Cardioprotective and Antihypertensive Effects of Topical Capsaicin in a Rat Model
Abstract
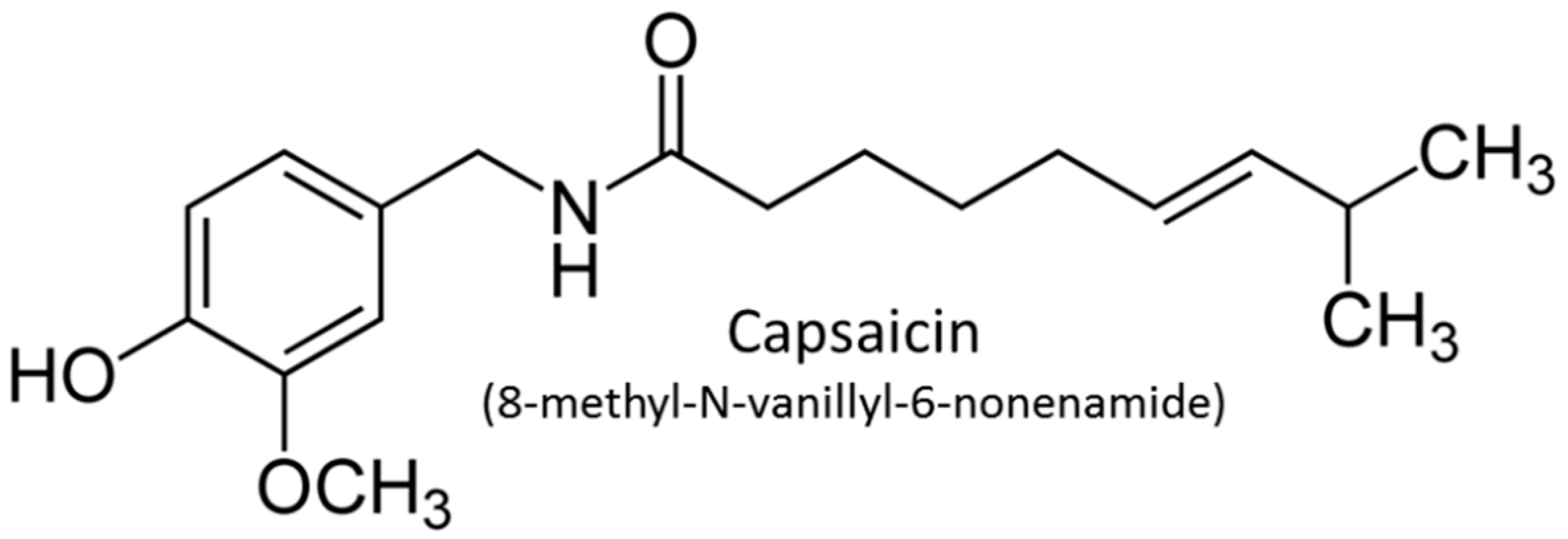
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Cream with Capsaicin
2.3. Animals
2.4. Experimental Groups
2.5. Systemic Arterial Hypertension Induction
2.6. Determination of Capsaicin
2.7. Treatment
2.8. Samples
2.9. Measurement of Mechanical Activity of the Heart
2.10. Biomarker Measurement
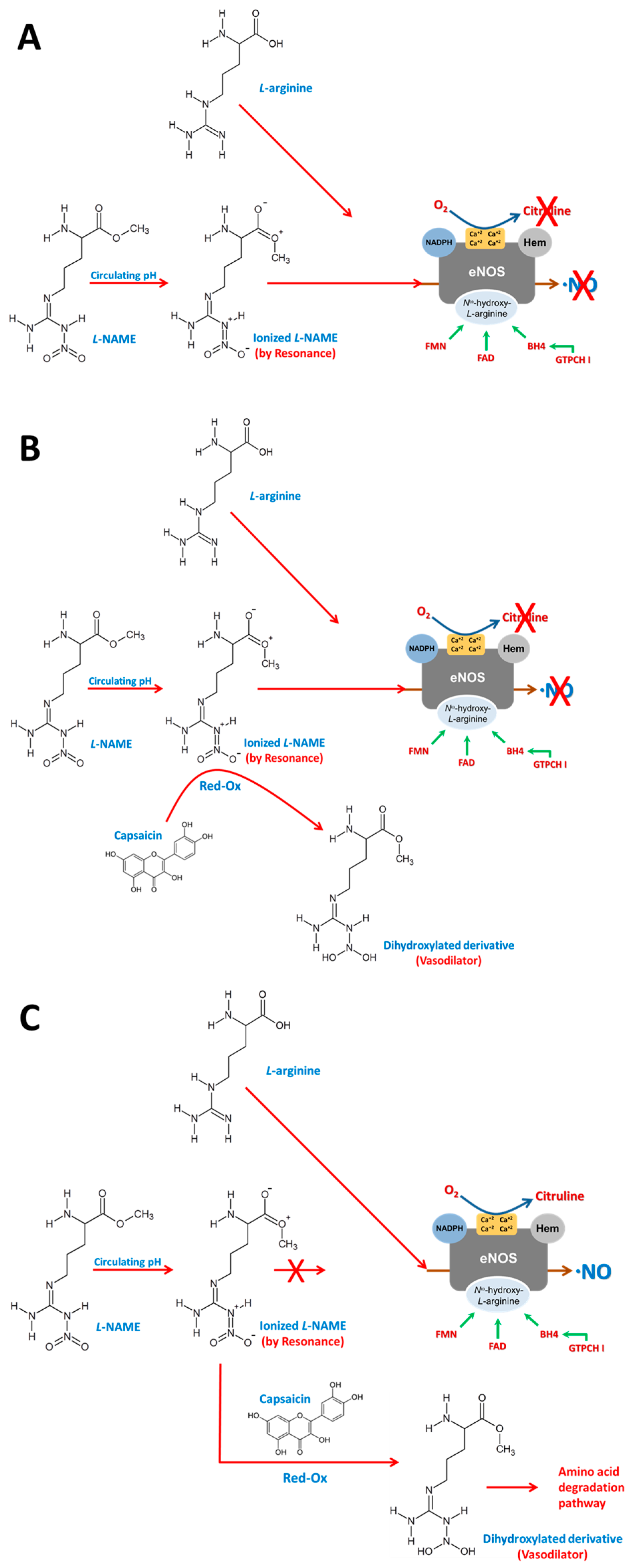
2.11. Nitric Oxide
2.12. Tetrahydrobiopterin and Dihydrobiopterin
2.13. Total Antioxidant Capacity
2.14. Oxidizing Capacity
2.15. Interleukin-6
2.16. Alpha Tumor Necrosis Factor
2.17. Malondialdehyde and Malonate
2.18. 8-Hidroxy-2-deoxiguanosine
2.19. Calcitonin Gene-Related Peptide Quantification
2.20. Protein Expression for TRPV1, eNOS, SOD, and Catalase
2.21. Histological Analysis
2.22. Statistical Analysis
3. Results
3.1. Absorption of Capsaicin
3.2. Mean Arterial Pressure
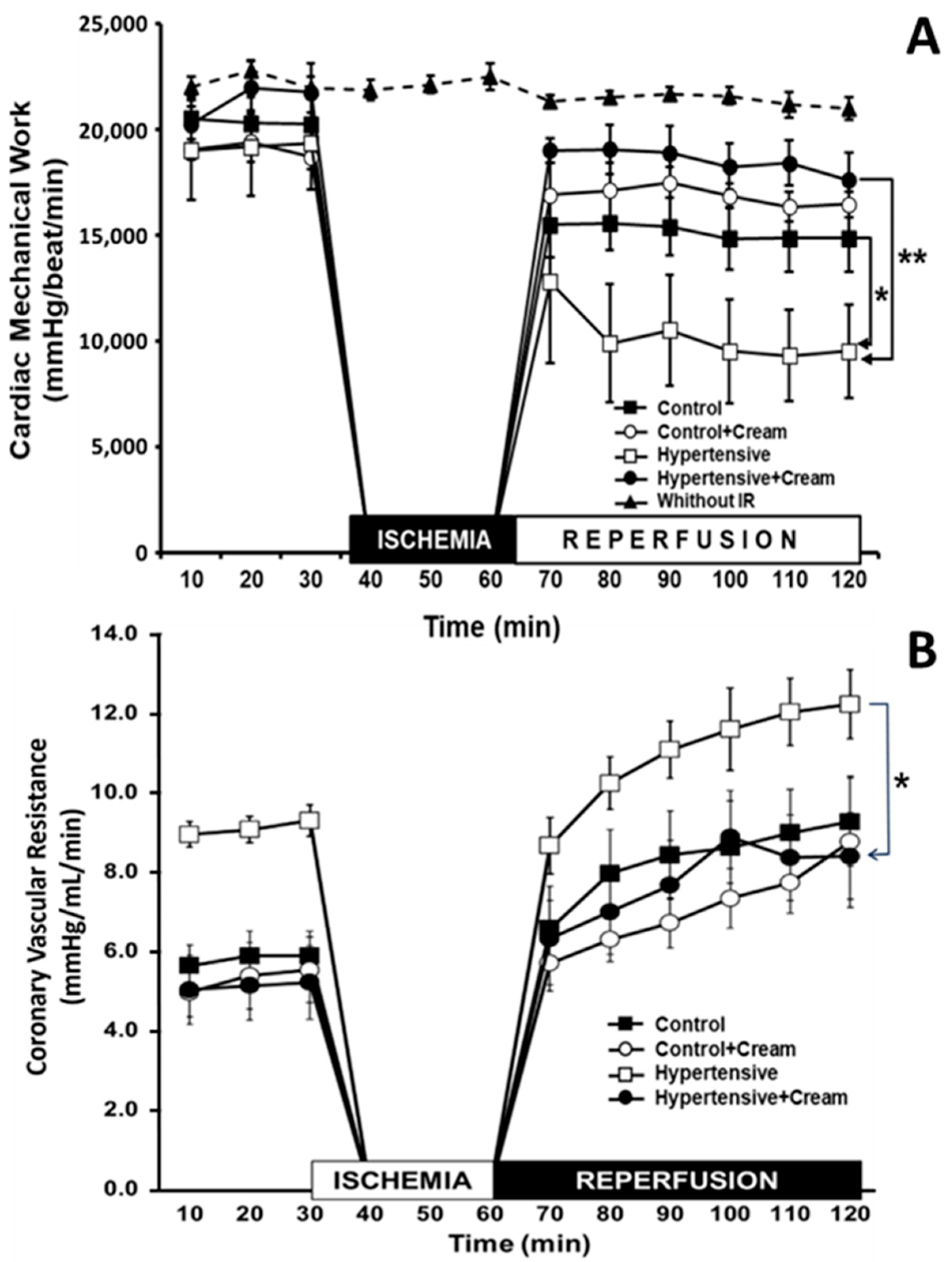
3.3. Cardiac Mechanical Work
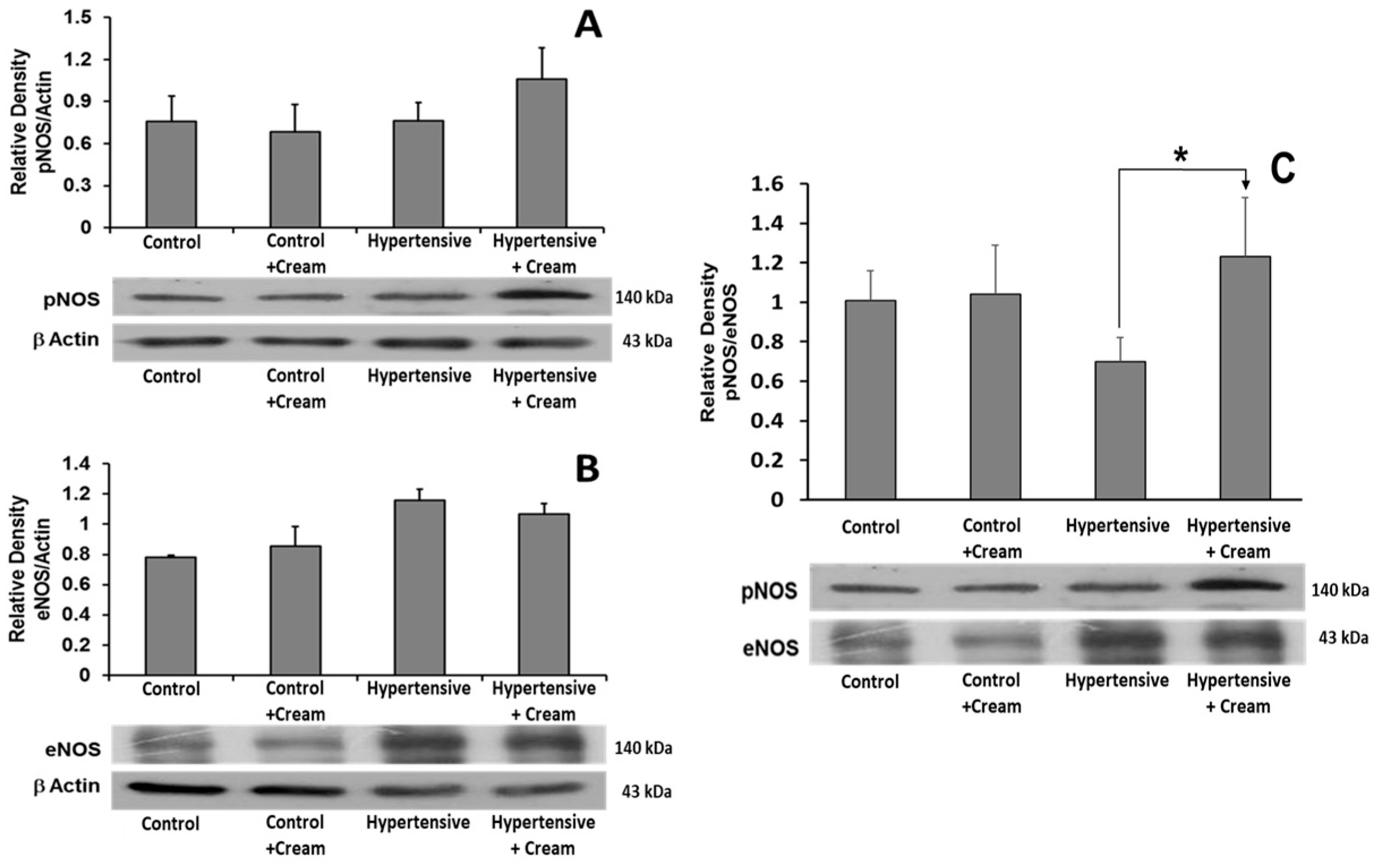
3.4. Effects of CS on Oxidation of BH4 to BH2 and on Production of NO, TAC, and OxCap
3.5. Effect of CS at a Systemic Level on Cell Damage Molecules (MDA and MTO) and DNA Damage (8HO2dG)
3.6. Effect of CS on Inflammatory Biomarkers Such as Tumor Necrosis Factor (TNF-α) and Interleukin-6 (IL-6) in Ventricular Tissue
3.7. Effect of CS in Left Ventricular Tissue of Hypertensive Rats
3.8. Effect of CS on Cell Damage Molecules Such as MDA, MTO, and DNA Damage, Such as 8HO2dG in Ventricular Tissue
3.9. Effect of CS on CGRP Levels in Ventricular Tissue
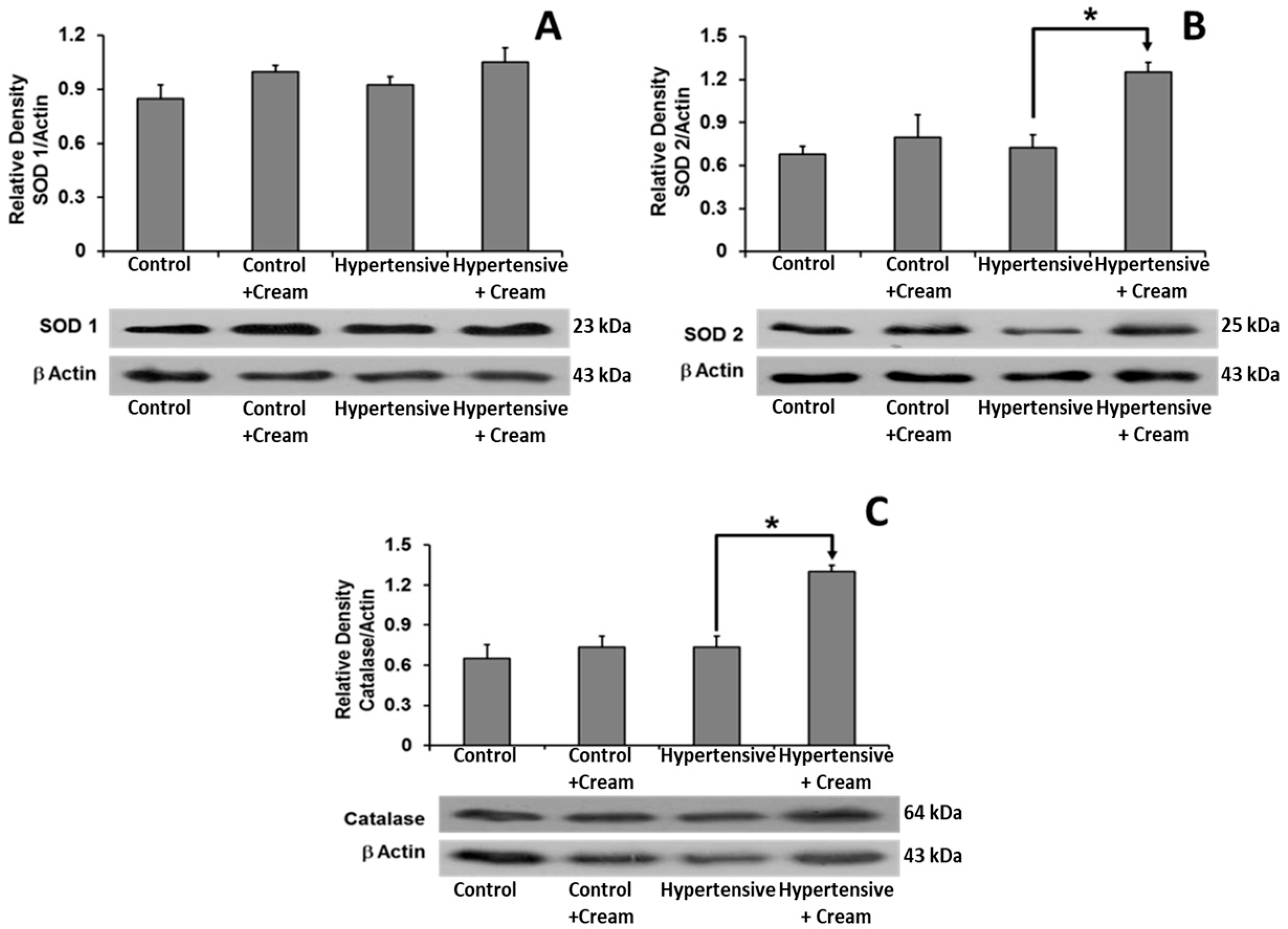
3.10. Expression of Molecules Related to Control of Oxidative Stress
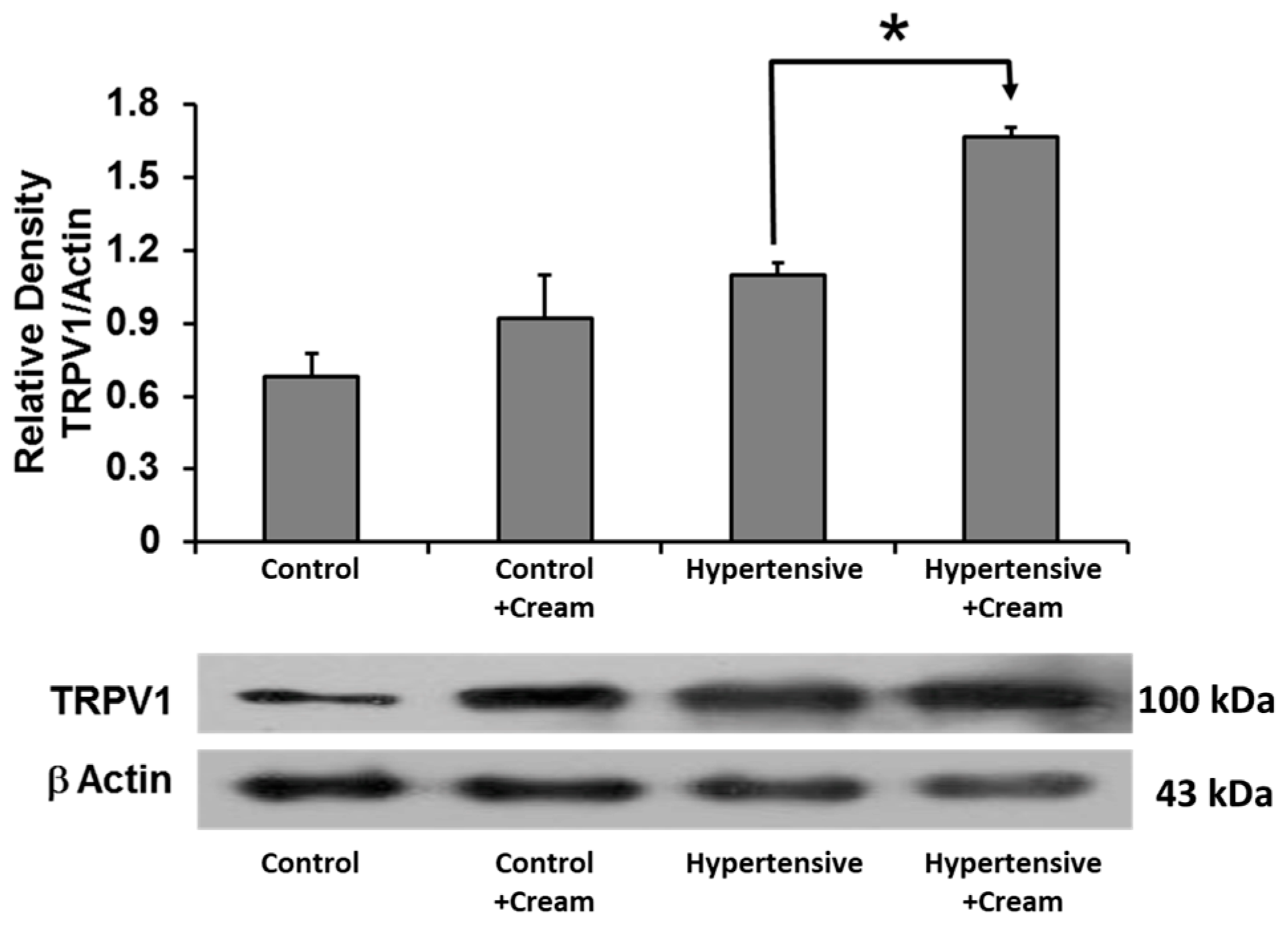
3.11. TRPV1 Expression in Ventricular Tissue
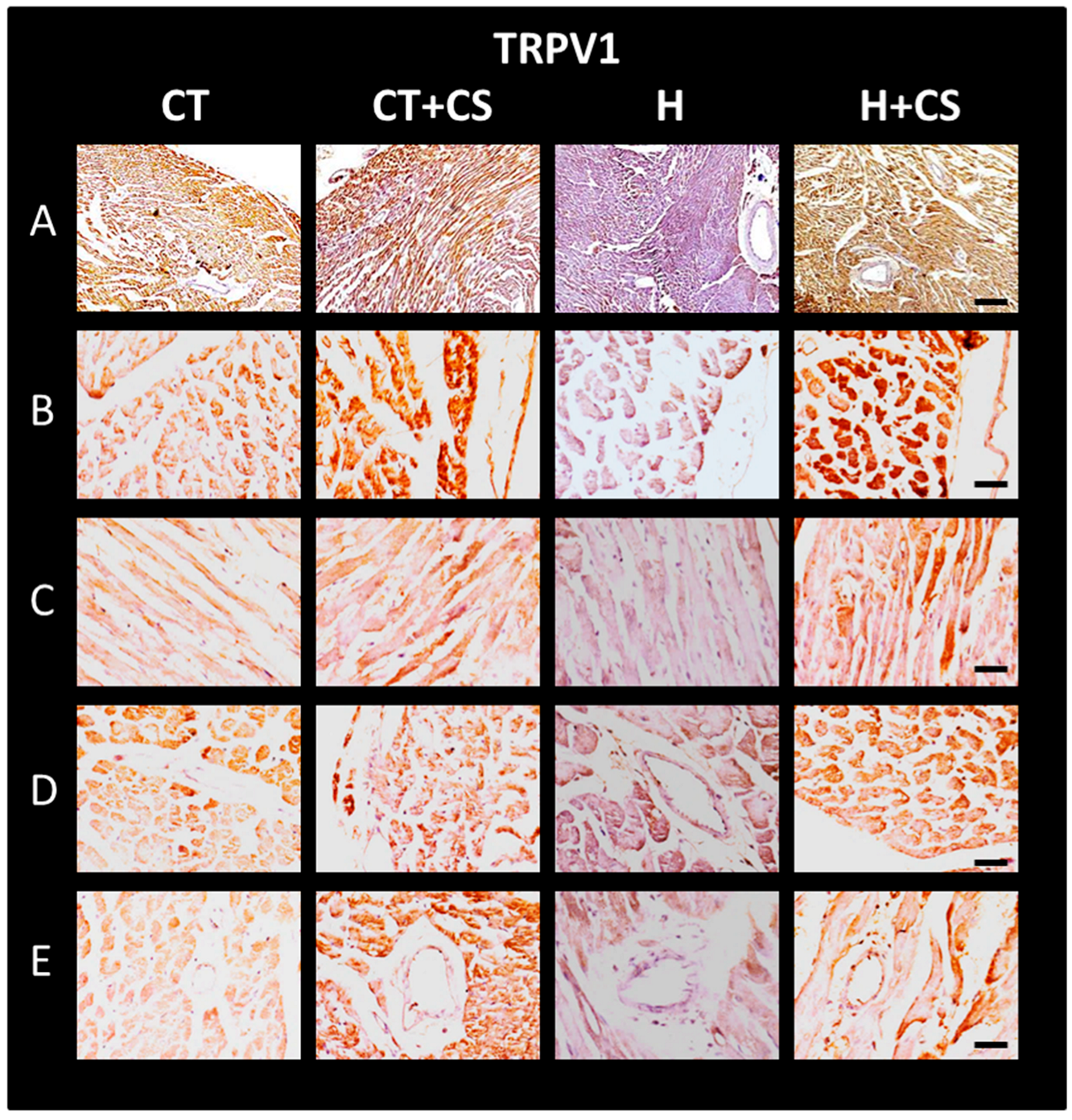
3.12. Immunolocalization of TRPV1 in Cardiac Tissue
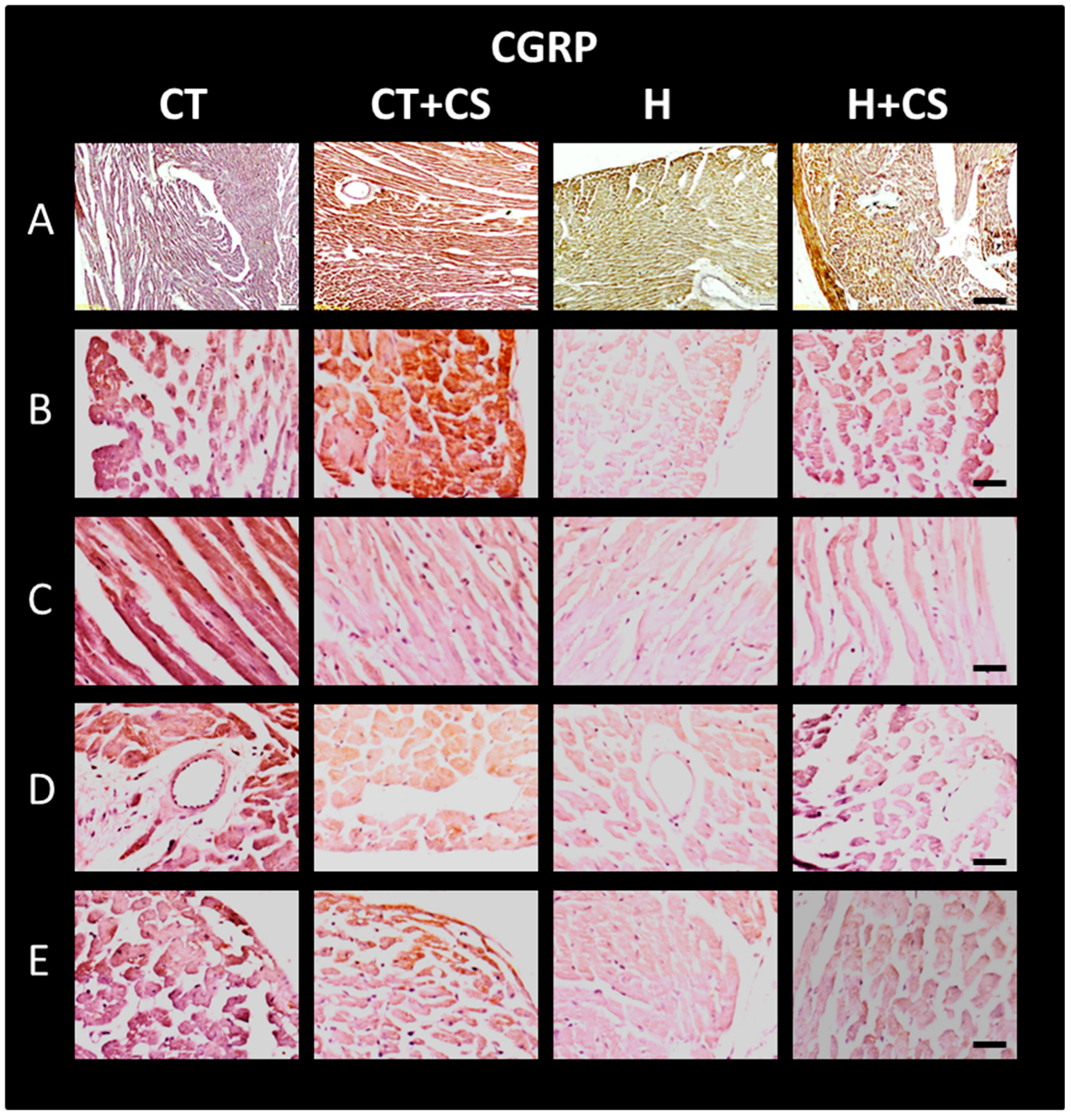
3.13. Immunolocalization of CGPR in Cardiac Tissue
4. Discussion
5. Conclusions
6. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szallasi, A. The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension? Int. J. Mol. Sci 2023, 24, 8769. [Google Scholar] [CrossRef] [PubMed]
- Gomes Silva, I.V.; Carvalho de Figueiredo, R.; Carvalho de Figueiredo, R.; Alves Rios, D.R. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Montezano, A.C.; Camargo, L.L. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardio 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Ramprasath, T.; Zou, M.H. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid. Redox Signal. 2021, 34, 750–764. [Google Scholar] [CrossRef]
- van der Pol, A.; Wiek, H.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escriva, C.; Dromant, M.; Borras, C.; Vina, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys 2021, 709, 108941. [Google Scholar] [CrossRef]
- Tonnesen, T.P.; Hjortbak, V.M.; Lassen, R.T.; Seefeldt, M.J.; Hans, E.B.; Jespersen, R.N. Myocardial salvage by succinate dehydrogenase inhibition in ischemia–reperfusion injury depends on diabetes stage in rats. Mol. Cell. Biochem. 2021, 476, 2675–2684. [Google Scholar] [CrossRef]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N.D. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Ramos, K.S.; Luciënne, B.L.; Wiersma, M.; Lanters, E.A.H.; Bogers, J.J.C.; de Groot, N.M.S.; Brundel, B.J.J. Blood-based 8-hydroxy-20-deoxyguanosine level: A potential diagnostic biomarker for atrial fibrillation. Heart Rhythm 2021, 18, 271–277. [Google Scholar] [CrossRef]
- Premer, C.; Kanelidis, A.J.; Hare, J.M.; Schulman, I.H. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 1–13. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef]
- Laklouk, M.; Baranidharan, G. Profile of the capsaicin 8% patch for the management of neuropathic pain associated with postherpetic neuralgia: Safety, efficacy, and patient acceptability. Patient Prefer. Adherence 2016, 10, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Markert, L.; Bennis, J.; Zimmerman, L.; Fox, J.; Thyagarajan, B. Assessment of Pharmacology, Safety, and Metabolic activity of Capsaicin Feeding in Mice. Sci. Rep. 2019, 9, 8588. [Google Scholar] [CrossRef] [PubMed]
- Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Q. Research Progress of Flavonoids Regulating Endothelial Function. Pharmaceuticals 2023, 16, 1201. [Google Scholar] [CrossRef]
- Lozano, C.; Córdova, C.; Marchant, I.; Zúñiga, R.; Ochova, P.; Ramírez-Barrantes, R.; González-Arriagada, W.A.; Rodríguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer Targets Ther. 2018, 10, 161–168. [Google Scholar] [CrossRef]
- Juárez-Contreras, R.; Méndez-Reséndiz, K.A.; Rosenbaum, T.; González-Ramírez, T.; Morales-Lázaro, S.L. TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 8882. [Google Scholar] [CrossRef]
- Gorbunov, S.A.; Maslov, L.N.; Jaggi, A.S.; Singh, N.; De Petrocellis, L.; Boshchenko, A.A.; Roohbakhsh, A.; Bezuglov, V.V.; Oeltgen, P.R. Physiological and Pathological Role of TRPV1, TRPV2 and TRPV4 Channels in Heart. Curr. Cardiol. Rev 2019, 15, 244–251. [Google Scholar] [CrossRef]
- Argunhan, F.; Brain, D.S. The Vascular-Dependent and Independent Actions of Calcitonin Gene-Related Peptide in Cardiovascular Disease. Front. Physiol. 2022, 13, 833645. [Google Scholar] [CrossRef]
- Torres-Narváez, J.; Pérez-Torres, I.; Castrejón-Téllez, V.; Varela-López, E.; Oidor-Chan, V.; Guarner-Lans, V.; Vargas-González, Á.; Martínez-Memije, R.; Flores-Chávez, P.; Cervantes-Yañez, E.; et al. The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats. Int. J. Environ. Res. Public Health 2019, 16, 3576. [Google Scholar] [CrossRef]
- Castrejón-Téllez, V.; Valle-Mondragón, L.; Pérez-Torres, I.; Guarner-Lans, V.; Pastelín-Hernández, G.; Ruiz-Ramírez, A.; Díaz-Juárez, J.; Varela-López, E.; Oidor-Chan, V.; Vargas-González, A.; et al. TRPV1 Contributes to Modulate the Nitric Oxide Pathway and Oxidative Stress in the Isolated and Perfused Rat Heart during Ischemia and Reperfusion. Molecules 2022, 27, 1031. [Google Scholar] [CrossRef]
- Medina, C.J.M.L.; Colado, V.J.; Gómez, V.N.L.; Mailloux, S.P.; Perez, T.I.; Aranda, F.A.; Carvajal, K.; Bravo, G. Effects of topical capsaicin combined with moderate exercise on insulin resistance, body weight and oxidative stress in hypoestrogenic obese rats. Int. J. Obes. 2017, 41, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Munjuluri, S.; Wilkenson, D.A.; Sooch, G.; Chen, X.; White, F.A.; Obulkhov, A.G. Capsaicin and TRPV1Channels in the Cardiovascular System: The Role of Inflammation. Cells 2022, 11, 18. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Bell, R.M.; Mocanu, M.M.; Yello, D.M. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. J. Mol. Cell. Cardiol. 2011, 50, 940–950. [Google Scholar] [CrossRef]
- Tenorio, L.F.A.; del Valle, M.L.; Pastelín, H.G. Validación de un método analítico espectrofotométrico para la cuantificación de metabolitos estables de óxido nítrico en fluidos biológicos. Rev. Mex. Cienc. Farmac. 2005, 36, 31–41. Available online: https://www.redalyc.org/pdf/579/57936106.pdf#:~:text=Validaci%C3%B3n%20de%20un%20m%C3%A9todo%20anal%C3%ADtico%20espectrofotom%C3%A9trico%20para%20la%20cuantificaci%C3%B3n%20de (accessed on 24 July 2025).
- Han, F.; Huynh, B.H.; Shi, H.; Lin, B.; Ma, Y. Pteridine analysis in urine by capillary electrophoresis using laser-induced fluorescence detection. Anal. Chem. 1999, 71, 1265–1269. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López, F.E.; Casado, Á. Evaluation of the copper (II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC–BCS assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Claeson, K.; Aberg, F.; Karlberg, B. Free malondialdehyde determination in rat brain tissue by capillary zone electrophoresis: Evaluation of two protein removal procedures. J. Chromatog. B 2000, 740, 87–92. [Google Scholar] [CrossRef]
- Kvasnicova, V.; Samcova, E.; Jursova, A.; Jelınek, I. Determination of 8-hydroxy-2′-deoxyguanosine in untreated urine by capillary electrophoresis with UV detection. J. Chromatog. A 2003, 985, 513–517. [Google Scholar] [CrossRef]
- Seon, A.A.; Pierre, T.N.; Redeker, V.; Lacombe, C.; Delfour, A.; Pierre, N.P.; Amiche, M. Isolation, Structure, Synthesis, and Activity of a New Member of the Calcitonin Gene-related Peptide Family from Frog Skin and Molecular Cloning of Its Precursor. J. Biol. Chem. 2000, 275, 5934–5940. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; William, G.; Hunter, J.; Hunter, S. Estadística para Investigadores. Diseño, Innovación y Descubrimiento, 2nd ed.; Editorial Reverte: Barcelona, Spain, 2008; p. 345. ISBN 978-84-291-5044-5. Available online: https://www.academia.edu/34386593/Estad%C3%ADstica_para_Investigadores_Segunda_edici%C3%B3n_Dise%C3%B1o_innovaci%C3%B3n_y_descubrimiento#:~:text=El%20uso%20y%20an%C3%A1lisis%20de%20arreglos%20experimentales%20complejos;%20en%20particular (accessed on 24 July 2025).
- Novakovskaya, Y.V. The nature of hydrogen bonds and conjugation in H-bonded systems. Russ. J. Phys. Chem. B 2012, 86, 1385–1398. [Google Scholar] [CrossRef]
- Edwards, J.M.; McCarthy, C.G.; Wenceslau, C.F. The Obligatory Role of the Acetylcholine-Induced Endothelium-Dependent Contraction in Hypertension: Can Arachidonic Acid Resolve This Inflammation? Curr. Pharm. Des. 2020, 26, 3723–3732. [Google Scholar] [CrossRef]
- Hurt, M.C.; Lu, Y.; Stary, M.C.; Piplani, H.; Small, A.B.; Urban, J.T.; Qvit, N.; Gross, J.G.; Mochly, R.D.; Gross, R.E. Transient Receptor Potential Vanilloid 1 Regulates Mitochondrial Membrane Potential and Myocardial Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003774. [Google Scholar] [CrossRef]
- He, H.; Zhou, Y.; Huang, J.; Wu, Z.; Liao, Z.; Liu, D.; Yin, D.; He, M. Capsaicin Protects Cardiomyocytes against Anoxia/Reoxygenation Injury via Preventing Mitochondrial Dysfunction Mediated by SIRT1. Oxid. Med. Cell. Longev. 2017, 2017, 1035702. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Jiang, L.; Wang, L.; Yang, Y.; Wang, Q.; Qian, X.; Zeng, W.; Yang, W.; Liang, R.; et al. Oxytocin ameliorates high glucose- and ischemia/reperfusion-induced myocardial injury by suppressing pyroptosis via AMPK signaling pathway. Biomed. Pharmacother. 2022, 153, 113498. [Google Scholar] [CrossRef]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-Based Inhibitors of Nitric Oxide Synthase: Therapeutic Potential and Challenges. Mediat. Inflamm. 2012, 2012, 318087. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]



| Control | Control+Cream | Hypertensive | Hypertensive+Cream | |
|---|---|---|---|---|
| Biomarkers of Nitric Oxide Bioavailability | ||||
| NO (pmol/mL) | 28.05 ± 2.72 | 18.90 ± 2.44 * | 9.99 ± 0.76 ** | 21.67 ± 0.88 *** |
| BH4 (pmol/mL) | 6.43 ± 0.51 | 5.02 ± 0.14 * | 3.46 ± 0.11 ** | 5.64 ± 0.34 *** |
| BH2 (pmol/mL) | 4.30 ± 0.14 | 6.33 ± 0.64 * | 7.87 ± 0.60 ** | 5.11 ± 0.20 *** |
| BH4/BH2 | 1.49 ± 0.09 | 0.78 ± 0.06 * | 0.44 ± 0.07 ** | 1.10 ± 0.19 *** |
| BH2/BH4 | 0.67 ± 0.06 | 1.28 ± 0.15 * | 2.27 ± 0.13 ** | 0.91 ± 0.28 *** |
| TAC (mmol/mL) | 285.30 ± 32.67 | 233.79 ± 8.86 * | 93.45 ± 22.07 ** | 171.48 ± 30.40 *** |
| OxCap (pmol) | 0.191 ± 0.035 | 0.201 ± 0.012 | 0.471 ± 0.076 ** | 0.339 ± 0.053 *** |
| Damage Molecules | ||||
| MDA (pmol/mL) | 0.163 ± 0.011 | 0.172 ± 0.010 | 0.69 ± 0.09 ** | 0.42 ± 0.01 *** |
| MTO (pmol/mL) | 0.062 ± 0.005 | 0.056 ± 0.006 | 0.233 ± 0.031 ** | 0.173 ± 0.021 *** |
| 8HO2dG (pM) | 1.914 ± 0.141 | 0.99 ± 0.23 * | 4.82 ± 0.32 ** | 3.16 ± 0.29 *** |
| Inflammatory Molecules | ||||
| TNF-α (pg/mL) | 28.33 ± 5.16 | 54.43 ± 5.85 * | 116.94 ± 7.53 ** | 34.24 ± 8.05 *** |
| IL-6 (pg/mL) | 106.01 ± 10.88 | 100.84 ± 8.02 | 169.91 ± 10.38 ** | 131.61 ± 7.50 *** |
| Control | Control+Cream | Hypertensive | Hypertensive+Cream | |
|---|---|---|---|---|
| Biomarkers of Nitric Oxide Bioavailability | ||||
| NO (pmol/mL) | 38.79 ± 6.23 | 36.36 ± 5.52 | 4.93 ± 1.06 ** | 17.06 ± 2.39 *** |
| BH4 (pmol/mL) | 3.88 ± 0.18 | 3.74 ± 0.09 | 2.80 ± 0.02 ** | 3.17 ± 0.05 *** |
| BH2 (pmol/mL) | 3.44 ± 0.23 | 3.39 ± 0.82 | 11.53 ± 1.30 ** | 4.60 ± 0.37 *** |
| BH4/BH2 | 1.13 ± 0.22 | 0.85 ± 0.36 | 0.24 ± 0.21 ** | 0.69 ± 0.29 *** |
| BH2/BH4 | 0.89 ± 0.31 | 1.18 ± 0.44 | 4.11 ± 1.02 ** | 1.45 ± 1.26 *** |
| TAC (mmol/mL) | 501.62 ± 33.26 | 512.49 ± 37.36 | 158.42 ± 22.52 ** | 586.61 ± 48.74 *** |
| OxCap (pmol) | 0.1001 ± 0.0056 | 0.092 ± 0.066 | 0.302 ± 0.058 ** | 0.140 ± 0.031 *** |
| Damage Molecules | ||||
| MDA (pmol/mL) | 0.0241 ± 0.0008 | 0.0232 ± 0.0012 | 0.0681 ± 0.0121 ** | 0.0382 ± 0.0020 *** |
| MTO (pmol/mL) | 0.00115639 ± 0.00003008 | 0.01128842 ± 0.00001449 | 0.00179634 ± 0.00013201 ** | 0.00120057 ± 0.00001192 *** |
| 8HO2dG (pM) | 0.00110238 ± 0.00003053 | 0.00109327 ± 0.00001243 | 0.01713044 ± 0.00001322 ** | 0.00127837 ± 0.00001981 *** |
| Inflammatory Molecules | ||||
| TNF-α (pg/mL) | 10.70 ± 0.83 | 27.05 ± 7.33 * | 122.97 ± 27.40 ** | 38.21 ± 3.60 *** |
| IL-6 (pg/mL) | 3.73 ± 0.87 | 13.96 ± 2.11 * | 22.81 ± 1.02 ** | 7.02 ± 1.31 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Narváez, J.C.; Castrejón-Téllez, V.; Sánchez-Aguilar, M.; Cano-Martínez, A.; Soria-Castro, E.; Díaz-Juárez, J.A.; Pérez-Torres, I.; Guarner-Lans, V.; Varela-López, E.; Ibarra-Lara, M.d.l.L.; et al. Cardioprotective and Antihypertensive Effects of Topical Capsaicin in a Rat Model. Antioxidants 2025, 14, 966. https://doi.org/10.3390/antiox14080966
Torres-Narváez JC, Castrejón-Téllez V, Sánchez-Aguilar M, Cano-Martínez A, Soria-Castro E, Díaz-Juárez JA, Pérez-Torres I, Guarner-Lans V, Varela-López E, Ibarra-Lara MdlL, et al. Cardioprotective and Antihypertensive Effects of Topical Capsaicin in a Rat Model. Antioxidants. 2025; 14(8):966. https://doi.org/10.3390/antiox14080966
Chicago/Turabian StyleTorres-Narváez, Juan Carlos, Vicente Castrejón-Téllez, María Sánchez-Aguilar, Agustina Cano-Martínez, Elizabeth Soria-Castro, Julieta Anabell Díaz-Juárez, Israel Pérez-Torres, Verónica Guarner-Lans, Elvira Varela-López, María de la Luz Ibarra-Lara, and et al. 2025. "Cardioprotective and Antihypertensive Effects of Topical Capsaicin in a Rat Model" Antioxidants 14, no. 8: 966. https://doi.org/10.3390/antiox14080966
APA StyleTorres-Narváez, J. C., Castrejón-Téllez, V., Sánchez-Aguilar, M., Cano-Martínez, A., Soria-Castro, E., Díaz-Juárez, J. A., Pérez-Torres, I., Guarner-Lans, V., Varela-López, E., Ibarra-Lara, M. d. l. L., Zarco-Olvera, G., Vargas-González, A., Flores-Chávez, P. L., & del Valle-Mondragón, L. (2025). Cardioprotective and Antihypertensive Effects of Topical Capsaicin in a Rat Model. Antioxidants, 14(8), 966. https://doi.org/10.3390/antiox14080966









