A Genome-Wide Association Study Reveals QTLs and Candidate Genes Associated with the Carotenoid Content in the Flesh of Cucurbita pepo L. Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm and Genomic Data Used for GWAS
2.2. Carotenoid Extraction and Quantification
2.3. Genome-Wide Association Study
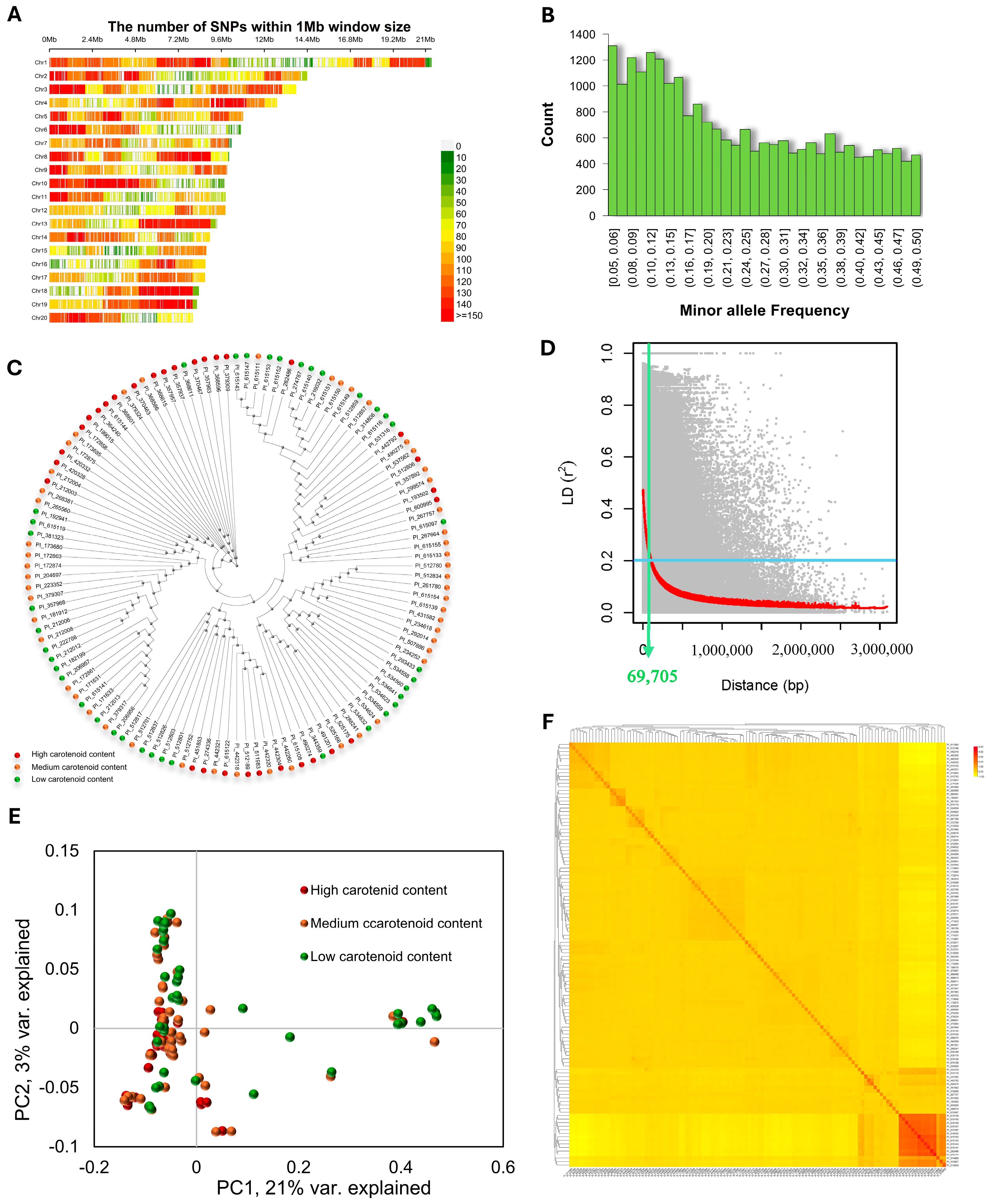
2.4. Population Structure Analysis and Linkage Disequilibrium (LD) Estimation
2.5. Identification and Analysis of Candidate Genes
2.6. Statistical Analyses
3. Results
3.1. Phenotypic Variation, Frequency Distribution, and Correlation Analysis of Carotenoid Content
3.2. Genetic Variation and Population Structure
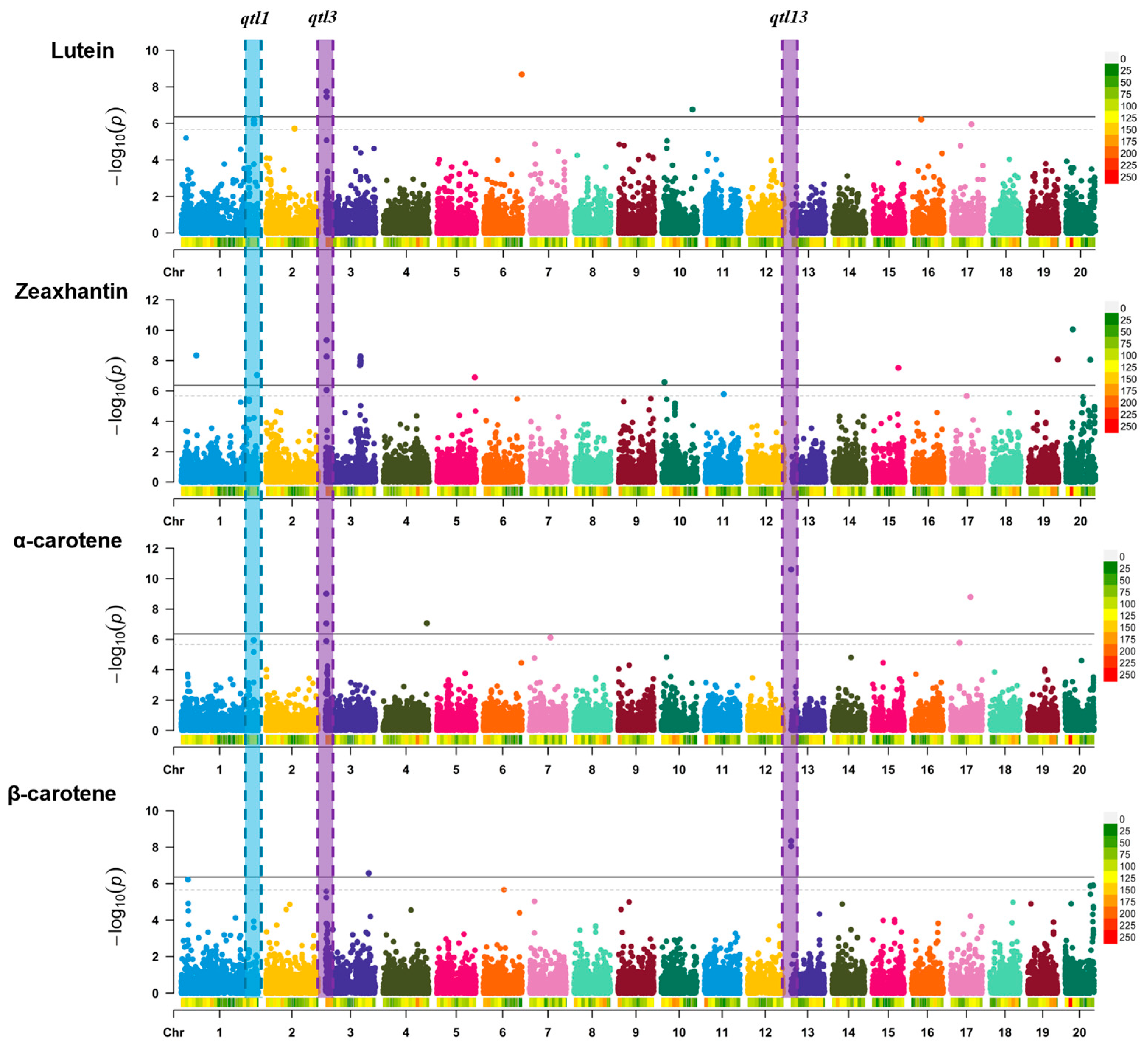
3.3. Genome-Wide Association Study of Carotenoid Content
3.4. Estimation of Local LD and Identification of Candidate Genes
3.5. Expression Profiles of the Candidate Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids; Birkhäuser: Basel, Switzerland, 2008; Volume 4, ISBN 978-3-7643-7498-3. [Google Scholar]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary Sources, Health Functions, Biofortification, Marketing Trend and Affecting Factors—A Review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Reddy, U.K.; Lopez-Ortiz, C.; Talavera-Caro, A.G.; Natarajan, P.; Tomason, Y.; Alaparthi, S.; Levi, A.; Nimmakayala, P. GWAS Resolves Molecular Mechanisms Underlying Natural Variation for Carotenoids in Cucurbita maxima Duchesne. Sci. Hortic. 2023, 312, 111881. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, L. Carotenoid Metabolism: Biosynthesis, Regulation, and Beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.-H.; Rajesh Banu, J.; Kumar, G. Technological Advances in the Production of Carotenoids and Their Applications—A Critical Review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef]
- Nakkanong, K.; Yang, J.H.; Zhang, M.F. Carotenoid Accumulation and Carotenogenic Gene Expression during Fruit Development in Novel Interspecific Inbred Squash Lines and Their Parents. J. Agric. Food Chem. 2012, 60, 5936–5944. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and Domestication of Cucurbitaceae Crops: Insights from Phylogenies, Genomics and Archaeology. New Phytol. 2019, 226, 1240–1255. [Google Scholar] [CrossRef]
- Paris, H.S. Complementary Genes for Orange Fruit Flesh Color in Cucurbita pepo. HortScience 1988, 23, 601–603. [Google Scholar] [CrossRef]
- Kaźmińska, K.; Hallmann, E.; Rusaczonek, A.; Korzeniewska, A.; Sobczak, M.; Filipczak, J.; Kuczerski, K.S.; Steciuk, J.; Sitarek-Andrzejczyk, M.; Gajewski, M.; et al. Genetic Mapping of Ovary Colour and Quantitative Trait Loci for Carotenoid Content in the Fruit of Cucurbita maxima Duchesne. Mol. Breed. 2018, 38, 114. [Google Scholar] [CrossRef]
- Martínez, C.; Valenzuela, J.L.; Jamilena, M. Genetic and Pre- and Postharvest Factors Influencing the Content of Antioxidants in Cucurbit Crops. Antioxidants 2021, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.S. Genetic Analysis and Breeding of Pumpkins and Squash for High Carotene Content. In Vegetables and Vegetable Products; Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 16, pp. 93–115. ISBN 978-3-642-84832-2. [Google Scholar]
- Hirschberg, J. Carotenoid Biosynthesis in Flowering Plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bonina-Noseworthy, J.; Loy, J.B.; Curran-Celentano, J.; Sideman, R.; Kopsell, D.A. Carotenoid Concentration and Composition in Winter Squash: Variability Associated with Different Cultigens, Harvest Maturities, and Storage Times. HortScience 2016, 51, 472–480. [Google Scholar] [CrossRef]
- Kreck, M.; Kürbel, P.; Ludwig, M.; Paschold, P.J.; Dietrich, H. Identification and Quantification of Carotenoids in Pumpkin Cultivars (Cucurbita maxima L.) and Their Juices by Liquid Chromatography with Ultraviolet-Diode Array Detection. J. Appl. Bot. Food Qual. 2006, 80, 93–99. [Google Scholar]
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Compos. Anal. 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid Metabolism and Regulation in Horticultural Crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. Genes and Enzymes of Carotenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Fraser, P.; Bramley, P.M. The Biosynthesis and Nutritional Uses of Carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Cuevas, H.E.; Rosa-Valentin, G.; Hayes, C.M.; Rooney, W.L.; Hoffmann, L. Genomic Characterization of a Core Set of the USDA-NPGS Ethiopian Sorghum Germplasm Collection: Implications for Germplasm Conservation, Evaluation, and Utilization in Crop Improvement. BMC Genom. 2017, 18, 108. [Google Scholar] [CrossRef]
- Suwarno, W.B.; Pixley, K.V.; Palacios-Rojas, N.; Kaeppler, S.M.; Babu, R. Genome-Wide Association Analysis Reveals New Targets for Carotenoid Biofortification in Maize. Theor. Appl. Genet. 2015, 128, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A Unified Mixed-Model Method for Association Mapping That Accounts for Multiple Levels of Relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef]
- Yu, J.; Buckler, E.S. Genetic Association Mapping and Genome Organization of Maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gore, M.; Buckler, E.S.; Yu, J. Status and Prospects of Association Mapping in Plants. Plant Genome 2008, 1, 5–20. [Google Scholar] [CrossRef]
- Gázquez, J.C.; Pérez, C.; Meca, D.E.; Segura, M.D.; Domene, M.A.; De La Cruz, E.; López, J.C.; Buendía, D. Comparative Study of Tomato Production Strategies for Long-Cycle Crop in Enarenado and for Inter-Planting in Different Substrates Systems in the Mediterranean Area. Acta Hortic. 2017, 1170, 773–776. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N. Voedingsoplossingen voor groenten en bloemen geteeld in water of substraten = Nutrient solutions for vegetables and flowers grown in water or substrates. Naaldwijk etc.: Proefstation voor Tuinbouw onder Glas. 1994. Available online: https://edepot.wur.nl/237302 (accessed on 2 September 2025).
- Wald, J.P.; Nohr, D.; Biesalski, H.K. Rapid and Easy Carotenoid Quantification in Ghanaian Starchy Staples Using RP-HPLC-PDA. J. Food Compos. Anal. 2018, 67, 119–127. [Google Scholar] [CrossRef]
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest Treatment with Abscisic Acid Alleviates Chilling Injury in Zucchini Fruit by Regulating Phenolic Metabolism and Non-Enzymatic Antioxidant System. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Remington, D.L.; Thornsberry, J.M.; Matsuoka, Y.; Wilson, L.M.; Whitt, S.R.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S. Structure of Linkage Disequilibrium and Phenotypic Associations in the Maize Genome. Proc. Natl. Acad. Sci. USA 2001, 98, 11479–11484. [Google Scholar] [CrossRef]
- Margolin, A.A.; Nemenman, I.; Basso, K.; Wiggins, C.; Stolovitzky, G.; Favera, R.D.; Califano, A. ARACNE: An Algorithm for the Reconstruction of Gene Regulatory Networks in a Mammalian Cellular Context. BMC Bioinform. 2006, 7, S7. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a Central Hub for Transcription Factors and Regulatory Interactions in Plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; García, A.; Gamarra, G.; Benítez, Á.; Iglesias-Moya, J.; Martínez, C.; Jamilena, M. An miR164-Resistant Mutation in the Transcription Factor Gene CpCUC2B Enhances Carpel Arrest and Ectopic Boundary Specification in Cucurbita pepo Flower Development. J. Exp. Bot. 2024, 75, 1948–1966. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Zhao, F.; Sun, G.; Xu, M.; Fu, A.; Lan, W.; Luan, S. A SPX Domain Vacuolar Transporter Links Phosphate Sensing to Homeostasis in Arabidopsis. Mol. Plant 2022, 15, 1590–1601. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The Emerging Importance of the SPX Domain-containing Proteins in Phosphate Homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Z.; Li, J.; Wang, J.; Yu, L.; Lin, S. SPX-Related Genes Regulate Phosphorus Homeostasis in the Marine Phytoplankton, Phaeodactylum tricornutum. Commun. Biol. 2021, 4, 797. [Google Scholar] [CrossRef]
- Zhou, X.; Welsch, R.; Yang, Y.; Álvarez, D.; Riediger, M.; Yuan, H.; Fish, T.; Liu, J.; Thannhauser, T.W.; Li, L. Arabidopsis OR Proteins Are the Major Posttranscriptional Regulators of Phytoene Synthase in Controlling Carotenoid Biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 3558–3563. [Google Scholar] [CrossRef]
- D’Andrea, L.; Rodriguez-Concepcion, M. Manipulation of Plastidial Protein Quality Control Components as a New Strategy to Improve Carotenoid Contents in Tomato Fruit. Front. Plant Sci. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Wyatt, L.E.; Strickler, S.R.; Mueller, L.A.; Mazourek, M. Comparative Analysis of Cucurbita pepo Metabolism throughout Fruit Development in Acorn Squash and Oilseed Pumpkin. Hortic. Res. 2016, 3, 16045. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Feng, Y.; Niu, X.; Giovannoni, J.; Liu, Y. Altered Plastid Levels and Potential for Improved Fruit Nutrient Content by Downregulation of the Tomato DDB1-interacting Protein CUL4. Plant J. 2008, 55, 89–103. [Google Scholar] [CrossRef]
- Obrero, Á.; González-Verdejo, C.I.; Die, J.V.; Gómez, P.; Del Río-Celestino, M.; Román, B. Carotenogenic Gene Expression and Carotenoid Accumulation in Three Varieties of Cucurbita pepo during Fruit Development. J. Agric. Food Chem. 2013, 61, 6393–6403. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Wang, M.; Wang, Y.; Xu, W.; Han, H.; Wang, Z.; Zhong, Y.; Huang, H.; Qu, S. Accumulation of Carotenoids and Expression of Carotenoid Biosynthesis Genes in Fruit Flesh during Fruit Development in Two Cucurbita maxima Inbred Lines. Hortic. Plant J. 2021, 7, 529–538. [Google Scholar] [CrossRef]
- Gur, A.; Tzuri, G.; Meir, A.; Sa’ar, U.; Portnoy, V.; Katzir, N.; Schaffer, A.A.; Li, L.; Burger, J.; Tadmor, Y. Genome-Wide Linkage-Disequilibrium Mapping to the Candidate Gene Level in Melon (Cucumis melo). Sci. Rep. 2017, 7, 9770. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hao, J.; Lv, M.; Liu, P.; Ge, Q.; Zhang, S.; Yang, J.; Niu, H.; Wang, Y.; Xue, Y.; et al. A Genome-Wide Association Study Identifies Genes Associated with Cuticular Wax Metabolism in Maize. Plant Physiol. 2024, 194, 2616–2630. [Google Scholar] [CrossRef]
- García, A.; Castro-Cegrí, A.; López, A.; Segura, M.; Benítez, Á.; Garrido, D.; Palma, F.; Martínez, C.; Jamilena, M. A QTL on Chromosome 17 Identified by Genome-Wide Association Mapping Controls Postharvest Cold Tolerance of Cucurbita pepo L. Physiol. Plant. 2024, 176, e14602. [Google Scholar] [CrossRef]
- Richardson, L.G.L.; Schnell, D.J. Origins, Function, and Regulation of the TOC–TIC General Protein Import Machinery of Plastids. J. Exp. Bot. 2020, 71, 1226–1238. [Google Scholar] [CrossRef]
- Nishimura, K.; Kato, Y.; Sakamoto, W. Chloroplast Proteases: Updates on Proteolysis within and across Suborganellar Compartments. Plant Physiol. 2016, 171, 2280–2293. [Google Scholar] [CrossRef]
- Pulido, P.; Llamas, E.; Llorente, B.; Ventura, S.; Wright, L.P.; Rodríguez-Concepción, M. Specific Hsp100 Chaperones Determine the Fate of the First Enzyme of the Plastidial Isoprenoid Pathway for Either Refolding or Degradation by the Stromal Clp Protease in Arabidopsis. PLoS Genet. 2016, 12, e1005824. [Google Scholar] [CrossRef]
- Welsch, R.; Zhou, X.; Yuan, H.; Álvarez, D.; Sun, T.; Schlossarek, D.; Yang, Y.; Shen, G.; Zhang, H.; Rodriguez-Concepcion, M.; et al. Clp Protease and OR Directly Control the Proteostasis of Phytoene Synthase, the Crucial Enzyme for Carotenoid Biosynthesis in Arabidopsis. Mol. Plant 2018, 11, 149–162. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, S.; Ren, Y.; Zhang, H.; Gong, G.; Zhou, M.; Wang, G.; Zong, M.; He, H.; Liu, F.; et al. High-level Expression of a Novel Chromoplast Phosphate Transporter ClPHT4;2 Is Required for Flesh Color Development in Watermelon. New Phytol. 2017, 213, 1208–1221. [Google Scholar] [CrossRef]
- Lu, P.; Wang, S.; Grierson, D.; Xu, C. Transcriptomic Changes Triggered by Carotenoid Biosynthesis Inhibitors and Role of Citrus Sinensis Phosphate Transporter 4;2 (CsPHT4;2) in Enhancing Carotenoid Accumulation. Planta 2019, 249, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.-L.; Zhou, J.-Y.; Huang, Y.-N.; Wang, H.-R.; Li, X.-H.; Guo, H.-L.; Liu, J.-X. Roles of Plastid-Located Phosphate Transporters in Carotenoid Accumulation. Front. Plant Sci. 2022, 13, 1059536. [Google Scholar] [CrossRef] [PubMed]
- Lurin, C.; Andreés, C.; Aubourg, S.; Bellaoui, M.; Bitton, F.; Bruyère, C.; Caboche, M.; Debast, C.; Gualberto, J.; Hoffmann, B.; et al. Genome-Wide Analysis of Arabidopsis Pentatricopeptide Repeat Proteins Reveals Their Essential Role in Organelle Biogenesis. Plant Cell 2004, 16, 2089–2103. [Google Scholar] [CrossRef]
- Galpaz, N.; Gonda, I.; Shem-Tov, D.; Barad, O.; Tzuri, G.; Lev, S.; Fei, Z.; Xu, Y.; Mao, L.; Jiao, C.; et al. Deciphering Genetic Factors That Determine Melon Fruit-quality Traits Using RNA -Seq-based High-resolution QTL and eQTL Mapping. Plant J. 2018, 94, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mao, L.; Yang, B.; Cui, Q.; Dai, Y.; Li, X.; Chen, Y.; Dai, X.; Zou, X.; Ou, L.; et al. A Multi-Omics Approach Identifies bHLH71-like as a Positive Regulator of Yellowing Leaf Pepper Mutants Exposed to High-Intensity Light. Hortic. Res. 2023, 10, uhad098. [Google Scholar] [CrossRef]
- Park, G.; Shahwar, D.; Jang, G.; Shin, J.; Kwon, G.; Kim, Y.; Hong, C.O.; Jin, B.; Kim, H.; Lee, O.; et al. Identification of a Novel Locus C2 Controlling Canary Yellow Flesh Color in Watermelons. Front. Genet. 2023, 14, 1256627. [Google Scholar] [CrossRef]
- Subburaj, S.; Tu, L.; Lee, K.; Park, G.-S.; Lee, H.; Chun, J.-P.; Lim, Y.-P.; Park, M.-W.; McGregor, C.; Lee, G.-J. A Genome-Wide Analysis of the Pentatricopeptide Repeat (PPR) Gene Family and PPR-Derived Markers for Flesh Color in Watermelon (Citrullus lanatus). Genes 2020, 11, 1125. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, Z.; Hu, G.; Yuan, L.; Liu, M.; Zhang, X.; Wei, C.; Dai, Z.; Yang, Z.; Wang, C.; et al. Clpf Encodes Pentatricopeptide Repeat Protein (PPR5) and Regulates Pink Flesh Color in Watermelon (Citrullus lanatus L.). Theor. Appl. Genet. 2024, 137, 126. [Google Scholar] [CrossRef]

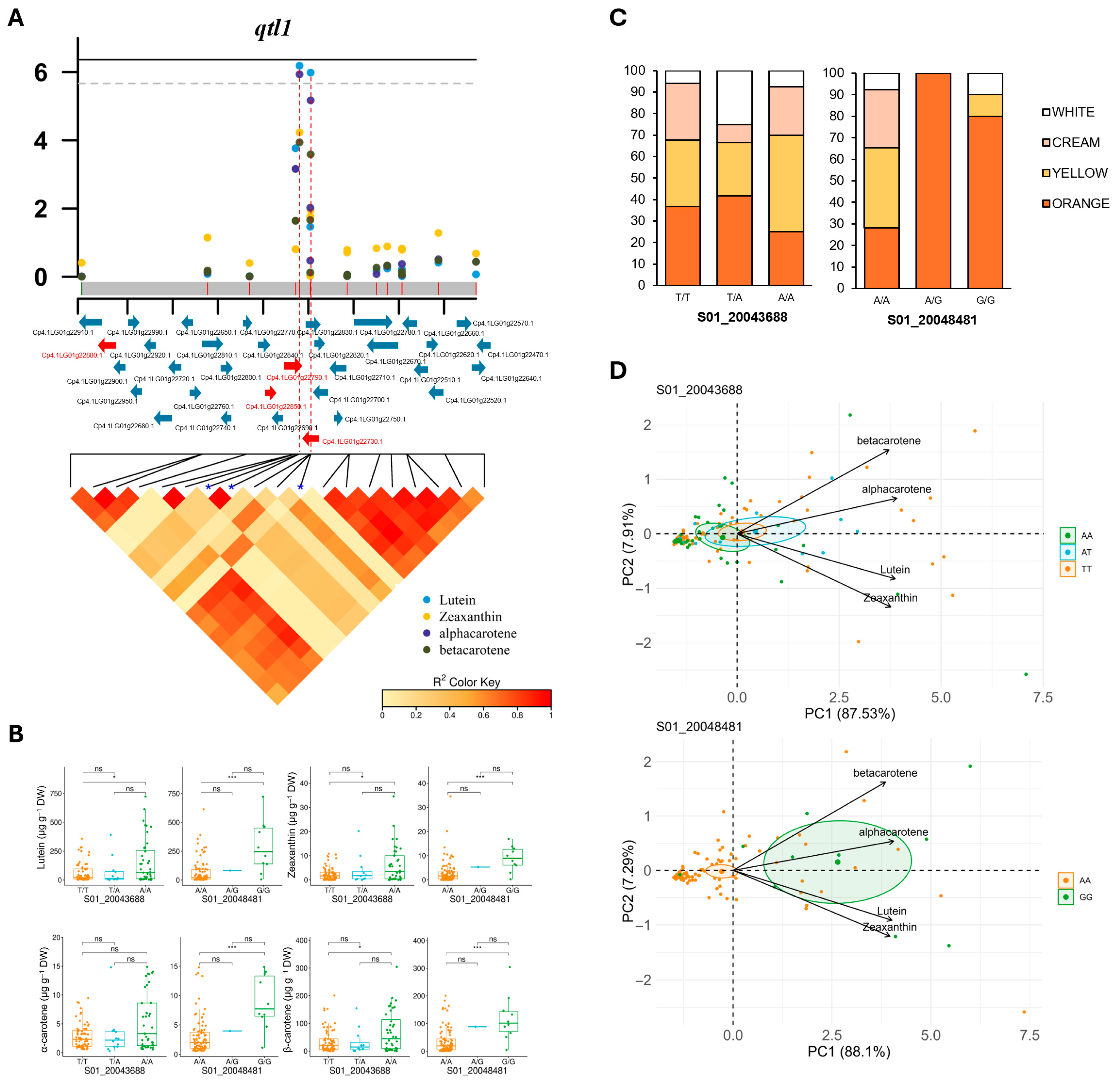
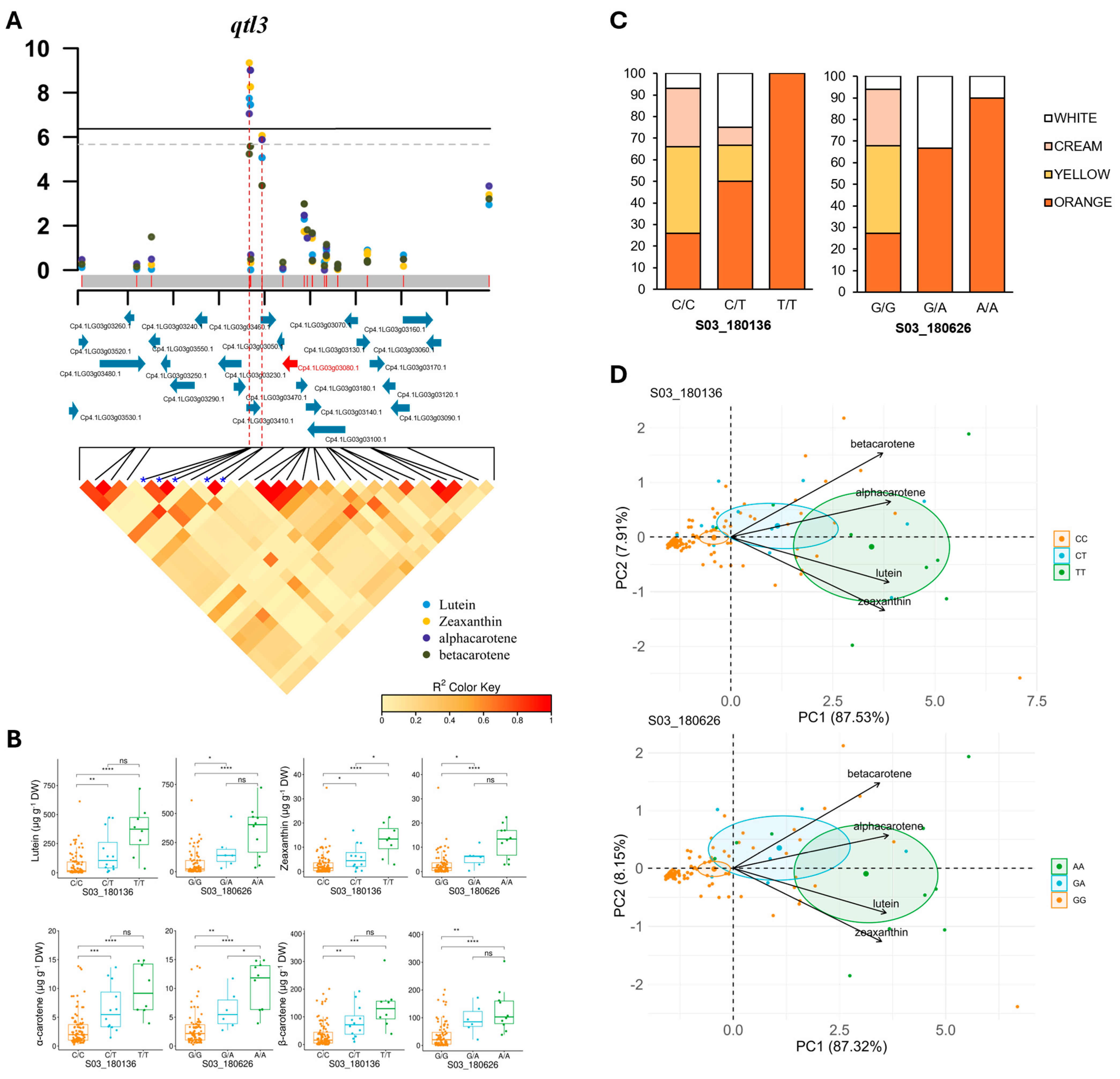
| Locus | SNP | Alleles | Lutein | Zeaxanthin | α-Carotene | β-Carotene | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | PVE | p-Value | PVE | p-Value | PVE | p-Value | PVE | |||
| qtl1 | S01_20043687 | C/T | 6.50 × 10−7 | 0.18 | 5.86 × 10−5 | 0.14 | 1.16 × 10−6 | 0.20 | 1.15 × 10−4 | 0.18 |
| S01_20043688 | T/A | 6.50 × 10−7 | 0.18 | 5.86 × 10−5 | 0.14 | 1.16 × 10−6 | 0.20 | 1.15 × 10−4 | 0.18 | |
| S01_20048481 | A/G | 1.04 × 10−6 | 0.21 | 1.11 × 10−2 | 0.09 | 6.75 × 10−6 | 0.21 | 2.59 × 10−4 | 0.20 | |
| qtl3 | S03_180136 | C/T | 1.80 × 10−8 | 0.24 | 4.53 × 10−10 | 0.29 | 8.96 × 10−8 | 0.23 | 5.83 × 10−6 | 0.23 |
| S03_180626 | G/A | 3.51 × 10−8 | 0.28 | 5.49 × 10−9 | 0.25 | 9.81 × 10−10 | 0.34 | 2.65 × 10−6 | 0.29 | |
| S03_180668 | C/T | 3.51 × 10−8 | 0.28 | 5.49 × 10−9 | 0.31 | 9.81 × 10−10 | 0.34 | 2.65 × 10−6 | 0.29 | |
| S03_185870 | C/T | 8.56 × 10−6 | 0.16 | 8.71 × 10−7 | 0.20 | 1.32 × 10−6 | 0.20 | 1.56 × 10−4 | 0.18 | |
| S03_185886 | T/C | 8.56 × 10−6 | 0.16 | 8.71 × 10−7 | 0.20 | 1.32 × 10−6 | 0.20 | 1.56 × 10−4 | 0.18 | |
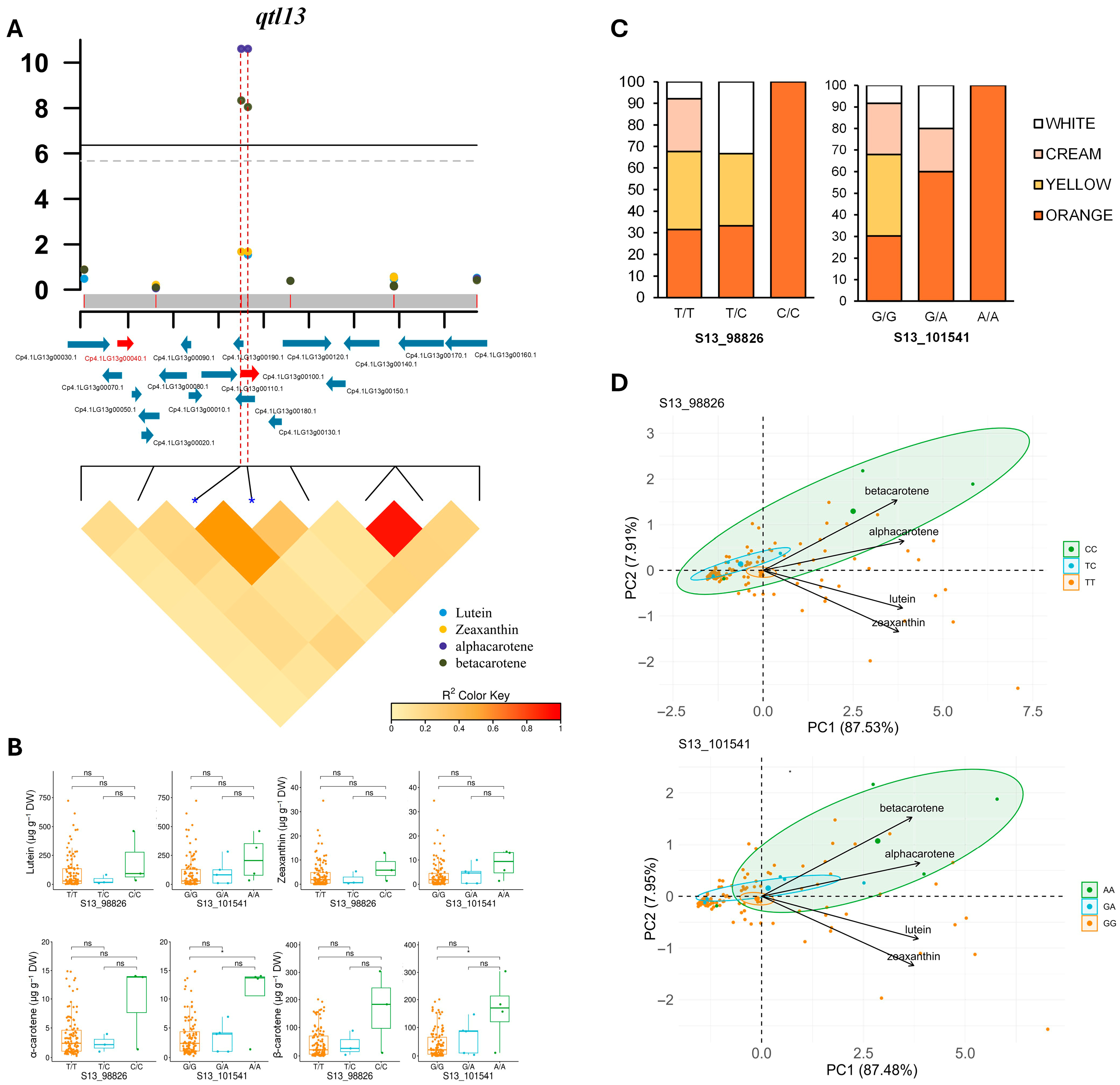
| qtl13 | S13_98826 | T/C | 2.85 × 10−2 | 0.05 | 2.10 × 10−2 | 0.06 | 2.48 × 10−11 | 0.37 | 4.59 × 10−9 | 0.36 |
| S13_101541 | G/A | 2.85 × 10−2 | 0.05 | 2.10 × 10−2 | 0.06 | 2.48 × 10−11 | 0.37 | 8.91 × 10−9 | 0.36 | |
| QTL | Gene | Description | Trait |
|---|---|---|---|
| qtl1 | Cp4.1LG01g22880 | Protease Do-like 8, chloroplast (DegP8/PDL8) | Lutein |
| Cp4.1LG01g22850 | Transcription factor MYB106-like | Zeaxanthin | |
| Cp4.1LG01g22790 | SPX domain-containing protein | β-carotene | |
| Cp4.1LG01g22730 | Phosphate transporter PHO1 homolog 9-like | α-carotene | |
| qtl3 | Cp4.1LG03g03080 | Protein TIC 56, chloroplast | Lutein |
| Zeaxanthin | |||
| β-carotene | |||
| α-carotene | |||
| qtl13 | Cp4.1LG13g00040 | Heat shock protein 70, chloroplast | β-carotene |
| Cp4.1LG13g00100 | Pentatricopeptide repeat (PPR) proteins | α-carotene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, A.; García, A.; Castro-Cegrí, A.; Segura, M.; Benítez, Á.; Palma, F.; Garrido, D.; Martínez, C.; Jamilena, M. A Genome-Wide Association Study Reveals QTLs and Candidate Genes Associated with the Carotenoid Content in the Flesh of Cucurbita pepo L. Fruit. Antioxidants 2025, 14, 1090. https://doi.org/10.3390/antiox14091090
López A, García A, Castro-Cegrí A, Segura M, Benítez Á, Palma F, Garrido D, Martínez C, Jamilena M. A Genome-Wide Association Study Reveals QTLs and Candidate Genes Associated with the Carotenoid Content in the Flesh of Cucurbita pepo L. Fruit. Antioxidants. 2025; 14(9):1090. https://doi.org/10.3390/antiox14091090
Chicago/Turabian StyleLópez, Alba, Alicia García, Alejandro Castro-Cegrí, María Segura, Álvaro Benítez, Francisco Palma, Dolores Garrido, Cecilia Martínez, and Manuel Jamilena. 2025. "A Genome-Wide Association Study Reveals QTLs and Candidate Genes Associated with the Carotenoid Content in the Flesh of Cucurbita pepo L. Fruit" Antioxidants 14, no. 9: 1090. https://doi.org/10.3390/antiox14091090
APA StyleLópez, A., García, A., Castro-Cegrí, A., Segura, M., Benítez, Á., Palma, F., Garrido, D., Martínez, C., & Jamilena, M. (2025). A Genome-Wide Association Study Reveals QTLs and Candidate Genes Associated with the Carotenoid Content in the Flesh of Cucurbita pepo L. Fruit. Antioxidants, 14(9), 1090. https://doi.org/10.3390/antiox14091090







