Chronic Carbonate Alkalinity Exposure Induces Dysfunction in Ovary and Testis Development in Largemouth Bass Micropterus salmoides by Oxidative Damage and Sex-Specific Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Carbonate Alkalinity Exposure
2.2. Sample Collection
2.3. Antioxidant Indicators and Sex Hormone Measurements
2.4. HE Staining
2.5. TUNEL Staining
2.6. TEM Analysis
2.7. Transcriptome Sequencing
2.7.1. RNA Extraction, Library Construction, and Sequencing
2.7.2. Differential Gene Expression Screening and Functional Enrichment Analysis
2.7.3. Protein–Protein Interaction (PPI) Network Construction and Hub Gene Screening
2.7.4. Gene Set Enrichment Analysis
2.8. RT-qPCR Analysis
2.9. Data Analysis
3. Results
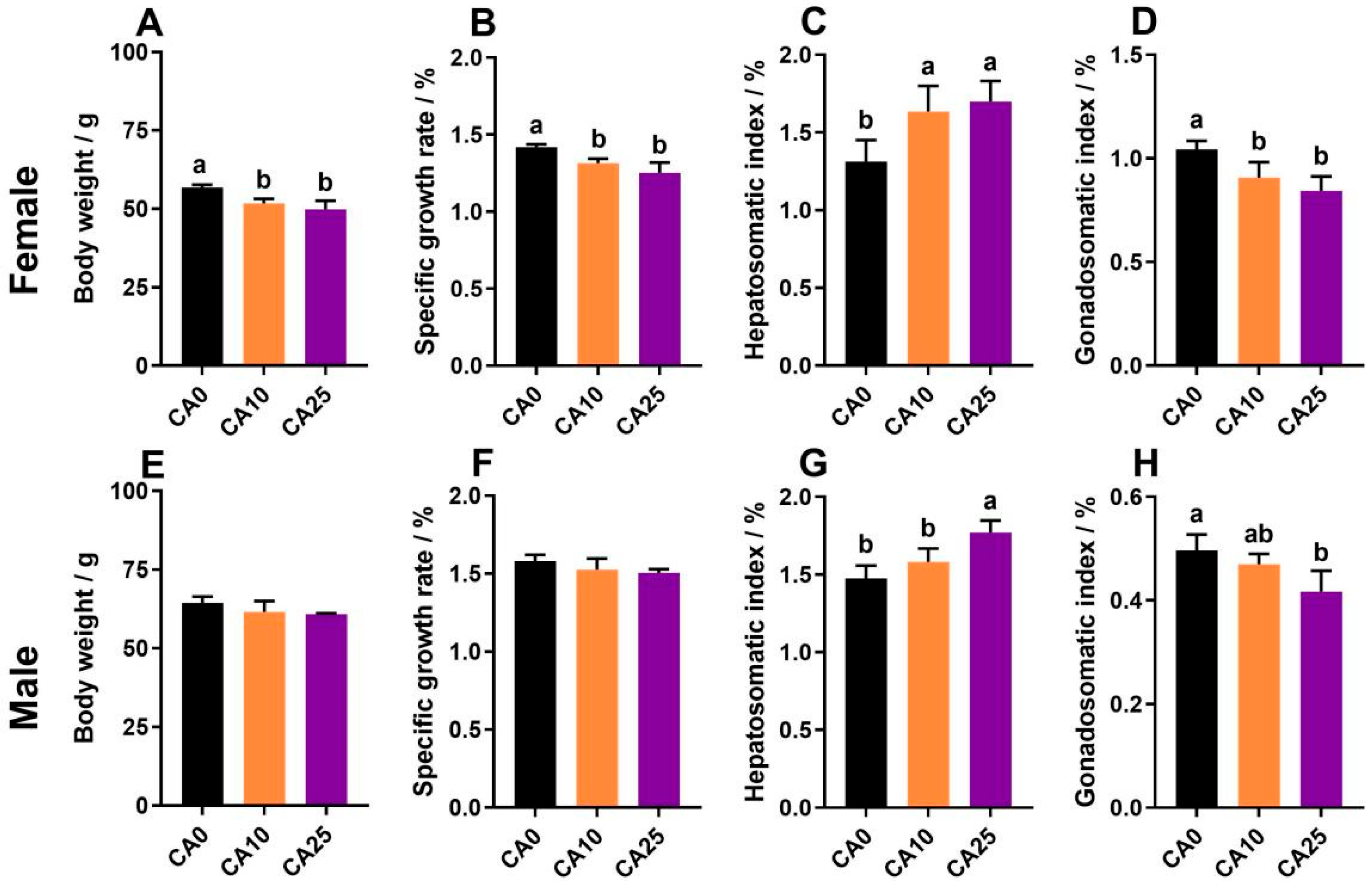
3.1. Comparison of Growth Performance
3.2. Antioxidant Indices and Variation in Sex Hormone Levels
3.3. Gonadal Histology and Apoptosis Analysis
3.4. Electron Microscopy of Gonadal Tissues After Carbonate Alkalinity Exposure
3.5. Transcriptome Sequencing Analysis
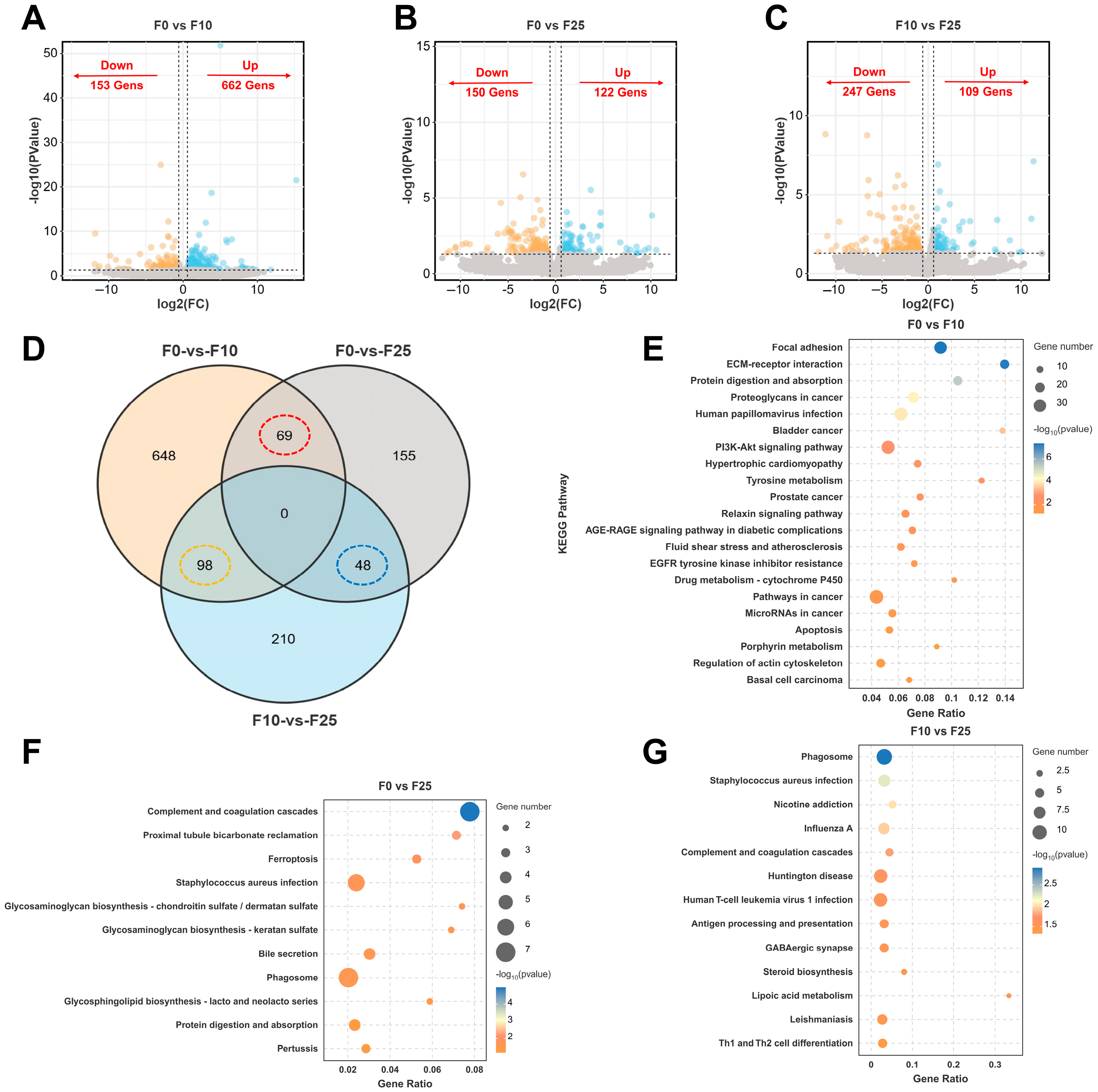
3.6. Identification and Functional Enrichment Analysis of DEGs in Ovary
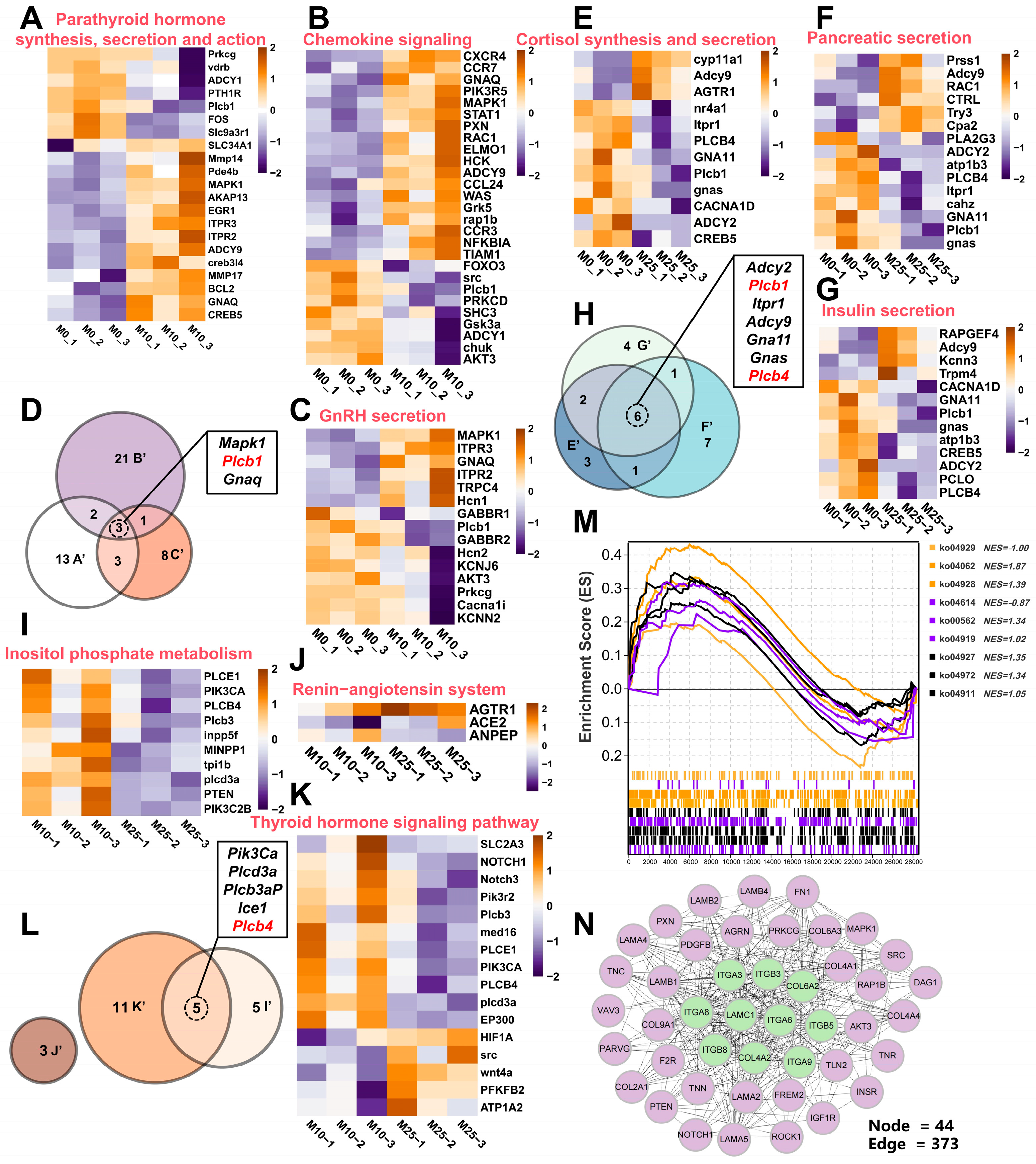
3.7. Identification and Functional Enrichment Analysis of DEGs in Testis
3.8. RT-qPCR Validation of DEGs in Relation to Indicators of Gonadal Developmental
4. Discussion
4.1. Physiological Changes in Largemouth Black Bass in Response to Carbonate Alkalinity Exposure
4.2. Carbonate Alkalinity Exposure Leads to Oxidative Stress
4.3. Carbonate Alkalinity Exposure Leads to Delayed Gonadal Development
4.4. Molecular Mechanisms of Gonadal Developmental Regulation Under Carbonate Alkalinity Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, N. Adapting legal regimes: Ensuring access, equity, and protection of genetic resources in Chinese aquaculture. Aquaculture 2025, 600, 742245. [Google Scholar] [CrossRef]
- Li, C.; Qian, S.; Liu, J.; Wang, Y. Analysis on the decoupling of China’s economic development and water resource utilization under the rigid constraint of water resources. Front. Sustain. Food Syst. 2025, 9, 1498807. [Google Scholar] [CrossRef]
- Shang, X.; Geng, L.; Yang, J.; Zhang, Y.; Xu, W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotoxicol. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef]
- Yao, Z.L.; Lai, Q.F.; Zhou, K.; Rizalita, R.E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in gill of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 2024, 586, 740766. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109487. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-B.; Chen, X.-Q.; Wang, S.; Chen, X.-C.; Wang, Y.-X.; Chang, J.P.; Du, J.-Z. Growth hormone and insulin-like growth factor of naked carp (Gymnocypris przewalskii) in Lake Qinghai: Expression in different water environments. Gen. Comp. Endocrinol. 2009, 161, 400–406. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, J.; Lv, H.; Zhou, T.; Zhao, J.; Bai, H.; Pu, F.; Xu, P.; Gossmann, T. The adaptive evolution of Leuciscus waleckii in Lake Dali Nur and convergent evolution of Cypriniformes fishes inhabiting extremely alkaline environments. Genome Biol. Evol. 2023, 15, evad082. [Google Scholar] [CrossRef]
- Wei, H.; Geng, L.; Shang, X.; Li, W.; Guo, K.; Xu, W. Metabolomics analysis reveals the mechanism for improving the growth performance and flesh quality of Aral barbel Luciobarbus brachycephalus by environmental alkalinity. J. Agric. Food Res. 2025, 19, 101670. [Google Scholar] [CrossRef]
- Kong, L.; Abudu, A.; Yang, L.; Ye, M.; Zhang, Y.; Hong, Y.; Chen, H.; Li, G.; Shi, G.; Tian, C. Acute alkalinity stress triggers kidney histopathology, oxidative-immune dysregulation, and MAPK/TGF-β signaling pathways activation in Scatophagus argus. Aquaculture 2025, 606, 742601. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, T.; Zhang, T.; Sun, M.; Ning, Z.; Chen, Y.; Mu, W. Effects of chronic saline-alkaline stress on gill, liver and intestinal histology, biochemical, and immune indexes in Amur minnow (Phoxinus lagowskii). Aquaculture 2024, 579, 740153. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, W.; Li, J.; Feng, W.; Kamunga, E.M.; Zhang, Z.; Tang, Y. Physiological and molecular responses of juvenile silver crucian carp (Carassius gibelio) to long-term high alkaline stress: Growth performance, histopathology, and transcriptomic analysis. Aquac. Rep. 2024, 39, 102393. [Google Scholar] [CrossRef]
- Yang, L.; Abudu, A.; Zhu, K.; Han, T.; Duan, C.; Chen, Y.; Li, X.; Shi, G.; Zhu, C.; Li, G.; et al. Acute alkalinity stress induces functional damage and alters immune metabolic pathways in the gill tissue of spotted scat (Scatophagus argus). Aquaculture 2025, 599, 742186. [Google Scholar] [CrossRef]
- Galat, D.L.; Post, G.; Keefe, T.J.; Bouck, G.R. Histological changes in the gill, kidney and liver of Lahontan cutthroat trout, Salmo clarki henshawi, living in lakes of different salinity-alkalinity. J. Fish Biol. 2006, 27, 533–552. [Google Scholar] [CrossRef]
- Ali, M.J.; Tao, Y.; Li, Y.; Sayouh, M.A.; Lu, S.; Qiang, J.; Xu, P. Modulation of chronic hypoxia on ovarian structure, oxidative stress, and apoptosis in female Nile Tilapia (Oreochromis niloticus). Aquaculture 2024, 590, 741081. [Google Scholar] [CrossRef]
- Alsubaie, N.; Mohamed, A.A.-R.; Metwally, M.M.M.; Khamis, T.; Osman, A.; Alotaibi, B.S.; Eskandrani, A.A.; Abuzahrah, S.S.; Abd-Elhakim, Y.M.; El-Murr, A.; et al. Alkalinity exposure induced growth inhibition, intestinal histopathological changes, and down-regulated nutrient transporter expression in Nile Tilapia: The ameliorative role of dietary camel whey protein hydrolysates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2025, 277, 111074. [Google Scholar] [CrossRef] [PubMed]
- Sashaw, J.; Nawata, M.; Thompson, S.; Wood, C.M.; Wright, P.A. Rhesus glycoprotein and urea transporter genes in rainbow trout embryos are upregulated in response to alkaline water (pH 9.7) but not elevated water ammonia. Aquat. Toxicol. 2010, 96, 308–313. [Google Scholar] [CrossRef]
- Tao, S.; Li, X.; Wang, J.; Bai, Y.; Wang, J.; Yang, Y.; Zhao, Z. Examination of the relationship of carbonate alkalinity stress and ammonia metabolism disorder-mediated apoptosis in the Chinese mitten crab, Eriocheir sinensis: Potential involvement of the ROS/MAPK signaling pathway. Aquaculture 2024, 579, 740179. [Google Scholar] [CrossRef]
- Liu, S.-T.; Horng, J.-L.; Chen, P.-Y.; Hwang, P.-P.; Lin, L.-Y. Salt secretion is linked to acid-base regulation of ionocytes in seawater-acclimated medaka: New insights into the salt-secreting mechanism. Sci. Rep. 2016, 6, 31433. [Google Scholar] [CrossRef]
- Hwang, P.-P.; Lee, T.-H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, J.; Zhang, Y.; Zhang, G.; Wang, T.; Zhang, K.; Yin, S. Effect of long-term hypoxia on the reproductive systems of female and male yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2023, 267, 110864. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Jin, C.X.; Wu, H.; He, W.; Wu, Z.X.; Wang, Z.T.; Hong, Y.Z.; Yang, Z.H.; Yang, S.; et al. Study on the potential impact of sustained high temperatures during non-breeding season on largemouth bass. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 55, 101501. [Google Scholar] [CrossRef]
- Wang, T.; Kong, H.; Shang, Y.; Dupont, S.; Peng, J.; Wang, X.; Deng, Y.; Peng, J.; Hu, M.; Wang, Y. Ocean acidification but not hypoxia alters the gonad performance in the thick shell mussel Mytilus coruscus. Mar. Pollut. Bull. 2021, 167, 112282. [Google Scholar] [CrossRef]
- Zhao, C.; Song, Y.; Yan, J.; Yang, Z.; Wang, S.; Liu, Y.; Wang, T.; Zhang, G.; Yin, S. Multi-omics analysis revealed the dysfunction of ovary and testis induced by chronic hypoxia in Pelteobagrus fulvidraco. Aquaculture 2024, 584, 740668. [Google Scholar] [CrossRef]
- Peng, X.; Sun, X.; Yu, M.; Fu, W.; Chen, H.; Chen, J. Chronic exposure to environmental concentrations of phenanthrene impairs zebrafish reproduction. Ecotoxicol. Environ. Saf. 2019, 182, 109376. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wang, C.; Li, W.; Ge, Q.; Qin, Z.; Li, J.; Li, J. Effects of long-term high carbonate alkalinity stress on the ovarian development in Exopalaemon carinicauda. Water 2022, 14, 3690. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Li, J.i.; Ge, Q.; Ge, H.; Shen, M. Effects of carbonate alkalinity stress on the survival, growth, reproduction, and immune enzyme activities of Exopalaemon carinicauda. J. Fish. Sci. China 2016, 23, 1137–1147. [Google Scholar]
- Ding, M.; Tao, Y.; Hua, J.; Dong, Y.; Lu, S.; Qiang, J.; He, J. Genome-wide association study reveals growth-related SNPs and candidate genes in largemouth bass (Micropterus salmoides) adapted to hypertonic environments. Int. J. Mol. Sci. 2025, 26, 1834. [Google Scholar] [CrossRef]
- Hua, J.; Tao, Y.; Lu, S.; Li, Y.; Dong, Y.; Jiang, B.; Xi, B.; Qiang, J. Integration of transcriptome, histopathology, and physiological indicators reveals regulatory mechanisms of largemouth bass (Micropterus salmoides) in response to carbonate alkalinity stress. Aquaculture 2025, 596, 741883. [Google Scholar] [CrossRef]
- Sun, S.; You, Q.; Wang, P.; He, H.; Cao, X.; Zhao, Y.; Wang, Q.; Gao, J. Effects of different dietary protein sources on the growth performance, digestive capacity and amino acid profiles of largemouth bass (Micropterus salmoides) larvae. Anim. Feed Sci. Technol. 2025, 325, 116343. [Google Scholar] [CrossRef]
- Tao, Y.-F.; Qiang, J.; Dagoudo, M.; Zhu, H.-J.; Bao, J.-W.; Ma, J.-L.; Li, M.-X.; Xu, P. Transcriptome profiling reveals differential expression of immune-related genes in gills of hybrid yellow catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂) under hypoxic stress: Potential NLR-mediated immune response. Fish Shellfish Immunol. 2021, 119, 409–419. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, S.; Hua, J.; Tao, Y.; Zhuge, Y.; Chen, W.; Duan, X.; Qiang, J. Regulatory patterns of testis and ovary maturation in largemouth bass (Micropterus salmoides) revealed by transcriptome profiling. Aquac. Rep. 2025, 42, 102761. [Google Scholar] [CrossRef]
- Pi, X.; Yan, D.; Xu, Y.; Pan, M.; Wang, Z.; Chang, M.; Qi, Z. TLRs signaling pathway regulation, antibacterial and apoptotic activity of largemouth bass ECSIT during Edwardsiella piscicida infection. Aquaculture 2025, 595, 741615. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, K.; Yang, P.; Che, C.; Gao, S.; Fan, Z.; Li, W.; Wang, C.; Mu, C.; Wang, H. Comparative analysis of the non-volatile flavor of mud crab (Scylla paramamosain) cultured in sea water and coastal low saline-alkali water. J. Food Compos. Anal. 2025, 142, 107530. [Google Scholar] [CrossRef]
- Duan, Y.; Xing, Y.; Zhu, X.; Li, H.; Wang, Y.; Nan, Y. Integration of transcriptomic and metabolomic reveals carbonate alkalinity stress responses in the hepatopancreas of Litopenaeus vannamei. Aquat. Toxicol. 2023, 260, 106569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, P.; Zhou, K.; Yao, Z.; Sun, Z.; Qin, H.; Lai, Q. Effects of saline and alkaline stresses on the survival, growth, and physiological responses in juvenile mandarin fish (Siniperca chuatsi). Aquaculture 2024, 591, 741143. [Google Scholar] [CrossRef]
- Pellegrin, L.; Nitz, L.F.; Maltez, L.C.; Copatti, C.E.; Garcia, L. Alkaline water improves the growth and antioxidant responses of pacu juveniles (Piaractus mesopotamicus). Aquaculture 2020, 519, 734713. [Google Scholar] [CrossRef]
- Hua, J.; Tao, Y.; Jiao, X.; Fan, D.; Lu, S.; Dong, Y.; Wang, W.; Xi, B.; Qiang, J. Muscle quality enhancement of largemouth bass (Micropterus salmoides) by alkaline water cultured environment: A comprehensive evaluation of physical features, nutrient composition, and flavor characteristics. Food Chem. 2025, 490, 145086. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, S.; Tao, Y.; Hua, J.; Zhuge, Y.; Chen, W.; Qiang, J. Characteristic muscle quality parameters of male largemouth bass (Micropterus salmoides) distinguished from female and physiological variations revealed by transcriptome profiling. Biology 2024, 13, 1029. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Hwang, P.-P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 419–429. [Google Scholar] [CrossRef]
- Che, X.; Shang, X.; Xu, W.; Xing, M.; Wei, H.; Li, W.; Li, Z.; Teng, X.; Geng, L. Selenium-enriched Lactiplantibacillus plantarum alleviates alkalinity stress-induced selective hepatic insulin resistance in common carp. Int. J. Biol. Macromol. 2025, 305, 141204. [Google Scholar] [CrossRef]
- Rahman, M.L.; Zahangir, M.M.; Kitahashi, T.; Shahjahan, M.; Ando, H. Effects of high and low temperature on expression of GnIH, GnIH receptor, GH and PRL genes in the male grass puffer during breeding season. Gen. Comp. Endocrinol. 2019, 282, 113200. [Google Scholar] [CrossRef]
- Shang, X.; Geng, L.; Wei, H.J.; Liu, T.; Che, X.; Li, W.; Liu, Y.; Shi, X.d.; Li, J.; Teng, X.; et al. Analysis revealed the molecular mechanism of oxidative stress-autophagy-induced liver injury caused by high alkalinity: Integrated whole hepatic transcriptome and metabolome. Front. Immunol. 2024, 15, 1431224. [Google Scholar] [CrossRef]
- Liu, X.-L.; Xi, Q.-Y.; Yang, L.; Li, H.-Y.; Jiang, Q.-Y.; Shu, G.; Wang, S.-B.; Gao, P.; Zhu, X.-T.; Zhang, Y.-L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, F.; Li, R.; Li, E.; Qin, J.; Chen, L.; Wang, X. Growth performance, antioxidant capacity, intestinal microbiota, and metabolomics analysis of Nile tilapia (Oreochromis niloticus) under carbonate alkalinity stress. Aquaculture 2025, 595, 741675. [Google Scholar] [CrossRef]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The origins of the vertebrate hypothalamic–pituitary–gonadal (HPG) and hypothalamic–pituitary–thyroid (HPT) endocrine systems: New insights from lampreys. Gen. Comp. Endocrinol. 2009, 161, 20–29. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, Y.W.; Chen, Q.L.; Liu, Z.H. Long-term exposure of xenoestrogens with environmental relevant concentrations disrupted spermatogenesis of zebrafish through altering sex hormone balance, stimulating germ cell proliferation, meiosis and enhancing apoptosis. Environ. Pollut. 2019, 244, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, G.; Wang, J.; Lu, L.; Li, X.; Zheng, Y.; Zhang, Z.; Ru, S. Microplastics increase the accumulation of phenanthrene in the ovaries of marine medaka (Oryzias melastigma) and its transgenerational toxicity. J. Hazard. Mater. 2022, 424, 127754. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.L.; da Silva Castro, J.; Val, A.L. Copper and cadmium impair sperm performance, fertilization and hatching of oocytes from Amazonian fish Colossoma macropomum. Chemosphere 2021, 266, 128957. [Google Scholar] [CrossRef] [PubMed]
- Schwindt, A.R.; Feist, G.W.; Schreck, C.B. Stress does not inhibit induced vitellogenesis in juvenile rainbow trout. Environ. Biol. Fishes 2006, 80, 453–463. [Google Scholar] [CrossRef]
- Lu, X.; Yu, R.M.K.; Murphy, M.B.; Lau, K.; Wu, R.S.S. Hypoxia disrupts gene modulation along the brain–pituitary–gonad (BPG)–liver axis. Ecotoxicol. Environ. Saf. 2014, 102, 70–78. [Google Scholar] [CrossRef]
- Su, M.; Duan, Z.; Shi, H.; Zhang, J. The effects of salinity on reproductive development and egg and larvae survival in the spotted scat Scatophagus argus under controlled conditions. Aquac. Res. 2019, 50, 1782–1794. [Google Scholar] [CrossRef]
- Corriero, A.; Zupa, R.; Bello, G.; Mylonas, C.C.; Deflorio, M.; Genovese, S.; Basilone, G.; Buscaino, G.; Buffa, G.; Pousis, C.; et al. Evidence that severe acute stress and starvation induce rapid atresia of ovarian vitellogenic follicles in Atlantic bluefin tuna, Thunnus thynnus (L.) (Osteichthyes: Scombridae). J. Fish Dis. 2011, 34, 853–860. [Google Scholar] [CrossRef]
- Milla, S.; Wang, N.; Mandiki, S.N.M.; Kestemont, P. Corticosteroids: Friends or foes of teleost fish reproduction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef]
- Morais, R.D.V.S.; Thomé, R.G.; Lemos, F.S.; Bazzoli, N.; Rizzo, E. Autophagy and apoptosis interplay during follicular atresia in fish ovary: A morphological and immunocytochemical study. Cell Tissue Res. 2012, 347, 467–478. [Google Scholar] [CrossRef]
- Qin, Z.; Ge, Q.; Wang, J.; Li, M.; Liu, P.; Li, J.; Li, J. Comparative Transcriptomic and Proteomic Analysis of Exopalaemon carinicauda in Response to Alkalinity Stress. Front. Mar. Sci. 2021, 8, 759923. [Google Scholar] [CrossRef]
- Shen, C.; Feng, G.; Zhao, F.; Huang, X.; Wang, M.; Wang, H. Integration of transcriptomics and proteomics analysis reveals the molecular mechanism of Eriocheir sinensis gills exposed to heat stress. Antioxidants 2023, 12, 2020. [Google Scholar] [CrossRef]
- Wang, D.; Li, M.; Liao, M.; Tian, Y.; Wu, Q.; Xie, S.; Luo, W.; Zou, J.; Shi, J.; Du, Z. Molecular mechanisms of cold stress-induced energy stress, cholesterol metabolic disorders, and apoptosis in Pangasianodon hypophthalmus. Aquaculture 2026, 610, 742886. [Google Scholar] [CrossRef]
- Wada, H.; Okuyama, M.; Satoh, N.; Zhang, S. Molecular evolution of fibrillar collagen in chordates, with implications for the evolution of vertebrate skeletons and chordate phylogeny. Evol. Dev. 2006, 8, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Zhang, L.; Liu, Z.; Song, X. Abundant expression of ferroptosis-related SAT1 is related to unfavorable outcome and immune cell infiltration in low-grade glioma. BMC Cancer 2022, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Choi, N.; Kim, W.-S.; Oh, S.H.; Sung, J.-H. HB-EGF improves the hair regenerative potential of adipose-derived stem cells via ROS generation and Hck phosphorylation. Int. J. Mol. Sci. 2019, 21, 122. [Google Scholar] [CrossRef]
- Su, H.; Ma, D.; Fan, J.; Zhong, Z.; Li, Y.; Zhu, H. Metabolism response mechanism in the gill of Oreochromis mossambicus under salinity, alkalinity and saline-alkalinity stresses. Ecotoxicol. Environ. Saf. 2023, 251, 114523. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Jiang, W.-D.; Wu, P.; Liu, Y.; Ma, Y.-B.; Ren, H.-M.; Jin, X.-W.; Zhang, L.; Mi, H.-F.; Feng, L.; et al. Phosphorus mitigates nitrite stress-induced liver damage in adult grass carp (Ctenopharyngodon idellus) by alleviating mitochondrial dysfunction and inhibiting apoptosis. Aquaculture 2025, 608, 742755. [Google Scholar] [CrossRef]
- Iida, M.; Asano, A. Effects of glucagon-like peptide-1 receptor agonists on spermatogenesis-related gene expression in mouse testis and testis-derived cell lines. J. Vet. Med. Sci. 2024, 86, 555–562. [Google Scholar] [CrossRef]
- Hamed, M.; Said, R.E.M.; Martyniuk, C.J.; Soliman, H.A.M.; Sayed, A.E.-D.H.; Osman, A.G.M. Reproductive and endocrine-disrupting toxicity of pyrogallol in catfish (Clarias gariepinus). Environ. Pollut. 2024, 352, 124104. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, J.; Tao, Y.; Wang, W.; Sun, H.; Zhu, T.; Lu, S.; Xi, B.; Qiang, J. Chronic Carbonate Alkalinity Exposure Induces Dysfunction in Ovary and Testis Development in Largemouth Bass Micropterus salmoides by Oxidative Damage and Sex-Specific Pathways. Antioxidants 2025, 14, 1042. https://doi.org/10.3390/antiox14091042
Hua J, Tao Y, Wang W, Sun H, Zhu T, Lu S, Xi B, Qiang J. Chronic Carbonate Alkalinity Exposure Induces Dysfunction in Ovary and Testis Development in Largemouth Bass Micropterus salmoides by Oxidative Damage and Sex-Specific Pathways. Antioxidants. 2025; 14(9):1042. https://doi.org/10.3390/antiox14091042
Chicago/Turabian StyleHua, Jixiang, Yifan Tao, Wen Wang, Hui Sun, Taide Zhu, Siqi Lu, Bingwen Xi, and Jun Qiang. 2025. "Chronic Carbonate Alkalinity Exposure Induces Dysfunction in Ovary and Testis Development in Largemouth Bass Micropterus salmoides by Oxidative Damage and Sex-Specific Pathways" Antioxidants 14, no. 9: 1042. https://doi.org/10.3390/antiox14091042
APA StyleHua, J., Tao, Y., Wang, W., Sun, H., Zhu, T., Lu, S., Xi, B., & Qiang, J. (2025). Chronic Carbonate Alkalinity Exposure Induces Dysfunction in Ovary and Testis Development in Largemouth Bass Micropterus salmoides by Oxidative Damage and Sex-Specific Pathways. Antioxidants, 14(9), 1042. https://doi.org/10.3390/antiox14091042





