances Anticancer Effect in Prostate Cancer via Inhibition of Glyoxalase-1. Prostate 2025, 85, 463–470. [Google Scholar] [CrossRef]
- Hsia, Y.-J.; Lin, Z.-M.; Zhang, T.; Chou, T.-C. Butyrate Increases Methylglyoxal Production through Regulation of the JAK2/Stat3/Nrf2/Glo1 Pathway in Castration-resistant Prostate Cancer Cells. Oncol. Rep. 2024, 51, 71. [Google Scholar] [CrossRef] [PubMed]
- Anaga, N.; Lekshmy, K.; Purushothaman, J. (+)-Catechin Mitigates Impairment in Insulin Secretion and Beta Cell Damage in Methylglyoxal-Induced Pancreatic Beta Cells. Mol. Biol. Rep. 2024, 51, 434. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xie, K.; Zheng, C.; Qiu, B.; Jiang, Z.; Luo, C.; Diao, Y.; Luo, J.; Yao, X.; Shen, Y. Exosomal MALAT1 Promotes the Proliferation of Esophageal Squamous Cell Carcinoma through Glyoxalase 1-Dependent Methylglyoxal Removal. Noncoding RNA Res. 2024, 9, 330–340. [Google Scholar] [CrossRef]
- Antognelli, C.; Marinucci, L.; Frosini, R.; Macchioni, L.; Talesa, V.N. Metastatic Prostate Cancer Cells Secrete Methylglyoxal-Derived MG-H1 to Reprogram Human Osteoblasts into a Dedifferentiated, Malignant-like Phenotype: A Possible Novel Player in Prostate Cancer Bone Metastases. Int. J. Mol. Sci. 2021, 22, 10191. [Google Scholar] [CrossRef]
- Antognelli, C.; Mandarano, M.; Prosperi, E.; Sidoni, A.; Talesa, V.N. Glyoxalase-1-Dependent Methylglyoxal Depletion Sustains PD-L1 Expression in Metastatic Prostate Cancer Cells: A Novel Mechanism in Cancer Immunosurveillance Escape and a Potential Novel Target to Overcome PD-L1 Blockade Resistance. Cancers 2021, 13, 2965. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, P.; Selke, P.; Scheller, C.; Strauss, C.; Horstkorte, R.; Leisz, S.; Scheer, M. Glycation Leads to Increased Invasion of Glioblastoma Cells. Cells 2023, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Wang, Z.; Wang, J. Role of the Glyoxalase System in Breast Cancer and Gynecological Cancer-Implications for Therapeutic Intervention: A Review. Front. Oncol. 2022, 12, 857746. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Rabbani, N. Glyoxalase in Tumourigenesis and Multidrug Resistance. Semin. Cell Dev. Biol. 2011, 22, 318–325. [Google Scholar] [CrossRef]
- Romaldi, B.; Scirè, A.; Minnelli, C.; Frontini, A.; Casari, G.; Cianfruglia, L.; Mobbili, G.; De Bari, L.; Antognelli, C.; Pallardó, F.V.; et al. Overexpression of Glyoxalase 2 in Human Breast Cancer Cells: Implications for Cell Proliferation and Doxorubicin Resistance. Int. J. Mol. Sci. 2024, 25, 10888. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, M. Glyoxalase System in Breast and Ovarian Cancers: Role of MEK/ERK/SMAD1 Pathway. Biomolecules 2024, 14, 584. [Google Scholar] [CrossRef]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Romaldi, B.; Laudadio, E.; Galeazzi, R.; Antognelli, C.; Armeni, T. Glyoxalase 2: Towards a Broader View of the Second Player of the Glyoxalase System. Antioxidants 2022, 11, 2131. [Google Scholar] [CrossRef]
- Manfredelli, D.; Armeni, T.; de Bari, L.; Scirè, A.; Talesa, V.N.; Antognelli, C.; Pariano, M. Acetylcholine Sustains LNCaP Prostate Cancer Cell Migration, Invasion and Proliferation Through Glyoxalase 1/MG-H1 Axis with the Involvement of Osteopontin. Int. J. Mol. Sci. 2025, 26, 4107. [Google Scholar] [CrossRef]
- Moradpour, Z.; Barik, A.; Jorjani, G.; Taherian, M.R.; Tousizadeh, S.; Halimi, A.; Soleimani, Y.; Karimian, M.; Khavari, T.; Kalankari, F.A.; et al. Prostate Cancer Incidence and Mortality Linked to Metalworking Fluid Exposure: A Systematic Review and Meta-Analysis. Front. Oncol. 2024, 14, 1491159. [Google Scholar] [CrossRef]
- Maekawa, S.; Takata, R.; Obara, W. Molecular Mechanisms of Prostate Cancer Development in the Precision Medicine Era: A Comprehensive Review. Cancers 2024, 16, 523. [Google Scholar] [CrossRef]
- Kulac, I.; Roudier, M.P.; Haffner, M.C. Molecular Pathology of Prostate Cancer. Clin. Lab. Med. 2024, 44, 161–180. [Google Scholar] [CrossRef]
- Rounds, L.; Nagle, R.B.; Muranyi, A.; Jandova, J.; Gill, S.; Vela, E.; Wondrak, G.T. Glyoxalase 1 Expression as a Novel Diagnostic Marker of High-Grade Prostatic Intraepithelial Neoplasia in Prostate Cancer. Cancers 2021, 13, 3608. [Google Scholar] [CrossRef]
- Bergez-Hernández, F.; Irigoyen-Arredondo, M.; Martínez-Camberos, A. A Systematic Review of Mechanisms of PTEN Gene Down-Regulation Mediated by miRNA in Prostate Cancer. Heliyon 2024, 10, e34950. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Oyovwi, M.O.; Akano, O.P.; Akanbi, G.B.; Adisa, F.B. Molecular Pathways in Reproductive Cancers: A Focus on Prostate and Ovarian Cancer. Cancer Cell Int. 2025, 25, 33. [Google Scholar] [CrossRef]
- Wali, A.F.; Talath, S.; El Tanani, M.; Rashid Rangraze, I.; Babiker, R.; Shafi, S.; Bansal, R. PI3K/AKT/mTOR Pathway in Breast Cancer Pathogenesis and Therapy: Insights into Phytochemical-Based Therapeutics. Nutr. Cancer 2025, 77, 938–958. [Google Scholar] [CrossRef]
- Maxwell, P.J.; Neisen, J.; Messenger, J.; Waugh, D.J.J. Tumor-Derived CXCL8 Signaling Augments Stroma-Derived CCL2-Promoted Proliferation and CXCL12-Mediated Invasion of PTEN-Deficient Prostate Cancer Cells. Oncotarget 2014, 5, 4895–4908. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, M.E.; Soung, P.; Perera, S.; Kaplan, I.; Loda, M.; Sellers, W.R. Loss of PTEN Expression in Paraffin-Embedded Primary Prostate Cancer Correlates with High Gleason Score and Advanced Stage. Cancer Res. 1999, 59, 4291–4296. [Google Scholar] [PubMed]
- Wu, S.; Cao, R.; Tao, B.; Wu, P.; Peng, C.; Gao, H.; Liang, J.; Yang, W. Pyruvate Facilitates FACT-Mediated γH2AX Loading to Chromatin and Promotes the Radiation Resistance of Glioblastoma. Adv. Sci. 2022, 9, e2104055. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.K.; Voss, D.M.; Scharner, J.; Costa, A.S.H.; Lin, K.-T.; Jeon, H.Y.; Wilkinson, J.E.; Jackson, M.; Rigo, F.; Bennett, C.F.; et al. ASO-Based PKM Splice-Switching Therapy Inhibits Hepatocellular Carcinoma Growth. Cancer Res. 2022, 82, 900–915. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zheng, J.; Song, J.; Liu, Y.; Ruan, X.; Shen, S.; Shao, L.; Yang, C.; Wang, D.; et al. Lin28A Promotes IRF6-Regulated Aerobic Glycolysis in Glioma Cells by Stabilizing SNHG14. Cell Death Dis. 2020, 11, 447. [Google Scholar] [CrossRef]
- Jemal, M.; Getinet, M.; Amare, G.A.; Tegegne, B.A.; Baylie, T.; Mengistu, E.F.; Osman, E.E.; Chura Waritu, N.; Adugna, A. Non-Metabolic Enzyme Function of Pyruvate Kinase M2 in Breast Cancer. Front. Oncol. 2024, 14, 1450325. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wu, S.; Pan, Q.; Zhang, Y.; He, L.; Yao, Q.; Chen, J.; Li, J.; Xu, Y. CHAC1 Blockade Suppresses Progression of Lung Adenocarcinoma by Interfering with Glucose Metabolism via Hijacking PKM2 Nuclear Translocation. Cell Death Dis. 2024, 15, 728. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, Z.; Li, S.; Luo, Y. Non-Metabolic Enzyme Function of PKM2 in Hepatocellular Carcinoma: A Review. Medicine 2023, 102, e35571. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Emerging Roles of PKM2 in Cell Metabolism and Cancer Progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, S.; Yeh, Y.; Shi, Y.; Henderson, N.; Price, D.; Gu, X.; Yu, X. The Expression of PKM1 and PKM2 in Developing, Benign, and Cancerous Prostatic Tissues. Front. Oncol. 2024, 14, 1392085. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, J.; Finetti, F.; Woodby, B.; Belmonte, G.; Miracco, C.; Valacchi, G.; Trabalzini, L. KRIT1 as a Possible New Player in Melanoma Aggressiveness. Arch. Biochem. Biophys. 2020, 691, 108483. [Google Scholar] [CrossRef]
- Cici, M.; Dilmac, S.; Aytac, G.; Tanriover, G. Cerebral Cavernous Malformation Proteins, CCM1, CCM2 and CCM3, Are Decreased in Metastatic Lesions in a Murine Breast Carcinoma Model. Biotech. Histochem. 2024, 99, 76–83. [Google Scholar] [CrossRef]
- Glading, A.J. KRIT1 in Vascular Biology and Beyond. Biosci. Rep. 2024, 44, BSR20231675. [Google Scholar] [CrossRef]
- Waltregny, D.; Bellahcène, A.; Van Riet, I.; Fisher, L.W.; Young, M.; Fernandez, P.; Dewé, W.; de Leval, J.; Castronovo, V. Prognostic Value of Bone Sialoprotein Expression in Clinically Localized Human Prostate Cancer. J. Natl. Cancer Inst. 1998, 90, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine Induces Apoptosis in Human Osteoblasts via a Novel Mechanism Driven by H2O2 and Entailing Glyoxalase 1-Dependent MG-H1 Accumulation Leading to TG2-Mediated NF-kB Desensitization: Implication for Smokers-Related Osteoporosis. Free Radic. Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Ge, Q.; Yan, Z.; Tian, Q.; Zhang, J.; Li, J.; Cai, F.; Zhang, L.; Zhu, Y. Xanthohumol Regulates Mitophagy in Osteosarcoma Cells via AMPK-ULK1-FUNDC1 Signaling Pathway. Phytother. Res. 2025, 39, 2393–2406. [Google Scholar] [CrossRef]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR Signaling in Prostate Cancer: Still a Potential Druggable Target? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Ma, J.; Peng, H.; Wang, F.; Zha, X.; Wang, Y.; Jing, Y.; Yang, H.; Chen, R.; et al. Mammalian Target of Rapamycin Up-Regulation of Pyruvate Kinase Isoenzyme Type M2 Is Critical for Aerobic Glycolysis and Tumor Growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4129–4134. [Google Scholar] [CrossRef]
- Ma, A.; Yang, Z.; He, Q.; Wang, W.; Ren, H.; Zhai, C.; Lan, J. Glyphosate Targets FYN to Regulate Glycolysis and Promote Glioblastoma Proliferation: A Network Toxicology Study. Int. J. Biol. Macromol. 2025, 321, 146486. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 Splice Isoform of Pyruvate Kinase Is Important for Cancer Metabolism and Tumour Growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Wong, N.; Yan, J.; Ojo, D.; De Melo, J.; Cutz, J.-C.; Tang, D. Changes in PKM2 Associate with Prostate Cancer Progression. Cancer Investig. 2014, 32, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Taddei, M.L.; Morandi, A.; Comito, G.; Calvani, M.; Bianchini, F.; Richichi, B.; Raugei, G.; Wong, N.; Tang, D.; et al. Targeting Stromal-Induced Pyruvate Kinase M2 Nuclear Translocation Impairs Oxphos and Prostate Cancer Metastatic Spread. Oncotarget 2015, 6, 24061–24074. [Google Scholar] [CrossRef]
- Chakravarty, D.; Sboner, A.; Nair, S.S.; Giannopoulou, E.; Li, R.; Hennig, S.; Mosquera, J.M.; Pauwels, J.; Park, K.; Kossai, M.; et al. The Oestrogen Receptor Alpha-Regulated lncRNA NEAT1 Is a Critical Modulator of Prostate Cancer. Nat. Commun. 2014, 5, 5383. [Google Scholar] [CrossRef]
- Xue, J.; Ray, R.; Singer, D.; Böhme, D.; Burz, D.S.; Rai, V.; Hoffmann, R.; Shekhtman, A. The Receptor for Advanced Glycation End Products (RAGE) Specifically Recognizes Methylglyoxal-Derived AGEs. Biochemistry 2014, 53, 3327–3335. [Google Scholar] [CrossRef]
- Antognelli, C.; Perrelli, A.; Armeni, T.; Nicola Talesa, V.; Retta, S.F. Dicarbonyl Stress and S-Glutathionylation in Cerebrovascular Diseases: A Focus on Cerebral Cavernous Malformations. Antioxidants 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, Y.; Zeng, Z.; Yin, Q.; Hong, Y.; Hou, X.; Liu, X. RAGE-Specific Inhibitor FPS-ZM1 Attenuates AGEs-Induced Neuroinflammation and Oxidative Stress in Rat Primary Microglia. Neurochem. Res. 2017, 42, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Shen, C.; Yin, Q.; Sun, M.; Ma, Y.; Liu, X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem. Res. 2016, 41, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Ji, Q.; An, P.; Wang, X.; Ju, Y.; Li, R.; Ruan, Y.; Zhao, J.; Cao, M.; et al. Supplementation with N-Acetyl-L-Cysteine during in Vitro Maturation Improves Goat Oocyte Developmental Competence by Regulating Oxidative Stress. Theriogenology 2025, 235, 221–230. [Google Scholar] [CrossRef]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 Is a Novel Regulator of the Epithelial to Mesenchymal Transition (EMT) in Prostate Cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-Kinase Pathway in Cancer. Annu. Rev. Pathol. 2009, 4, 127–150. [Google Scholar] [CrossRef]
- Choudhury, A.D. PTEN-PI3K Pathway Alterations in Advanced Prostate Cancer and Clinical Implications. Prostate 2022, 82 (Suppl. S1), S60–S72. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Guo, W.; Zhang, Z.; Zhao, F.; Zhao, Y.; Jia, D.; Ding, J.; Wang, H.; Yao, M.; et al. TRIM35 Interacts with Pyruvate Kinase Isoform M2 to Suppress the Warburg Effect and Tumorigenicity in Hepatocellular Carcinoma. Oncogene 2015, 34, 3946–3956. [Google Scholar] [CrossRef]
- Kumar, B.; Bamezai, R.N.K. Moderate DNA Damage Promotes Metabolic Flux into PPP via PKM2 Y-105 Phosphorylation: A Feature That Favours Cancer Cells. Mol. Biol. Rep. 2015, 42, 1317–1321. [Google Scholar] [CrossRef]
- Hitosugi, T.; Kang, S.; Vander Heiden, M.G.; Chung, T.-W.; Elf, S.; Lythgoe, K.; Dong, S.; Lonial, S.; Wang, X.; Chen, G.Z.; et al. Tyrosine Phosphorylation Inhibits PKM2 to Promote the Warburg Effect and Tumor Growth. Sci. Signal 2009, 2, ra73. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Yang, J.J.; Liu, X.; Liu, Z.-R. Pyruvate Kinase M2 Regulates Gene Transcription by Acting as a Protein Kinase. Mol. Cell 2012, 45, 598–609. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Yang, J.J.; Chen, J.; Jie, J.; Li, L.; Zhang, Y.; Liu, Z.-R. Reciprocal Regulation of Protein Kinase and Pyruvate Kinase Activities of Pyruvate Kinase M2 by Growth Signals. J. Biol. Chem. 2013, 288, 15971–15979. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Mohammad, M.A.; Diaz-Arrastia, C.R.; Kamel, M.W.; Kilic, G.S.; Ndofor, B.T.; Abdel-Baki, M.S.; Theiler, S.K. Estradiol-17β Upregulates Pyruvate Kinase M2 Expression to Coactivate Estrogen Receptor-α and to Integrate Metabolic Reprogramming with the Mitogenic Response in Endometrial Cells. J. Clin. Endocrinol. Metab. 2014, 99, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, I.; Lawrence, M.G.; Balanathan, P.; Rebello, R.; Pearson, H.B.; Garg, E.; Pedersen, J.; Pouliot, N.; Nadon, R.; Watt, M.J.; et al. Estrogen Receptor Alpha Drives Proliferation in PTEN-Deficient Prostate Carcinoma by Stimulating Survival Signaling, MYC Expression and Altering Glucose Sensitivity. Oncotarget 2015, 6, 604–616. [Google Scholar] [CrossRef]
- Semenas, J.; Wang, T.; Sajid Syed Khaja, A.; Firoj Mahmud, A.; Simoulis, A.; Grundström, T.; Fällman, M.; Persson, J.L. Targeted Inhibition of ERα Signaling and PIP5K1α/Akt Pathways in Castration-Resistant Prostate Cancer. Mol. Oncol. 2021, 15, 968–986. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Zhang, L.; Hu, Q.; Guo, Y.; Jiang, H.; Shi, S.; Zhang, X. Association of Estrogen Receptor α PvuII and XbaI Polymorphisms with Prostate Cancer Susceptibility and Risk Stratification: A Meta-Analysis from Case-Control Studies. Onco Targets Ther. 2017, 10, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt Pathway in Human Cancer: Rationale and Promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; de Oliveira, M.G.; Mónica, F.Z.; Antunes, E. Methylglyoxal and Advanced Glycation End Products (AGEs): Targets for the Prevention and Treatment of Diabetes-Associated Bladder Dysfunction? Biomedicines 2024, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-K.; Jeong, S.-R.; Pyo, M.C.; Ha, S.-K.; Nam, M.-H.; Lee, K.-W. Methylglyoxal-Derived Advanced Glycation End Products (AGE4) Promote Cell Proliferation and Survival in Renal Cell Carcinoma Cells through the RAGE/Akt/ERK Signaling Pathways. Biol. Pharm. Bull. 2021, 44, 1697–1706. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Perrelli, A.; Fornelli, C.; Magini, A.; Gorbi, S.; Salzano, A.M.; Antognelli, C.; Retta, F.; Benedetti, V.; Cassoni, P.; et al. KRIT1 Loss-Of-Function Associated with Cerebral Cavernous Malformation Disease Leads to Enhanced S-Glutathionylation of Distinct Structural and Regulatory Proteins. Antioxidants 2019, 8, 27. [Google Scholar] [CrossRef]
- He, Q.; Ye, A.; Ye, W.; Liao, X.; Qin, G.; Xu, Y.; Yin, Y.; Luo, H.; Yi, M.; Xian, L.; et al. Cancer-Secreted Exosomal miR-21-5p Induces Angiogenesis and Vascular Permeability by Targeting KRIT1. Cell Death Dis. 2021, 12, 576. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredelli, D.; Torcoli, C.; Pariano, M.; Bellezza, G.; Baroni, T.; Talesa, V.N.; Sidoni, A.; Antognelli, C. PTEN/PKM2/ERα-Driven Glyoxalase 1 Overexpression Sustains PC3 Prostate Cancer Cell Growth Through MG-H1/RAGE Pathway Desensitization Leading to H2O2-Dependent KRIT1 Downregulation. Antioxidants 2025, 14, 1120. https://doi.org/10.3390/antiox14091120
Manfredelli D, Torcoli C, Pariano M, Bellezza G, Baroni T, Talesa VN, Sidoni A, Antognelli C. PTEN/PKM2/ERα-Driven Glyoxalase 1 Overexpression Sustains PC3 Prostate Cancer Cell Growth Through MG-H1/RAGE Pathway Desensitization Leading to H2O2-Dependent KRIT1 Downregulation. Antioxidants. 2025; 14(9):1120. https://doi.org/10.3390/antiox14091120
Chicago/Turabian StyleManfredelli, Dominga, Camilla Torcoli, Marilena Pariano, Guido Bellezza, Tiziano Baroni, Vincenzo N. Talesa, Angelo Sidoni, and Cinzia Antognelli. 2025. "PTEN/PKM2/ERα-Driven Glyoxalase 1 Overexpression Sustains PC3 Prostate Cancer Cell Growth Through MG-H1/RAGE Pathway Desensitization Leading to H2O2-Dependent KRIT1 Downregulation" Antioxidants 14, no. 9: 1120. https://doi.org/10.3390/antiox14091120
APA StyleManfredelli, D., Torcoli, C., Pariano, M., Bellezza, G., Baroni, T., Talesa, V. N., Sidoni, A., & Antognelli, C. (2025). PTEN/PKM2/ERα-Driven Glyoxalase 1 Overexpression Sustains PC3 Prostate Cancer Cell Growth Through MG-H1/RAGE Pathway Desensitization Leading to H2O2-Dependent KRIT1 Downregulation. Antioxidants, 14(9), 1120. https://doi.org/10.3390/antiox14091120






