Polynucleotide HPTTM-Based Hydrogels Exhibit Scavenging Activity Against Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Polynucleotide High Purification Technology (PN HPTTM)
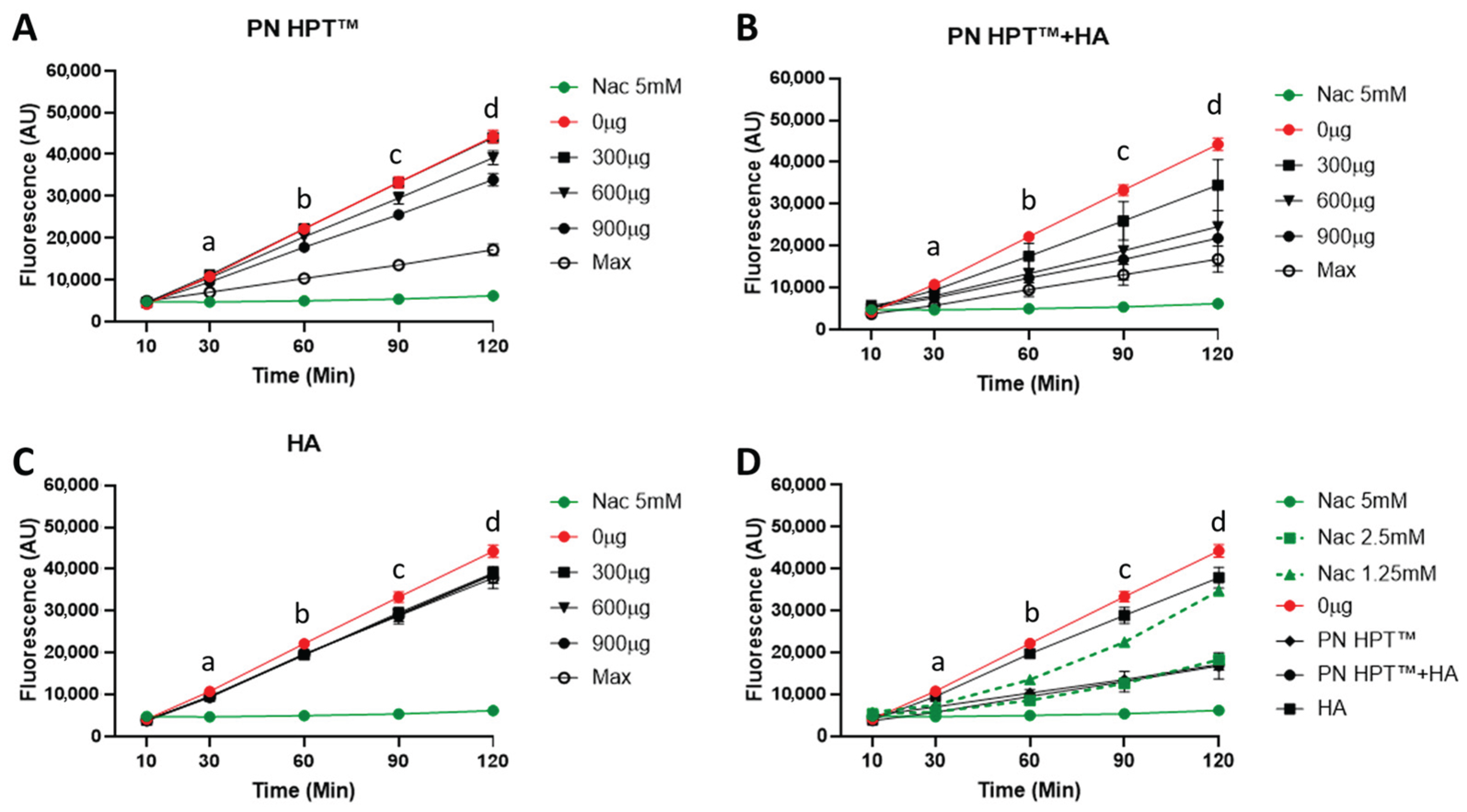
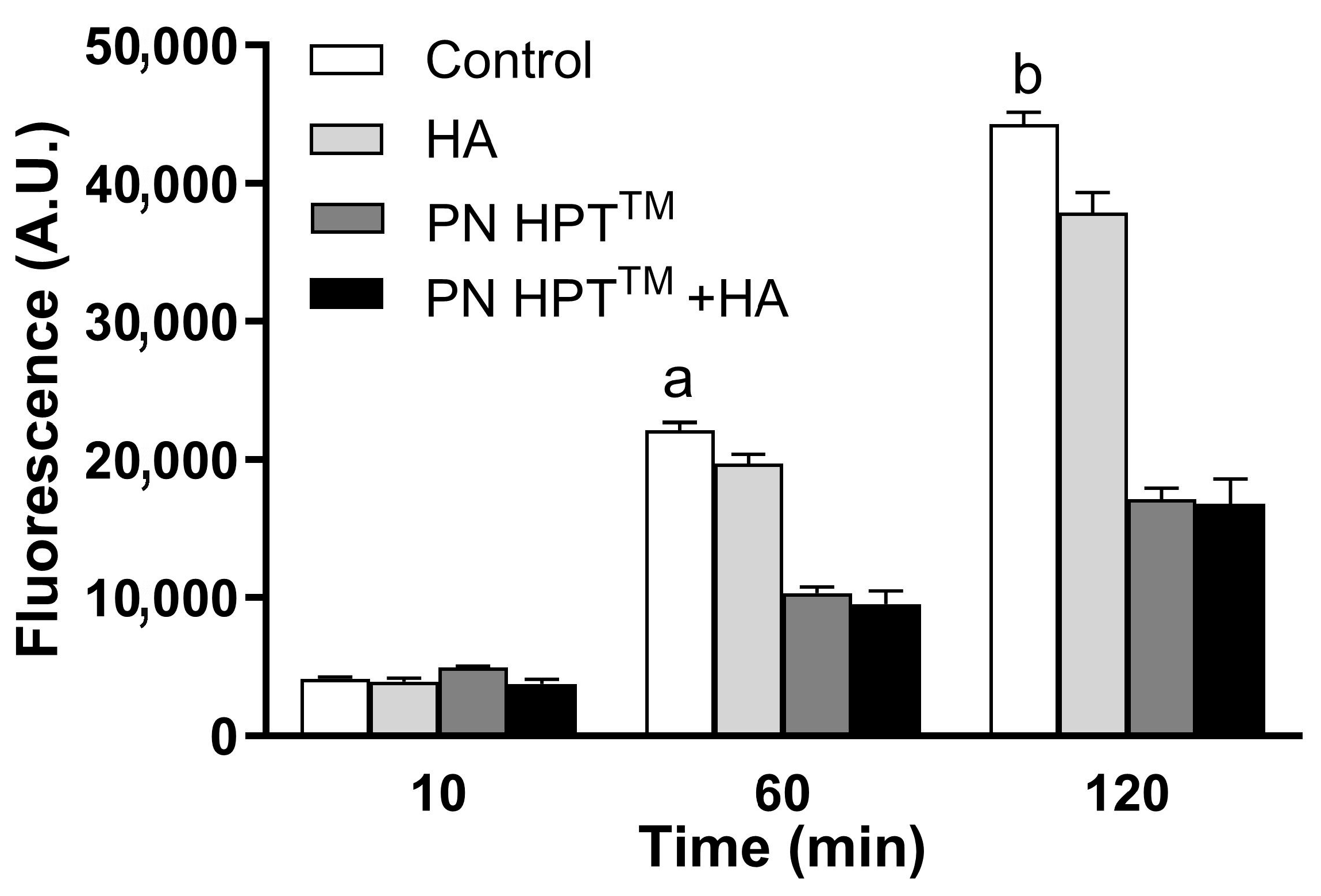
2.2. H2O2 Oxidative Stress Assay
2.3. ORAC Assay
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
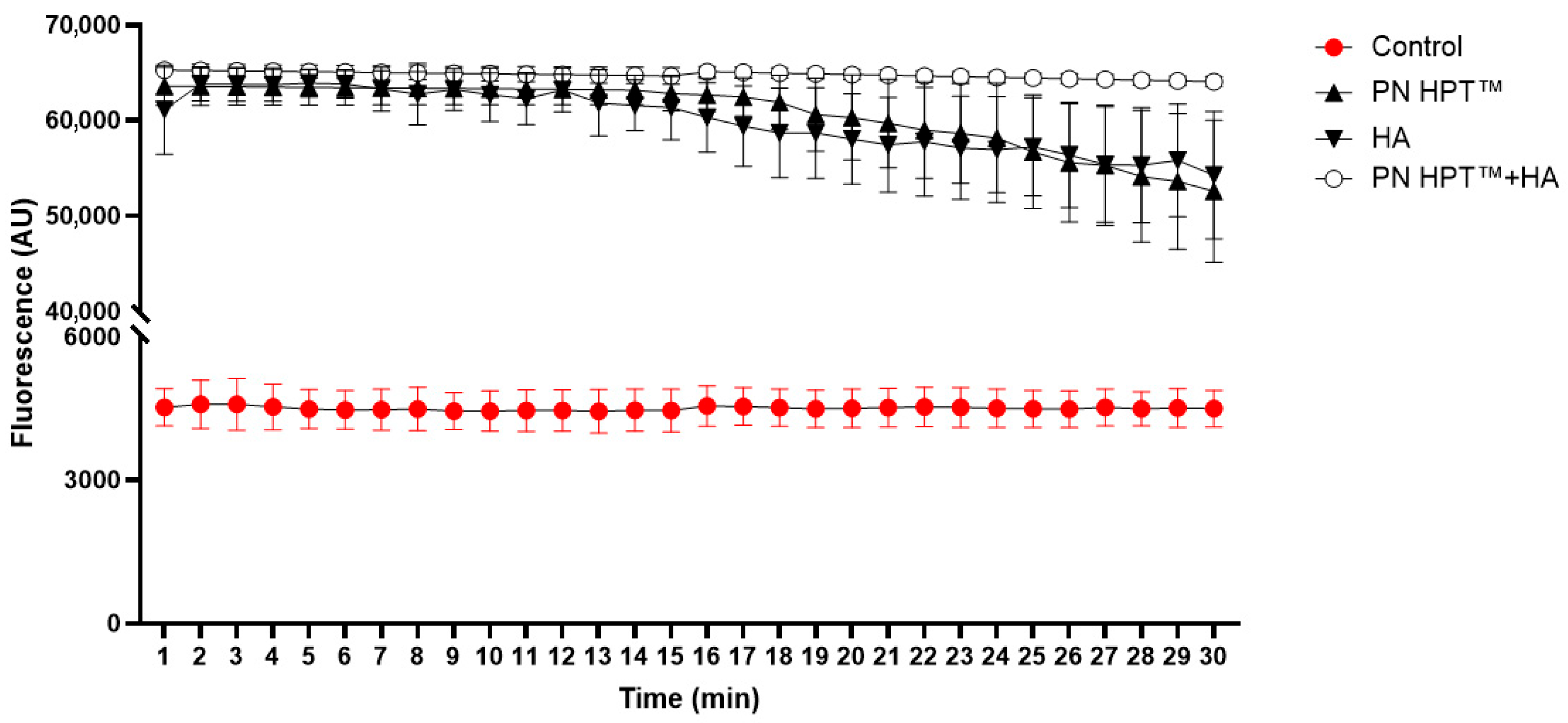
| Treatment | AUC |
|---|---|
| Control | 130,609 ± 12,069 |
| HA | 1,743,405 ± 97,672 |
| PN HPT™ | 1,763,275 ± 93,434 |
| PN HPT™ + HA | 1,878,686 ± 13,199 |
References
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Bateman, J.F.; Boot-Handford, R.P.; Lamandé, S.R. Genetic Diseases of Connective Tissues: Cellular and Extracellular Effects of ECM Mutations. Nat. Rev. Genet. 2009, 10, 173–183. [Google Scholar] [CrossRef]
- Jones, M.J.; Jones, M.C. Cell Cycle Control by Cell-Matrix Interactions. Curr. Opin. Cell Biol. 2024, 86, 102288. [Google Scholar] [CrossRef] [PubMed]
- Novoseletskaya, E.S.; Evdokimov, P.V.; Efimenko, A.Y. Extracellular Matrix-Induced Signaling Pathways in Mesenchymal Stem/Stromal Cells. Cell Commun. Signal. 2023, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kietzmann, T. Reactive Oxygen Species and Fibrosis: Further Evidence of a Significant Liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking Oxidative Stress and DNA Damage to Changes in the Expression of Extracellular Matrix Components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Lin, X.; Moreno, I.Y.; Nguyen, L.; Gesteira, T.F.; Coulson-Thomas, V.J. ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing. Biomolecules 2023, 13, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kennett, E.C.; Chuang, C.Y.; Degendorfer, G.; Whitelock, J.M.; Davies, M.J. Mechanisms and Consequences of Oxidative Damage to Extracellular Matrix. Biochem. Soc. Trans. 2011, 39, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Marangio, A.; Biccari, A.; D’Angelo, E.; Sensi, F.; Spolverato, G.; Pucciarelli, S.; Agostini, M. The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation? Cancers 2022, 14, 5903. [Google Scholar] [CrossRef]
- Moseley, R.; Waddington, R.J. Modification of Gingival Proteoglycans by Reactive Oxygen Species: Potential Mechanism of Proteoglycan Degradation during Periodontal Diseases. Free Radic. Res. 2021, 55, 970–981. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Kang, Y.; Wu, J.; Zhang, Z. Nanomaterial-Based Reactive Oxygen Species Scavengers for Osteoarthritis Therapy. Acta Biomater. 2023, 162, 1–19. [Google Scholar] [CrossRef]
- Wang, R.M.; Mesfin, J.M.; Hunter, J.; Cattaneo, P.; Guimarães-Camboa, N.; Braden, R.L.; Luo, C.; Hill, R.C.; Dzieciatkowska, M.; Hansen, K.C.; et al. Myocardial Matrix Hydrogel Acts as a Reactive Oxygen Species Scavenger and Supports a Proliferative Microenvironment for Cardiomyocytes. Acta Biomater. 2022, 152, 47–59. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, X.; Liu, J.; Hou, M.; Liu, Y.; Zhou, Z.; Xu, Y.; He, F.; Yang, H.; Zhang, Y.; et al. Cartilage-Inspired Self-Assembly Glycopeptide Hydrogels for Cartilage Regeneration via ROS Scavenging. Bioact. Mater. 2024, 32, 319–332. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Q.; Li, T.; Wang, W.; Li, Z.; Ji, Y.; Zhang, S.; Li, Y.; Liu, W.; Yu, Y. ECM-Mimetic Glucomannan Hydrogel Promotes Pressure Ulcer Healing by Scavenging ROS, Promoting Angiogenesis and Regulating Macrophages. Int. J. Biol. Macromol. 2024, 280, 135776. [Google Scholar] [CrossRef]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into Oxidative Stress in Bone Tissue and Novel Challenges for Biomaterials. Mater. Sci. Eng. C 2021, 130, 112433. [Google Scholar] [CrossRef] [PubMed]
- Buzoglu, H.D.; Burus, A.; Bayazıt, Y.; Goldberg, M. Stem Cell and Oxidative Stress-Inflammation Cycle. Curr. Stem Cell Res. Ther. 2023, 18, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Keskin-Erdogan, Z.; Sawadkar, P.; Nik Sharifulden, N.S.A.; Shannon, M.R.; Patel, M.; Silva, L.B.; Patel, R.; Chau, D.Y.S.; Knowles, J.C.; et al. Oxidative Stress Modulating Nanomaterials and Their Biochemical Roles in Nanomedicine. Nanoscale Horiz. 2024, 9, 1630–1682. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue ngineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Bin Abdullah, M.F.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental Properties of Smart Hydrogels for Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of Hydrogels for Regenerative Engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel Scaffolds in Bone Regeneration: Their Promising Roles in Angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Kamel, S. Polysaccharides-Based Injectable Hydrogels: Preparation, Characteristics, and Biomedical Applications. Colloids Interfaces 2022, 6, 78. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Guizzardi, S.; Laschera, L.; Meleti, M.; Galli, C. The Effects of Polynucleotides-Based Biomimetic Hydrogels in Tissue Repair: A 2D and 3D in Vitro Study. bioRxiv 2025. [Google Scholar] [CrossRef]
- Ye, S.; Wei, B.; Zeng, L. Advances on Hydrogels for Oral Science Research. Gels 2022, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing Functional Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, R.; Lu, W.; Shi, S.; Chen, Y. Framework Nucleic Acids as Promising Reactive Oxygen Species Scavengers for Anti-Inflammatory Therapy. Nanoscale 2024, 16, 7363–7377. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Govoni, P.; Belletti, S.; Squadrito, F.; Guizzardi, S.; Galli, C. Polynucleotide Biogel Enhances Tissue Repair, Matrix Deposition and Organization. J. Biol. Regul. Homeost. Agents 2021, 35, 355–362. [Google Scholar] [CrossRef]
- Maioli, L. Polynucleotides Highly Purified Technology and Nucleotides for the Acceleration and Regulation of Normal Wound Healing. Aesthetic Med. 2020, 6, 48–52. [Google Scholar]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.-H. Application of Hyaluronic Acid in Tissue Engineering, Regenerative Medicine, and Nanomedicine: A Review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes Wound Healing in a Primary Gingival Fibroblast Model. Appl. Sci. 2021, 11, 4405. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Vicedomini, M.L.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts. Appl. Sci. 2023, 13, 743. [Google Scholar] [CrossRef]
- Guizzardi, S.; Uggeri, J.; Belletti, S.; Cattarini, G. Hyaluronate Increases Polynucleotides Effect on Human Cultured Fibroblasts. J. Cosmet. Dermatol. Sci. Appl. 2013, 03, 124–128. [Google Scholar] [CrossRef]
- De Caridi, G.; Massara, M.; Acri, I.; Zavettieri, S.; Grande, R.; Butrico, L.; de Franciscis, S.; Serra, R. Trophic Effects of Polynucleotides and Hyaluronic Acid in the Healing of Venous Ulcers of the Lower Limbs: A Clinical Study. Int. Wound J. 2016, 13, 754–758. [Google Scholar] [CrossRef]
- Segreto, F.; Carotti, S.; Marangi, G.F.; Francesconi, M.; Scaramuzzino, L.; Gratteri, M.; Caldaria, E.; Morini, S.; Persichetti, P. The Use of Acellular Porcine Dermis, Hyaluronic Acid and Polynucleotides in the Treatment of Cutaneous Ulcers: Single Blind Randomised Clinical Trial. Int. Wound J. 2020, 17, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Di Stefano, A.; Capogrosso, F.; Carniel, R.; Haidar Hassan, K.; Bellomo, R.G. Viscosupplementation with Hyaluronic Acid or Polynucleotides: Results and Hypothesis for Condro-Synchronization. J. Clin. Trials 2014, 4, 2167–2870. [Google Scholar]
- Migliore, A.; Graziano, E.; Martín, L.S.M.; Sorbino, A.; Raichi, M.; Boni, G. Three-Year Management of Hip Osteoarthritis with Intra-Articular Polynucleotides: A Real-Life Cohort Retrospective Study. J. Biol. Regul. Homeost. Agents 2021, 35, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Palmieri, I.P.; Papagni, M. Value and Benefits of the Polynucleotides HPTTM Dermal Priming Paradigm: A Consensus on Practice Guidance for Aesthetic Medicine Practitioners and Future Research. Clin. Exp. Dermatol. Ther. 2024, 9, 224. [Google Scholar]
- Cenzato, N.; Crispino, R.; Russillo, A.; Del Fabbro, M.; Tartaglia, G.M. Clinical Effectiveness of Polynucleotide TMJ Injection Compared with Physiotherapy: A 3-Month Randomised Clinical Trial. Br. J. Oral Maxillofac. Surg. 2024, 62, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, M.; Fabbrini, R.; Guelfi, M.G. Intra-Articular Treatment of Knee and Ankle Osteoarthritis with Polynucleotides: Prospective Case Record Cohort vs Historical Controls. J. Biol. Regul. Homeost. Agents 2020, 34, 1949–1953. [Google Scholar] [CrossRef]
- Dallari, D.; Sabbioni, G.; Del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Fini, M. Efficacy of Intra-Articular Polynucleotides Associated with Hyaluronic Acid versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Clin. J. Sport Med. 2020, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Uggeri, J.; Gatti, R.; Belletti, S.; Scandroglio, R.; Corradini, R.; Rotoli, B.M.; Orlandini, G. Calcein-AM Is a Detector of Intracellular Oxidative Activity. Histochem. Cell Biol. 2000, 122, 499–505. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.; de la Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling—Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Buskiewicz, I.A.; Montgomery, T.; Yasewicz, E.C.; Huber, S.A.; Murphy, M.P.; Hartley, R.C.; Kelly, R.; Crow, M.K.; Perl, A.; Budd, R.C.; et al. Reactive Oxygen Species Induce Virus-Independent MAVS Oligomerization in Systemic Lupus Erythematosus. Sci. Signal. 2016, 9, ra115. [Google Scholar] [CrossRef]
- Rohnstock, A.; Lehmann, L. Evaluation of the Probe Dihydrocalcein Acetoxymethylester as an Indicator of Reactive Oxygen Species Formation and Comparison with Oxidative DNA Base Modification Determined by Modified Alkaline Elution Technique. Toxicol. Vitr. 2007, 21, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis Is an Inflammatory Disease of Oxidative Stress: We Should Treat It That Way. Periodontol. 2000 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Liu, H.; Zheng, Y.; Zhang, Z. Role of Oxidative Stress in the Relationship between Periodontitis and Systemic Diseases. Front. Physiol. 2023, 14, 1210449. [Google Scholar] [CrossRef] [PubMed]
- Matyushkin, A.I.; Ivanova, E.A.; Voronina, T.A. New Directions in the Development of Pharmacotherapy for Osteoarthrosis Based on Modern Concepts of the Disease Pathogenesis (A Review). Pharm. Chem. J. 2022, 55, 1282–1287. [Google Scholar] [CrossRef]
- Arra, M.; Abu-Amer, Y. Cross-Talk of Inflammation and Chondrocyte Intracellular Metabolism in Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1012–1021. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Si, H.; Wu, Y.; Zhou, S.; Shen, B. The Role Played by Ferroptosis in Osteoarthritis: Evidence Based on Iron Dyshomeostasis and Lipid Peroxidation. Antioxidants 2022, 11, 1668. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Guo, Z.; Wang, G.; Xu, J.; Zheng, Z.; Sun, K.; Guo, F. Lipid Peroxidation in Osteoarthritis: Focusing on 4-Hydroxynonenal, Malondialdehyde, and Ferroptosis. Cell Death Discov. 2023, 9, 320. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The Role of Oxidative Stress in the Development of Knee Osteoarthritis: A Comprehensive Research Review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Pia Palmieri, I.; Raichi, M. Biorevitalization of Postmenopausal Labia Majora, the Polynucleotide/Hyaluronic Acid Option. Obstet. Gynecol. Rep. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of Intra-Articular Polynucleotides in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; Del Piccolo, N.; Rani, N.; Govoni, M.; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, Double-Blind Comparison of a Fixed Co-Formulation of Intra-Articular Polynucleotides and Hyaluronic Acid versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: Two-Year Follow-Up. BMC Musculoskelet. Disord. 2021, 22, 773. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Cavalcanti, R.; Barbato, L.; Nieri, M.; Castelluzzo, W.; di Martino, M.; Pilloni, A. Polynucleotides and Hyaluronic Acid (PN-HA) Mixture With or Without Deproteinized Bovine Bone Mineral as a Novel Approach for the Treatment of Deep Infra-Bony Defects: A Retrospective Case-Series. Int. J. Periodontics Restor. Dent. 2024, 45, 1–24. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colangelo, M.T.; Belletti, S.; Guizzardi, S.; Galli, C. Polynucleotide HPTTM-Based Hydrogels Exhibit Scavenging Activity Against Reactive Oxygen Species. Antioxidants 2025, 14, 1089. https://doi.org/10.3390/antiox14091089
Colangelo MT, Belletti S, Guizzardi S, Galli C. Polynucleotide HPTTM-Based Hydrogels Exhibit Scavenging Activity Against Reactive Oxygen Species. Antioxidants. 2025; 14(9):1089. https://doi.org/10.3390/antiox14091089
Chicago/Turabian StyleColangelo, Maria Teresa, Silvana Belletti, Stefano Guizzardi, and Carlo Galli. 2025. "Polynucleotide HPTTM-Based Hydrogels Exhibit Scavenging Activity Against Reactive Oxygen Species" Antioxidants 14, no. 9: 1089. https://doi.org/10.3390/antiox14091089
APA StyleColangelo, M. T., Belletti, S., Guizzardi, S., & Galli, C. (2025). Polynucleotide HPTTM-Based Hydrogels Exhibit Scavenging Activity Against Reactive Oxygen Species. Antioxidants, 14(9), 1089. https://doi.org/10.3390/antiox14091089







