Hydrogen Sulfide Deficiency Contributes to Tubular Damage and Calcium Oxalate Crystal Formation in Hyperoxaluria Nephropathy: Role of Osteopontin and Tamm–Horsfall Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Hyperoxaluria
2.2. Preparation of a Mini-Osmotic Pump for Chronic H2S Donor Delivery
2.3. Metabolic Cage Study
2.4. Urinalysis for Crystalluria
2.5. CaOx Crystal Deposition in Rat Kidneys
2.6. Tissue Localization of H2S-Producing Enzymes
2.7. Determination of Tubular Damage Markers, THP, and OPN in Urine
2.8. Renal Tubular Cell Culture and Drug Treatment
2.9. Evaluation of Cytotoxicity and Cell Viability
2.10. Kinetics of CaOx Formation in Culture System
2.11. Measurement of cAMP Levels and PKA Activity
2.12. Cellular Distribution of OPN and THP
2.13. Determination of H2S Levels in Urine and Cell Culture Medium
2.14. Western Blot Analysis
2.15. Determination of Sp1 Activity
2.16. Quantitative Real-Time PCR for H2S-Producing Enzyme Expression
2.17. Statistics
3. Results
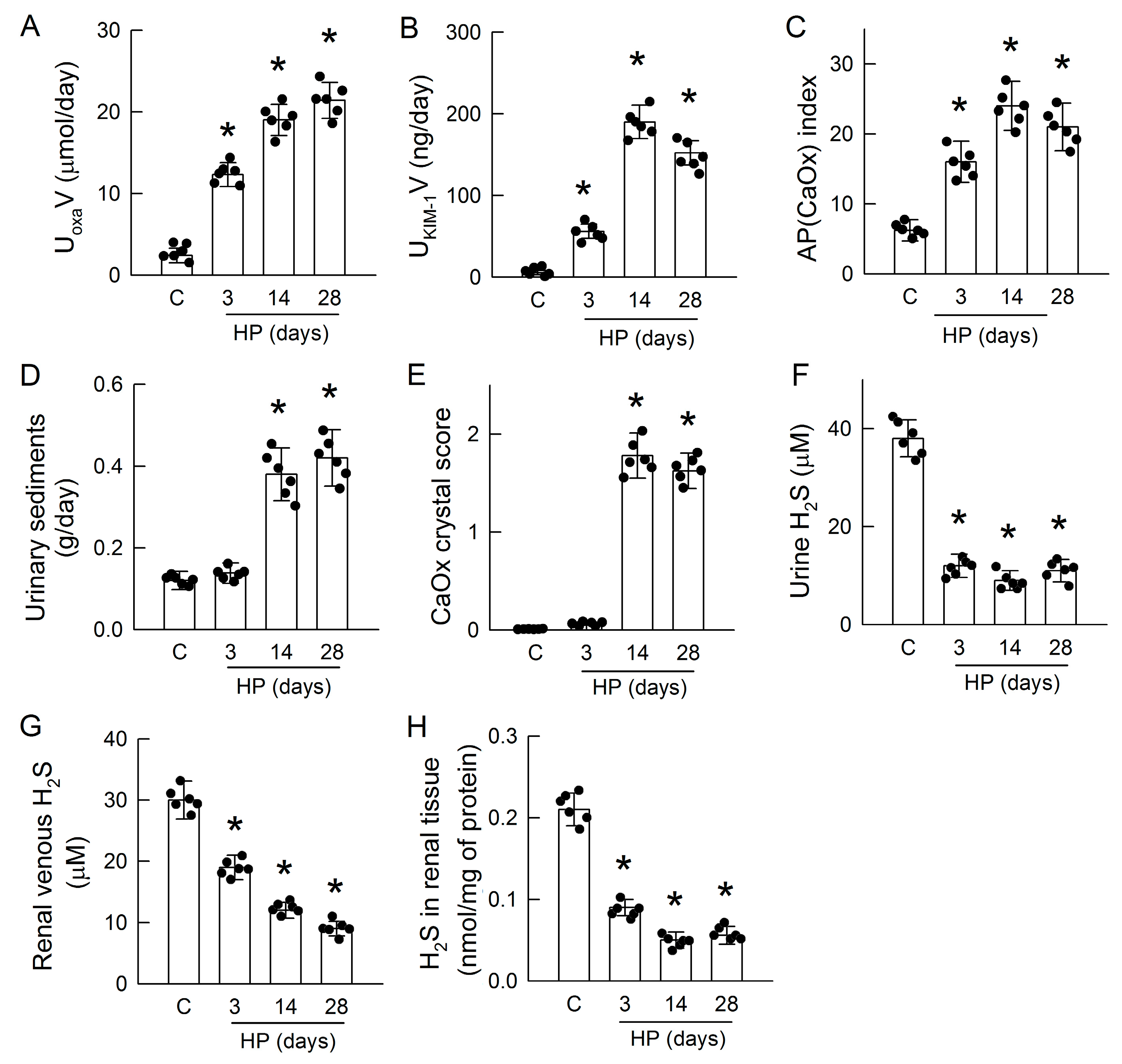
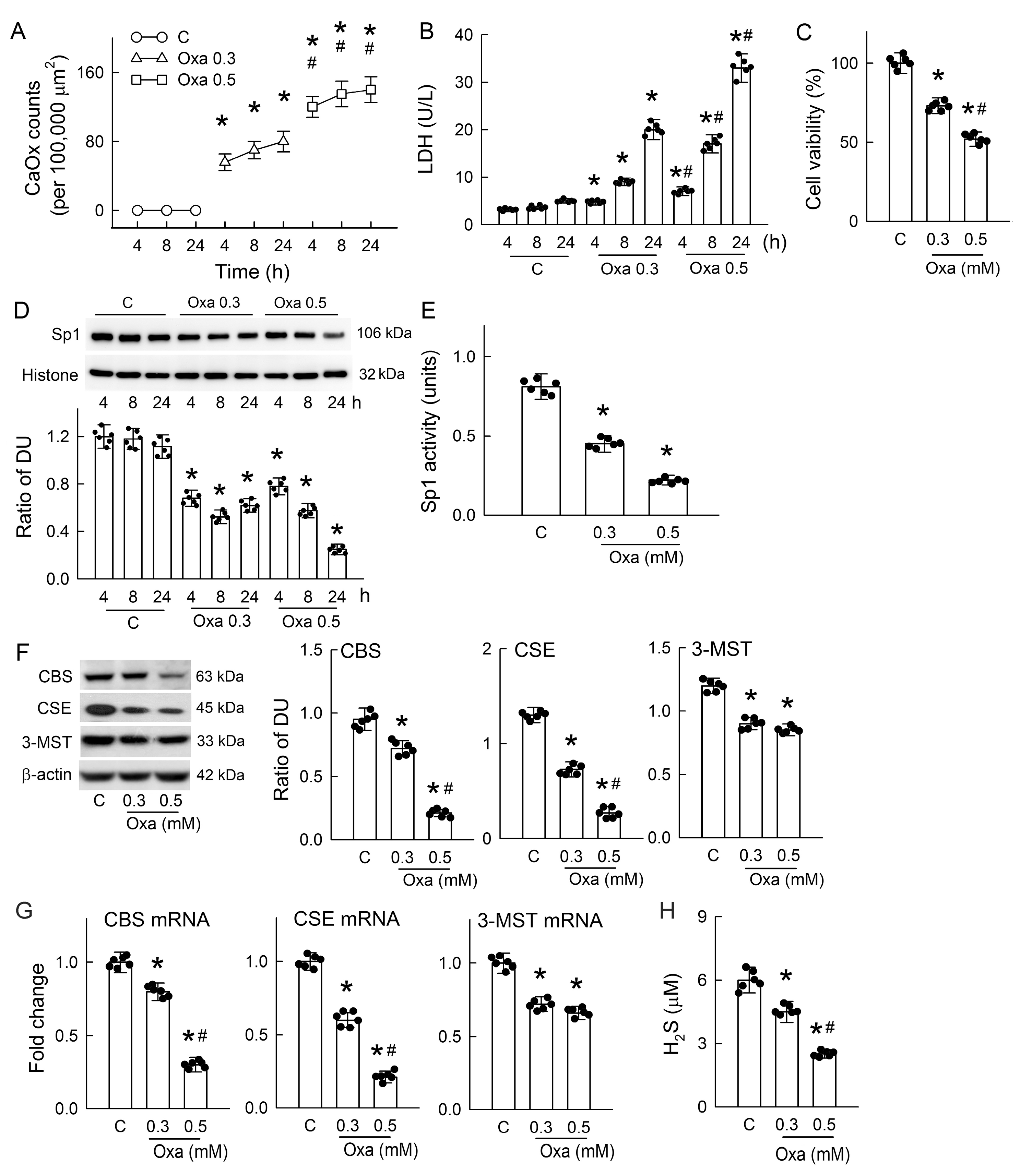
3.1. Hyperoxaluria Induces Kidney Injury, CaOx Crystal Deposition, and H2S Deficiency
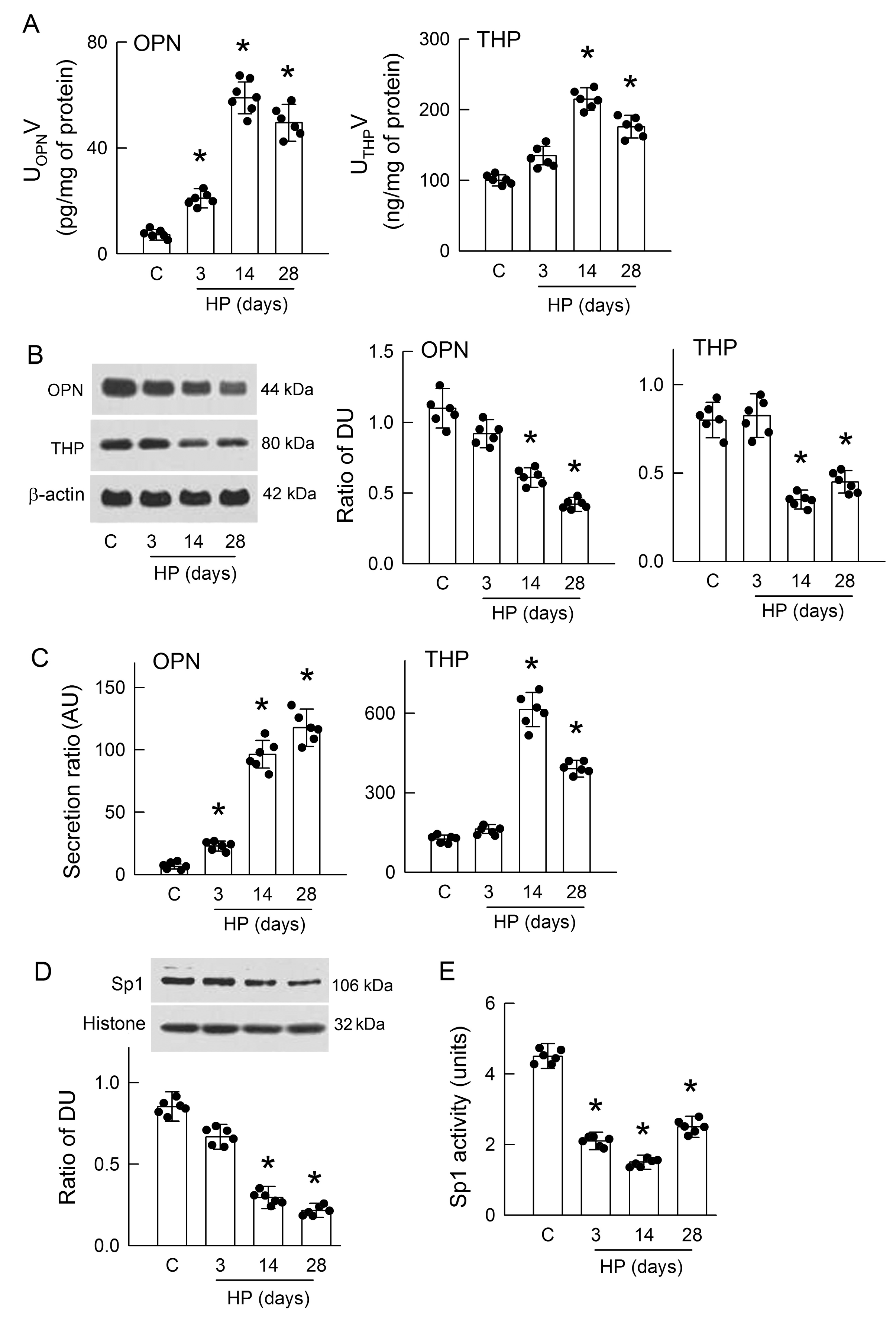
3.2. Hyperoxaluria Enhances Renal OPN and THP Excretion and Decreases Sp1 Activity
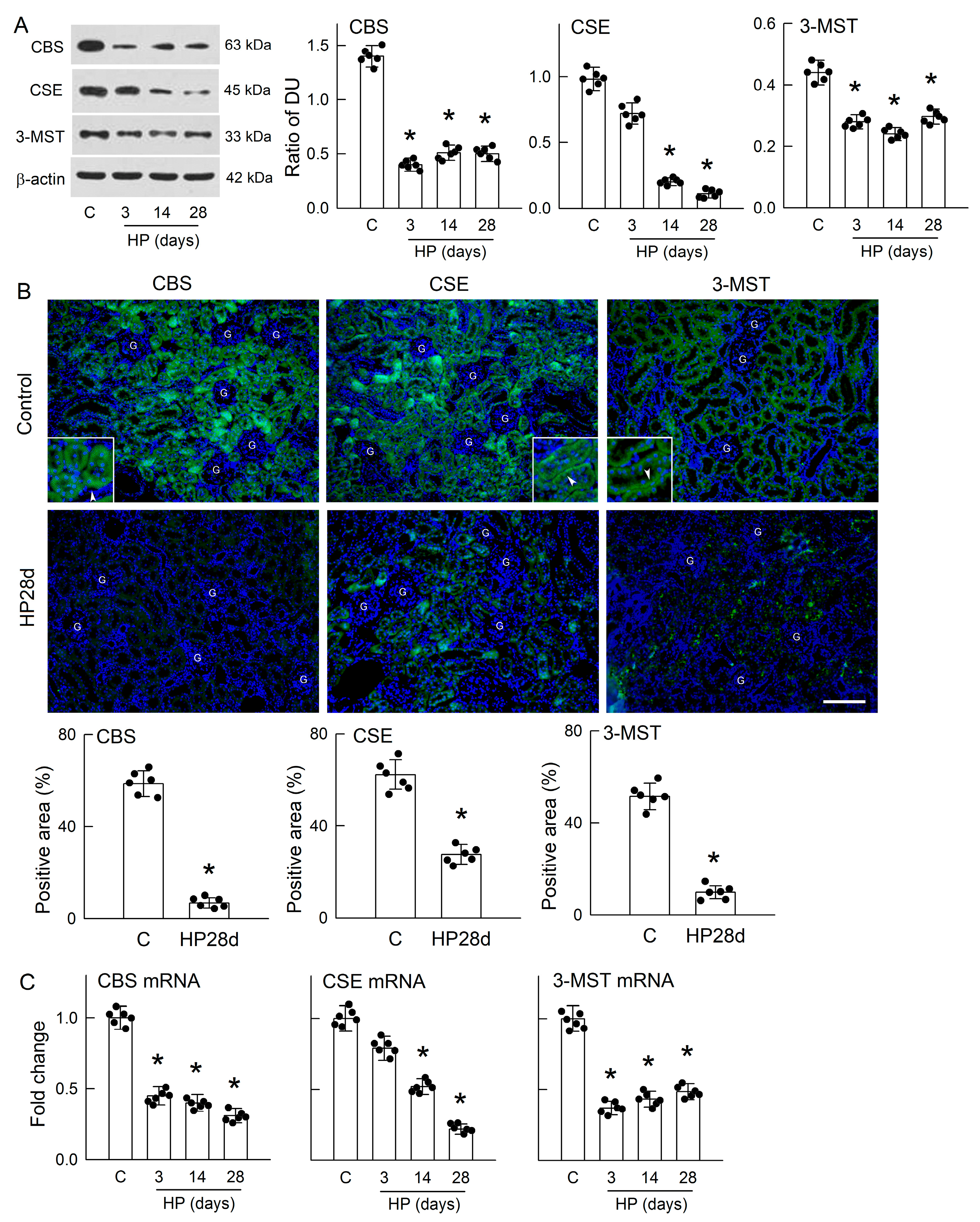
3.3. Hyperoxaluria Is Harmful for H2S-Producing Enzyme Expression
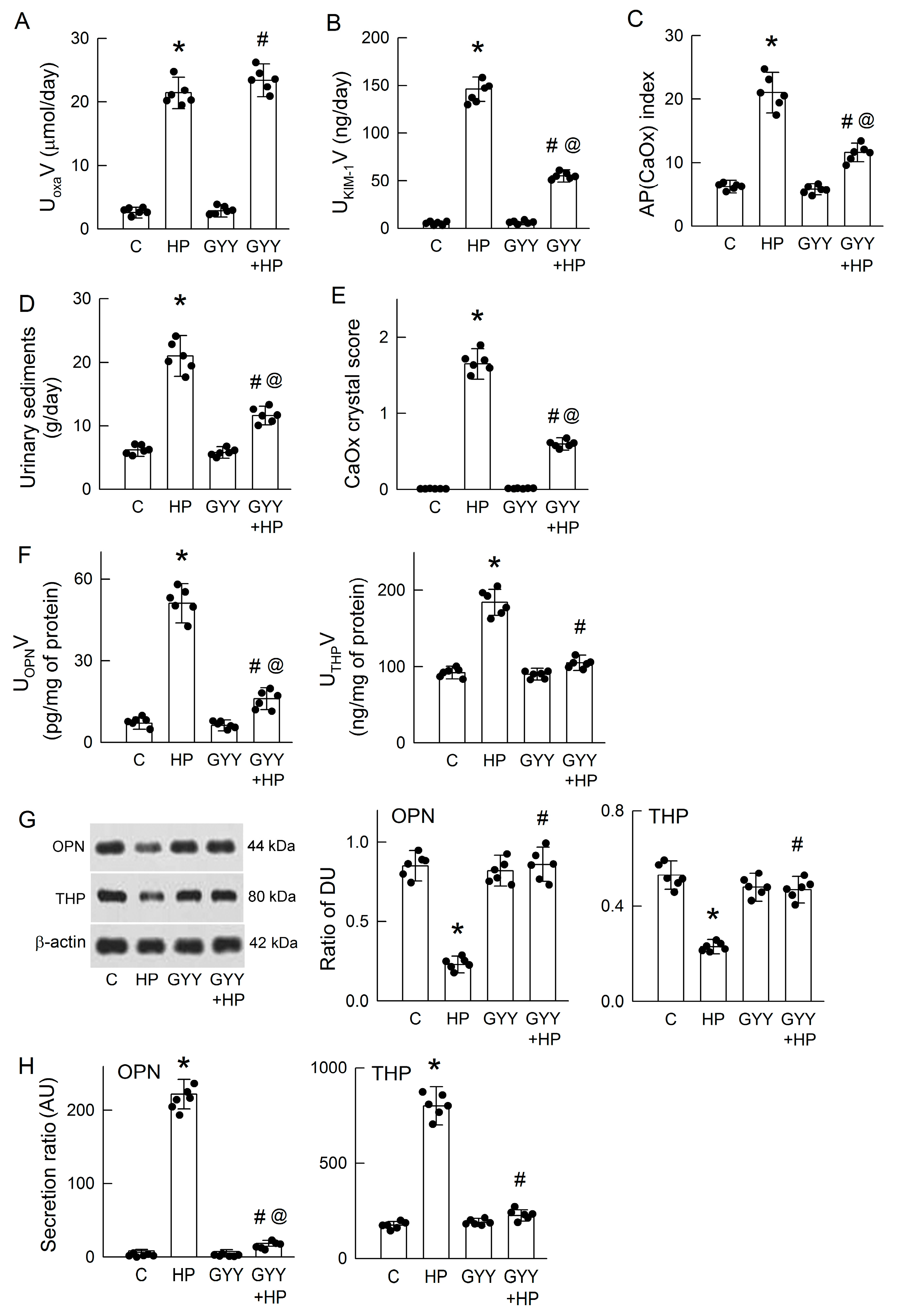
3.4. Chronic H2S Supplementation Ameliorates Hyperoxaluria-Induced Tubular Damage, CaOx Deposition, and the Secretion of OPN and THP
3.5. Oxalate-Induced Cytotoxicity and CaOx Formation in Renal Tubular Cells
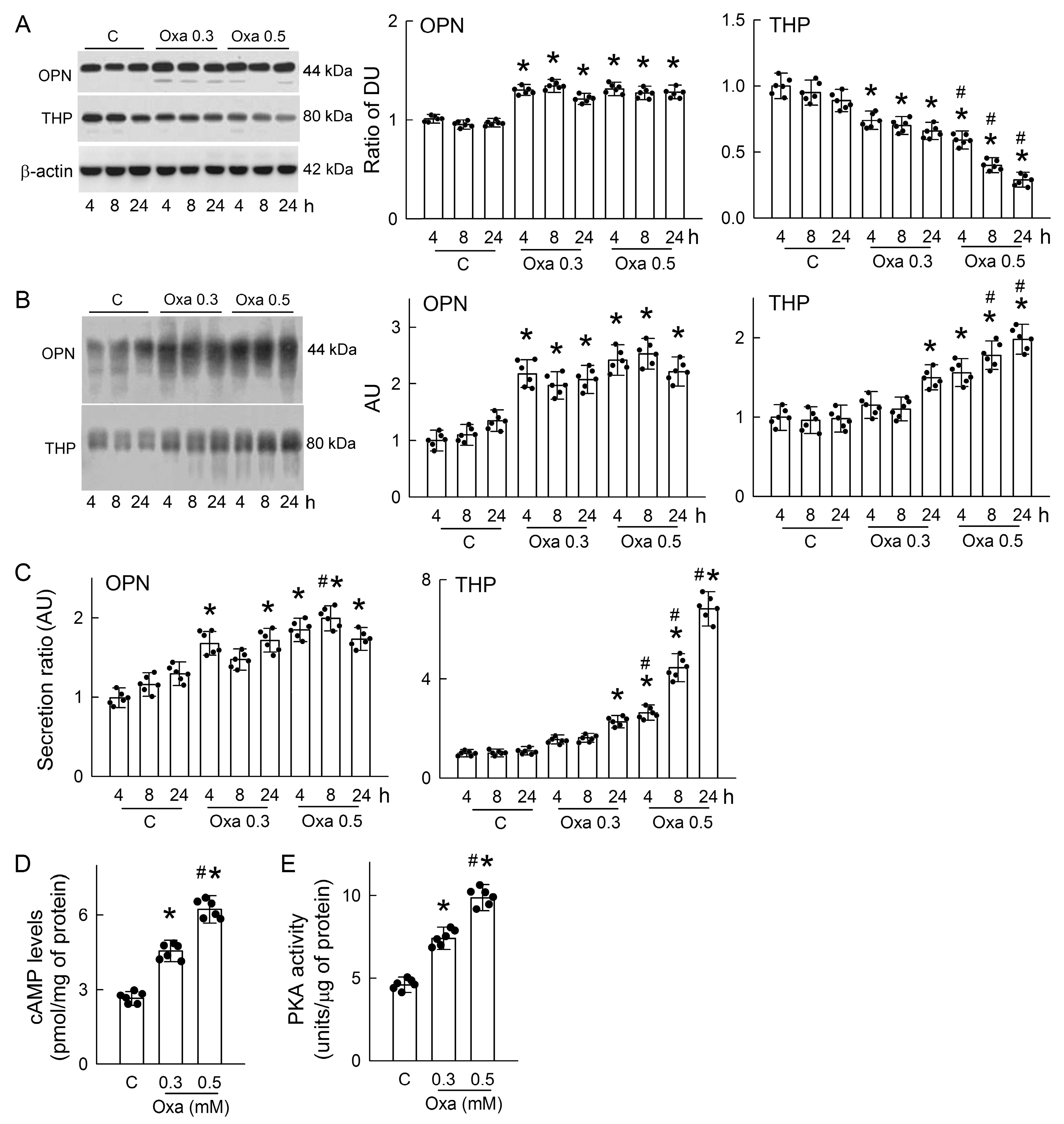
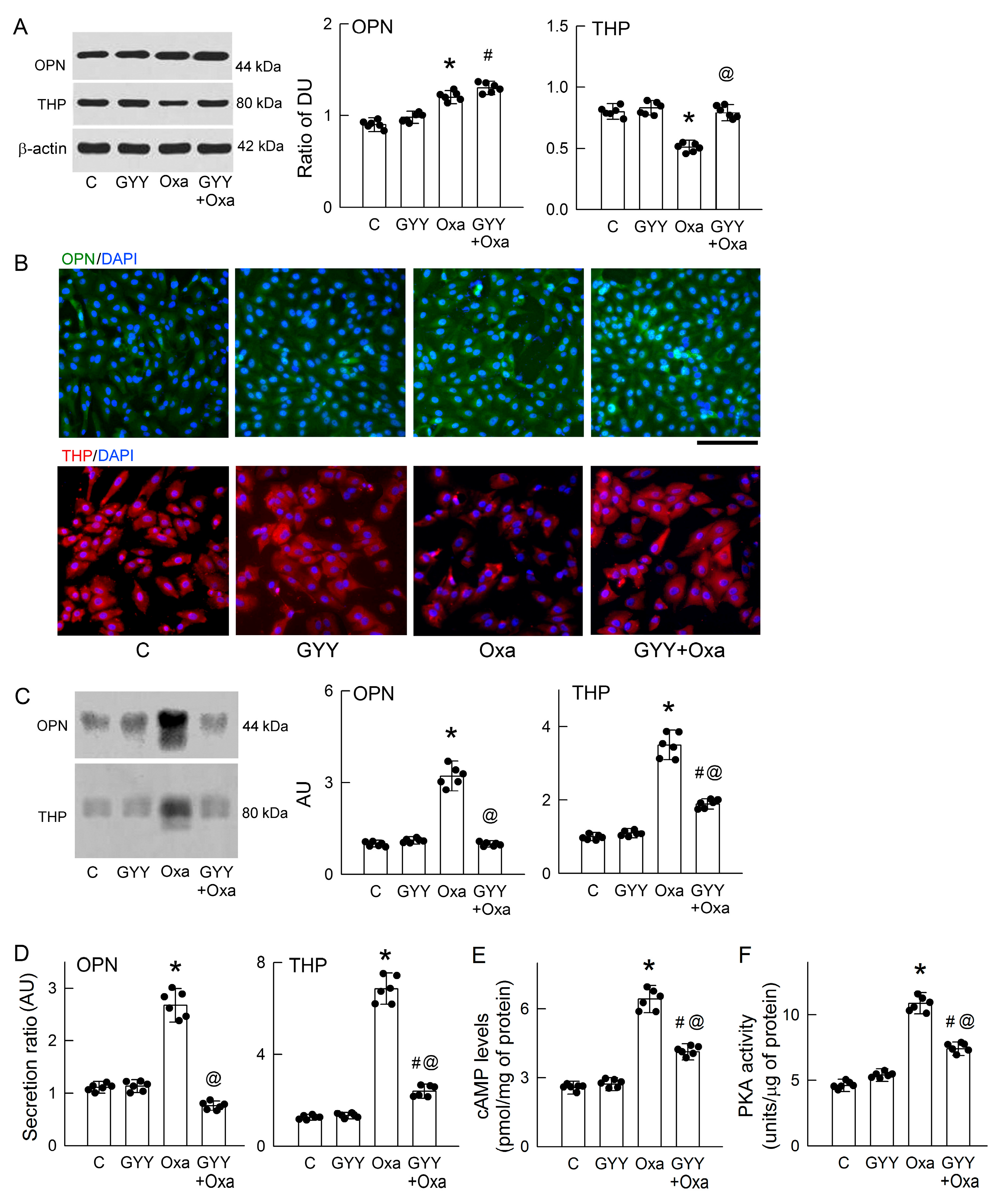
3.6. Oxalate Increases OPN and THP Secretion and Activates Protein Kinase A (PKA)
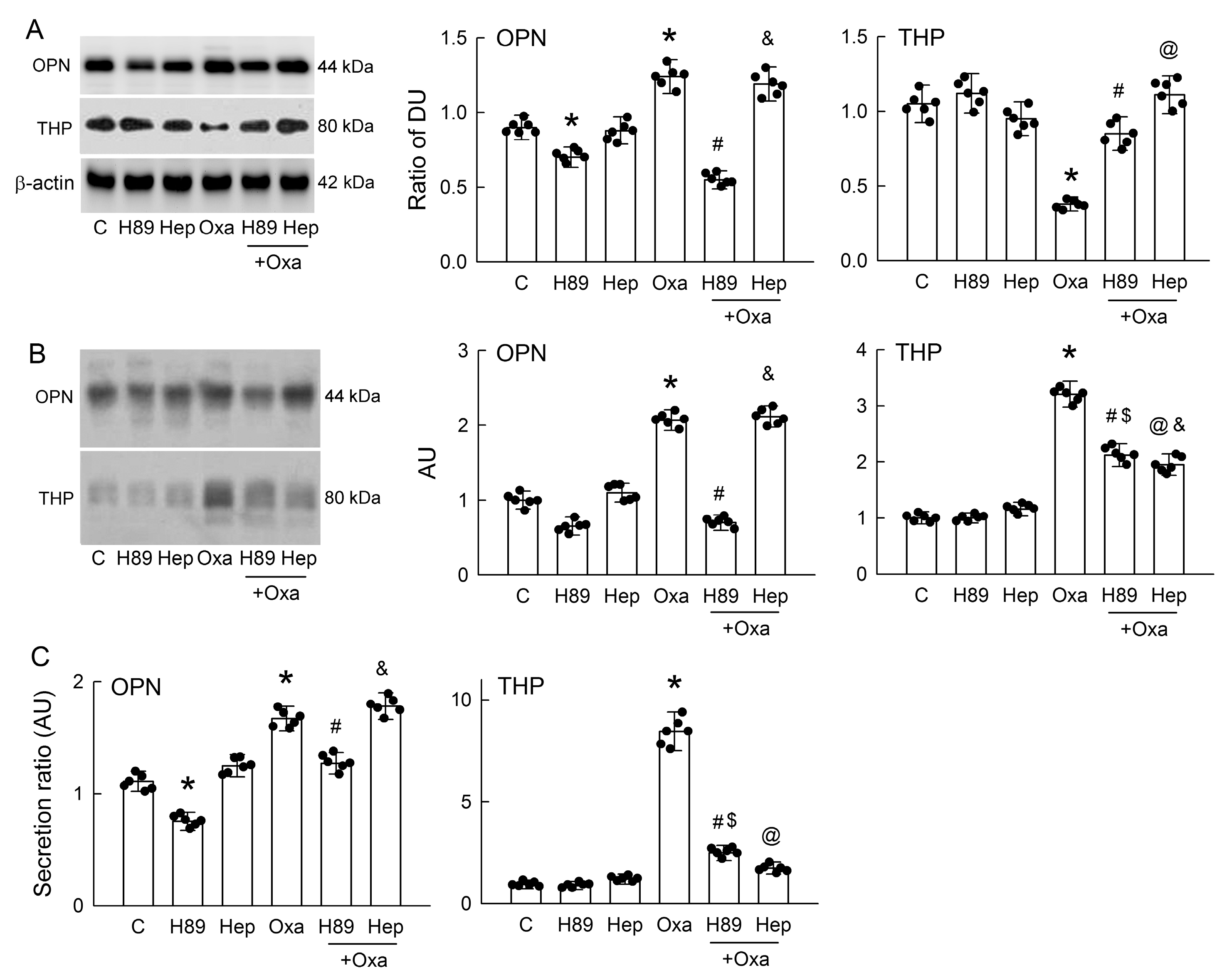
3.7. PKA Inhibition or Hepsin Blockade Attenuates Oxalate-Mediated THP and OPN Secretion
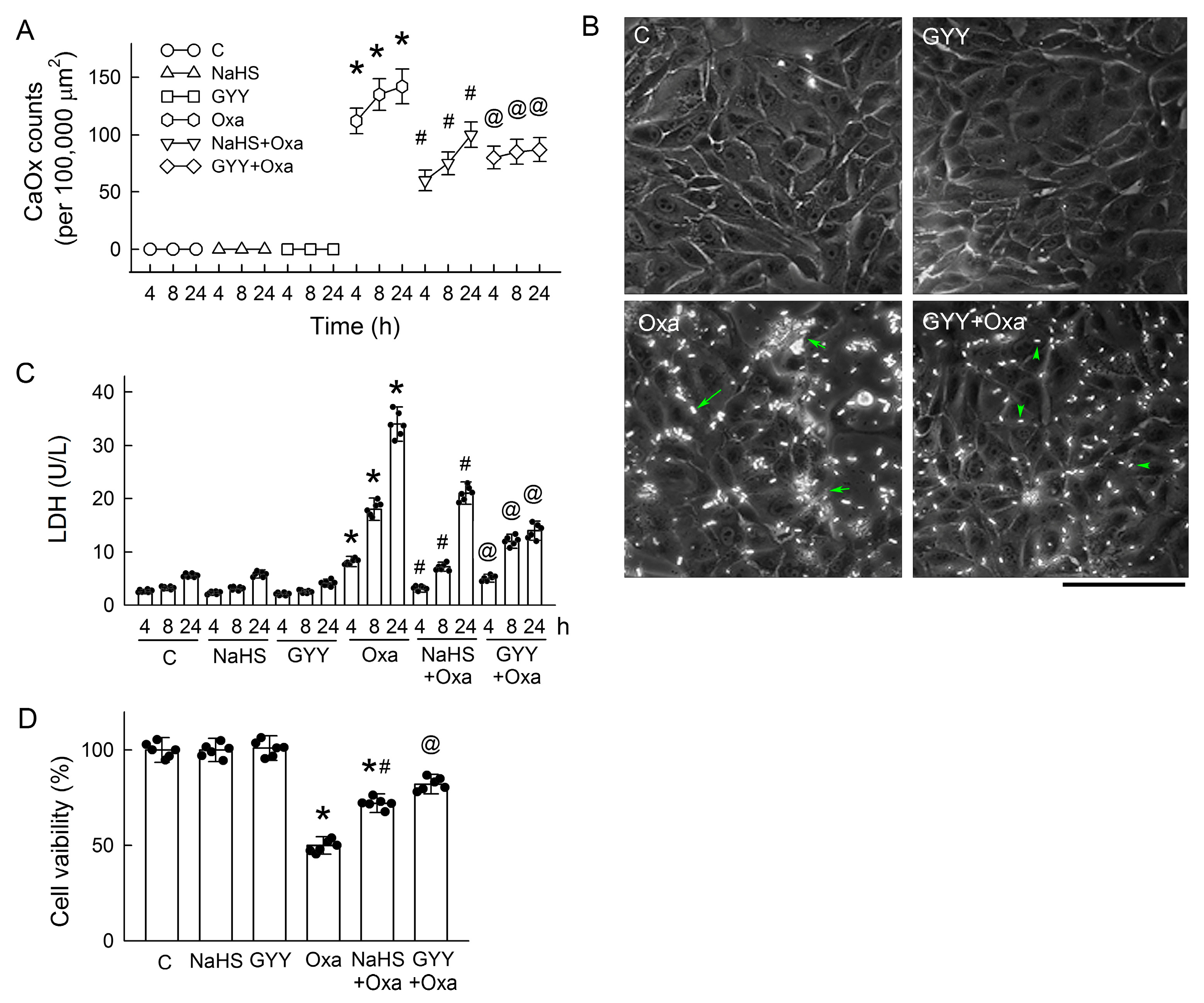
3.8. H2S Donors Attenuate CaOx Crystal Formation and Protect Tubular Cell Against Oxalate
3.9. Exogenous H2S Supplement Attenuates OPN and THP Secretion in Tubular Cells
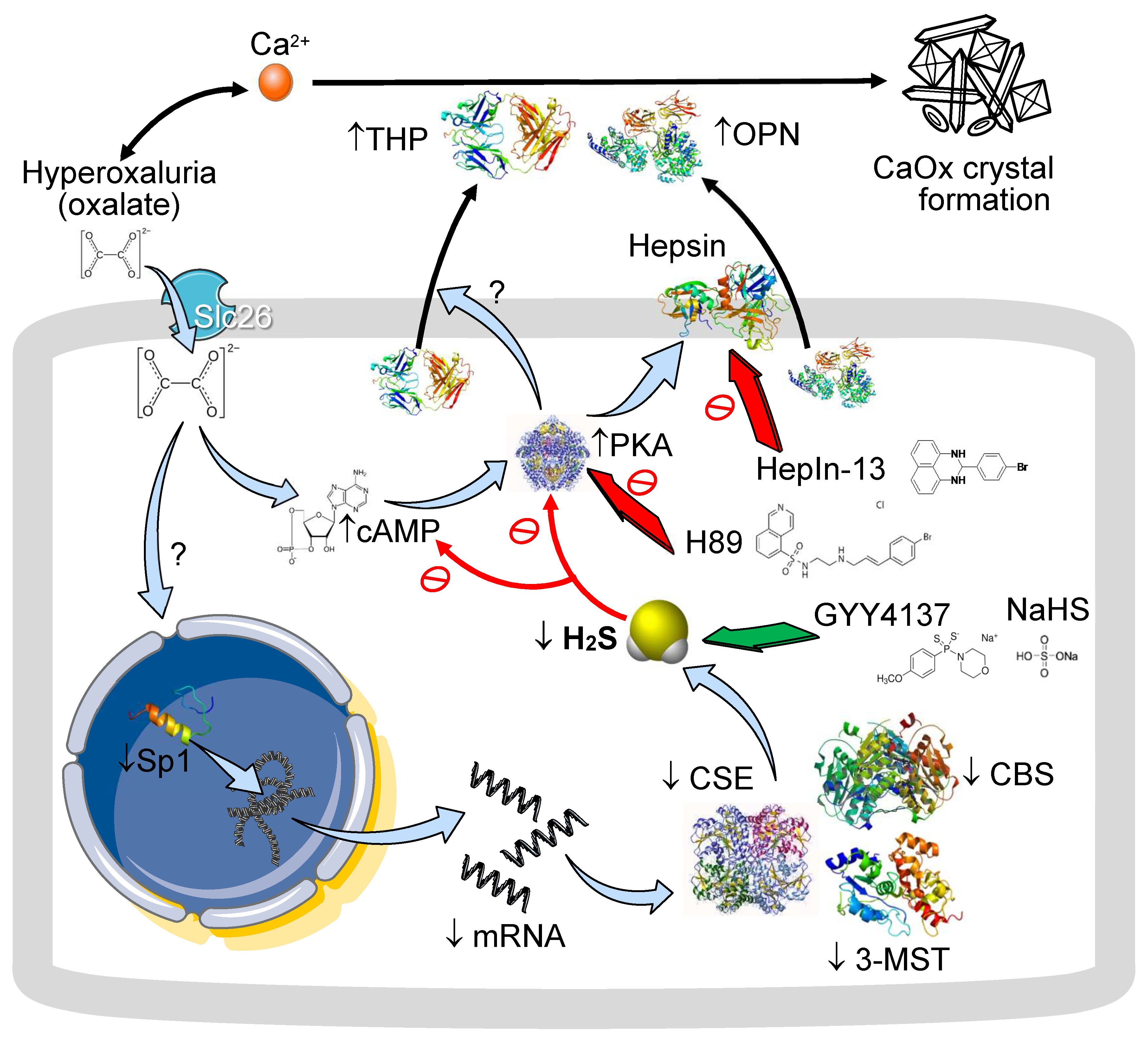
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-MST | 3-mercaptopyruvate sulfurtransferase |
| AP(CaOx) | Ion activity product of calcium oxalate |
| [Ca2+]i | Intracellular calcium ion concentration |
| CaOx | Calcium oxalate |
| cAMP | Cyclic adenosine monophosphate |
| CBS | Cystathionine β-synthase |
| CSE | Cystathionine γ-lyase |
| COD | Calcium oxalate dihydrate |
| COM | Calcium oxalate monohydrate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ERK | Extracellular signal-regulated kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GYY | GYY4137 |
| GPCR | G protein-coupled receptor |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| H2S | Hydrogen sulfide |
| H89 | PKA inhibitor |
| Hep | HepIn-13 |
| HP | Hydroxy-L-proline |
| HRP | Horseradish peroxidase |
| I/R | Ischemia/reperfusion |
| JNK | c-Jun N-terminal kinase |
| KIM-1 | Kidney injury molecule-1 |
| LDH | Lactate dehydrogenase |
| LLC-PK1 | Lilly Laboratories cell-porcine kidney 1 |
| MAPK | Mitogen-activated protein kinase |
| MDCK | Madin–Darby Canine Kidney |
| MTT | 3-(4,5)-2,5-diphenyltetrazolium bromide |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| NaHS | Sodium hydrosulfide |
| NO | Nitric oxide |
| OPN | Osteopontin |
| PBS | Phosphate-buffered saline |
| PKA | Protein kinase A |
| qPCR | Quantitative real-time polymerase chain reaction |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| Sp1 | Specificity protein 1 |
| THP | Tamm–Horsfall protein |
References
- Owino, C.; Mutugi, A.; Tang, J. Hyperoxaluria—A Major Metabolic Risk for Kidney Stone Disease. Rhode Isl. Med. J. 2023, 106, 14–19. [Google Scholar]
- Ziemba, J.B.; Matlaga, B.R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 2017, 58, 299–306. [Google Scholar] [CrossRef]
- Pfau, A.; Knauf, F. Update on Nephrolithiasis: Core Curriculum 2016. Am. J. Kidney Dis. 2016, 68, 973–985. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef]
- Rule, A.D.; Krambeck, A.E.; Lieske, J.C. Chronic kidney disease in kidney stone formers. Clin. J. Am. Soc. Nephrol. 2011, 6, 2069–2075. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol. Res. 2005, 33, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Ma, M.C.; Chen, J. Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am. J. Physiol. Ren. Physiol. 2009, 296, F34–F45. [Google Scholar] [CrossRef]
- Huang, H.S.; Ma, M.C.; Chen, J. Chronic L-arginine administration increases oxidative and nitrosative stress in rat hyperoxaluric kidneys and excessive crystal deposition. Am. J. Physiol. Ren. Physiol. 2008, 295, F388–F396. [Google Scholar] [CrossRef]
- Huang, H.S.; Chen, J.; Chen, C.F.; Ma, M.C. Vitamin E attenuates crystal formation in rat kidneys: Roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006, 70, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef]
- Miyake, O.; Yoshioka, T.; Yoshimura, K.; Honda, M.; Yamaguchi, S.; Koide, T.; Okuyama, A. Expression of Tamm-Horsfall protein in stone-forming rat models. Br. J. Urol. 1998, 81, 14–19. [Google Scholar] [CrossRef]
- Ophascharoensuk, V.; Giachelli, C.M.; Gordon, K.; Hughes, J.; Pichler, R.; Brown, P.; Liaw, L.; Schmidt, R.; Shankland, S.J.; Alpers, C.E.; et al. Obstructive uropathy in the mouse: Role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999, 56, 571–580. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Ren. Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef]
- Brunati, M.; Perucca, S.; Han, L.; Cattaneo, A.; Consolato, F.; Andolfo, A.; Schaeffer, C.; Olinger, E.; Peng, J.; Santambrogio, S.; et al. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. eLife 2015, 4, e08887. [Google Scholar] [CrossRef]
- Nanamatsu, A.; Mori, T.; Ando, F.; Furusho, T.; Mandai, S.; Susa, K.; Sohara, E.; Rai, T.; Uchida, S. Vasopressin Induces Urinary Uromodulin Secretion By Activating PKA (Protein Kinase A). Hypertension 2021, 77, 1953–1963. [Google Scholar] [CrossRef]
- Arvans, D.; Alshaikh, A.; Bashir, M.; Weber, C.; Hassan, H. Activation of the PKA signaling pathway stimulates oxalate transport by human intestinal Caco2-BBE cells. Am. J. Physiol. Cell Physiol. 2020, 318, C372–C379. [Google Scholar] [CrossRef]
- Yamate, T.; Kohri, K.; Umekawa, T.; Amasaki, N.; Amasaki, N.; Isikawa, Y.; Iguchi, M.; Kurita, T. The effect of osteopontin on the adhesion of calcium oxalate crystals to Madin-Darby canine kidney cells. Eur. Urol. 1996, 30, 388–393. [Google Scholar] [CrossRef]
- Mo, L.; Huang, H.Y.; Zhu, X.H.; Shapiro, E.; Hasty, D.L.; Wu, X.R. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004, 66, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [PubMed]
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen Sulfide in Renal Physiology and Disease. Antioxid. Redox Signal. 2016, 25, 720–731. [Google Scholar] [CrossRef]
- Lu, C.L.; Tseng, Y.S.; Wu, W.B.; Liao, C.H.; Ma, M.C. Blockade of Aryl Hydrocarbon Receptor Ameliorates Functional Insufficiency in 5/6 Nephrectomized Rat Kidneys by Restoring Hydrogen Sulfide Formation. Antioxid. Redox Signal. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Lu, C.L.; Liao, C.H.; Wu, W.B.; Zheng, C.M.; Lu, K.C.; Ma, M.C. Uremic Toxin Indoxyl Sulfate Impairs Hydrogen Sulfide Formation in Renal Tubular Cells. Antioxidants 2022, 11, 361. [Google Scholar] [CrossRef]
- Beierwaltes, W.H. Hydrogen sulfide, renin, and regulating the second messenger cAMP. Focus on “Hydrogen sulfide regulates cAMP homeostasis and renin degranulation in As4.1 and rat renin-rich kidney cell”. Am. J. Physiol. Cell Physiol. 2012, 302, C21–C23. [Google Scholar] [CrossRef][Green Version]
- Bos, E.M.; Leuvenink, H.G.; Snijder, P.M.; Kloosterhuis, N.J.; Hillebrands, J.L.; Leemans, J.C.; Florquin, S.; van Goor, H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2009, 20, 1901–1905. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 498–504. [Google Scholar] [CrossRef]
- Vaitheeswari, S.; Sriram, R.; Brindha, P.; Kurian, G.A. Studying inhibition of calcium oxalate stone formation: An in vitro approach for screening hydrogen sulfide and its metabolites. Int. Braz. J. Urol. 2015, 41, 503–510. [Google Scholar] [CrossRef]
- Lai, Y.; Liang, X.; Zhong, F.; Wu, W.; Zeng, T.; Huang, J.; Duan, X.; Li, S.; Zeng, G.; Wu, W. Allicin attenuates calcium oxalate crystal deposition in the rat kidney by regulating gap junction function. J. Cell. Physiol. 2019, 234, 9640–9651. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Teng, T.Y.; Liao, M.T.; Ma, M.C. TRPV1 Hyperfunction Contributes to Renal Inflammation in Oxalate Nephropathy. Int. J. Mol. Sci. 2021, 22, 6204. [Google Scholar] [CrossRef]
- Lu, C.L.; Liao, C.H.; Lu, K.C.; Ma, M.C. TRPV1 Hyperfunction Involved in Uremic Toxin Indoxyl Sulfate-Mediated Renal Tubular Damage. Int. J. Mol. Sci. 2020, 21, 6212. [Google Scholar] [CrossRef] [PubMed]
- Mohamaden, W.; Wang, H.; Guan, H.; Meng, X.; Li, J. Immunohistochemical localization and mRNA quantification of osteopontin and Tamm-Horsfall protein in canine renal tissue after potassium oxalate injection. BMC Vet. Res. 2014, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Liaw, L.; Evan, A.P.; Sommer, A.J.; Lieske, J.C.; Wu, X.R. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am. J. Physiol. Ren. Physiol. 2007, 293, F1935–F1943. [Google Scholar] [CrossRef]
- Cai, Y.; Teng, X.; Pan, C.S.; Duan, X.H.; Tang, C.S.; Qi, Y.F. Adrenomedullin up-regulates osteopontin and attenuates vascular calcification via the cAMP/PKA signaling pathway. Acta Pharmacol. Sin. 2010, 31, 1359–1366. [Google Scholar] [CrossRef][Green Version]
- Huang, H.S.; Ma, M.C. High Sodium-Induced Oxidative Stress and Poor Anticrystallization Defense Aggravate Calcium Oxalate Crystal Formation in Rat Hyperoxaluric Kidneys. PLoS ONE 2015, 10, e0134764. [Google Scholar] [CrossRef]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Jin, S.; Cheng, R.X.; Cai, W.J.; Xue, W.L.; Zhang, Q.Q.; Yang, L.J.; Zhu, Q.; Li, M.Y.; Lin, G.; et al. Hydrogen sulfide functions as a micro-modulator bound at the copper active site of Cu/Zn-SOD to regulate the catalytic activity of the enzyme. Cell Rep. 2023, 42, 112750. [Google Scholar] [CrossRef]
- Dugbartey, G.J. Physiological role of hydrogen sulfide in the kidney and its therapeutic implications for kidney diseases. Biomed. Pharmacother. 2023, 166, 115396. [Google Scholar] [CrossRef]
- Kishikawa, S.; Murata, T.; Kimura, H.; Shiota, K.; Yokoyama, K.K. Regulation of transcription of the Dnmt1 gene by Sp1 and Sp3 zinc finger proteins. Eur. J. Biochem. 2002, 269, 2961–2970. [Google Scholar] [CrossRef]
- Wu, N.; Siow, Y.L.; O, K. Ischemia/reperfusion reduces transcription factor Sp1-mediated cystathionine beta-synthase expression in the kidney. J. Biol. Chem. 2010, 285, 18225–18233. [Google Scholar] [CrossRef]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200. [Google Scholar] [CrossRef] [PubMed]
- Kapiloff, M.; Mathis, J.; Nelson, C.; Lin, C.; Rosenfeld, M. Calcium/calmodulin-dependent protein kinase mediates a pathway for transcriptional regulation. Proc. Natl. Acad. Sci. USA 1991, 88, 3710–3714. [Google Scholar] [CrossRef] [PubMed]
- Wiegert, S.; Bading, H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: A link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium 2011, 49, 296–305. [Google Scholar] [CrossRef]
- Pando, P.; Vattamparambil, A.S.; Sheth, S.; Landry, G.M. Acute lead (Pb2+) exposure increases calcium oxalate crystallization in the inner medullary collecting duct, and is ameliorated by Ca2+/Mg2+-ATPase inhibition, as well as Capa receptor and SPoCk C knockdown in a Drosophila melanogaster model of nephrolithiasis. Chem. Biol. Interact. 2024, 402, 111201. [Google Scholar] [CrossRef]
- Yanagawa, M.; Koul, H.; Honeyman, T.; Malhotra, R.; Scheid, C.; Menon, M. Oxalate-Induced Changes in Intracellular Calcium Levels in Renal Papillary Cells. In Oxalate Metabolism in Relation to Urinary Stone; Khan, S.R., Ed.; Springer: Boston, MA, USA, 1994; pp. 117–119. [Google Scholar] [CrossRef]
- D’Addario, M.; Arora, P.D.; McCulloch, C.A. Role of p38 in stress activation of Sp1. Gene 2006, 379, 51–61. [Google Scholar] [CrossRef]
- Ma, W.; Lim, W.; Gee, K.; Aucoin, S.; Nandan, D.; Kozlowski, M.; Diaz-Mitoma, F.; Kumar, A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 2001, 276, 13664–13674. [Google Scholar] [CrossRef]
- Chu, S.; Ferro, T.J. Identification of a hydrogen peroxide-induced PP1-JNK1-Sp1 signaling pathway for gene regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L983–L992. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Wang, Y.T.; Yeh, S.H.; Liu, Y.W.; Chang, W.C.; Hung, J.J. Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Mol. Biol. Cell 2008, 19, 1139–1151. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, J.; Chen, Z. MicroRNA-204 attenuates oxidative stress damage of renal tubular epithelial cells in calcium oxalate kidney-stone formation via MUC4-mediated ERK signaling pathway. Urolithiasis 2022, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, L.S.; Koul, S.; Sekhon, A.; Bhandari, A.; Menon, M.; Koul, H.K. Oxalate selectively activates p38 mitogen-activated protein kinase and c-Jun N-terminal kinase signal transduction pathways in renal epithelial cells. J. Biol. Chem. 2002, 277, 13321–13330. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells. Sci. Rep. 2013, 3, 1041. [Google Scholar] [CrossRef]
- Khan, S.R.; Canales, B.K.; Dominguez-Gutierrez, P.R. Randall’s plaque and calcium oxalate stone formation: Role for immunity and inflammation. Nat. Rev. Nephrol. 2021, 17, 417–433. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Chang, W.C.; Hung, J.J. Hydrogen peroxide induces Sp1 methylation and thereby suppresses cyclin B1 via recruitment of Suv39H1 and HDAC1 in cancer cells. Free Radic. Biol. Med. 2011, 51, 2309–2318. [Google Scholar] [CrossRef]
- Thielemans, R.; Speeckaert, R.; Delrue, C.; De Bruyne, S.; Oyaert, M.; Speeckaert, M.M. Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases. Diagnostics 2023, 13, 3077. [Google Scholar] [CrossRef]
- Wang, K.; Ge, J.; Han, W.; Wang, D.; Zhao, Y.; Shen, Y.; Chen, J.; Chen, D.; Wu, J.; Shen, N.; et al. Risk factors for kidney stone disease recurrence: A comprehensive meta-analysis. BMC Urol. 2022, 22, 62. [Google Scholar] [CrossRef]
- Wesson, J.A.; Worcester, E.M.; Wiessner, J.H.; Mandel, N.S.; Kleinman, J.G. Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int. 1998, 53, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Nishi, S.; Iguchi, S.; Imai, N.; Sakatsume, M.; Saito, A.; Ikegame, M.; Iino, N.; Shimada, H.; Ueno, M.; et al. Expression of osteopontin in gentamicin-induced acute tubular necrosis and its recovery process. Kidney Int. 2001, 59, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Wang, W.; Khan, S.R. Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: Inflammatory changes are mainly associated with crystal deposition. PLoS ONE 2017, 12, e0185009. [Google Scholar] [CrossRef]
- Hong, S.Y.; Qin, B.L. The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation. Biology 2024, 13, 814. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. Biomed. Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef]
- Han, H.J.; Lim, M.J.; Lee, Y.J. Oxalate inhibits renal proximal tubule cell proliferation via oxidative stress, p38 MAPK/JNK, and cPLA2 signaling pathways. Am. J. Physiol. Cell Physiol. 2004, 287, C1058–C1066. [Google Scholar] [CrossRef] [PubMed]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Oxalate-induced activation of PKC-alpha and -delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 2009, 297, F1399–F1410. [Google Scholar] [CrossRef]
- Yu, L.; Gan, X.; Liu, X.; An, R. Calcium oxalate crystals induces tight junction disruption in distal renal tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling pathway. Ren. Fail. 2017, 39, 440–451. [Google Scholar] [CrossRef]
- Hou, B.; Liu, M.; Chen, Y.; Ni, W.; Suo, X.; Xu, Y.; He, Q.; Meng, X.; Hao, Z. Cpd-42 protects against calcium oxalate nephrocalcinosis-induced renal injury and inflammation by targeting RIPK3-mediated necroptosis. Front. Pharmacol. 2022, 13, 1041117. [Google Scholar] [CrossRef]
- Isobe, K.; Jung, H.J.; Yang, C.R.; Claxton, J.; Sandoval, P.; Burg, M.B.; Raghuram, V.; Knepper, M.A. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, E8875–E8884. [Google Scholar] [CrossRef]
- Luo, P.; Chen, T.; Zheng, L.; Zou, J.; Zou, J.; Li, W.; Chen, Q.; Cheng, L.; Qian, B. Calcium sensing receptor regulate claudin-14 via PKA-STAT3 pathway in rat model of nephrolithiasis. Front. Pharmacol. 2024, 15, 1477122. [Google Scholar] [CrossRef]
- Khongsti, K.; Das, K.B.; Das, B. MAPK pathway and SIRT1 are involved in the down-regulation of secreted osteopontin expression by genistein in metastatic cancer cells. Life Sci. 2021, 265, 118787. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H.G. The effect of pH on the urinary inhibition of calcium oxalate crystal growth. Br. J. Urol. 1981, 53, 470–474. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017, 7, 3541. [Google Scholar] [CrossRef]
- Lee, Z.; Zhou, J.; Chen, C.; Zhao, Y.; Tan, C.H.; Li, L.-X.; Moore, P.; Deng, L. The Slow-Releasing Hydrogen Sulfide Donor, GYY4137, Exhibits Novel Anti-Cancer Effects In Vitro and In Vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef]
- Li, S.; Ping, N.N.; Cao, L.; Mi, Y.N.; Cao, Y.X. H2S induces vasoconstriction of rat cerebral arteries via cAMP/adenylyl cyclase pathway. Toxicol. Appl. Pharmacol. 2015, 289, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Wu, Z.Y.; Wood, M.; Whiteman, M.; Bian, J.S. Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway. Antioxid. Redox Signal. 2014, 20, 31–41. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Xie, Z.Z.; Hua, F.; Xie, L.; Gao, J.H.; Koh, Y.H.; Bian, J.S. Hydrogen sulfide prevents heart failure development via inhibition of renin release from mast cells in isoproterenol-treated rats. Antioxid. Redox Signal. 2014, 20, 759–769. [Google Scholar] [CrossRef]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011, 79, 1277–1288. [Google Scholar] [CrossRef]
- Srinivasan, S.; Spear, J.; Chandran, K.; Joseph, J.; Kalyanaraman, B.; Avadhani, N. Oxidative Stress Induced Mitochondrial Protein Kinase A Mediates Cytochrome C Oxidase Dysfunction. PLoS ONE 2013, 8, e77129. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.; Zhang, S.; Jansson, K.; Fields, T.; Boulanger, J.; Liu, S.; Rowe, P. Critical Role of Osteopontin in Maintaining Urinary Phosphate Solubility in CKD. Kidney360 2022, 3, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Li, Y.; Ponsetto, J.; Zhu, C.; Bergwitz, C. Impaired urinary osteopontin excretion in Npt2a-/- mice. Am. J. Physiol. Ren. Physiol. 2017, 312, F77–F83. [Google Scholar] [CrossRef] [PubMed]
| Gene | GenBank Accession Number | Primer Sequence |
|---|---|---|
| CBS | XM_039080137 | 5′-TAGACGGCAGAGCCTTTCGA-3′ (forward) 5′-AATCCCCGGCCGTAGAAC-3′ (reverse) |
| CSE | NM_017074 | 5′-ACACTTCAGGAATGGGATGG-3′ (forward) 5′-TGAGCATGCTGCAGAGTACC-3′ (reverse) |
| 3-MST | NM_001013440 | 5′-CTGGGAAACGGGGAGCG-3′ (forward) 5′-GCTCGGAAAAGTTGCGGG-3′ (reverse) |
| GAPDH | XM_039097338 | 5′-TTAGCACCCCTGGCCAAGG-3′ (forward) 5′-CTTACTCCTTGGAGGCCATG-3′ (reverse) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-L.; Tseng, Y.-S.; Wu, W.-B.; Liao, C.-H.; Ma, M.-C. Hydrogen Sulfide Deficiency Contributes to Tubular Damage and Calcium Oxalate Crystal Formation in Hyperoxaluria Nephropathy: Role of Osteopontin and Tamm–Horsfall Protein. Antioxidants 2025, 14, 1088. https://doi.org/10.3390/antiox14091088
Lu C-L, Tseng Y-S, Wu W-B, Liao C-H, Ma M-C. Hydrogen Sulfide Deficiency Contributes to Tubular Damage and Calcium Oxalate Crystal Formation in Hyperoxaluria Nephropathy: Role of Osteopontin and Tamm–Horsfall Protein. Antioxidants. 2025; 14(9):1088. https://doi.org/10.3390/antiox14091088
Chicago/Turabian StyleLu, Chien-Lin, Yi-Shiou Tseng, Wen-Bin Wu, Chun-Hou Liao, and Ming-Chieh Ma. 2025. "Hydrogen Sulfide Deficiency Contributes to Tubular Damage and Calcium Oxalate Crystal Formation in Hyperoxaluria Nephropathy: Role of Osteopontin and Tamm–Horsfall Protein" Antioxidants 14, no. 9: 1088. https://doi.org/10.3390/antiox14091088
APA StyleLu, C.-L., Tseng, Y.-S., Wu, W.-B., Liao, C.-H., & Ma, M.-C. (2025). Hydrogen Sulfide Deficiency Contributes to Tubular Damage and Calcium Oxalate Crystal Formation in Hyperoxaluria Nephropathy: Role of Osteopontin and Tamm–Horsfall Protein. Antioxidants, 14(9), 1088. https://doi.org/10.3390/antiox14091088







