Synthetic Cyclic C5-Curcuminoids Increase Antioxidant Defense and Reduce Inflammation in 6-OHDA-Induced Retinoic Acid-Differentiated SH-SY5Y Cells
Abstract
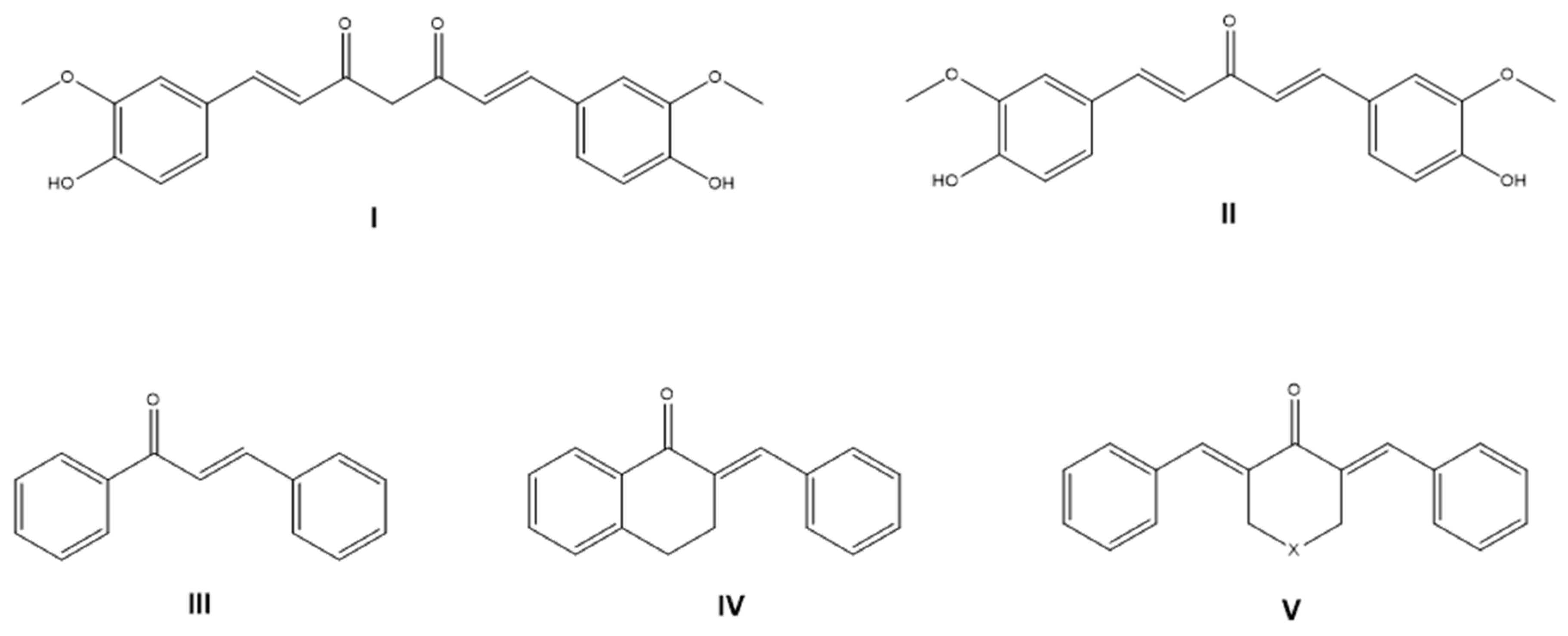
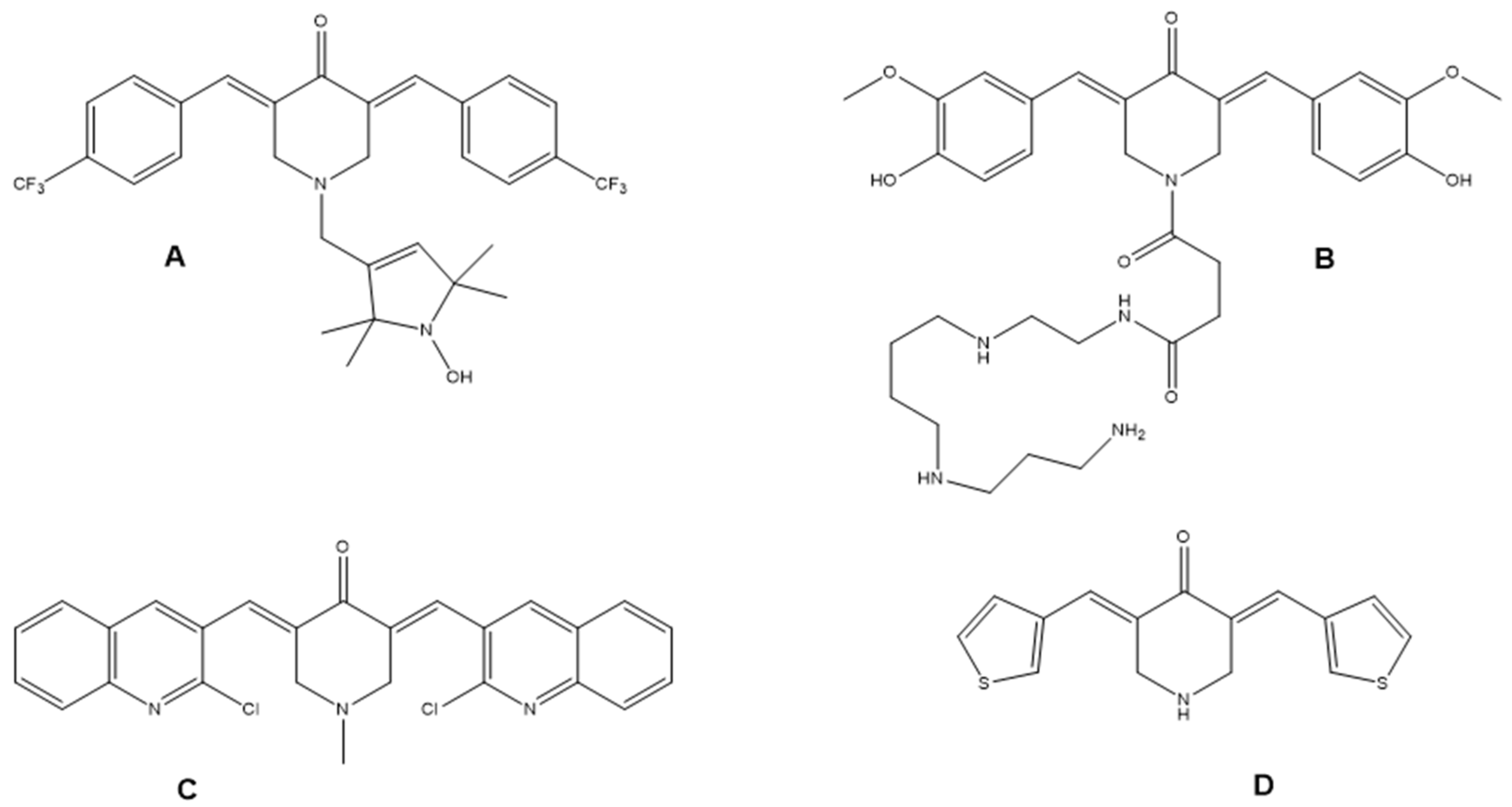
1. Introduction
2. Materials and Methods
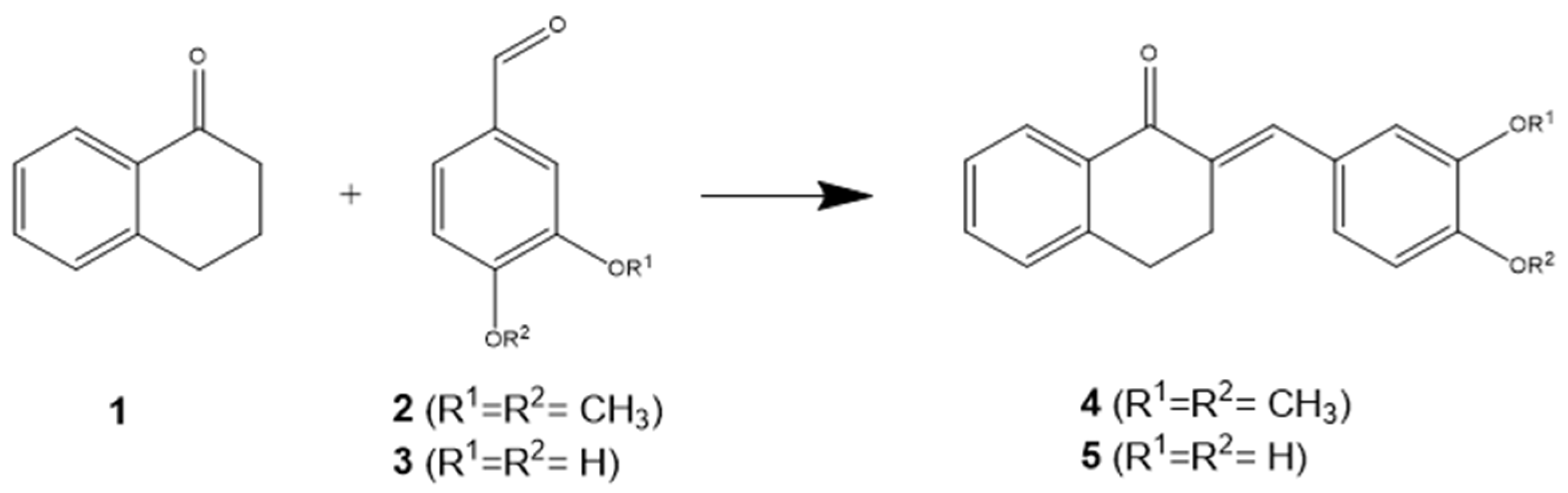
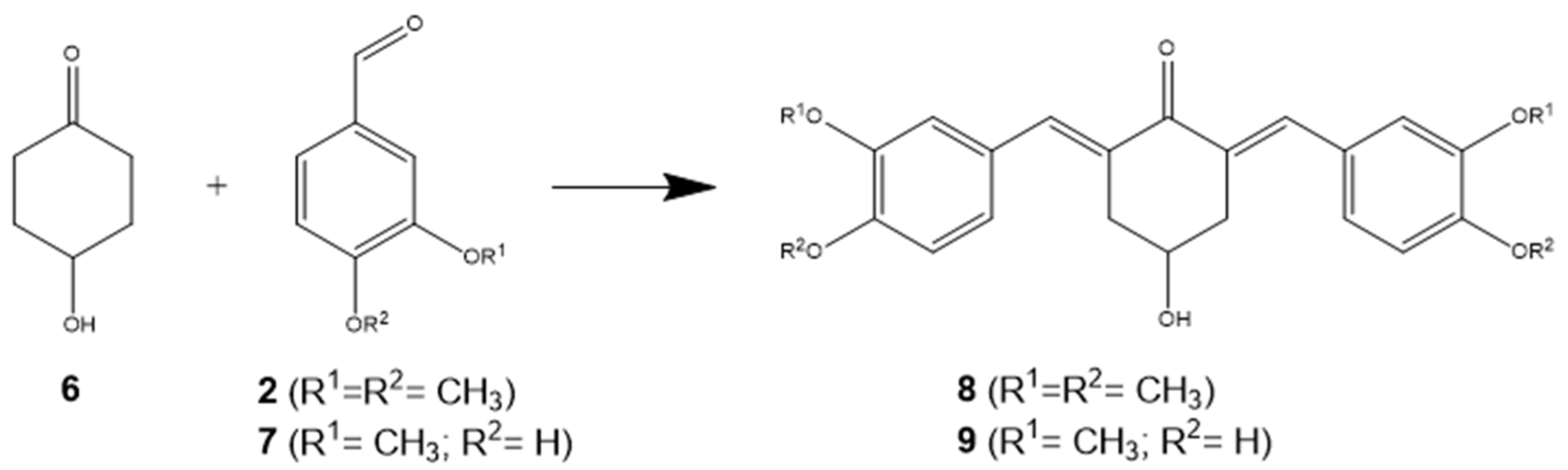
2.1. Chemical Experimental Protocol
2.2. Cell Culture and Treatment
2.3. Viability Measurements
2.4. Determination of the Reactive Oxygen Species (ROS)
2.5. Total Antioxidant Capacity (TAC) Measurement
2.6. Glutathione Peroxidase (GPx) Activity Measurement
2.7. Superoxide Dismutase (SOD) Activity Determination
2.8. Total Intracellular Iron Content Determination
2.9. Thiol Concentration Measurement
2.10. Malondialdehyde (MDA) Detection
2.11. ATP Concentration Determination
2.12. Cytochrome c Measurement
2.13. Caspase-3 Activity Assay
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Statistical Analysis
3. Results
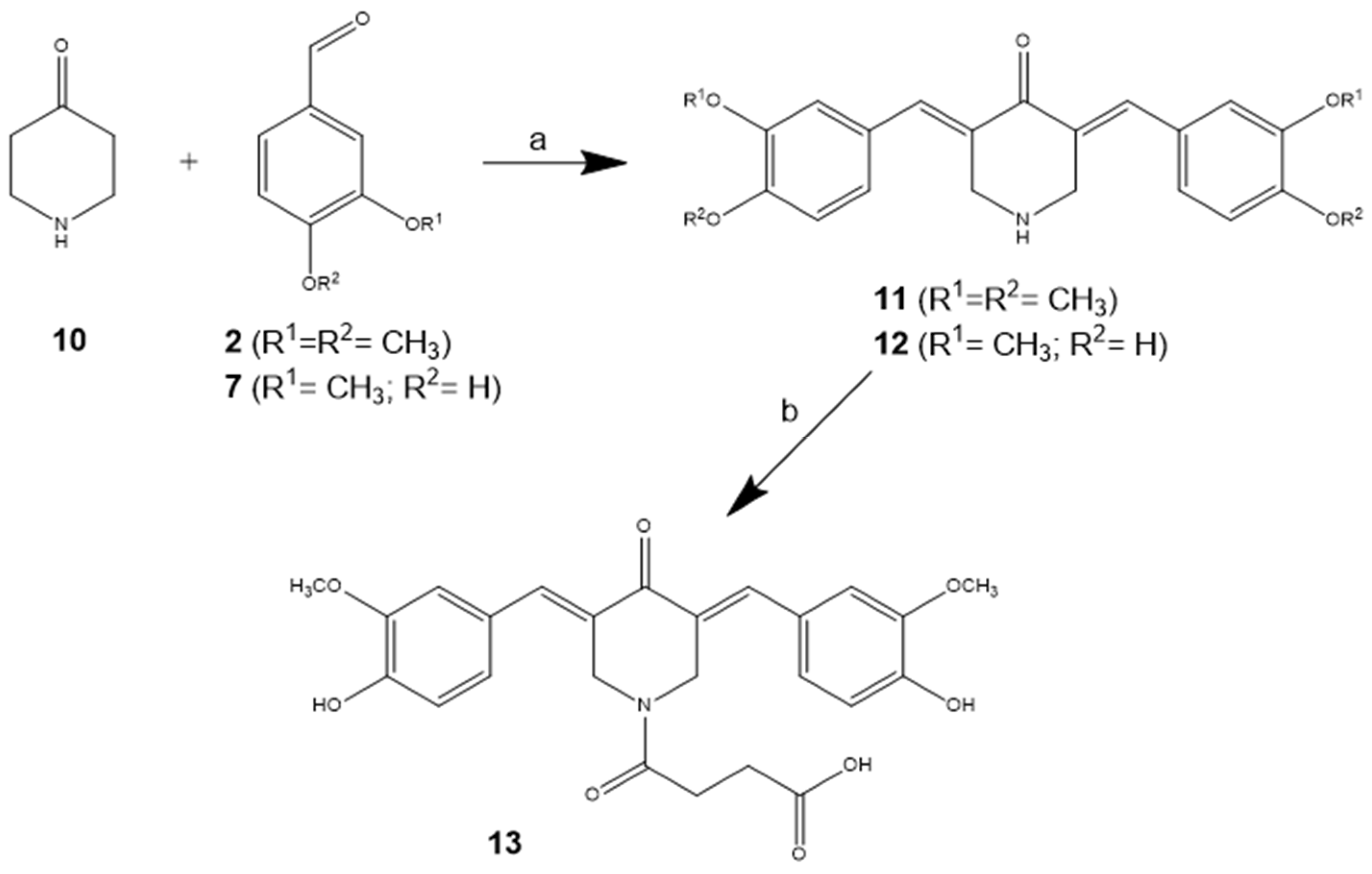
3.1. Chemical Synthesis
3.2. IC50 Values
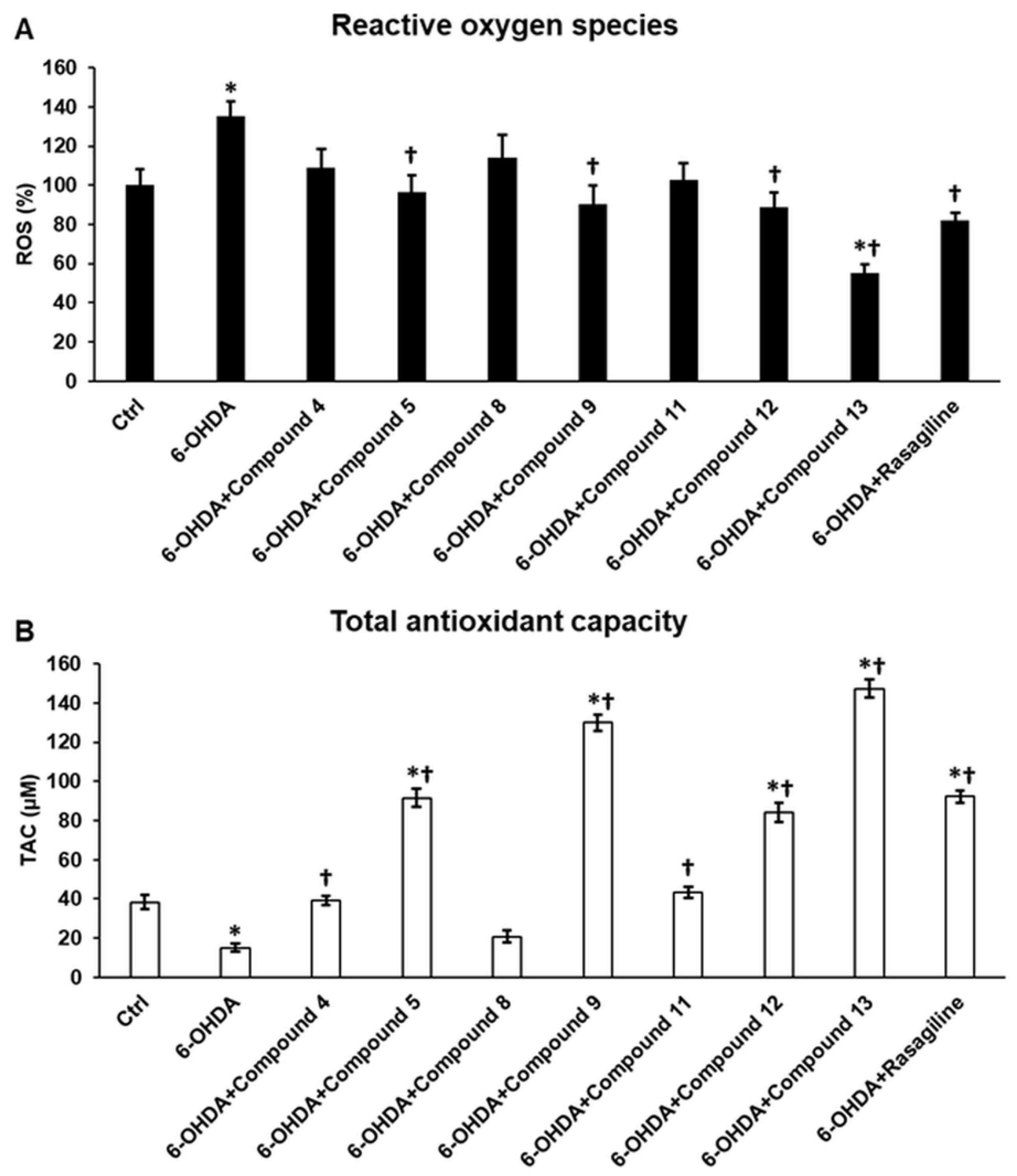
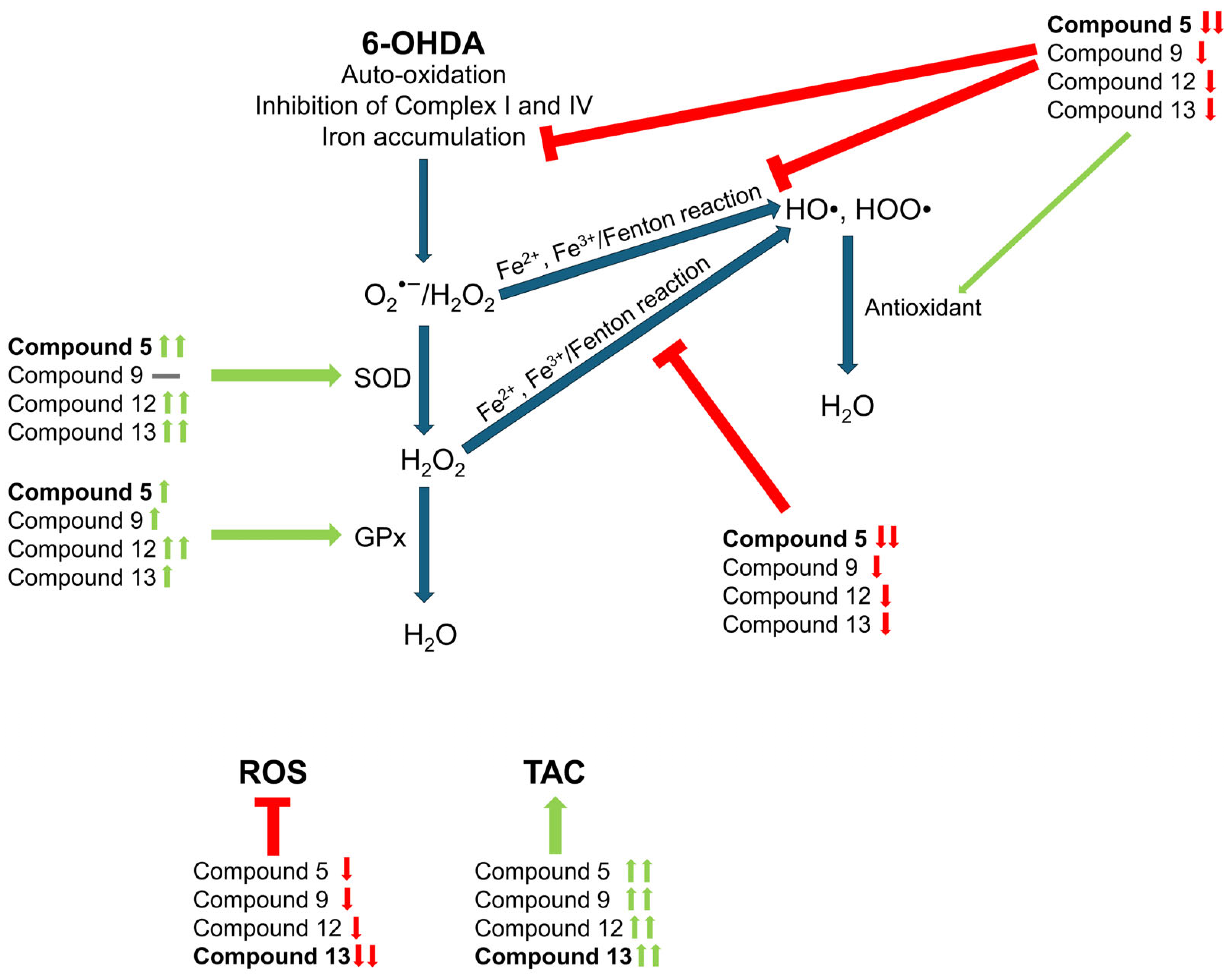
3.3. Cyclic C5-Curcuminoids and Chalcones Decrease the Production of ROS by Increasing Antioxidant Defense
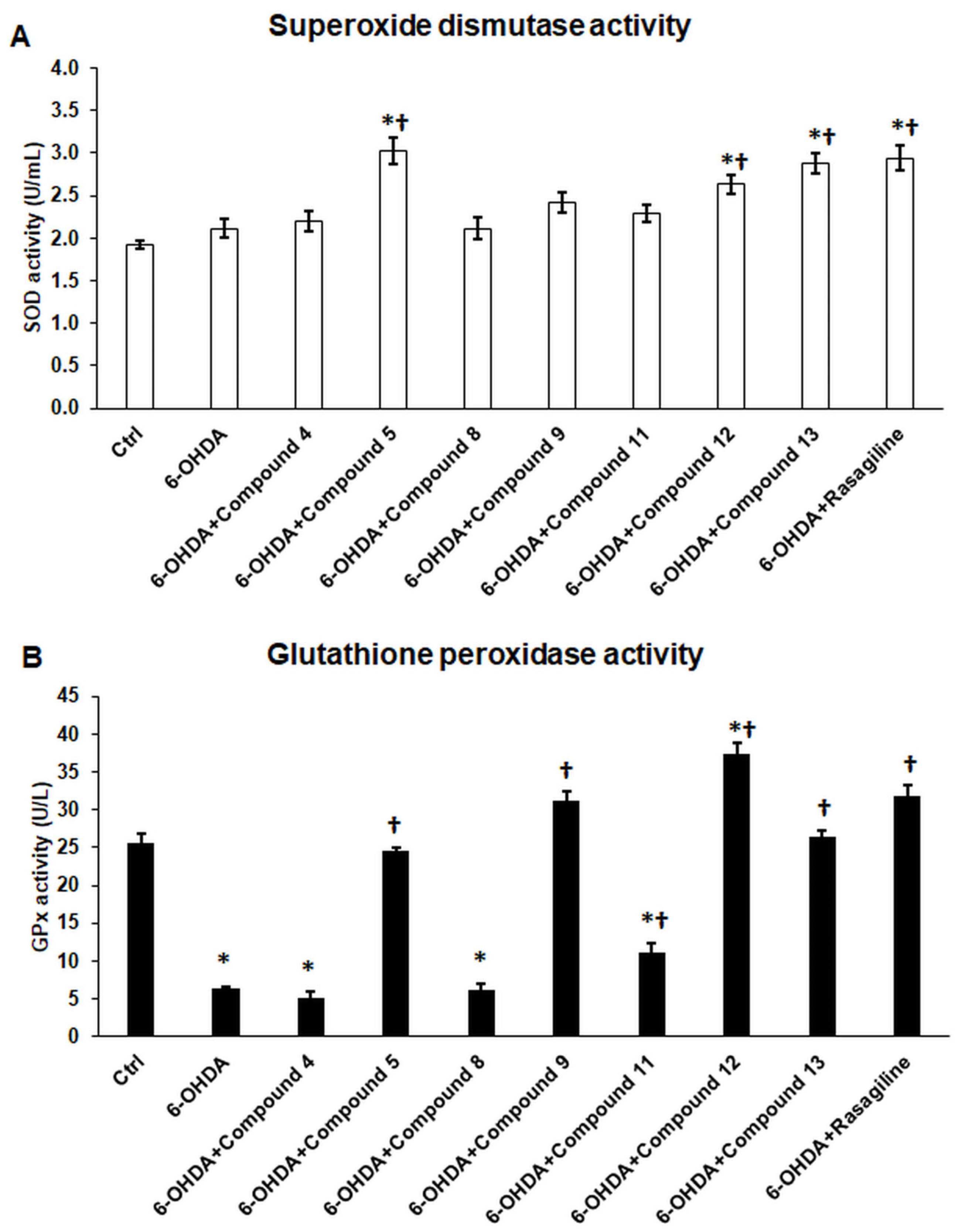
3.4. Cyclic C5-Curcuminoid and Chalcone Derivatives Influence the Activity of the Antioxidant Enzymes GPx and SOD
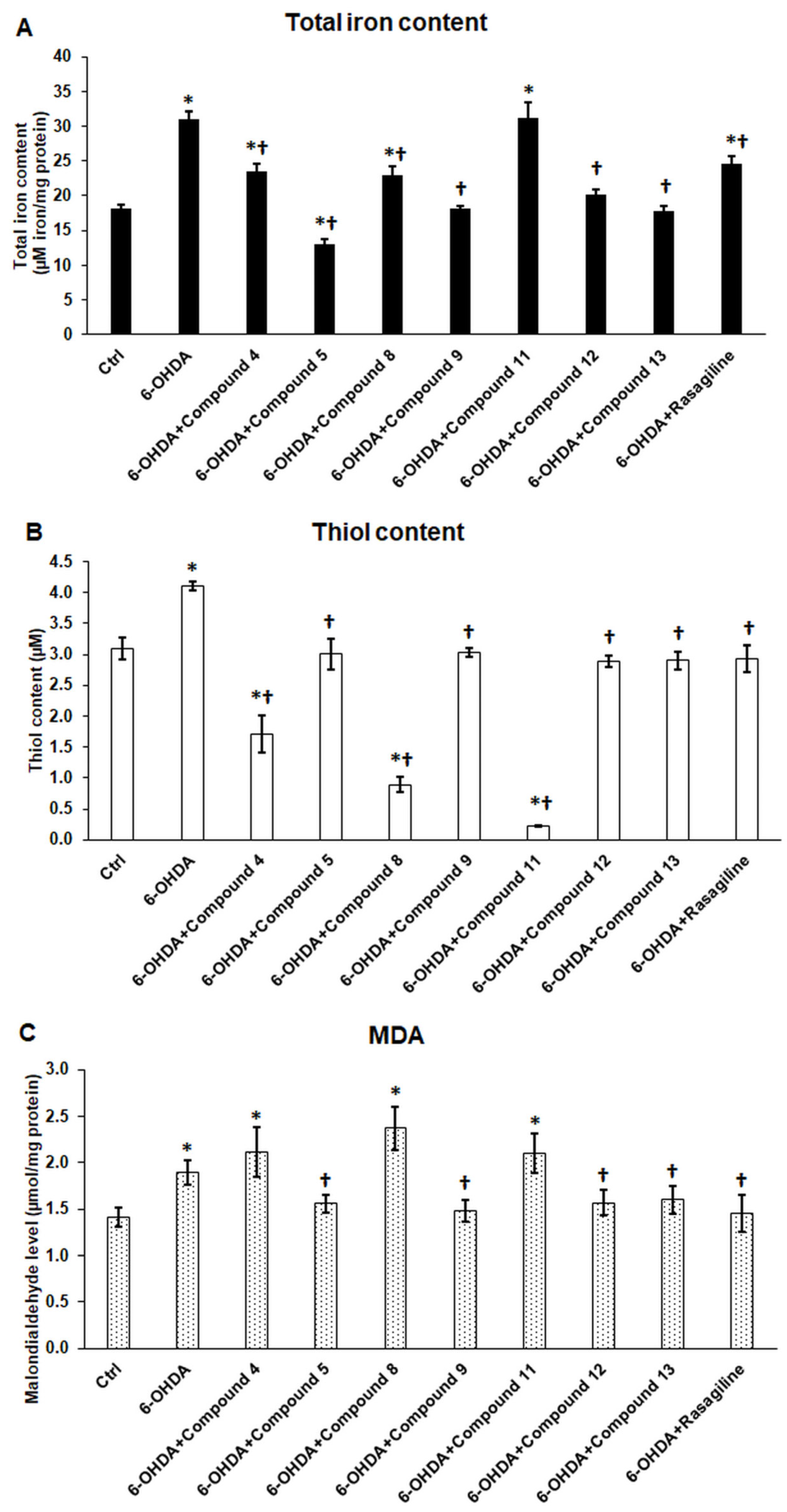
3.5. Cyclic C5-Curcuminoids 9, 12, 13 and Cyclic Chalcone 5 Inhibit 6-OHDA-Induced Iron Accumulation and Lipid Peroxidation
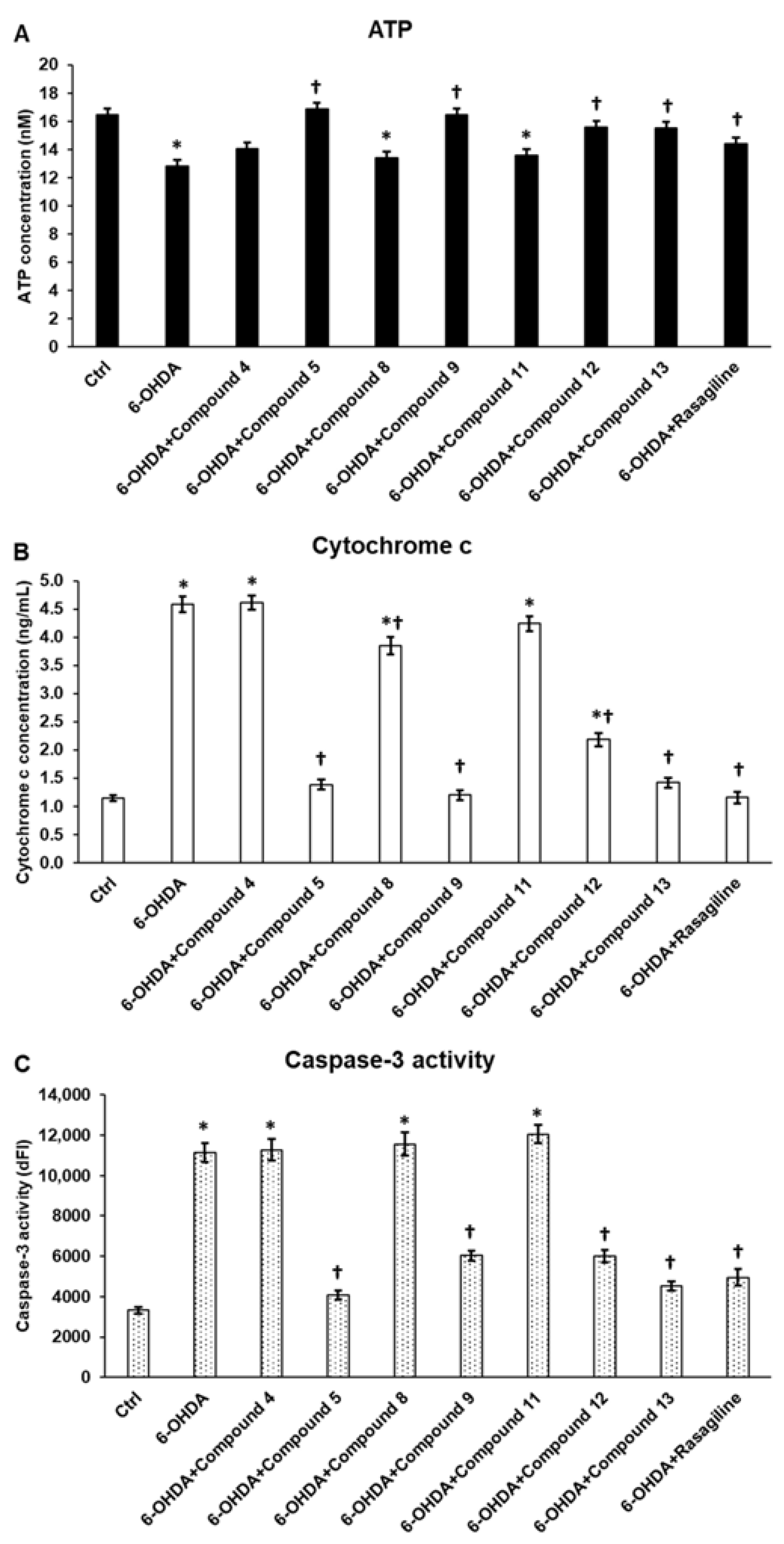
3.6. Cyclic C5-Curcuminoids and Cyclic Chalcones Affect Mitochondrial Function and Inhibit Apoptosis
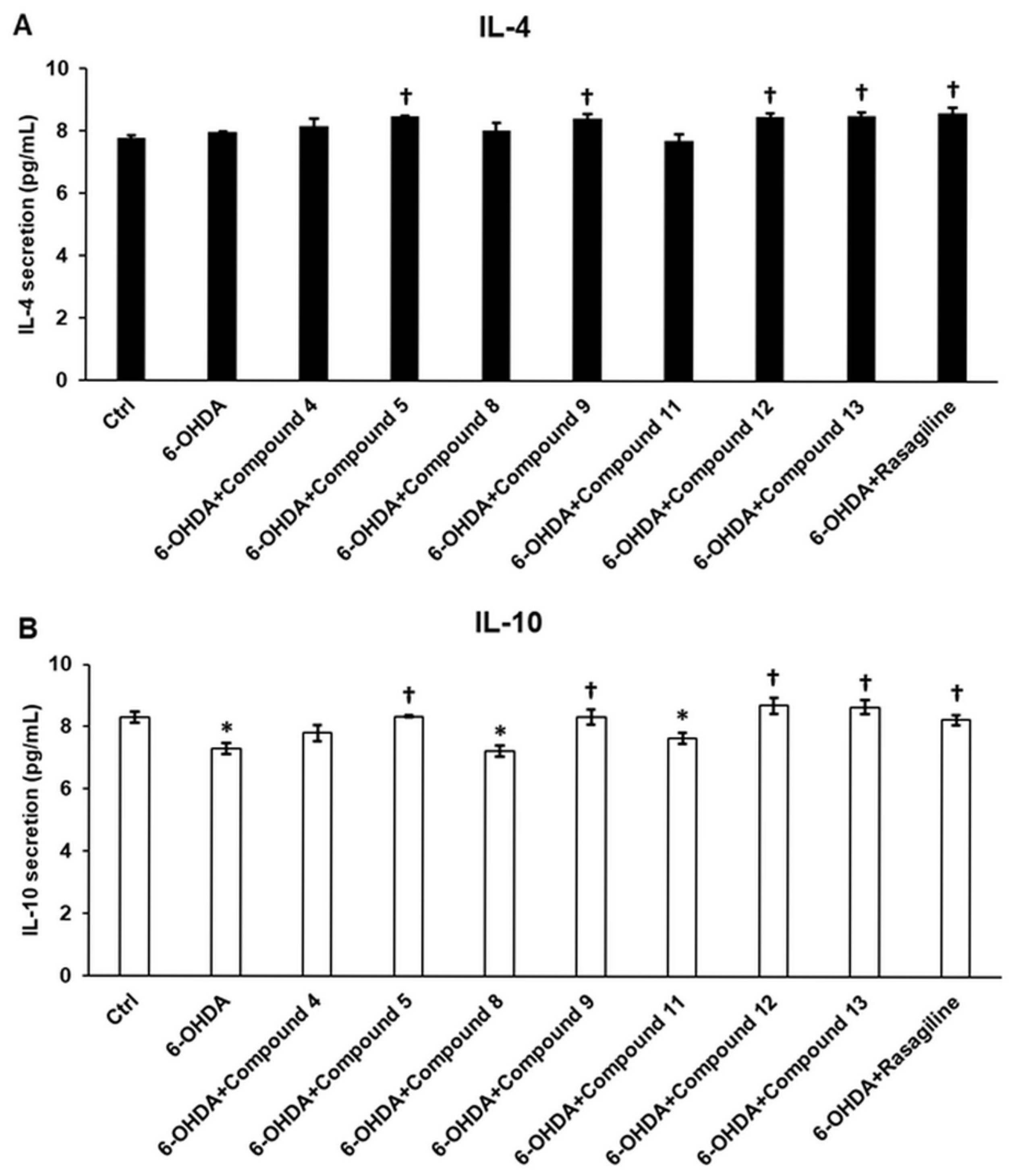
3.7. Cyclic C5-Curcuminoids 9, 12, 13 and Cyclic Chalcone 5 Exhibit Minimal Influence on Anti-Inflammatory Cytokine Production of the OHDA-Induced Differentiated SH-SY5Y Cells
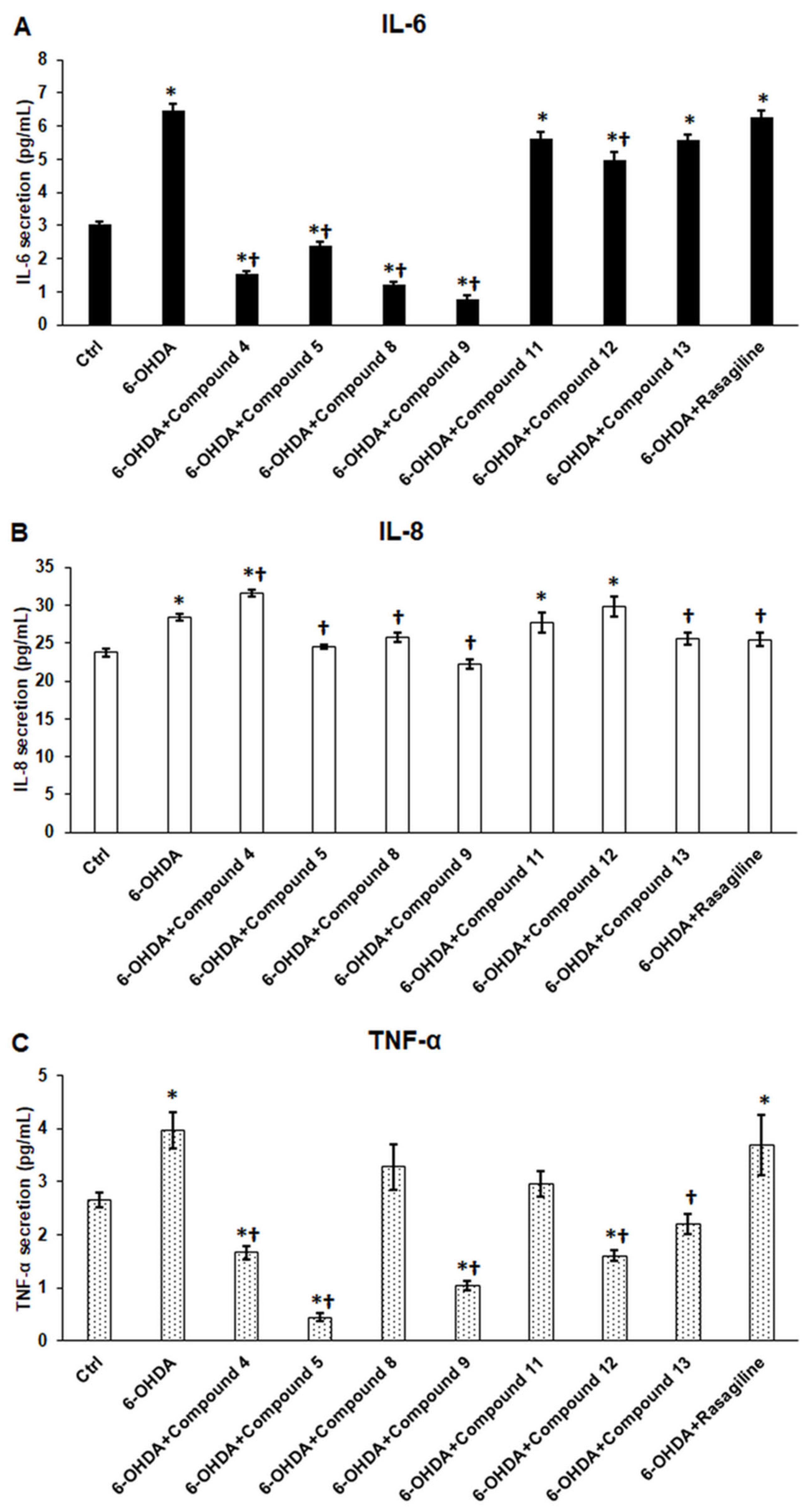
3.8. Cyclic C5-Curcuminoids and Cyclic Chalcones Reduce Pro-Inflammatory Cytokine Release of the OHDA-Induced Differentiated SH-SY5Y Cells
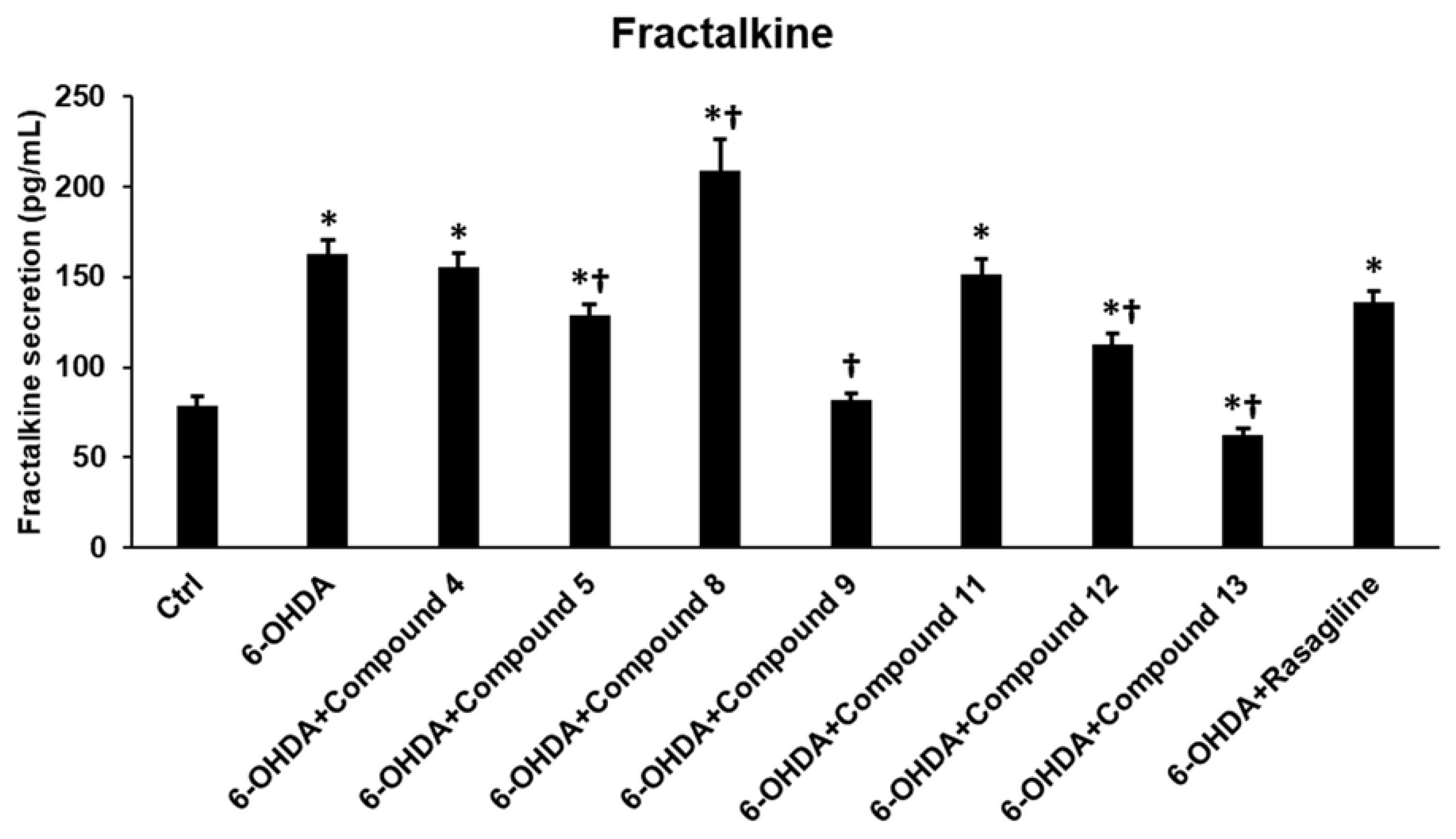
3.9. Cyclic C5-Curcuminoids 9, 12, 13, and Cyclic Chalcone 5 Reduce the Release of Fractalkine from the OHDA-Induced Differentiated SH-SY5Y Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| ATRA | all-trans retinoic acid |
| CX3CR1 | fractalkine receptor |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| FI | fluorescence intensity |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| HO• | hydroxyl radical |
| HOO• | hydroperoxyl |
| HPLC | high-performance liquid chromatography |
| IL | interleukin |
| MAO-B | monoamine oxidase B |
| MDA | malondialdehyde |
| MDR | multiple-drug-resistance |
| MS | mass spectrometry |
| NMR | nuclear magnetic resonance |
| O2•− | superoxide anion |
| P/S | penicillin/streptomycin |
| PBS | phosphate-buffered saline |
| PD | Parkinson’s disease |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TAC | total antioxidant capacity |
| TNF-α | tumor necrosis factor alpha |
References
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic—A Call to Action. JAMA Neurol. 2018, 75, 9. [Google Scholar] [CrossRef] [PubMed]
- Adam, H.; Gopinath, S.C.B.; Md Arshad, M.K.; Adam, T.; Parmin, N.A.; Husein, I.; Hashim, U. An Update on Pathogenesis and Clinical Scenario for Parkinson’s Disease: Diagnosis and Treatment. 3 Biotech 2023, 13, 142. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Trevisan, L.; Gaudio, A.; Monfrini, E.; Avanzino, L.; Di Fonzo, A.; Mandich, P. Genetics in Parkinson’s Disease, State-of-the-Art and Future Perspectives. Br. Med. Bull. 2024, 149, 60–71. [Google Scholar] [CrossRef]
- Arya, R.; Haque, A.K.M.A.; Shakya, H.; Billah, M.M.; Parvin, A.; Rahman, M.-M.; Sakib, K.M.; Faruquee, H.M.; Kumar, V.; Kim, J.-J. Parkinson’s Disease: Biomarkers for Diagnosis and Disease Progression. Int. J. Mol. Sci. 2024, 25, 12379. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial Dysfunction in Parkinson’s Disease—A Key Disease Hallmark with Therapeutic Potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Kawabata, T. Iron-Induced Oxidative Stress in Human Diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef]
- Ding, X.; Gao, L.; Han, Z.; Eleuteri, S.; Shi, W.; Shen, Y.; Song, Z.; Su, M.; Yang, Q.; Qu, Y.; et al. Ferroptosis in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Potential. Ageing Res. Rev. 2023, 91, 102077. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Chen, C.-M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Asano, H.; Tian, Y.-S.; Hatabu, A.; Takagi, T.; Ueda, M.; Ikeda, K. Safety Comparisons among Monoamine Oxidase Inhibitors against Parkinson’s Disease Using FDA Adverse Event Reporting System. Sci. Rep. 2023, 13, 19272. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Recent Advances in Drug Therapy for Parkinson’s Disease. Intern. Med. 2023, 62, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Gurevich, T.; Djaldetti, R.; Adar, L.; Case, R.; Leibman-Barak, S.; Sasson, N.; Caraco, Y. ND0612 (Levodopa/Carbidopa for Subcutaneous Infusion) in Patients with Parkinson’s Disease and Motor Response Fluctuations: A Randomized, Placebo-Controlled Phase 2 Study. Park. Relat. Disord. 2021, 91, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Woitalla, D.; Buhmann, C.; Hilker-Roggendorf, R.; Höglinger, G.; Koschel, J.; Müller, T.; Weise, D. Role of Dopamine Agonists in Parkinson’s Disease Therapy. J. Neural. Transm. 2023, 130, 863–873. [Google Scholar] [CrossRef]
- Hariz, M.; Blomstedt, P. Deep Brain Stimulation for Parkinson’s Disease. J. Intern. Med. 2022, 292, 764–778. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA 2020, 323, 548. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- González, Y.; Mojica-Flores, R.; Moreno-Labrador, D.; Cubilla-Rios, L.; Rao, K.S.J.; Fernández, P.L.; Larionov, O.V.; Lakey-Beitia, J. Polyphenols with Anti-Inflammatory Properties: Synthesis and Biological Activity of Novel Curcumin Derivatives. Int. J. Mol. Sci. 2023, 24, 3691. [Google Scholar] [CrossRef]
- Bērziņa, L.; Mieriņa, I. Antiradical and Antioxidant Activity of Compounds Containing 1,3-Dicarbonyl Moiety: An Overview. Molecules 2023, 28, 6203. [Google Scholar] [CrossRef]
- Cozmin, M.; Lungu, I.I.; Gutu, C.; Stefanache, A.; Duceac, L.D.; Șoltuzu, B.D.; Damir, D.; Calin, G.; Bogdan Goroftei, E.R.; Grierosu, C.; et al. Turmeric: From Spice to Cure. A Review of the Anti-Cancer, Radioprotective and Anti-Inflammatory Effects of Turmeric Sourced Compounds. Front. Nutr. 2024, 11, 1399888. [Google Scholar] [CrossRef]
- Pál, P. Chalcones and Their Synthetic Analogs; Nova Science Publishers: Hauppauge, NY, USA, 2020. [Google Scholar]
- Kuzminska, J.; Szyk, P.; Mlynarczyk, D.T.; Bakun, P.; Muszalska-Kolos, I.; Dettlaff, K.; Sobczak, A.; Goslinski, T.; Jelinska, A. Curcumin Derivatives in Medicinal Chemistry: Potential Applications in Cancer Treatment. Molecules 2024, 29, 5321. [Google Scholar] [CrossRef]
- Vieira, T.M.; Tanajura, L.S.; Heleno, V.C.G.; Magalhães, L.G.; Crotti, A.E.M. Monoketone Curcuminoids: An Updated Review of Their Synthesis and Biological Activities. Future Pharmacol. 2024, 4, 54–77. [Google Scholar] [CrossRef]
- Roayapalley, P.K.; Sakagami, H.; Satoh, K.; Amano, S.; Bandow, K.; Aguilera, R.J.; Hernandez, K.G.C.; Schiaffino Bustamante, A.Y.; Dimmock, S.G.; Sharma, R.K.; et al. Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring. Medicines 2021, 8, 78. [Google Scholar] [CrossRef]
- ElNaggar, A.C.; Saini, U.; Naidu, S.; Wanner, R.; Sudhakar, M.; Fowler, J.; Nagane, M.; Kuppusamy, P.; Cohn, D.E.; Selvendiran, K. Anticancer Potential of Diarylidenyl Piperidone Derivatives, HO-4200 and H-4318, in Cisplatin Resistant Primary Ovarian Cancer. Cancer Biol. Ther. 2016, 17, 1107–1115. [Google Scholar] [CrossRef]
- Shi, L.; Gao, L.; Cai, S.; Xiong, Q.; Ma, Z. A Novel Selective Mitochondrial-Targeted Curcumin Analog with Remarkable Cytotoxicity in Glioma Cells. Eur. J. Med. Chem. 2021, 221, 113528. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Siddiqui, M.M.; Subhedar, D.D.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study. Chem. Biodivers. 2020, 17, e1900624. [Google Scholar] [CrossRef]
- Nivedha, J.; Kanimozhi, K.; Olikkavi, S.; Vidhyasagar, T.; Vijayakumar, N.; Uma, C.; Rajeswari, K.; Vennila, L. In Vitro Screening for Antioxidant and Antimicrobial Properties of 3,5-Bis(E-Thienylmethylene) Piperidin-4-One, a Curcumin Analogue. Pharmacogn. Res. 2022, 14, 276–283. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H.; Mustafa, Y.F. Curcumin and Its Derivatives: A Review of Their Biological Activities. Syst. Rev. Pharm. 2020, 11, 472–481. [Google Scholar] [CrossRef]
- Chang, Y.-H. Curcumin as a Potential Therapeutic Agent for Parkinson’s Disease: A Systematic Review. Front. Pharmacol. 2025, 16, 1593191. [Google Scholar] [CrossRef]
- Huber, I.; Pandur, E.; Sipos, K.; Barna, L.; Harazin, A.; Deli, M.A.; Tyukodi, L.; Gulyás-Fekete, G.; Kulcsár, G.; Rozmer, Z. Novel Cyclic C5-Curcuminoids Penetrating the Blood-Brain Barrier: Design, Synthesis and Antiproliferative Activity against Astrocytoma and Neuroblastoma Cells. Eur. J. Pharm. Sci. 2022, 173, 106184. [Google Scholar] [CrossRef]
- Al Nakib, T.; Bezjak, V.; Meegan, M.; Chandy, R. Synthesis and Antifungal Activity of Some 3-Benzylidenechroman-4-Ones, 3-Benzylidenethiochroman-4-Ones and 2-Benzylidene-1-Tetralones. Eur. J. Med. Chem. 1990, 25, 455–462. [Google Scholar] [CrossRef]
- Huber, I.; Zupkó, I.; Gyovai, A.; Horváth, P.; Kiss, E.; Gulyás-Fekete, G.; Schmidt, J.; Perjési, P. A Novel Cluster of C5-Curcuminoids: Design, Synthesis, In Vitro Antiproliferative Activity and DNA Binding of Bis(Arylidene)-4-Cyclanone Derivatives Based on 4-Hydroxycyclohexanone Scaffold. Res. Chem. Intermed. 2019, 45, 4711–4735. [Google Scholar] [CrossRef]
- Das, S.; Das, U.; Selvakumar, P.; Sharma, R.K.; Balzarini, J.; De Clercq, E.; Molnár, J.; Serly, J.; Baráth, Z.; Schatte, G.; et al. 3,5-Bis(Benzylidene)-4-oxo-1-phosphonopiperidines and Related Diethyl Esters: Potent Cytotoxins with Multi-Drug-Resistance Reverting Properties. ChemMedChem 2009, 4, 1831–1840. [Google Scholar] [CrossRef]
- Fawzy, I.M.; Youssef, K.M.; Ismail, N.S.M.; Gullbo, J.; Abouzid, K.A.M. Newly Designed and Synthesized Curcumin Analogs with in Vitro Cytotoxicity and Tubulin Polymerization Activity. Chem. Biol. Drug Des. 2015, 86, 80–90. [Google Scholar] [CrossRef]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef]
- Pandur, E.; Major, B.; Rák, T.; Sipos, K.; Csutak, A.; Horváth, G. Linalool and Geraniol Defend Neurons from Oxidative Stress, Inflammation, and Iron Accumulation in In Vitro Parkinson’s Models. Antioxidants 2024, 13, 917. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Fritz, F.R.; Nagy, T.; Agócs, A.; Deli, J. Protective Effects of 3′-Epilutein and 3′-Oxolutein against Glutamate-Induced Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 12008. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Pap, R.; Sipos, K. Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells. Int. J. Mol. Sci. 2024, 25, 9624. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric Ferrozine-Based Assay for the Quantitation of Iron in Cultured Cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Yeh, T.-H.; Lu, C.-S.; Huang, Y.-C.; Cheng, Y.-C.; Huang, Y.-Z.; Weng, Y.-H.; Liu, Y.-C.; Lai, S.-C.; Chen, Y.-L.; et al. PARK14 PLA2G6 Mutants Are Defective in Preventing Rotenone-Induced Mitochondrial Dysfunction, ROS Generation and Activation of Mitochondrial Apoptotic Pathway. Oncotarget 2017, 8, 79046–79060. [Google Scholar] [CrossRef]
- Huber, I.; Rozmer, Z.; Gyöngyi, Z.; Budán, F.; Horváth, P.; Kiss, E.; Perjési, P. Structure Activity Relationship Analysis of Antiproliferative Cyclic C5-Curcuminoids without DNA Binding: Design, Synthesis, Lipophilicity and Biological Activity. J. Mol. Struct. 2020, 1206, 127661. [Google Scholar] [CrossRef]
- Trainor, A.R.; MacDonald, D.S.; Penney, J. Microglia: Roles and Genetic Risk in Parkinson’s Disease. Front. Neurosci. 2024, 18, 1506358. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Pan, H.; Han, L. Global, Regional, and National Burden of Neurological Disorders in 204 Countries and Territories Worldwide. J. Glob. Health 2023, 13, 04160. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New Strategies of Neurodegenerative Disease Treatment with Extracellular Vesicles (EVs) Derived from Mesenchymal Stem Cells (MSCs). Theranostics 2023, 13, 4138–4165. [Google Scholar] [CrossRef]
- de la Fuente, A.G.; Pelucchi, S.; Mertens, J.; Di Luca, M.; Mauceri, D.; Marcello, E. Novel Therapeutic Approaches to Target Neurodegeneration. Br. J. Pharmacol. 2023, 180, 1651–1673. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. The Golden Spice for Life: Turmeric with the Pharmacological Benefits of Curcuminoids Components, Including Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumins. Curr. Org. Synth. 2024, 21, 665–683. [Google Scholar] [CrossRef]
- Di Martino, R.M.C.; Pruccoli, L.; Bisi, A.; Gobbi, S.; Rampa, A.; Martinez, A.; Pérez, C.; Martinez-Gonzalez, L.; Paglione, M.; Di Schiavi, E.; et al. Novel Curcumin-Diethyl Fumarate Hybrid as a Dualistic GSK-3β Inhibitor/Nrf2 Inducer for the Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Ramires, O.V., Jr.; Alves, B.d.S.; Barros, P.A.B.; Rodrigues, J.L.; Ferreira, S.P.; Monteiro, L.K.S.; Araújo, G.d.M.S.; Fernandes, S.S.; Vaz, G.R.; Dora, C.L.; et al. Nanoemulsion Improves the Neuroprotective Effects of Curcumin in an Experimental Model of Parkinson’s Disease. Neurotox. Res. 2021, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- El Nebrisi, E.; Javed, H.; Ojha, S.K.; Oz, M.; Shehab, S. Neuroprotective Effect of Curcumin on the Nigrostriatal Pathway in a 6-Hydroxydopmine-Induced Rat Model of Parkinson’s Disease Is Mediated by A7-Nicotinic Receptors. Int. J. Mol. Sci. 2020, 21, 7329. [Google Scholar] [CrossRef]
- Xu, L.; Hao, L.-P.; Yu, J.; Cheng, S.-Y.; Li, F.; Ding, S.-M.; Zhang, R. Curcumin Protects against Rotenone-Induced Parkinson’s Disease in Mice by Inhibiting Microglial NLRP3 Inflammasome Activation and Alleviating Mitochondrial Dysfunction. Heliyon 2023, 9, e16195. [Google Scholar] [CrossRef]
- Nebrisi, E. El Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical Composition of Turmeric (Curcuma longa L.) Ethanol Extract and Its Antimicrobial Activities and Free Radical Scavenging Capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Tai, S.; Zheng, Q.; Zhai, S.; Cai, T.; Xu, L.; Yang, L.; Jiao, L.; Zhang, C. Alpha-Lipoic Acid Mediates Clearance of Iron Accumulation by Regulating Iron Metabolism in a Parkinson’s Disease Model Induced by 6-OHDA. Front. Neurosci. 2020, 14, 612. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, Y.; Shuai, K.; Zhou, C.; Chen, L.; Zhu, L.; Wu, X.-M. The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2023, 24, 3709. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kanthasamy, A.G.; Jin, H.; Reddy, M.B. Hepcidin Plays a Key Role in 6-OHDA Induced Iron Overload and Apoptotic Cell Death in a Cell Culture Model of Parkinson’s Disease. Park. Dis. 2016, 2016, 8684130. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Patel, M. Role of Reactive Oxygen Species in the Neurotoxicity of Environmental Agents Implicated in Parkinson’s Disease. Free. Radic. Biol. Med. 2008, 44, 1873–1886. [Google Scholar] [CrossRef]
- Pantic, I.; Cumic, J.; Skodric, S.R.; Dugalic, S.; Brodski, C. Oxidopamine and Oxidative Stress: Recent Advances in Experimental Physiology and Pharmacology. Chem. Biol. Interact. 2021, 336, 109380. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nishio, K.; Ogawa, Y.; Kinumi, T.; Yoshida, Y.; Masuo, Y.; Niki, E. Molecular Mechanisms of 6-Hydroxydopamine-Induced Cytotoxicity in PC12 Cells: Involvement of Hydrogen Peroxide-Dependent and -Independent Action. Free. Radic. Biol. Med. 2007, 42, 675–685. [Google Scholar] [CrossRef]
- Glinka, Y.; Tipton, K.F.; Youdim, M.B.H. Nature of Inhibition of Mitochondrial Respiratory Complex I by 6-Hydroxydopamine. J. Neurochem. 1996, 66, 2004–2010. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Lecht, S.; Lahiani, A.; Klazas, M.; Naamneh, M.S.; Rubin, L.; Dong, J.; Zheng, W.; Lazarovici, P. Rasagiline Exerts Neuroprotection towards Oxygen–Glucose-Deprivation/Reoxygenation-Induced GAPDH-Mediated Cell Death by Activating Akt/Nrf2 Signaling. Biomedicines 2024, 12, 1592. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 11059. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Effect of 6-Hydroxydopamine Increase the Glutathione Level in SH-SY5Y Human Neuroblastoma Cells. Acta Biochim. Pol. 2023, 70, 457–464. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Wu, X.; Hoo, R.L.-C.; Lee, S.M.-Y.; Cheung, T.M.-Y.; Ho, B.S.-Y.; Leung, G.P.-H. Amauroderma rugosum Protects PC12 Cells against 6-OHDA-Induced Neurotoxicity through Antioxidant and Antiapoptotic Effects. Oxid. Med. Cell. Longev. 2021, 2021, 6683270. [Google Scholar] [CrossRef]
- Mercola, J. Reductive Stress and Mitochondrial Dysfunction: The Hidden Link in Chronic Disease. Free. Radic. Biol. Med. 2025, 233, 118–131. [Google Scholar] [CrossRef]
- Mladenov, M.; Sazdova, I.; Hadzi-Petrushev, N.; Konakchieva, R.; Gagov, H. The Role of Reductive Stress in the Pathogenesis of Endocrine-Related Metabolic Diseases and Cancer. Int. J. Mol. Sci. 2025, 26, 1910. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; da Cunha Lima, B.F.; Rodrigues, B.M.; et al. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Viedma-Poyatos, Á.; González-Jiménez, P.; Langlois, O.; Company-Marín, I.; Spickett, C.M.; Pérez-Sala, D. Protein Lipoxidation: Basic Concepts and Emerging Roles. Antioxidants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging Mechanisms of Lipid Peroxidation in Regulated Cell Death and Its Physiological Implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, H.G.; Hur, J.; Lee, G.H.; Won, J.P.; Kim, E.; Hwang, J.S.; Seo, H.G. PPARδ Activation Mitigates 6-OHDA-Induced Neuronal Damage by Regulating Intracellular Iron Levels. Antioxidants 2022, 11, 810. [Google Scholar] [CrossRef]
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Kükürt, A.; Gelen, V.; Faruk Başer, Ö.; Ahmet Deveci, H.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. In Accenting Lipid Peroxidation; IntechOpen: London, UK, 2021. [Google Scholar]
- Ates, I.; Kaplan, M.; Inan, B.; Alısık, M.; Erel, O.; Yilmaz, N.; Guler, S. How Does Thiol/Disulfide Homeostasis Change in Prediabetic Patients? Diabetes Res. Clin. Pract. 2015, 110, 166–171. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Bulbuloglu, E.; Kurutas, E.B.; Ciralik, H.; Kantarceken, B.; Buyukbese, M.A. Beneficial Effects of N-Acetylcysteine on Acetic Acid-Induced Colitis in Rats. Tohoku J. Exp. Med. 2005, 206, 131–139. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated with Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Luis, P.B.; Boeglin, W.E.; Schneider, C. Thiol Reactivity of Curcumin and Its Oxidation Products. Chem. Res. Toxicol. 2018, 31, 269–276. [Google Scholar] [CrossRef]
- Li, Y.; Toscano, M.; Mazzone, G.; Russo, N. Antioxidant Properties and Free Radical Scavenging Mechanisms of Cyclocurcumin. New J. Chem. 2018, 42, 12698–12705. [Google Scholar] [CrossRef]
- Rahul, R.; Arrivukkarasan, S.; Anhuradha, S. In Vitro Antioxidant and Free Radical Scavenging Activity of Curcuma Longa, Acorus Calamus and Camellia Sinensis. Food Nutr. Sci. 2022, 13, 750–760. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N.A. Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Lee, H.; Zhuang, L.; Gan, B. Energy Stress Inhibits Ferroptosis via AMPK. Mol. Cell. Oncol. 2020, 7, 1761242. [Google Scholar] [CrossRef]
- Gonçalves, D.F.; Courtes, A.A.; Hartmann, D.D.; da Rosa, P.C.; Oliveira, D.M.; Soares, F.A.A.; Dalla Corte, C.L. 6-Hydroxydopamine Induces Different Mitochondrial Bioenergetics Response in Brain Regions of Rat. Neurotoxicology 2019, 70, 1–11. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, Structure, and Function of the Mitochondrial Permeability Transition Pore: Controversies, Consensus, Recent Advances, and Future Directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Manica, D.; Silva, G.B.d.; Silva, A.P.d.; Marafon, F.; Maciel, S.F.V.d.O.; Bagatini, M.D.; Moreno, M. Curcumin Promotes Apoptosis of Human Melanoma Cells by Caspase 3. Cell Biochem. Funct. 2023, 41, 1295–1304. [Google Scholar] [CrossRef]
- Razali, N.S.C.; Lam, K.W.; Rajab, N.F.; Jamal, A.R.A.; Kamaludin, N.F.; Chan, K.M. Curcumin Piperidone Derivatives Induce Caspase-Dependent Apoptosis and Suppress MiRNA-21 Expression in LN-18 Human Glioblastoma Cells. Genes Environ. 2024, 46, 4. [Google Scholar] [CrossRef]
- Pakrashi, S.; Chakraborty, J.; Bandyopadhyay, J. Quercetin Alleviates 6-OHDA-Caused Apoptosis in SH-SY5Y Cells. Toxicol. Res. 2024, 13, tfae117. [Google Scholar] [CrossRef]
- Huber, I.; Zupkó, I.; Kovács, I.J.; Minorics, R.; Gulyás-Fekete, G.; Maász, G.; Perjési, P. Synthesis and Antiproliferative Activity of Cyclic Arylidene Ketones: A Direct Comparison of Monobenzylidene and Dibenzylidene Derivatives. Monatshefte Chem. Chem. Mon. 2015, 146, 973–981. [Google Scholar] [CrossRef]
- Banerjee, S.; Sadler, P.J. Transfer Hydrogenation Catalysis in Cells. RSC Chem. Biol. 2021, 2, 12–29. [Google Scholar] [CrossRef]
- Ludwig, R. Hydrogen-Transfer Reactions.Edited by J. T. Hynes, J.P. Klinman, H.-H. Limbach and R. L. Schowen. ChemPhysChem 2007, 8, 2539. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and Immune Dysfunction in Parkinson Disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and Astrocyte Dysfunction in Parkinson’s Disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huh, J.R.; Choi, G.B. One Messenger Shared by Two Systems: How Cytokines Directly Modulate Neurons. Curr. Opin. Neurobiol. 2023, 80, 102708. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Harms, A.S.; Boehringer, A.; Kordower, J.H. Decreased Neuronal and Increased Endothelial Fractalkine Expression Are Associated with Neuroinflammation in Parkinson’s Disease and Related Disorders. Front. Cell. Neurosci. 2025, 19, 1557645. [Google Scholar] [CrossRef]
- Dzamko, N. Cytokine Activity in Parkinson’s Disease. Neuronal Signal. 2023, 7, NS20220063. [Google Scholar] [CrossRef]
- Liu, J.; Liang, Y.; Meng, Q.; Chen, J.; Ma, J.; Zhu, H.; Cai, L.; Song, N.; Ding, J.; Fan, Y.; et al. Antagonism of β-Arrestins in IL-4–Driven Microglia Reactivity via the Samd4/MTOR/OXPHOS Axis in Parkinson’s Disease. Sci. Adv. 2024, 10, eadn4845. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef]
- Amin, R.; Quispe, C.; Docea, A.O.; Ydyrys, A.; Kulbayeva, M.; Durna Daştan, S.; Calina, D.; Sharifi-Rad, J. The Role of Tumour Necrosis Factor in Neuroinflammation Associated with Parkinson’s Disease and Targeted Therapies. Neurochem. Int. 2022, 158, 105376. [Google Scholar] [CrossRef]
- Zhan, L.; Qiu, M.; Zheng, J.; Lai, M.; Lin, K.; Dai, J.; Sun, W.; Xu, E. Fractalkine/CX3CR1 Axis Is Critical for Neuroprotection Induced by Hypoxic Postconditioning against Cerebral Ischemic Injury. Cell Commun. Signal. 2024, 22, 457. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Ouyang, X.; Wang, F.; Li, Q.; Xu, Z.; Ji, D.; Wu, Q.; Zhang, J.; Lu, C.; et al. The Effect of CX3CL1/ CX3CR1 Signal Axis on Microglia in Central Nervous System Diseases. J. Neurorestoratology 2023, 11, 100042. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Shaikh, M.F.; Piperi, C. Fractalkine (CX3CL1) Signaling and Neuroinflammation in Parkinson’s Disease: Potential Clinical and Therapeutic Implications. Pharmacol. Res. 2020, 158, 104930. [Google Scholar] [CrossRef] [PubMed]

| Test Compound | Concentrations (µM) |
|---|---|
| Compound 4 | 3; 5; 8 |
| Compound 5 | 5; 10; 15 |
| Compound 8 | 3; 4; 5 |
| Compound 9 | 5; 10; 14 |
| Compound 11 | 3; 5; 6 |
| Compound 12 | 3; 5; 7 |
| Compound 13 | 5; 10; 12 |
| IC50 (µM) | SH-SY5Y | Concentration Used in the Experiments (µM) |
|---|---|---|
| Compound 4 | 9.14 ± 1.13 | 5 |
| Compound 5 | 18.11 ± 2.11 | 10 |
| Compound 8 | 6.23 ± 1.09 | 5 |
| Compound 9 | 15.11 ± 1.03 | 10 |
| Compound 11 | 7.46 ± 1.14 | 5 |
| Compound 12 | 8.94 ± 1.23 | 5 |
| Compound 13 | 13.68 ± 1.22 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Gulyás-Fekete, G.; Kulcsár, G.; Huber, I. Synthetic Cyclic C5-Curcuminoids Increase Antioxidant Defense and Reduce Inflammation in 6-OHDA-Induced Retinoic Acid-Differentiated SH-SY5Y Cells. Antioxidants 2025, 14, 1057. https://doi.org/10.3390/antiox14091057
Pandur E, Gulyás-Fekete G, Kulcsár G, Huber I. Synthetic Cyclic C5-Curcuminoids Increase Antioxidant Defense and Reduce Inflammation in 6-OHDA-Induced Retinoic Acid-Differentiated SH-SY5Y Cells. Antioxidants. 2025; 14(9):1057. https://doi.org/10.3390/antiox14091057
Chicago/Turabian StylePandur, Edina, Gergely Gulyás-Fekete, Győző Kulcsár, and Imre Huber. 2025. "Synthetic Cyclic C5-Curcuminoids Increase Antioxidant Defense and Reduce Inflammation in 6-OHDA-Induced Retinoic Acid-Differentiated SH-SY5Y Cells" Antioxidants 14, no. 9: 1057. https://doi.org/10.3390/antiox14091057
APA StylePandur, E., Gulyás-Fekete, G., Kulcsár, G., & Huber, I. (2025). Synthetic Cyclic C5-Curcuminoids Increase Antioxidant Defense and Reduce Inflammation in 6-OHDA-Induced Retinoic Acid-Differentiated SH-SY5Y Cells. Antioxidants, 14(9), 1057. https://doi.org/10.3390/antiox14091057





