Inhibitory Infrared Light Attenuates Mitochondrial Hyperactivity and Accelerates Restoration of Mitochondrial Homeostasis in an Oxygen–Glucose Deprivation/Reoxygenation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
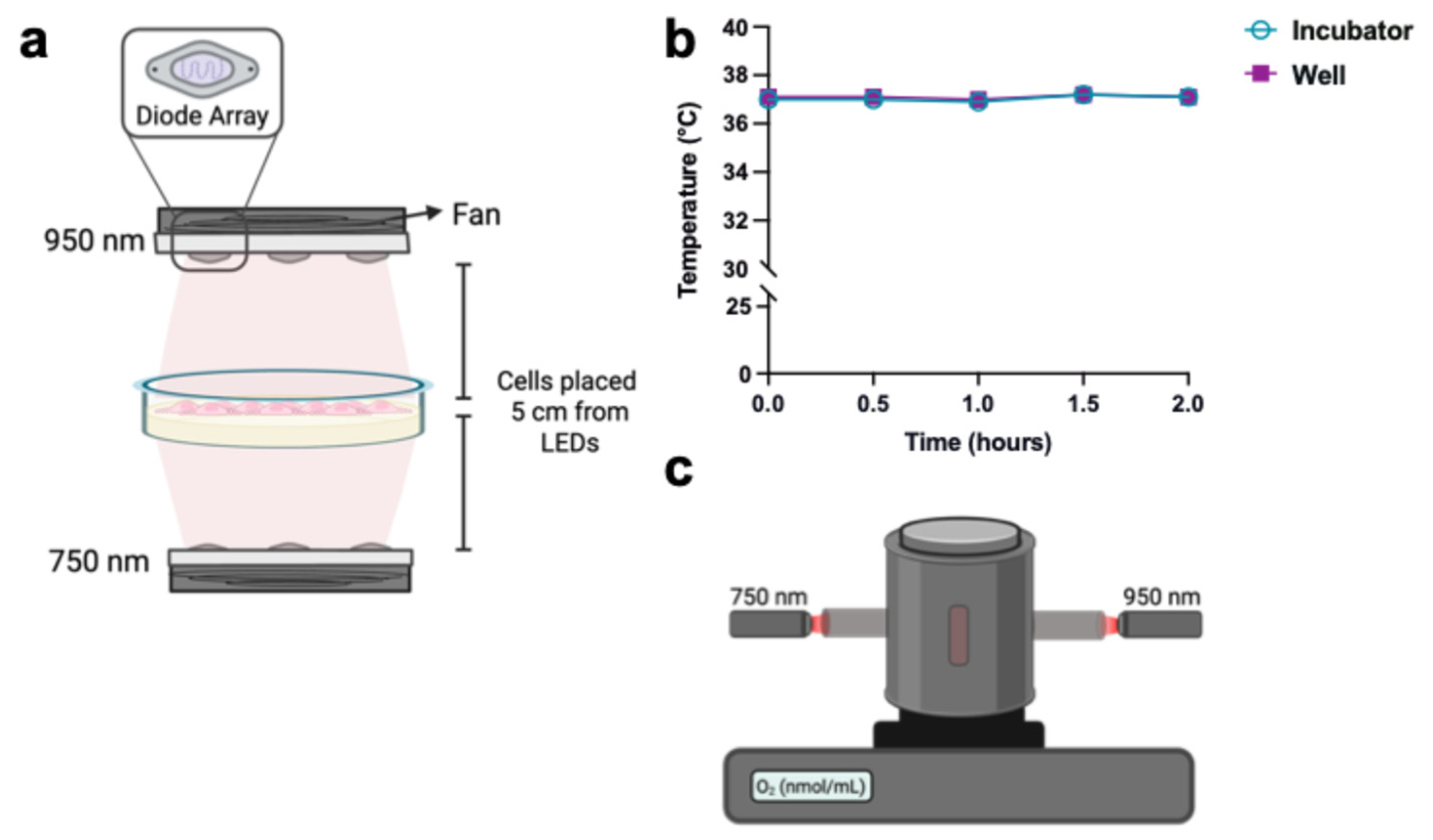
2.2. Oxygen–Glucose Deprivation/Reoxygenation (OGD/R) and IRL Treatment
2.3. Mitochondrial Membrane Potential (ΔΨm) and Superoxide Measurement
2.4. Cytochrome c Oxidase Activity Measurement
2.5. Live Cell Oxygen Consumption Rate Measurements
2.6. Western Blotting
2.7. Phosphorylated Protein Detection Using ProQ Diamond Phosphoprotein Stain
2.8. Annexin V/Propidium Iodide Staining and Fluorescence-Activated Cell Sorting
2.9. Statistical Analysis
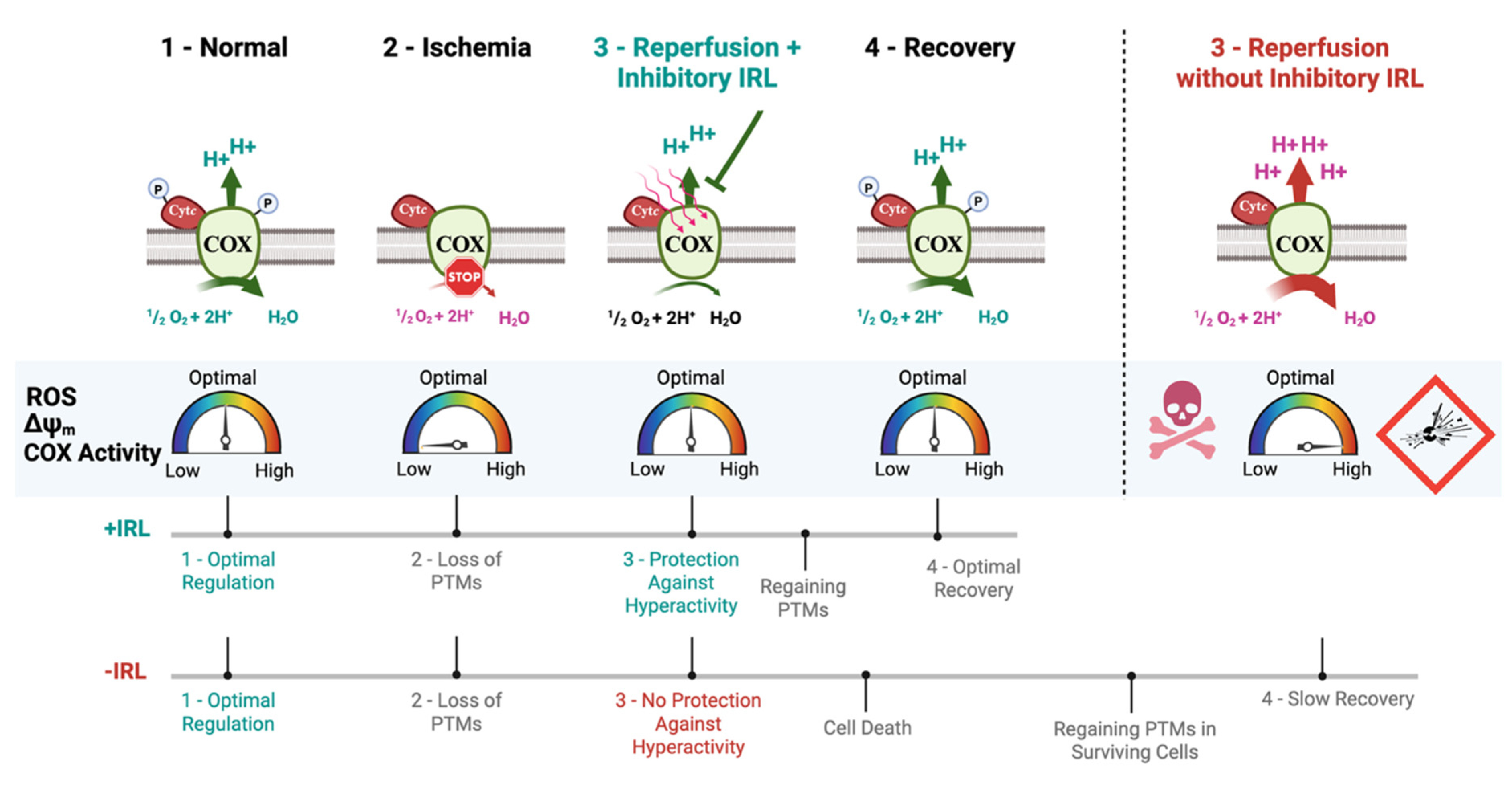
3. Results
3.1. Oxygen–Glucose Deprivation Leads to a Decrease in Mitochondrial Protein Phosphorylation and Increased COX Activity
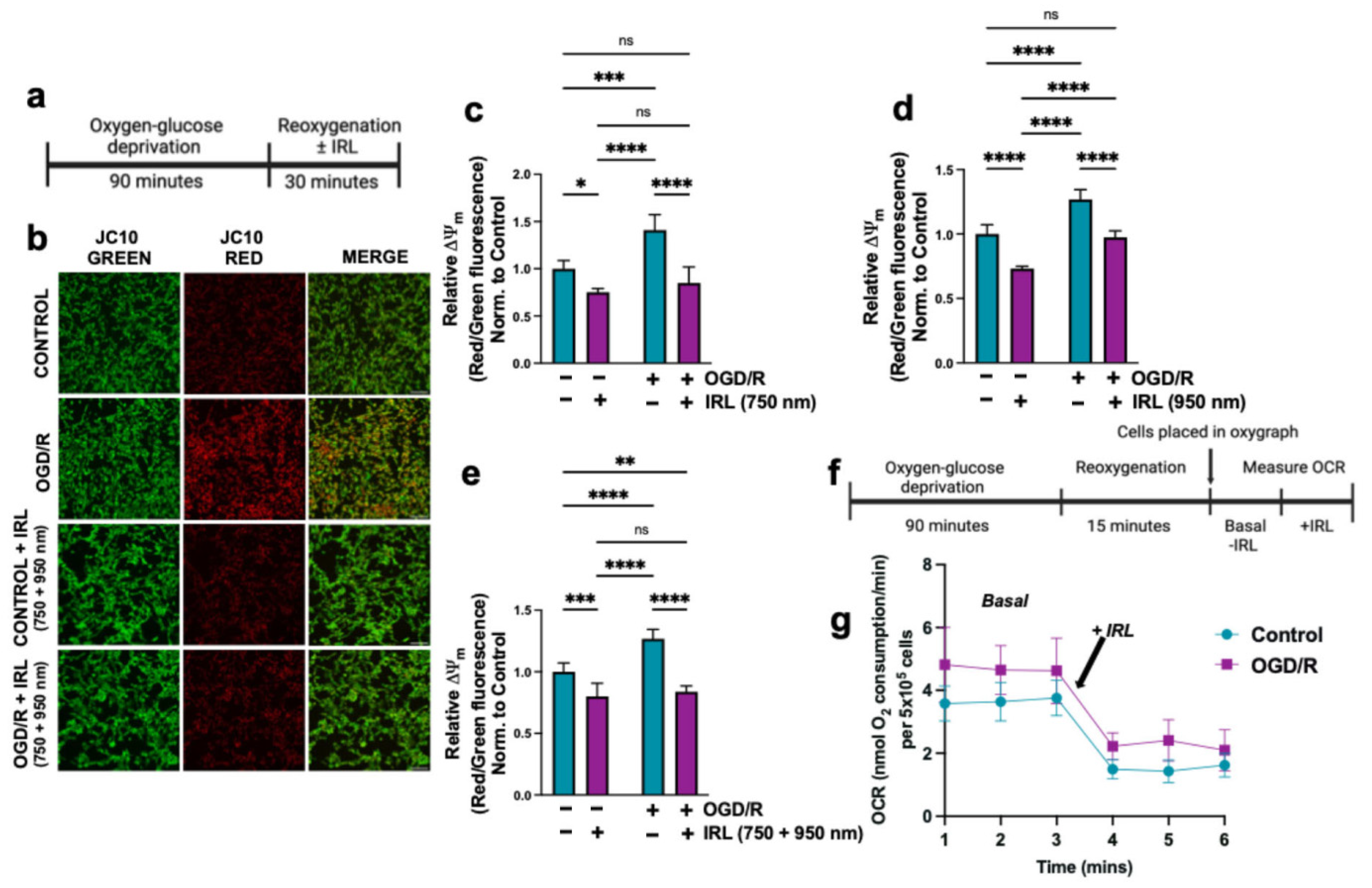
3.2. Inhibitory Infrared Light Treatment Prevents ΔΨm Hyperpolarization and Suppresses Mitochondrial Superoxide Production During Early Reperfusion
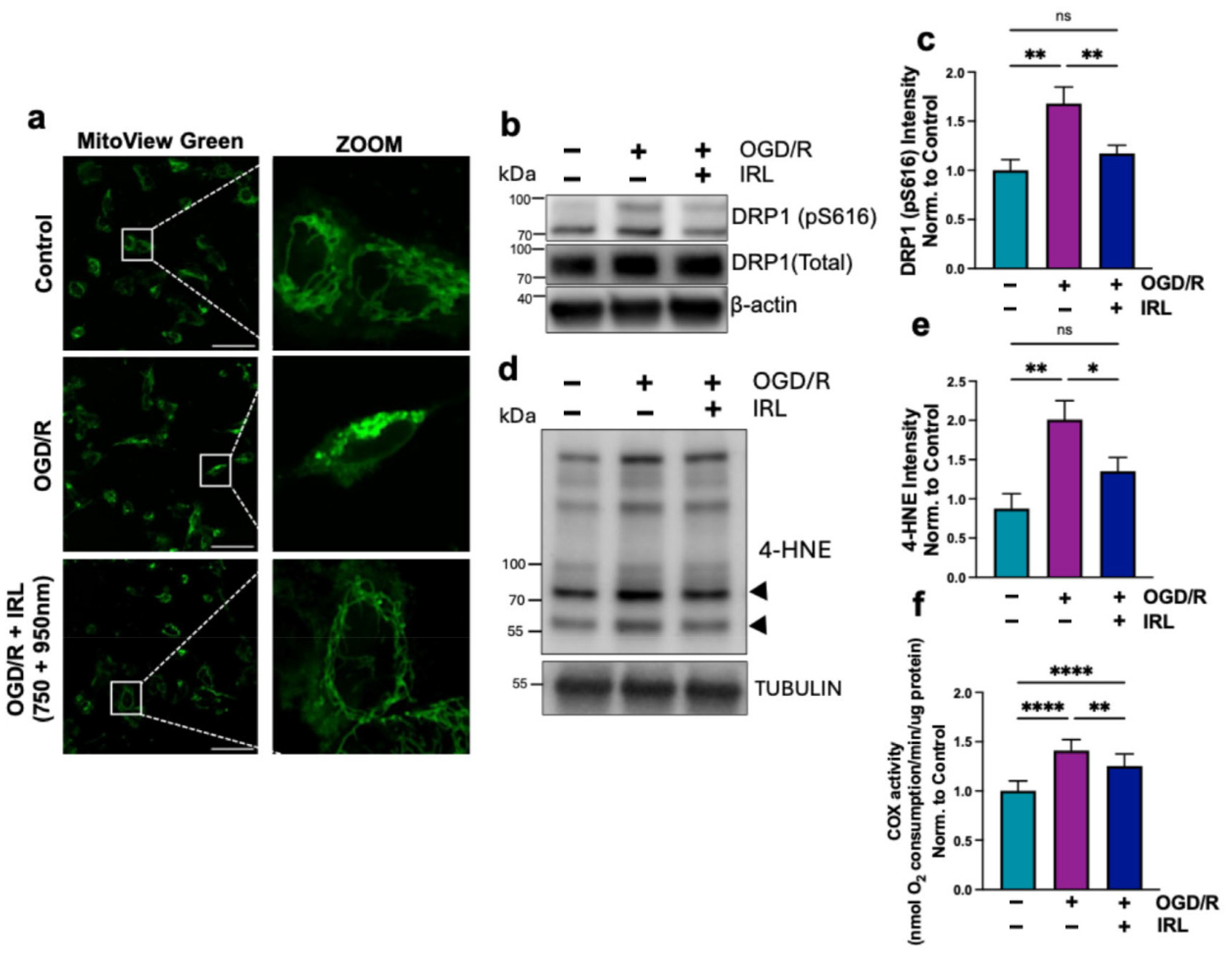
3.3. Inhibitory IRL Prevents Mitochondrial Fragmentation and Oxidative Damage
3.4. A 2 h Inhibitory IRL Treatment Allows Restoration of COX Activity Sooner
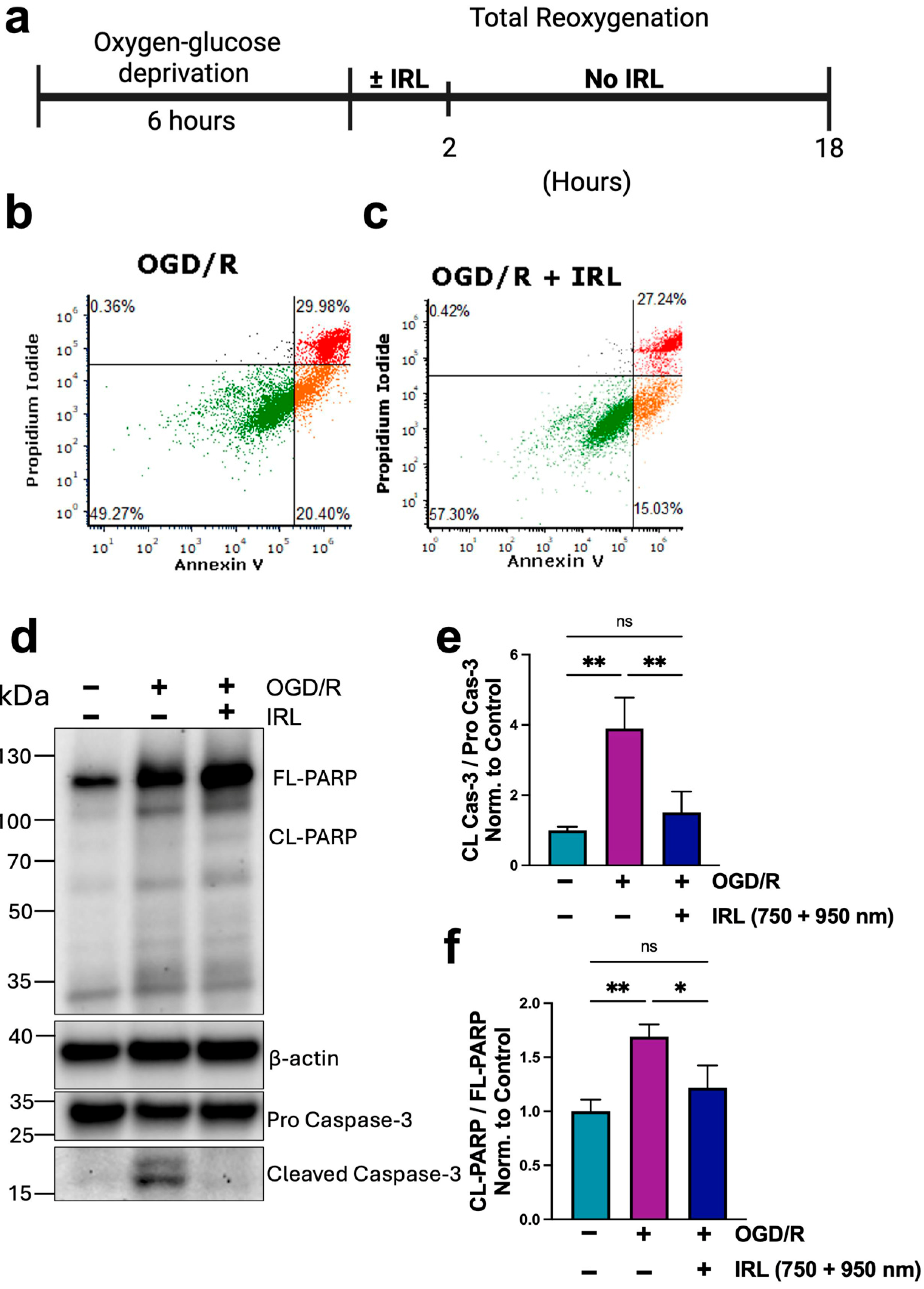
3.5. Inhibitory IRL Treatment Decreases Cell Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | Cytochrome c oxidase |
| Cytc | Cytochrome c |
| ROS | Reactive oxygen species |
| OGD/R | Oxygen–glucose deprivation/reoxygenation |
| I/R | Ischemia/reperfusion |
| IRL | Infrared light |
| ETC | Electron transport chain |
| PTM | Post-translation modifications |
| OCR | Oxygen consumption rate |
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [CrossRef]
- Morse, P.T.; Wan, J.; Bell, J.; Lee, I.; Goebel, D.J.; Malek, M.H.; Sanderson, T.H.; Hüttemann, M. Sometimes less is more: Inhibitory infrared light during early reperfusion calms hyperactive mitochondria and suppresses reperfusion injury. Biochem. Soc. Trans. 2022, 50, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S. Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain--superoxide generating and cycling mechanisms in mitochondria. J. Bioenerg. Biomembr. 1999, 31, 367–376. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- Liu, S.S. Mitochondrial Q cycle-derived superoxide and chemiosmotic bioenergetics. Ann. N. Y. Acad. Sci. 2010, 1201, 84–95. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Rottenberg, H.; Covian, R.; Trumpower, B.L. Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J. Biol. Chem. 2009, 284, 19203–19210. [Google Scholar] [CrossRef]
- Kaim, G.; Dimroth, P. ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J. 1999, 18, 4118–4127. [Google Scholar] [CrossRef]
- Dalmonte, M.E.; Forte, E.; Genova, M.L.; Giuffre, A.; Sarti, P.; Lenaz, G. Control of respiration by cytochrome c oxidase in intact cells: Role of the membrane potential. J. Biol. Chem. 2009, 284, 32331–32335. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E.; Lassnig, B.; Kuznetsov, A.; Rieger, G.; Margreiter, R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 1998, 201, 1129–1139. [Google Scholar] [CrossRef]
- Kunz, W.S.; Kudin, A.; Vielhaber, S.; Elger, C.E.; Attardi, G.; Villani, G. Flux control of cytochrome c oxidase in human skeletal muscle. J. Biol. Chem. 2000, 275, 27741–27745. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Scrima, R.; Boffoli, D.; Capitanio, N. Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem. J. 2006, 396, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Villani, G.; Attardi, G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc. Natl. Acad. Sci. USA 1997, 94, 1166–1171. [Google Scholar] [CrossRef]
- Wiedemann, F.R.; Kunz, W.S. Oxygen dependence of flux control of cytochrome c oxidase—Implications for mitochondrial diseases. FEBS Lett. 1998, 422, 33–35. [Google Scholar] [CrossRef]
- Pham, L.; Arroum, T.; Wan, J.; Pavelich, L.; Bell, J.; Morse, P.T.; Lee, I.; Grossman, L.I.; Sanderson, T.H.; Malek, M.H.; et al. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease—Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024, 78, 103426. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Salomon, A.R.; Ficarro, S.; Mathes, I.; Lottspeich, F.; Grossman, L.I.; Hüttemann, M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J. Biol. Chem. 2005, 280, 6094–6100. [Google Scholar] [CrossRef]
- Lee, I.; Salomon, A.R.; Yu, K.; Doan, J.W.; Grossman, L.I.; Hüttemann, M. New prospects for an old enzyme: Mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry 2006, 45, 9121–9128. [Google Scholar] [CrossRef]
- Yu, H.; Lee, I.; Salomon, A.R.; Yu, K.; Hüttemann, M. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim. Biophys. Acta 2008, 1777, 1066–1071. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Mahapatra, G.; Pecina, P.; Ji, Q.; Yu, K.; Sinkler, C.; Varughese, A.; Kumar, R.; Bukowski, M.J.; Tousignant, R.N.; et al. Cytochrome C is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS ONE 2013, 8, e78627. [Google Scholar] [CrossRef]
- Prabu, S.K.; Anandatheerthavarada, H.K.; Raza, H.; Srinivasan, S.; Spear, J.F.; Avadhani, N.G. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J. Biol. Chem. 2006, 281, 2061–2070. [Google Scholar] [CrossRef]
- Zaidan, E.; Sims, N.R. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J. Neurochem. 1994, 63, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Kristian, T.; Pivovarova, N.B.; Fiskum, G.; Andrews, S.B. Calcium-induced precipitate formation in brain mitochondria: Composition, calcium capacity, and retention. J. Neurochem. 2007, 102, 1346–1356. [Google Scholar] [CrossRef]
- Puka-Sundvall, M.; Gajkowska, B.; Cholewinski, M.; Blomgren, K.; Lazarewicz, J.W.; Hagberg, H. Subcellular distribution of calcium and ultrastructural changes after cerebral hypoxia-ischemia in immature rats. Brain Res. Dev. Brain Res. 2000, 125, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S. Cardiac energy metabolism homeostasis: Role of cytosolic calcium. J. Mol. Cell Cardiol. 2002, 34, 1259–1271. [Google Scholar] [CrossRef]
- Robb-Gaspers, L.D.; Burnett, P.; Rutter, G.A.; Denton, R.M.; Rizzuto, R.; Thomas, A.P. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998, 17, 4987–5000. [Google Scholar] [CrossRef] [PubMed]
- Ankarcrona, M.; Dypbukt, J.M.; Orrenius, S.; Nicotera, P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996, 394, 321–324. [Google Scholar] [CrossRef]
- Hopper, R.K.; Carroll, S.; Aponte, A.M.; Johnson, D.T.; French, S.; Shen, R.F.; Witzmann, F.A.; Harris, R.A.; Balaban, R.S. Mitochondrial matrix phosphoproteome: Effect of extra mitochondrial calcium. Biochemistry 2006, 45, 2524–2536. [Google Scholar] [CrossRef]
- Bender, E.; Kadenbach, B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000, 466, 130–134. [Google Scholar] [CrossRef]
- Lee, I.; Bender, E.; Kadenbach, B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol. Cell. Biochem. 2002, 234–235, 63–70. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Vaishnav, A.; Liu, J.; Varughese, A.; Wan, J.; Turner, A.A.; Ji, Q.; Zurek, M.P.; Kapralov, A.A.; Kagan, V.E.; et al. Serine-47 phosphorylation of cytochrome c in the mammalian brain regulates cytochrome c oxidase and caspase-3 activity. FASEB J. 2019, 33, 13503–13514. [Google Scholar] [CrossRef]
- Iijima, T.; Mishima, T.; Akagawa, K.; Iwao, Y. Neuroprotective effect of propofol on necrosis and apoptosis following oxygen-glucose deprivation--relationship between mitochondrial membrane potential and mode of death. Brain Res. 2006, 1099, 25–32. [Google Scholar] [CrossRef]
- Iijima, T.; Mishima, T.; Akagawa, K.; Iwao, Y. Mitochondrial hyperpolarization after transient oxygen-glucose deprivation and subsequent apoptosis in cultured rat hippocampal neurons. Brain Res. 2003, 993, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Marcillat, O.; Giulivi, C.; Ernster, L.; Davies, K.J. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990, 265, 16330–16336. [Google Scholar] [CrossRef] [PubMed]
- Kushnareva, Y.; Murphy, A.N.; Andreyev, A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002, 368, 545–553. [Google Scholar] [CrossRef]
- Kunimatsu, T.; Kobayashi, K.; Yamashita, A.; Yamamoto, T.; Lee, M.C. Cerebral reactive oxygen species assessed by electron spin resonance spectroscopy in the initial stage of ischemia-reperfusion are not associated with hypothermic neuroprotection. J. Clin. Neurosci. 2011, 18, 545–548. [Google Scholar] [CrossRef]
- Liu, R.R.; Murphy, T.H. Reversible cyclosporin A-sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo: A two-photon imaging study. J. Biol. Chem. 2009, 284, 36109–36117. [Google Scholar] [CrossRef]
- Pandya, J.D.; Pauly, J.R.; Sullivan, P.G. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 2009, 218, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Southworth, R.; Medina, R.A.; Davidson, S.M.; Duchen, M.R.; Shattock, M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 2006, 72, 313–321. [Google Scholar] [CrossRef]
- Choi, K.; Kim, J.; Kim, G.W.; Choi, C. Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr. Neurovasc. Res. 2009, 6, 213–222. [Google Scholar] [CrossRef]
- Chan, P.H.; Kawase, M.; Murakami, K.; Chen, S.F.; Li, Y.; Calagui, B.; Reola, L.; Carlson, E.; Epstein, C.J. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J. Neurosci. 1998, 18, 8292–8299. [Google Scholar] [CrossRef]
- Murakami, K.; Kondo, T.; Kawase, M.; Li, Y.; Sato, S.; Chen, S.F.; Chan, P.H. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J. Neurosci. 1998, 18, 205–213. [Google Scholar] [CrossRef]
- Fujimura, M.; Morita-Fujimura, Y.; Noshita, N.; Sugawara, T.; Kawase, M.; Chan, P.H. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J. Neurosci. 2000, 20, 2817–2824. [Google Scholar] [CrossRef]
- Christophe, M.; Nicolas, S. Mitochondria: A target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr. Pharm. Des. 2006, 12, 739–757. [Google Scholar] [CrossRef]
- Niizuma, K.; Endo, H.; Chan, P.H. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009, 109 (Suppl. S1), 133–138. [Google Scholar] [CrossRef]
- Weigl, M.; Tenze, G.; Steinlechner, B.; Skhirtladze, K.; Reining, G.; Bernardo, M.; Pedicelli, E.; Dworschak, M. A systematic review of currently available pharmacological neuroprotective agents as a sole intervention before anticipated or induced cardiac arrest. Resuscitation 2005, 65, 21–39. [Google Scholar] [CrossRef]
- Cemeli, E.; Baumgartner, A.; Anderson, D. Antioxidants and the Comet assay. Mutat. Res. 2009, 681, 51–67. [Google Scholar] [CrossRef]
- Hazeki, O.; Tamura, M. Quantitative analysis of hemoglobin oxygenation state of rat brain in situ by near-infrared spectrophotometry. J. Appl. Physiol. 1988, 64, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Afanas’eva, N.I. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light. Dokl. Akad. Nauk. 1995, 342, 693–695. [Google Scholar] [PubMed]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim. Biophys. Acta 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Wharton, D.C.; Tzagoloff, A. Studies on the Electron Transfer System. Lvii. The near Infrared Absorption Band of Cytochrome Oxidase. J. Biol. Chem. 1964, 239, 2036–2041. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Eells, J.T.; Wong-Riley, M.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Morse, P.T.; Goebel, D.J.; Wan, J.; Tuck, S.; Hakim, L.; Hüttemann, C.L.; Malek, M.H.; Lee, I.; Sanderson, T.H.; Hüttemann, M. Cytochrome c oxidase-modulatory near-infrared light penetration into the human brain: Implications for the noninvasive treatment of ischemia/reperfusion injury. IUBMB Life 2021, 73, 554–567. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Wider, J.M.; Lee, I.; Reynolds, C.A.; Liu, J.; Lepore, B.; Tousignant, R.; Bukowski, M.J.; Johnston, H.; Fite, A.; et al. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci. Rep. 2018, 8, 3481. [Google Scholar] [CrossRef] [PubMed]
- Wider, J.M.; Gruley, E.; Morse, P.T.; Wan, J.; Lee, I.; Anzell, A.R.; Fogo, G.M.; Mathieu, J.; Hish, G.; O’Neil, B.; et al. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest. Crit. Care 2023, 27, 491. [Google Scholar] [CrossRef]
- Strubakos, C.D.; Malik, M.; Wider, J.M.; Lee, I.; Reynolds, C.A.; Mitsias, P.; Przyklenk, K.; Hüttemann, M.; Sanderson, T.H. Non-invasive treatment with near-infrared light: A novel mechanisms-based strategy that evokes sustained reduction in brain injury after stroke. J. Cereb. Blood Flow. Metab. 2020, 40, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, D. Two-Dimensional Gel Electrophoresis and Pro-Q Diamond Phosphoprotein Stain-Based Plant Phosphosproteomics. Methods Mol. Biol. 2021, 2358, 159–168. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bukowski, M.J.; Wider, J.M.; Reynolds, C.A.; Calo, L.; Lepore, B.; Tousignant, R.; Jones, M.; Przyklenk, K.; Sanderson, T.H. Mitochondrial dynamics following global cerebral ischemia. Mol. Cell Neurosci. 2016, 76, 68–75. [Google Scholar] [CrossRef]
- Maneechote, C.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell Mol. Med. 2017, 21, 2643–2653. [Google Scholar] [CrossRef]
- Ong, S.B.; Subrayan, S.; Lim, S.Y.; Yellon, D.M.; Davidson, S.M.; Hausenloy, D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121, 2012–2022. [Google Scholar] [CrossRef]
- Liu, S.; Luo, W.; Wang, Y. Emerging role of PARP-1 and PARthanatos in ischemic stroke. J. Neurochem. 2022, 160, 74–87. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, Y.; Fang, Q.Y.; Wang, T.; Chen, C.; Gao, T.Y.; Wu, M.; Zhang, W.P.; Lu, Y.B. PARP-1 inhibitor alleviates cerebral ischemia/reperfusion injury by reducing PARylation of HK-1 and LDH in mice. Eur. J. Pharmacol. 2024, 967, 176377. [Google Scholar] [CrossRef]
- Wan, B.; Doumen, C.; Duszynski, J.; Salama, G.; Vary, T.C.; LaNoue, K.F. Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am. J. Physiol. 1993, 265, H453–H460. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.M.; Carson, R.C.; Mahmood, J.; Thomas, H.M.; Gibson, G.E. Assessment of membrane potentials of mitochondrial populations in living cells. Anal. Biochem. 2001, 298, 170–180. [Google Scholar] [CrossRef]
- Backus, M.; Piwnica-Worms, D.; Hockett, D.; Kronauge, J.; Lieberman, M.; Ingram, P.; LeFurgey, A. Microprobe analysis of Tc-MIBI in heart cells: Calculation of mitochondrial membrane potential. Am. J. Physiol. 1993, 265, C178–C187. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974, 50, 305–315. [Google Scholar] [CrossRef]
- Labajova, A.; Vojtiskova, A.; Krivakova, P.; Kofranek, J.; Drahota, Z.; Houstek, J. Evaluation of mitochondrial membrane potential using a computerized device with a tetraphenylphosphonium-selective electrode. Anal. Biochem. 2006, 353, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Robertson, J.D.; Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 2002, 99, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Racay, P.; Tatarkova, Z.; Chomova, M.; Hatok, J.; Kaplan, P.; Dobrota, D. Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem. Res. 2009, 34, 1469–1478. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Tang, S.; Liu, J.; Yan, J.; Chen, H.; Yan, X. Quercetin Protects H9c2 Cardiomyocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Oxidative Stress and Mitochondrial Apoptosis by Regulating the ERK1/2/DRP1 Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 7522175. [Google Scholar] [CrossRef]
- Ye, M.; Wu, H.; Li, S. Resveratrol alleviates oxygen/glucose deprivation/reoxygenation-induced neuronal damage through induction of mitophagy. Mol. Med. Rep. 2021, 23, 73. [Google Scholar] [CrossRef]
- Zhou, T.; Mo, J.; Xu, W.; Hu, Q.; Liu, H.; Fu, Y.; Jiang, J. Mild hypothermia alleviates oxygen-glucose deprivation/reperfusion-induced apoptosis by inhibiting ROS generation, improving mitochondrial dysfunction and regulating DNA damage repair pathway in PC12 cells. Apoptosis 2023, 28, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2(*-) generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer 2009, 63, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.T.; Tuck, S.; Kerns, M.; Goebel, D.J.; Wan, J.; Waddell, T.; Wider, J.M.; Hüttemann, C.L.; Malek, M.H.; Lee, I.; et al. Non-invasive treatment of ischemia/reperfusion injury: Effective transmission of therapeutic near-infrared light into the human brain through soft skin-conforming silicone waveguides. Bioeng. Transl. Med. 2023, 8, e10496. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, L.; Arroum, T.; Morse, P.T.; Bell, J.; Malek, M.H.; Sanderson, T.H.; Hüttemann, M. Inhibitory Infrared Light Attenuates Mitochondrial Hyperactivity and Accelerates Restoration of Mitochondrial Homeostasis in an Oxygen–Glucose Deprivation/Reoxygenation Model. Antioxidants 2025, 14, 1119. https://doi.org/10.3390/antiox14091119
Pham L, Arroum T, Morse PT, Bell J, Malek MH, Sanderson TH, Hüttemann M. Inhibitory Infrared Light Attenuates Mitochondrial Hyperactivity and Accelerates Restoration of Mitochondrial Homeostasis in an Oxygen–Glucose Deprivation/Reoxygenation Model. Antioxidants. 2025; 14(9):1119. https://doi.org/10.3390/antiox14091119
Chicago/Turabian StylePham, Lucynda, Tasnim Arroum, Paul T. Morse, Jamie Bell, Moh H. Malek, Thomas H. Sanderson, and Maik Hüttemann. 2025. "Inhibitory Infrared Light Attenuates Mitochondrial Hyperactivity and Accelerates Restoration of Mitochondrial Homeostasis in an Oxygen–Glucose Deprivation/Reoxygenation Model" Antioxidants 14, no. 9: 1119. https://doi.org/10.3390/antiox14091119
APA StylePham, L., Arroum, T., Morse, P. T., Bell, J., Malek, M. H., Sanderson, T. H., & Hüttemann, M. (2025). Inhibitory Infrared Light Attenuates Mitochondrial Hyperactivity and Accelerates Restoration of Mitochondrial Homeostasis in an Oxygen–Glucose Deprivation/Reoxygenation Model. Antioxidants, 14(9), 1119. https://doi.org/10.3390/antiox14091119










