Deinoxanthin-Enriched Extracellular Vesicles from Deinococcus radiodurans Drive IL-10–Dependent Tolerogenic Programming of Dendritic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. crtI Deletion Mutant Construction
2.3. Isolation of BEVs
2.4. Characterization of BEVs
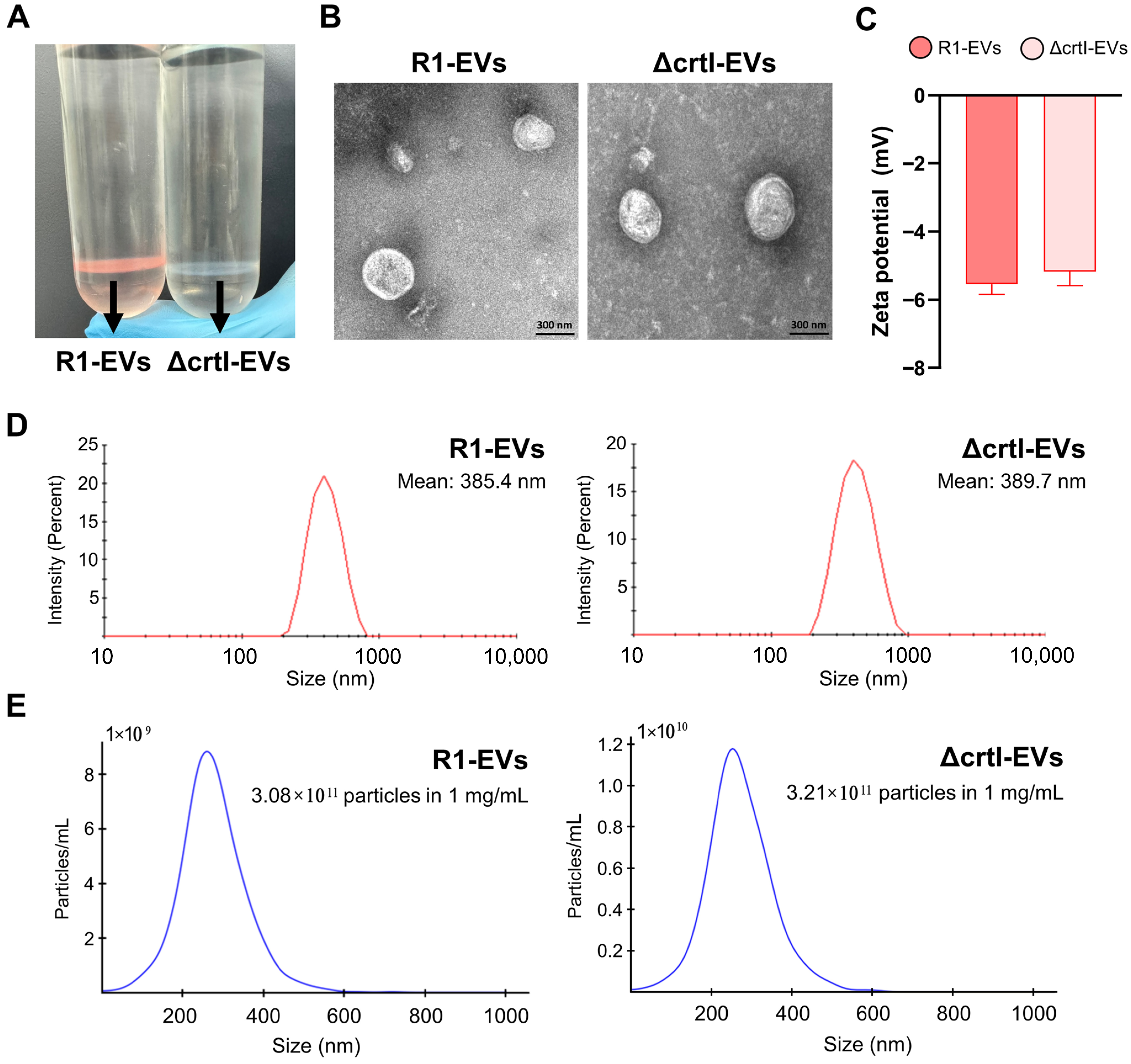
2.4.1. Particle Size and Zeta Potential Analysis
2.4.2. Transmission Electron Microscopy Analysis (TEM)
2.4.3. Nanoparticle Tracking Analysis (NTA)
2.5. Antibodies and Reagents
2.6. Ethics Statement and Mice
2.7. Treatment of BMDCs with R1-EVs and ΔcrtI-EVs
2.8. Annexin V and Propidium Iodide (PI) Staining
2.9. Analysis of Surface Molecules on BMDCs
2.10. Measurement of Extracellular Cytokine Levels
2.11. Detection of the Levels of Intracellular Cytokines in BMDCs
2.12. Analysis of the Antigen-Uptake Ability of BMDCs
2.13. Western Blotting Analysis
2.14. Allogeneic Mixed Lymphocyte Reaction
2.15. Inhibition of IL-10 by Neutralizing Antibody
2.16. Statistical Analysis
3. Results
3.1. Isolation and Characterization of EVs Derived from Deinococcus radiodurans (R1-EVs) and ΔcrtI Mutant D. radiodurans (ΔcrtI-EVs)
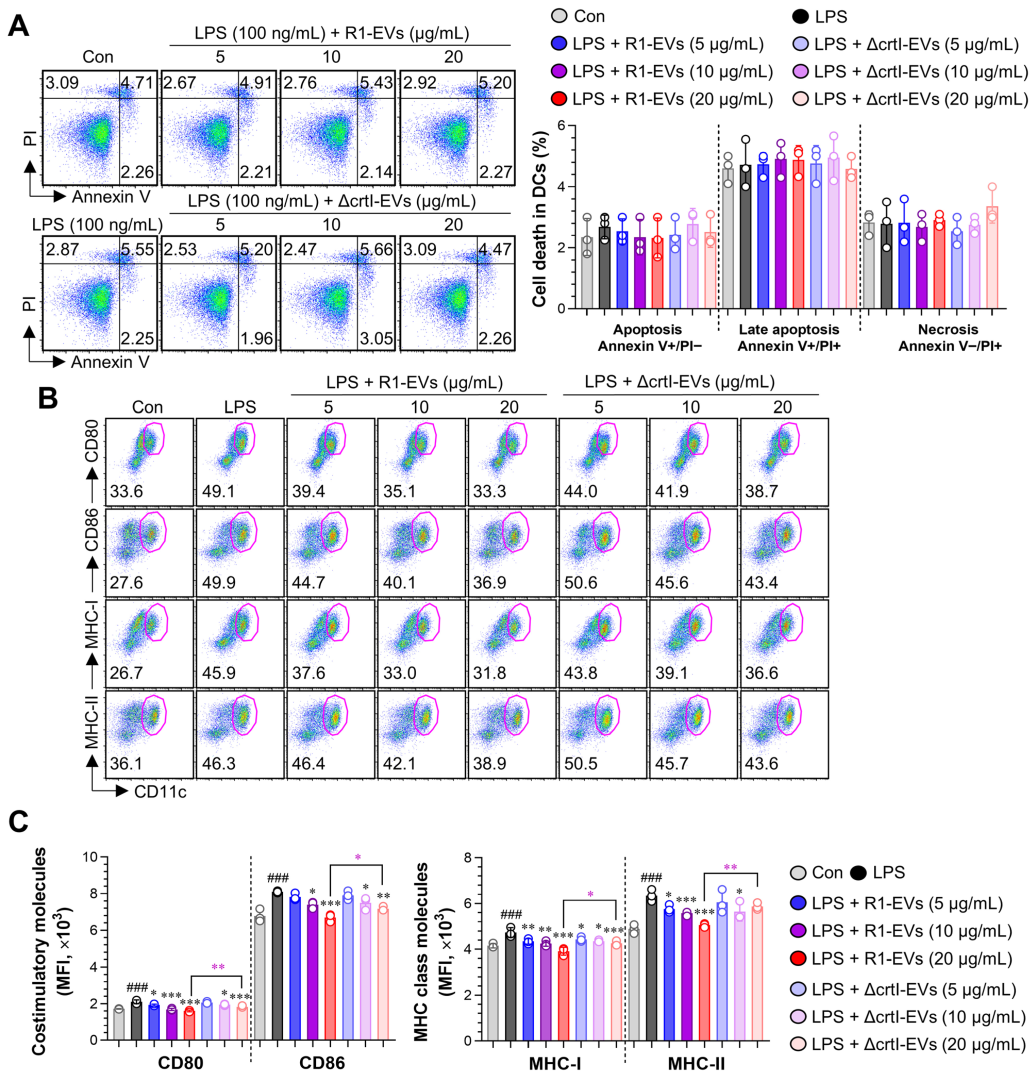
3.2. R1-EVs Attenuate LPS-Induced DC Maturation Compared with ΔcrtI-EVs
3.2.1. R1-EVs Suppress LPS-Induced Upregulation of Surface Costimulatory Molecules on the BMDCs More Effectively than ΔcrtI-EVs, Without Inducing Cytotoxicity
3.2.2. R1-EVs Suppress Pro-Inflammatory Cytokines, Induce IL-10 Secretion, and Preserve Antigen Uptake in LPS-Stimulated BMDCs More Effectively than ΔcrtI-EVs
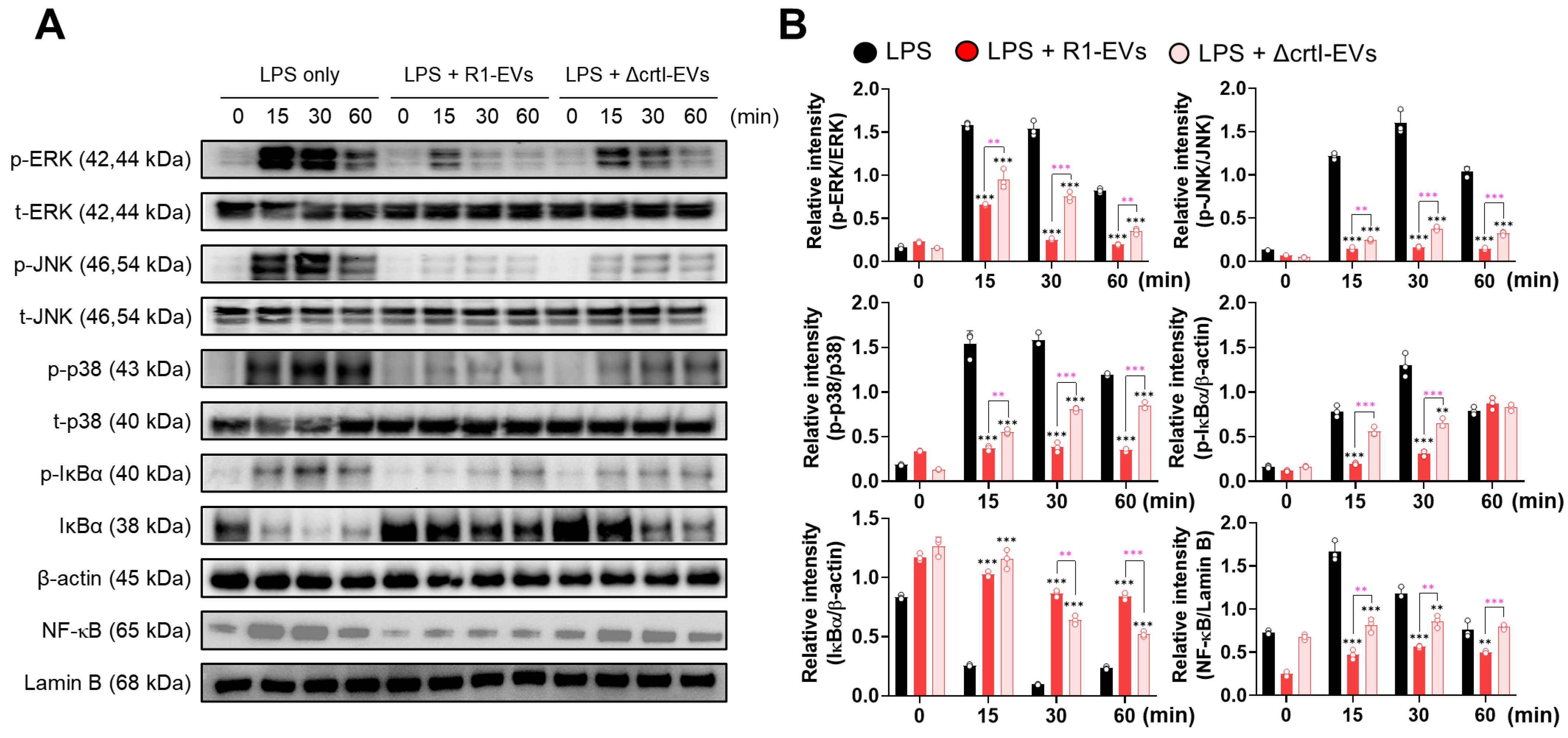
3.3. R1-EVs Inhibit DC Maturation via Attenuation of MAPK and NF-κB Signaling Pathways
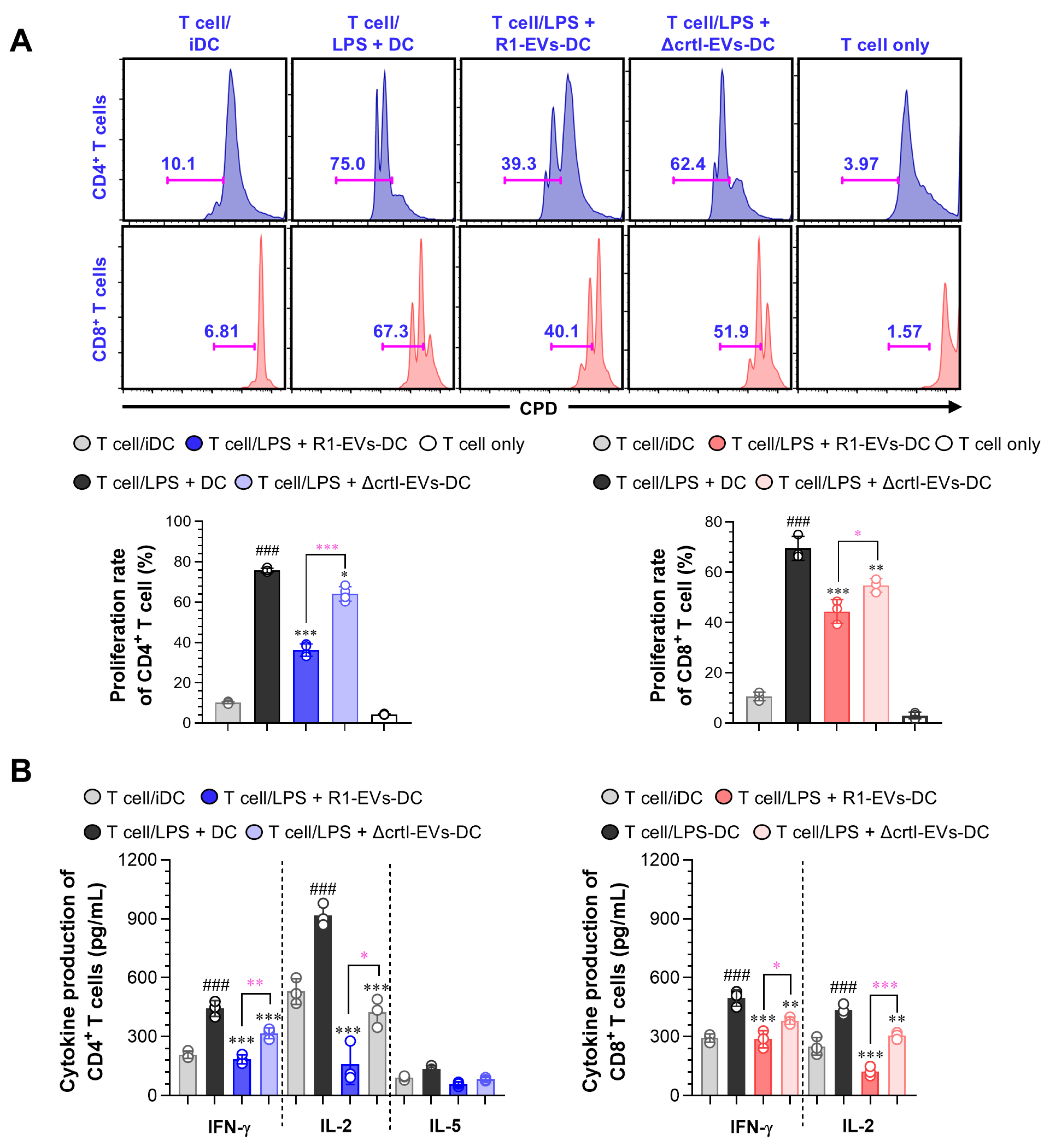
3.4. R1-EVs Attenuate Allogeneic T Cell Responses by Modulating DC–T Cell Interactions
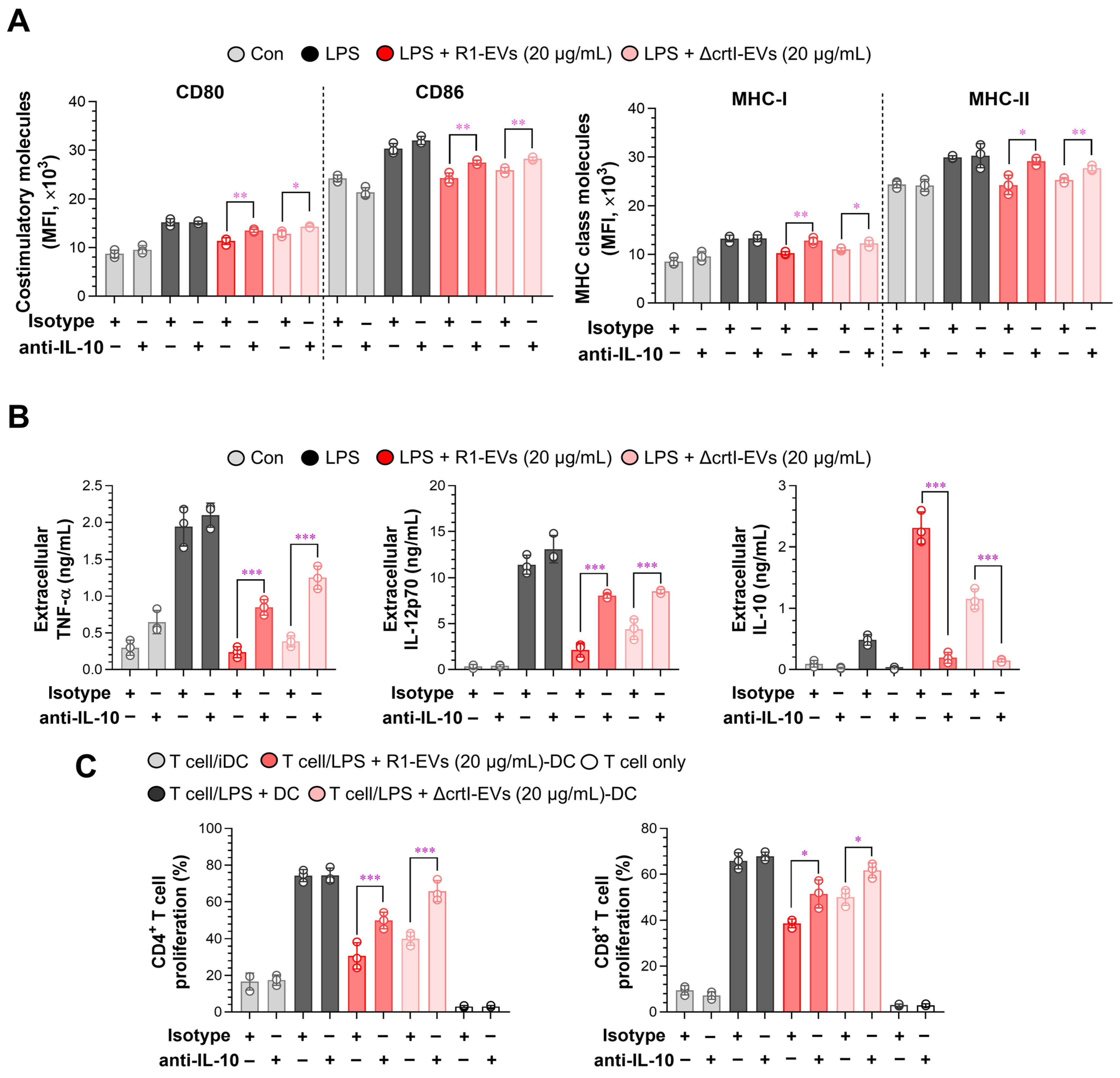
3.5. IL-10 Neutralization Reverses R1-EV–Mediated Tolerogenic Phenotypes in Dendritic Cells and T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| BEVs | bacterial EVs |
| BMDCs | bone marrow-derived DCs |
| CAT | catalase |
| DC | dendritic cell |
| DLS | dynamic light scattering |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DX | deinoxanthin |
| EVs | Extracellular vesicles |
| FBS | fetal bovine serum |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MFI | mean fluorescence intensity |
| MHC | major histocompatibility complex |
| MLR | mixed lymphocyte reaction |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NTA | nanoparticle tracking analysis |
| OMVs | outer membrane vesicles |
| PDI | polydispersity index |
| PI | propidium iodide |
| R1-EVs | D. radiodurans–derived EVs |
| rmGM | recombinant mouse granulocyte-macrophage |
| CSF | colony-stimulating factor |
| ROS | reactive oxygen species |
| RT | room temperature |
| SD | standard deviation |
| SDBC | S-layer deinoxanthin-binding complex |
| SOD | superoxide dismutase |
| TEM | transmission electron microscopy |
| TFF | tangential flow filtration |
| TGY | tryptone glucose yeast extract |
References
- Wiklander, O.P.B.; Brennan, M.A.; Lotvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.H. Bacterial extracellular vesicles: Emerging nanoplatforms for biomedical applications. Microb. Pathog. 2023, 183, 106308. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Dhurve, G.; Madikonda, A.K.; Jagannadham, M.V.; Siddavattam, D. Outer Membrane Vesicles of Acinetobacter baumannii DS002 Are Selectively Enriched with TonB-Dependent Transporters and Play a Key Role in Iron Acquisition. Microbiol. Spectr. 2022, 10, e0029322. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Giardino Torchia, M.L.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Liu, B.D.; Akbar, R.; Oliverio, A.; Thapa, K.; Wang, X.; Fan, G.C. Bacterial Extracellular Vesicles in the Regulation of Inflammatory Response and Host-Microbe Interactions. Shock 2024, 61, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Peregrino, E.S.; Castaneda-Casimiro, J.; Vazquez-Flores, L.; Estrada-Parra, S.; Wong-Baeza, C.; Serafin-Lopez, J.; Wong-Baeza, I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 6210. [Google Scholar] [CrossRef]
- Li, J.; Webster, T.J.; Tian, B. Functionalized Nanomaterial Assembling and Biosynthesis Using the Extremophile Deinococcus radiodurans for Multifunctional Applications. Small 2019, 15, e1900600. [Google Scholar] [CrossRef]
- Jeong, S.; Jung, J.H.; Kim, M.K.; de Groot, A.; Blanchard, L.; Ryu, S.; Bahn, Y.S.; Lim, S. Atypical Bacilliredoxin AbxC Plays a Role in Responding to Oxidative Stress in Radiation-Resistant Bacterium Deinococcus radiodurans. Antioxidants 2021, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jung, J.H.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Kiang, J.G.; Fukumoto, R.; Lee, D.Y.; Wehr, N.B.; Viteri, G.A.; Berlett, B.S.; Levine, R.L. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE 2010, 5, e12570. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [PubMed]
- Bentchikou, E.; Servant, P.; Coste, G.; Sommer, S. A major role of the RecFOR pathway in DNA double-strand-break repair through ESDSA in Deinococcus radiodurans. PLoS Genet. 2010, 6, e1000774. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta 2007, 1770, 902–911. [Google Scholar] [CrossRef]
- Ji, H.F. Insight into the strong antioxidant activity of deinoxanthin, a unique carotenoid in Deinococcus radiodurans. Int. J. Mol. Sci. 2010, 11, 4506–4510. [Google Scholar] [CrossRef]
- Kuzucu, M. Extremophilic Solutions: The Role of Deinoxanthin in Counteracting UV-Induced Skin Harm. Curr. Issues Mol. Biol. 2023, 45, 8372–8394. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.; Kim, J.; Kim, J.W.; Kim, S.Y.; Yeom, B.; Kim, H.; Choi, W.I.I.; Sung, D. Highly Water-Dispersed and Stable Deinoxanthin Nanocapsule for Effective Antioxidant and Anti-Inflammatory Activity. Int. J. Nanomed. 2023, 18, 4555–4565. [Google Scholar] [CrossRef]
- Han, J.M.; Song, H.Y.; Jung, J.H.; Lim, S.; Seo, H.S.; Kim, W.S.; Lim, S.T.; Byun, E.B. Deinococcus radiodurans-derived membrane vesicles protect HaCaT cells against H(2)O(2)-induced oxidative stress via modulation of MAPK and Nrf2/ARE pathways. Biol. Proced. Online 2023, 25, 17. [Google Scholar] [CrossRef]
- Han, J.M.; Mwiti, G.; Yeom, S.J.; Lim, J.; Kim, W.S.; Lim, S.; Lim, S.T.; Byun, E.B. Radiation-Resistant Bacteria Deinococcus radiodurans-Derived Extracellular Vesicles as Potential Radioprotectors. Adv. Healthc. Mater. 2025, 14, e2403192. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Joe, M.H.; Lee, K.H.; Lim, S.Y.; Im, S.H.; Song, H.P.; Lee, I.S.; Kim, D.H. Pigment-based whole-cell biosensor system for cadmium detection using genetically engineered Deinococcus radiodurans. Bioprocess Biosyst. Eng. 2012, 35, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Singh, H.; Jung, J.H.; Jung, K.W.; Ryu, S.; Lim, S. Comparative genomics of Deinococcus radiodurans: Unveiling genetic discrepancies between ATCC 13939K and BAA-816 strains. Front. Microbiol. 2024, 15, 1410024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nguyen, L.T.H.; Hickey, R.; Walters, N.; Wang, X.; Kwak, K.J.; Lee, L.J.; Palmer, A.F.; Reategui, E. Immunomagnetic sequential ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. Sci. Rep. 2021, 11, 8034. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, W.S.; Choi, H.G.; Jang, B.; Lee, K.; Park, J.H.; Kim, H.J.; Cho, S.N.; Shin, S.J. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J. Leukoc. Biol. 2013, 94, 733–749. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
- Lin, G.; Wang, J.; Yang, Y.G.; Zhang, Y.; Sun, T. Advances in dendritic cell targeting nano-delivery systems for induction of immune tolerance. Front. Bioeng. Biotechnol. 2023, 11, 1242126. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Rozman, P. Induction of Tolerogenic Dendritic Cells by Endogenous Biomolecules: An Update. Front. Immunol. 2018, 9, 2482. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Palucka, A.K.; Pascual, V.; Banchereau, J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008, 19, 41–52. [Google Scholar] [CrossRef]
- McBride, J.M.; Jung, T.; de Vries, J.E.; Aversa, G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002, 215, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Passeri, L.; Andolfi, G.; Bassi, V.; Russo, F.; Giacomini, G.; Laudisa, C.; Marrocco, I.; Cesana, L.; Di Stefano, M.; Fanti, L.; et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J. Autoimmun. 2023, 138, 103051. [Google Scholar] [CrossRef]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Thery, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Castiello, L.; Sabatino, M.; Jin, P.; Clayberger, C.; Marincola, F.M.; Krensky, A.M.; Stroncek, D.F. Monocyte-derived DC maturation strategies and related pathways: A transcriptional view. Cancer Immunol. Immunother. 2011, 60, 457–466. [Google Scholar] [CrossRef]
- Bhandarkar, V.; Dinter, T.; Spranger, S. Architects of immunity: How dendritic cells shape CD8(+) T cell fate in cancer. Sci. Immunol. 2025, 10, eadf4726. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Y.; Sun, Y.; Li, S.; Chen, L.; Jin, X.; Hou, X.; Liu, X.; Chen, Q.; Li, J.; et al. Knockdown of NEAT1 induces tolerogenic phenotype in dendritic cells by inhibiting activation of NLRP3 inflammasome. Theranostics 2019, 9, 3425–3442. [Google Scholar] [CrossRef]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Zou, D.; Li, X.C.; Chen, W. Beyond T-cell subsets: Stemness and adaptation redefining immunity and immunotherapy. Cell Mol. Immunol. 2025, 22, 957–974. [Google Scholar] [CrossRef]
- Morelli, A.E.; Thomson, A.W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007, 7, 610–621. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Ling, Z.; Li, J.; Hu, J.; He, F.; Chen, Q. The role of dendritic cells in the immunomodulation to implanted biomaterials. Int. J. Oral Sci. 2022, 14, 52. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef]
- Glocker, E.O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schaffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Eom, J.E.; Shin, D.U.; Kim, G.D.; Yoon, J.H.; Shin, H.S.; Lee, S.Y. Pediococcus pentosaceus KF159 alleviates house dust mite-induced atopic dermatitis by promoting IL10 production and regulatory T cell induction. Food Funct. 2024, 15, 6975–6987. [Google Scholar] [CrossRef]
- Malacco, N.L.; Michi, A.N.; Siciliani, E.; Madrigal, A.G.; Sternlieb, T.; Fontes, G.; King, I.L.; Cestari, I.; Jardim, A.; Stevenson, M.M.; et al. Helminth-derived metabolites induce tolerogenic functional, metabolic, and transcriptional signatures in dendritic cells that attenuate experimental colitis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of beta-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kappaB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hong, P.; Zheng, X. beta-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-kappaB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef]
- Bai, S.K.; Lee, S.J.; Na, H.J.; Ha, K.S.; Han, J.A.; Lee, H.; Kwon, Y.G.; Chung, C.K.; Kim, Y.M. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef]
- Han, J.M.; Lim, J.; Kang, H.; Kim, W.S.; Yoo, B.-G.; Byun, E.-H.; Lim, S.; Kim, J.; Byun, E.-B. Eutrema japonicum–derived exosome-like nanoparticles as an immunostimulatory nutraceutical candidate with anti-cancer potential. Food Res. Int. 2025, 221, 117296. [Google Scholar] [CrossRef]
- Kim, W.; Lee, E.J.; Bae, I.H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, K.H.; Pham, A.V.Q.; Ko, J.; Oh, S.H.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514. [Google Scholar] [CrossRef]
- Farci, D.; Slavov, C.; Piano, D. Coexisting properties of thermostability and ultraviolet radiation resistance in the main S-layer complex of Deinococcus radiodurans. Photochem. Photobiol. Sci. 2018, 17, 81–88. [Google Scholar] [CrossRef]
- Farci, D.; Aksoyoglu, M.A.; Farci, S.F.; Bafna, J.A.; Bodrenko, I.; Ceccarelli, M.; Kirkpatrick, J.; Winterhalter, M.; Kereiche, S.; Piano, D. Structural insights into the main S-layer unit of Deinococcus radiodurans reveal a massive protein complex with porin-like features. J. Biol. Chem. 2020, 295, 4224–4236. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.M.; Lim, J.; Kim, W.S.; Yoo, B.-G.; Jung, J.-H.; Lim, S.; Byun, E.-B. Deinoxanthin-Enriched Extracellular Vesicles from Deinococcus radiodurans Drive IL-10–Dependent Tolerogenic Programming of Dendritic Cells. Antioxidants 2025, 14, 1108. https://doi.org/10.3390/antiox14091108
Han JM, Lim J, Kim WS, Yoo B-G, Jung J-H, Lim S, Byun E-B. Deinoxanthin-Enriched Extracellular Vesicles from Deinococcus radiodurans Drive IL-10–Dependent Tolerogenic Programming of Dendritic Cells. Antioxidants. 2025; 14(9):1108. https://doi.org/10.3390/antiox14091108
Chicago/Turabian StyleHan, Jeong Moo, Jaeyoon Lim, Woo Sik Kim, Bo-Gyeong Yoo, Jong-Hyun Jung, Sangyong Lim, and Eui-Baek Byun. 2025. "Deinoxanthin-Enriched Extracellular Vesicles from Deinococcus radiodurans Drive IL-10–Dependent Tolerogenic Programming of Dendritic Cells" Antioxidants 14, no. 9: 1108. https://doi.org/10.3390/antiox14091108
APA StyleHan, J. M., Lim, J., Kim, W. S., Yoo, B.-G., Jung, J.-H., Lim, S., & Byun, E.-B. (2025). Deinoxanthin-Enriched Extracellular Vesicles from Deinococcus radiodurans Drive IL-10–Dependent Tolerogenic Programming of Dendritic Cells. Antioxidants, 14(9), 1108. https://doi.org/10.3390/antiox14091108





