CIRBP Stabilizes Slc7a11 mRNA to Sustain the SLC7A11/GPX4 Antioxidant Axis and Limit Ferroptosis in Doxorubicin-Induced Cardiotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Echocardiography
2.4. Histological Analysis
2.5. Transmission Electron Microscopy
2.6. Cell Cultures and In Vitro Treatment
2.7. Cell Counting and Viability Assay
2.8. Laser Confocal Detection of Mitochondrial Membrane Potential
2.9. Measurement of CIRBP, Markers of Myocardial Injury, and Ferroptosis
2.10. Flow Cytometry and Imaging Flow Cytometry
2.11. Western Blot Analyses
2.12. Quantitative Real-Time PCR
2.13. Datasets and Bioinformatic Analysis
2.14. Molecular Docking and Molecular Dynamics Simulations
2.15. Statistical Analysis
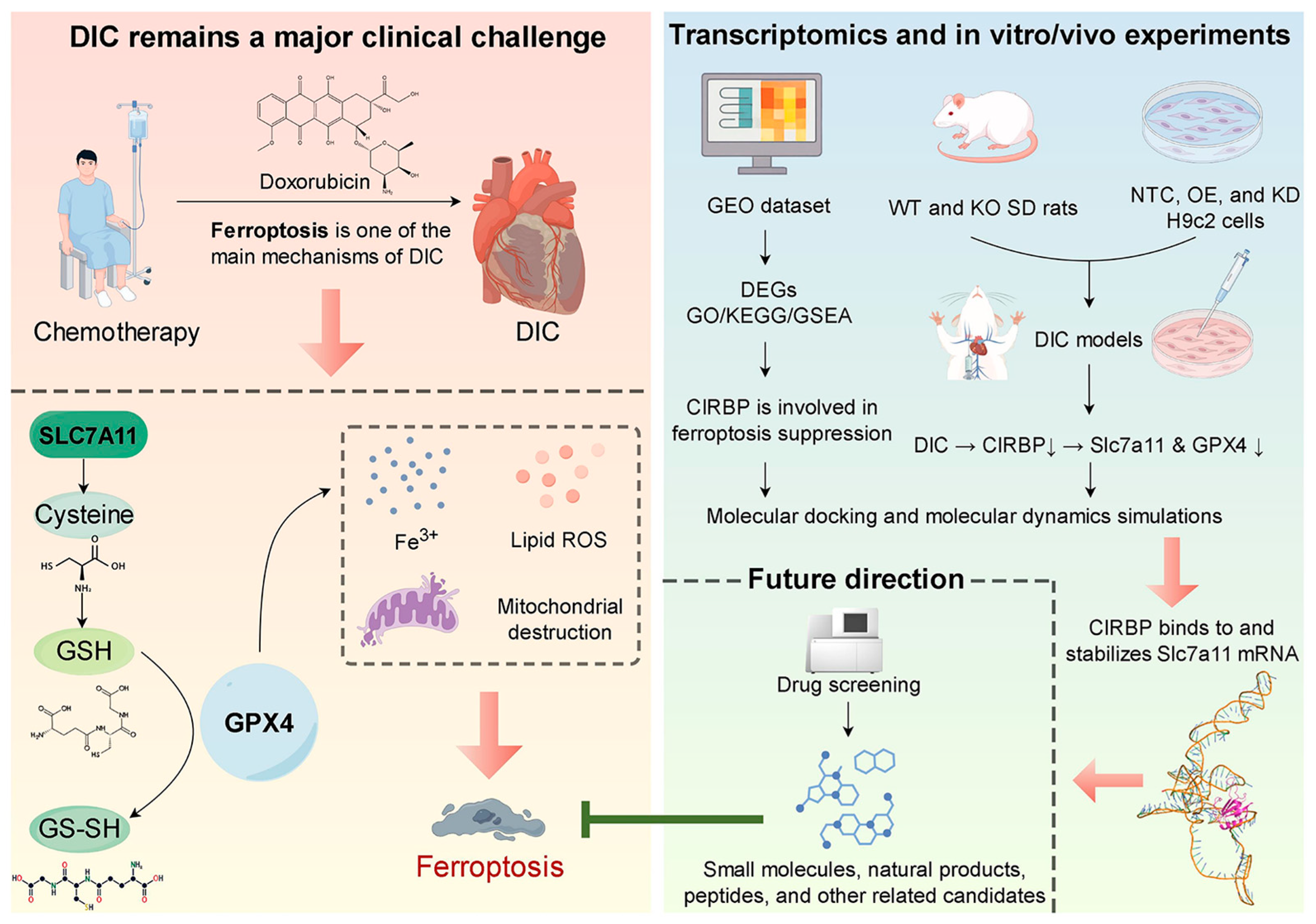
3. Results
3.1. CIRBP Expression Is Downregulated in DIC
3.2. CIRBP Deletion Aggravates DIC In Vivo
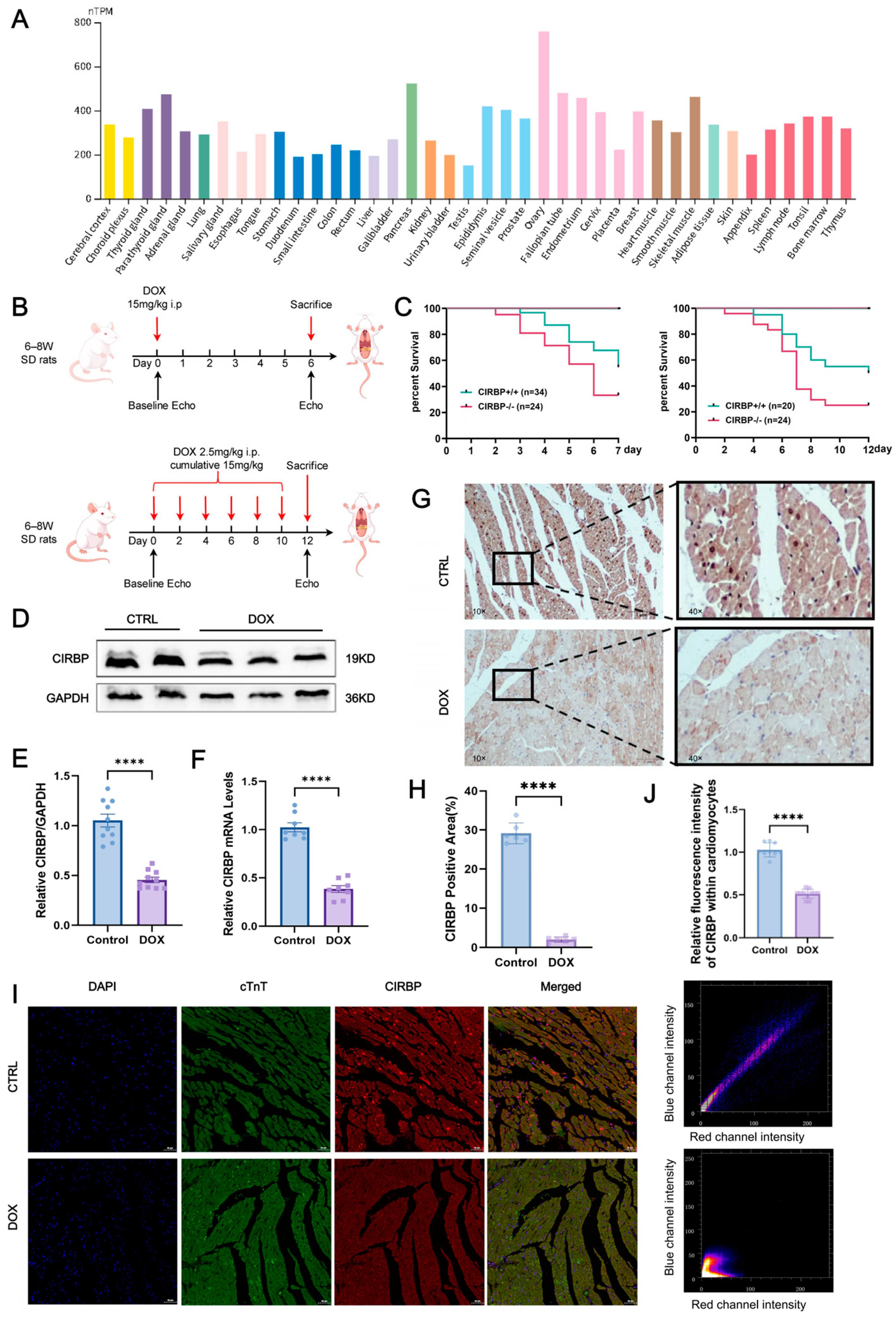
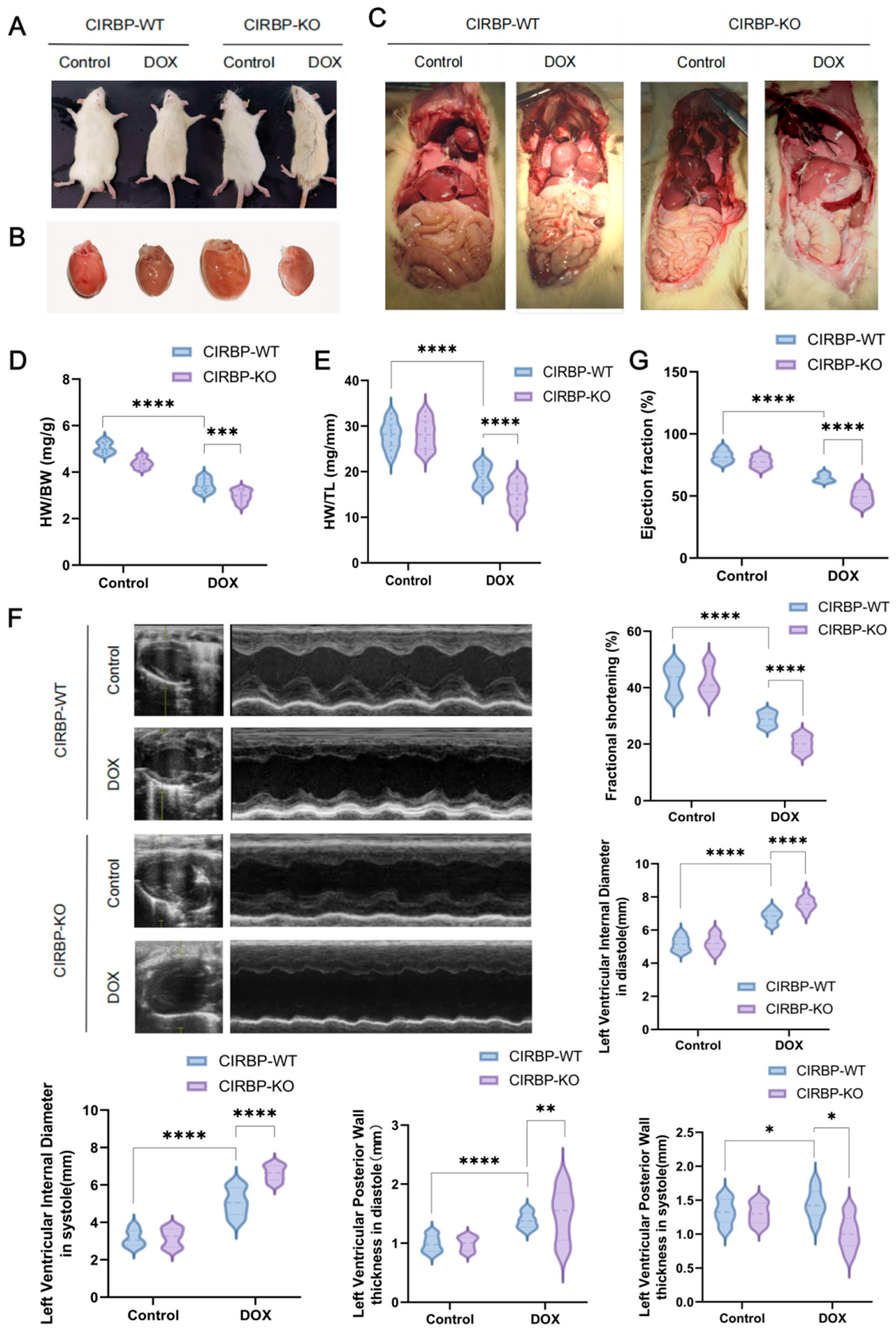
3.3. CIRBP Provides Cardioprotection Against DIC In Vivo
3.4. CIRBP Is Involved in Doxorubicin-Induced Ferroptosis

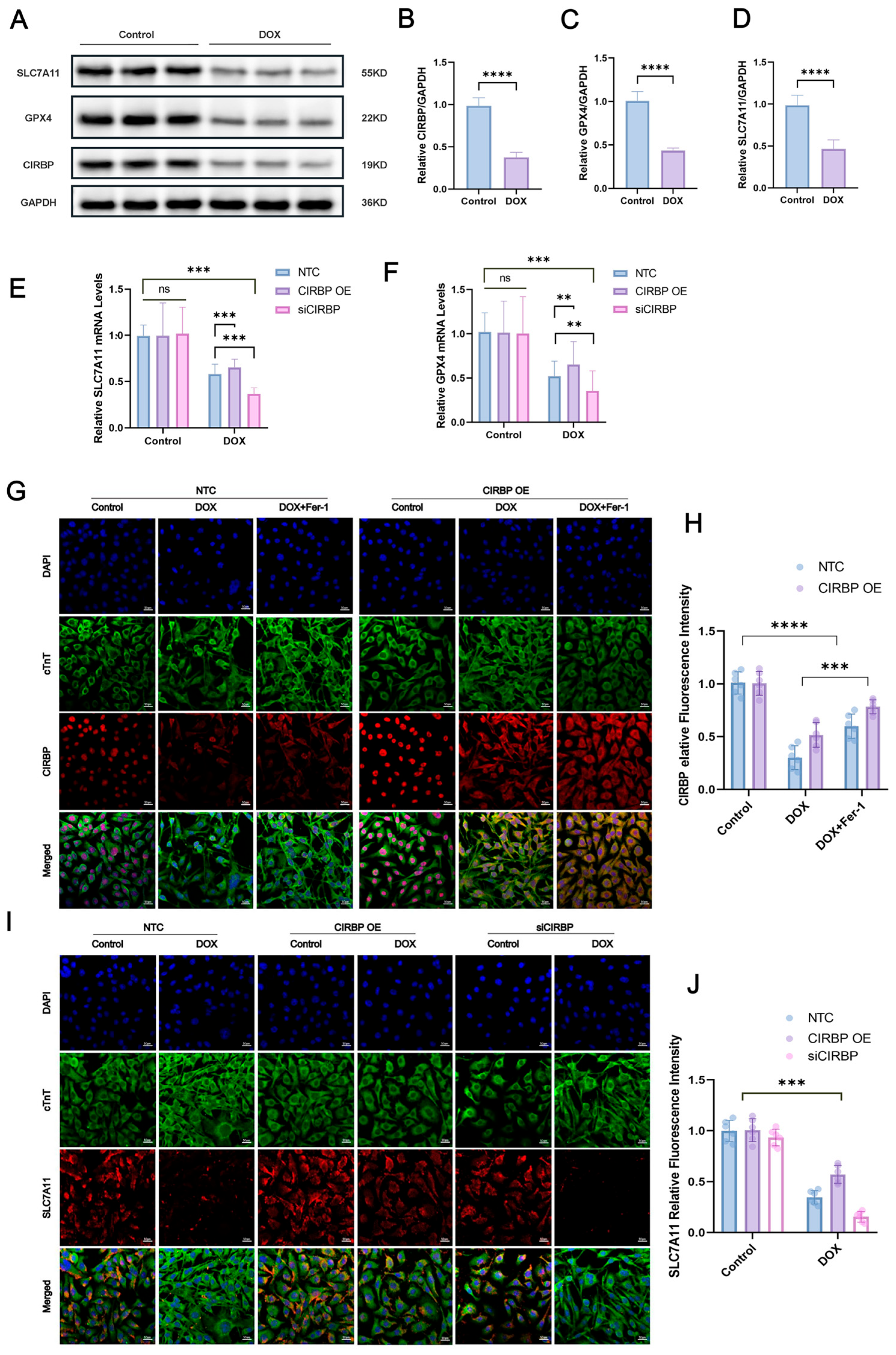
3.5. CIRBP Modulates Ferroptosis by Regulating the SLC7A11/GPX4 Axis In Vivo
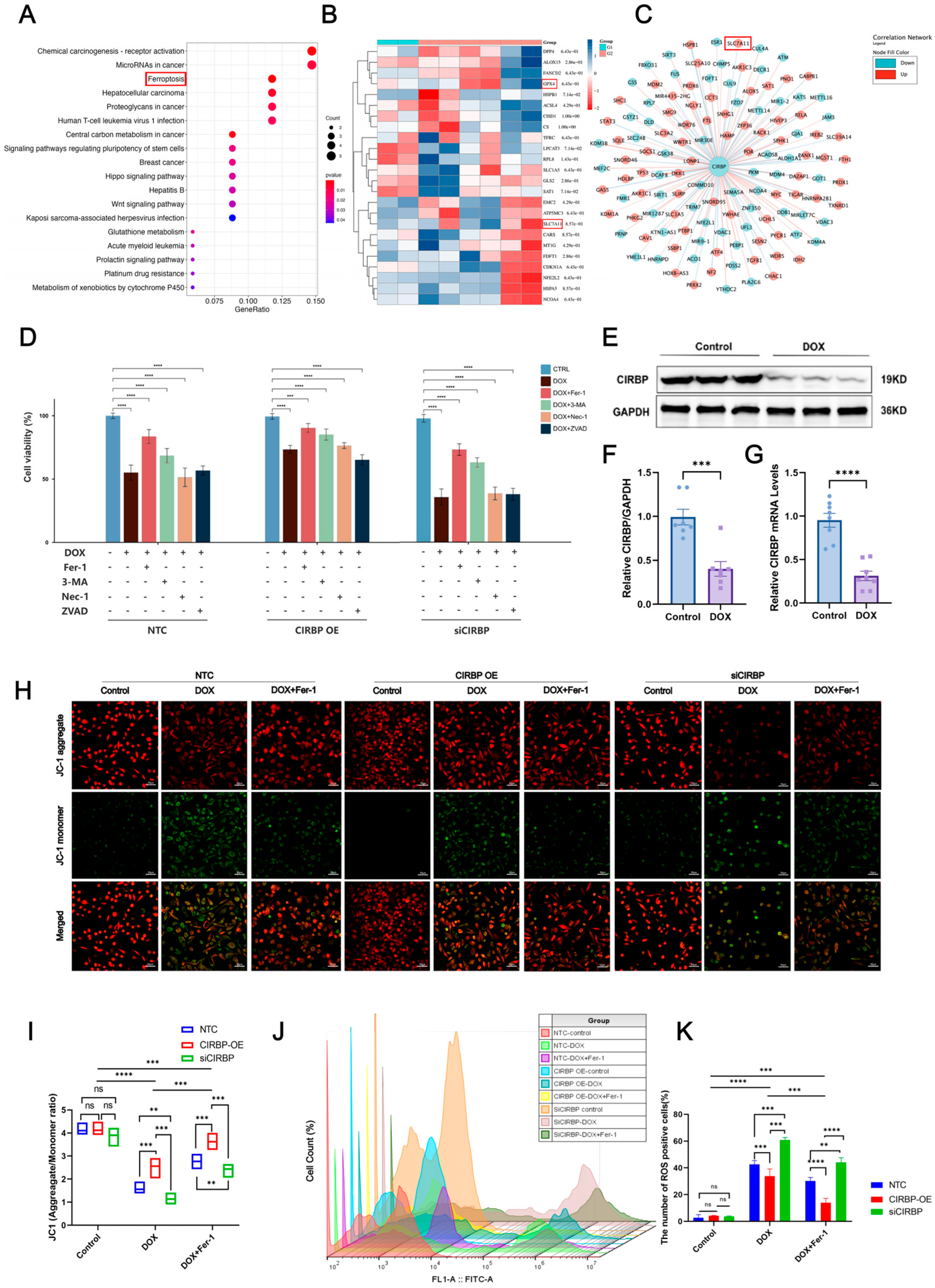
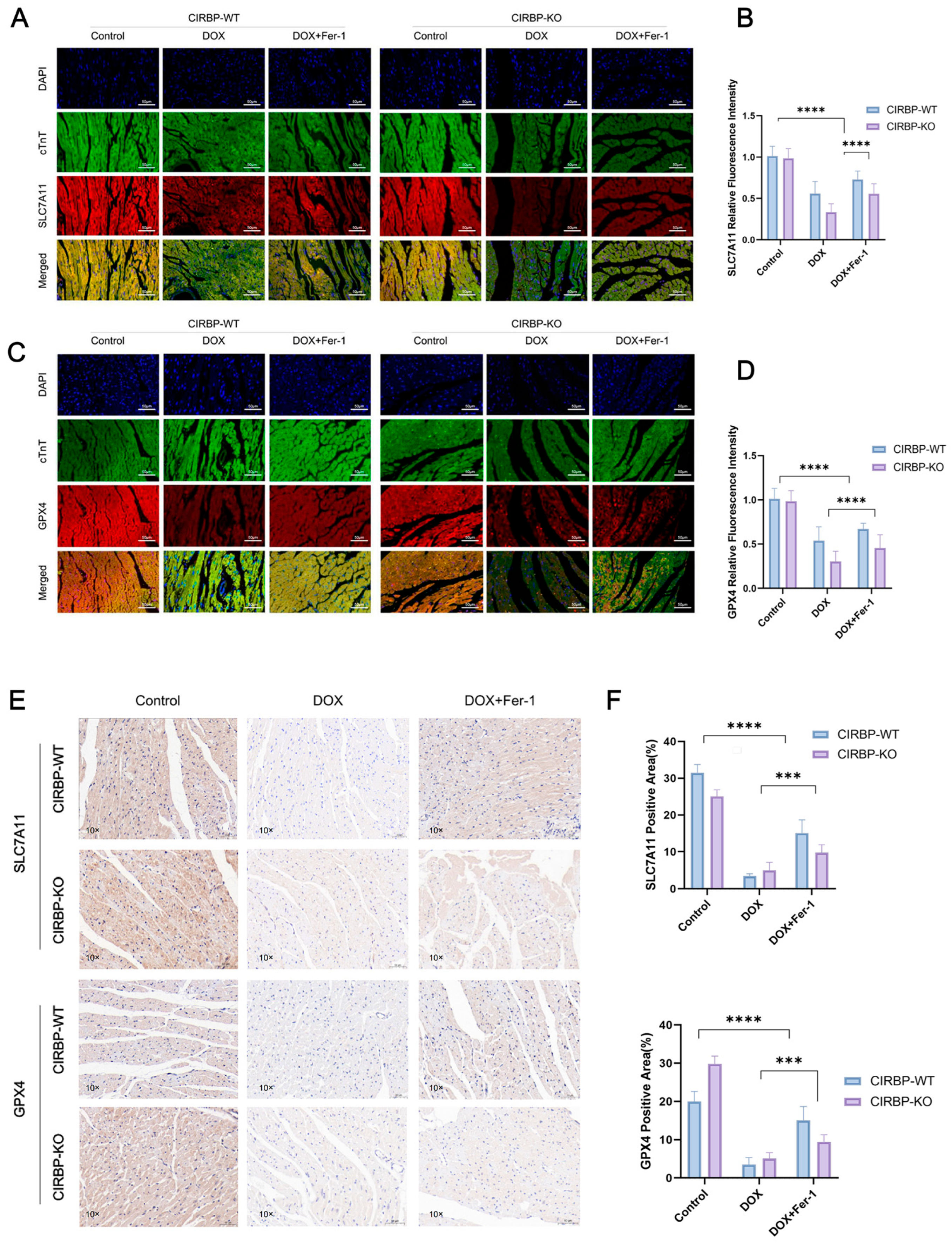
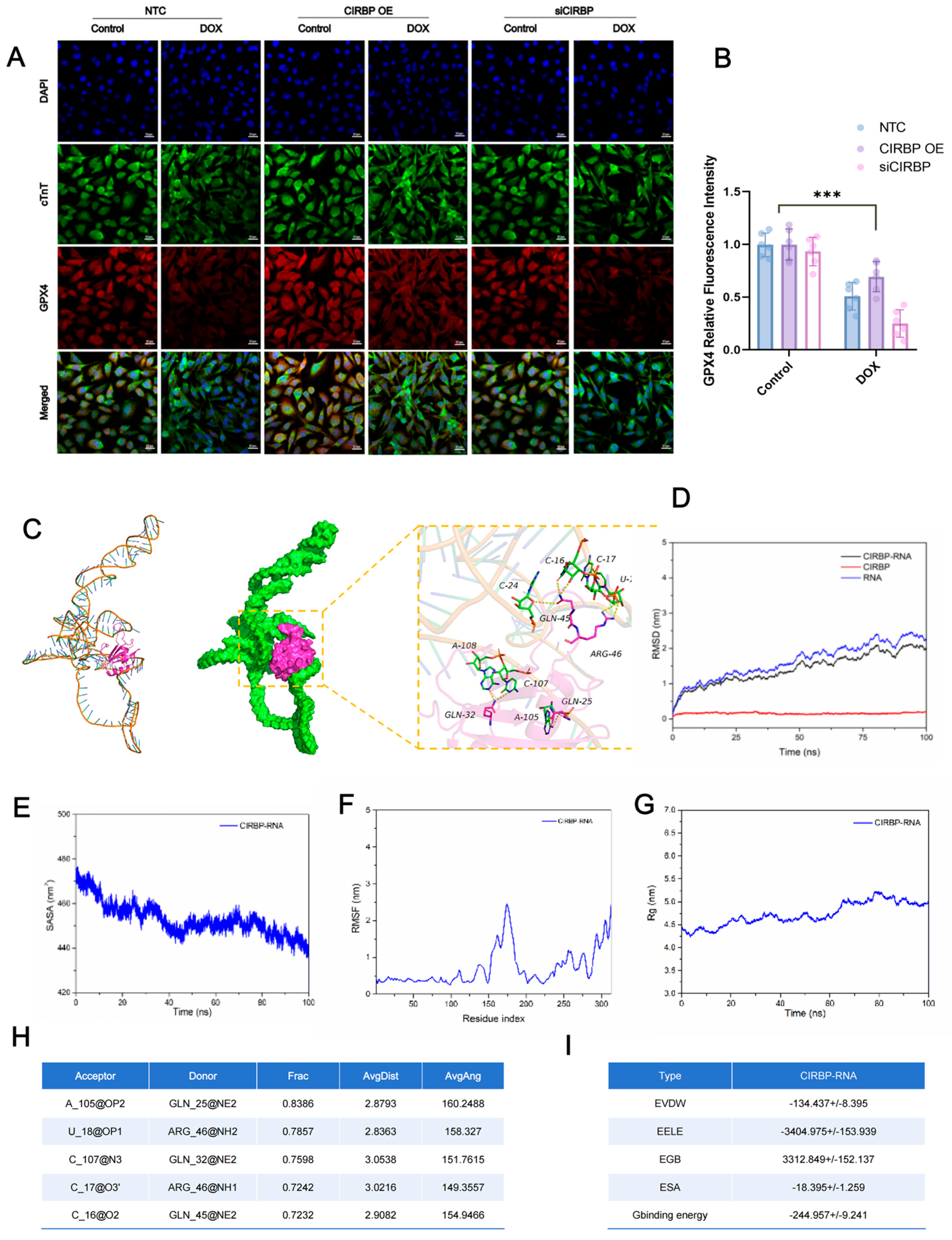
3.6. CIRBP Upregulates the SLC7A11/GPX4 Axis to Inhibit Ferroptosis In Vitro
3.7. CIRBP Directly Interacts with Slc7a11 mRNA to Stabilize Its Expression
4. Discussion
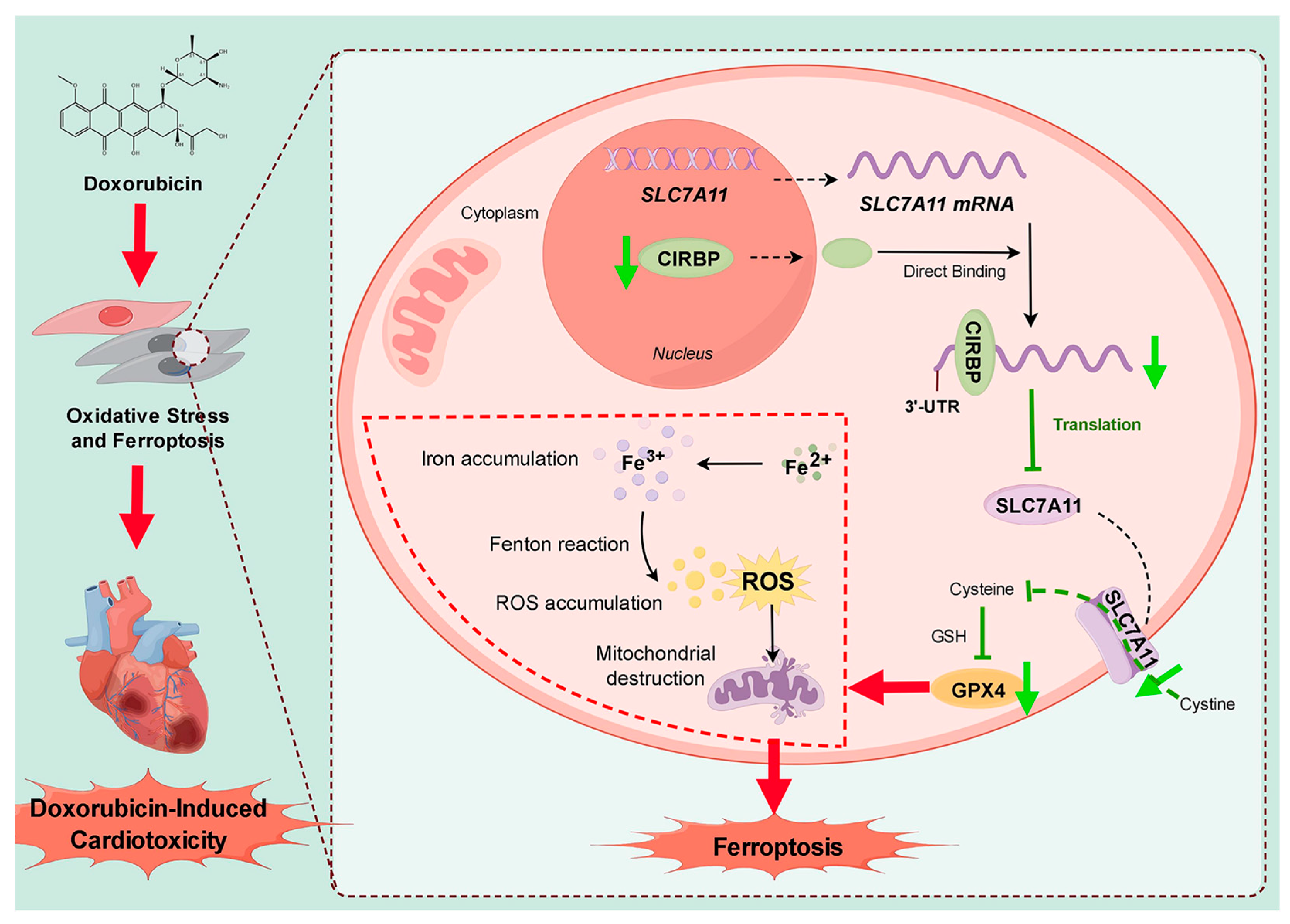
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine |
| ANOVA | Analysis of variance |
| BNP | B-type natriuretic peptide |
| cTnT | Cardiac troponin T |
| CCK-8 | Cell Counting Kit-8 |
| CIRBP | Cold-inducible RNA-binding protein |
| CK | Creatine kinase |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DCFH-DA | 2′,7′-Dichlorofluorescin diacetate |
| DEGs | Differentially expressed genes |
| DIC | Doxorubicin-induced cardiotoxicity |
| DOX | Doxorubicin |
| ELISA | Enzyme-linked immunosorbent assay |
| EF | Ejection fraction |
| Fer-1 | Ferrostatin-1 |
| FS | Fractional shortening |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GEO | Gene Expression Omnibus |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GO | Gene Ontology |
| GPX4 | Glutathione peroxidase 4 |
| H&E | Hematoxylin and eosin |
| H9c2 | Rat embryonic cardiomyoblast cell line |
| HW/BW | Heart-weight-to-body-weight ratio |
| HW/TL | Heart-weight-to-tibia-length ratio |
| i.p. | Intraperitoneal |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine iodide |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KO | Knockout |
| LDH | Lactate dehydrogenase |
| LV | Left ventricle/left-ventricular |
| MD | Molecular dynamics |
| MDA | Malondialdehyde |
| MMP | Mitochondrial membrane potential |
| mRNA | Messenger RNA |
| Ns | Not significant |
| OE | Over-expression |
| qPCR | Quantitative polymerase chain reaction |
| RIP | RNA immuno-precipitation |
| RMSD | Root-mean-square deviation |
| RMSF | Root-mean-square fluctuation |
| ROS | Reactive oxygen species |
| RT-qPCR | Reverse-transcription quantitative PCR |
| SLC7A11 | Solute carrier family 7 member 11 |
| siRNA | Small interfering RNA |
| SOD | Superoxide dismutase |
| TEM | Transmission electron microscopy |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| WGA | Wheat-germ agglutinin |
| WT | Wild type |
References
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef]
- Dempke, W.C.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-induced cardiotoxicity—Are we about to clear this hurdle? Eur. J. Cancer 2023, 185, 94–104. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Wang, K.; Li, J.; Chen, R.; Wu, X.; Ni, G.; Liu, C.; Das, S.; Sluijter, J.P.; et al. ADAR2 increases in exercised heart and protects against myocardial infarction and doxorubicin-induced cardiotoxicity. Mol. Ther. 2022, 30, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Tai, P.; Chen, X.; Jia, G.; Chen, G.; Gong, L.; Cheng, Y.; Li, Z.; Wang, H.; Chen, A.; Zhang, G.; et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 2023, 21, 823. [Google Scholar] [CrossRef]
- Wu, L.; Du, Y.; Wang, L.; Zhang, Y.; Ren, J. Inhibition of METTL3 ameliorates doxorubicin-induced cardiotoxicity through suppression of TFRC-mediated ferroptosis. Redox Biol. 2024, 72, 103157. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, T.; Xing, Z.; Shi, Q.; Gu, J.; Fan, Q.; Wang, H.; Chen, B.; Cheng, J.; Cai, R. Sirtuin 3 protects lung adenocarcinoma from ferroptosis by deacetylating and stabilizing mitochondrial glutamate transporter solute carrier family 25 member A22. Antioxidants 2025, 14, 403. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Wu, H.; Li, N.; Peng, S.; Fu, H.; Hu, Z.; Su, L. Maresin1 improves hippocampal neuroinflammation and cognitive function in septic rats by activating the SLC7A11/GPX4 ferroptosis signaling pathway. Int. Immunopharmacol. 2024, 131, 111792. [Google Scholar] [CrossRef]
- Corre, M.; Lebreton, A. Regulation of cold-inducible RNA-binding protein (CIRBP) in response to cellular stresses. Biochimie 2024, 217, 3–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, C.; He, J.; He, C.; Zhou, X.; Huang, X.; Shen, Y.; Wu, L.; Li, Y.; Feng, B.; et al. Cirbp suppression compromises DHODH-mediated ferroptosis defense and attenuates hypothermic cardioprotection in an aged donor transplantation model. J. Clin. Invest. 2024, 134, e175645. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Xing, J.; Li, J.; Li, Z.; Jian, D.; Wang, Y.; Wang, S.; Li, R.; Zhang, W.; et al. CIRBP-OGFR axis safeguards against cardiomyocyte apoptosis and cardiotoxicity induced by chemotherapy. Int. J. Biol. Sci. 2022, 18, 2882–2897. [Google Scholar] [CrossRef]
- Shimizu, J.; Murao, A.; Nofi, C.; Wang, P.; Aziz, M. Extracellular CIRP promotes GPX4-mediated ferroptosis in sepsis. Front. Immunol. 2022, 13, 903859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, Z.; Jacob, A.; Brenner, M.; Wang, P. An eCIRP inhibitor attenuates fibrosis and ferroptosis in ischemia and reperfusion induced chronic kidney disease. Mol. Med. 2025, 31, 11. [Google Scholar] [CrossRef]
- Sangweni, N.F.; Gabuza, K.; Huisamen, B.; Mabasa, L.; Van Vuuren, D.; Johnson, R. Molecular insights into the pathophysiology of doxorubicin-induced cardiotoxicity: A graphical representation. Arch. Toxicol. 2022, 96, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2023, 8, e169756. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L. Ferroptosis-induced cardiotoxicity and antitumor drugs. Curr. Med. Chem. 2024, 31, 4935–4957. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Bian, Z.; He, Y.; Chen, L.; Gong, L.; Hua, Y. Gut microbial metabolism in ferroptosis and colorectal cancer. Trends Cell Biol. 2025, 35, 341–351. [Google Scholar] [CrossRef]
- Brown, A.R.; Hirschhorn, T.; Stockwell, B.R. Ferroptosis—Disease perils and therapeutic promise. Science 2024, 386, 848–849. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.E.; Dewson, G.; Tait, S.W.G. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Z.; Lei, Z.; Zhang, H.T. RNA-binding proteins and cancer metastasis. Semin. Cancer Biol. 2022, 86, 748–768. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Nag, S.; Ray, P.S. Fates and functions of RNA-binding proteins under stress. Wiley Interdiscip. Rev. RNA 2024, 15, e1825. [Google Scholar] [CrossRef]
- Yan, Y.; Gan, J.; Tao, Y.; Okita, T.W.; Tian, L. RNA-binding proteins: The key modulator in stress granule formation and abiotic stress response. Front. Plant Sci. 2022, 13, 882596. [Google Scholar] [CrossRef]
- Sommer, G.; Heise, T. Role of the RNA-binding protein La in cancer pathobiology. RNA Biol. 2021, 18, 218–236. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, M.; Gu, Z.; Lou, Y.R. Role of long non-coding RNA polymorphisms in cancer chemotherapeutic response. J. Pers. Med. 2021, 11, 513. [Google Scholar] [CrossRef]
- Haniffa, S.; Narain, P.; Hughes, M.A.; Petković, A.; Šušić, M.; Mlambo, V.; Chaudhury, D. Chronic social stress blunts core body temperature and molecular rhythms of Rbm3 and Cirbp in mouse lateral habenula. Open Biol. 2023, 13, 220380. [Google Scholar] [CrossRef]
- Kitakata, H.; Endo, J.; Ikura, H.; Moriyama, H.; Shirakawa, K.; Katsumata, Y.; Sano, M. Therapeutic targets for DOX-induced cardiomyopathy: Role of apoptosis vs. ferroptosis. Int. J. Mol. Sci. 2022, 23, 1414. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.C.; Ding, Y.X.; Bai, J.; Cao, F.; Li, F. The crosstalk between ferroptosis and mitochondrial dynamic regulatory networks. Int. J. Biol. Sci. 2023, 19, 2756–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shen, Z.; Zheng, Z.; Xu, Y.; Zhang, J.; Liu, J.; Peng, S.; Wan, J.; Qin, J.J.; Wang, M. Cardiomyocyte LGR6 alleviates ferroptosis in diabetic cardiomyopathy via regulating mitochondrial biogenesis. Metabolism 2024, 159, 155979. [Google Scholar] [CrossRef]
- Ye, T.; Yang, W.; Gao, T.; Yu, X.; Chen, T.; Yang, Y.; Guo, J.; Li, Q.; Li, H.; Yang, L. Trastuzumab-induced cardiomyopathy via ferroptosis-mediated mitochondrial dysfunction. Free Radic. Biol. Med. 2023, 206, 143–161. [Google Scholar] [CrossRef]
- Ahola, S.; Langer, T. Ferroptosis in mitochondrial cardiomyopathy. Trends Cell Biol. 2024, 34, 150–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Li, Y.; Xie, Y.; Chen, J.; Ding, H.; Zhang, X. CIRBP Stabilizes Slc7a11 mRNA to Sustain the SLC7A11/GPX4 Antioxidant Axis and Limit Ferroptosis in Doxorubicin-Induced Cardiotoxicity. Antioxidants 2025, 14, 930. https://doi.org/10.3390/antiox14080930
Xie Y, Li Y, Xie Y, Chen J, Ding H, Zhang X. CIRBP Stabilizes Slc7a11 mRNA to Sustain the SLC7A11/GPX4 Antioxidant Axis and Limit Ferroptosis in Doxorubicin-Induced Cardiotoxicity. Antioxidants. 2025; 14(8):930. https://doi.org/10.3390/antiox14080930
Chicago/Turabian StyleXie, Yixin, Yongnan Li, Yafei Xie, Jianshu Chen, Hong Ding, and Xiaowei Zhang. 2025. "CIRBP Stabilizes Slc7a11 mRNA to Sustain the SLC7A11/GPX4 Antioxidant Axis and Limit Ferroptosis in Doxorubicin-Induced Cardiotoxicity" Antioxidants 14, no. 8: 930. https://doi.org/10.3390/antiox14080930
APA StyleXie, Y., Li, Y., Xie, Y., Chen, J., Ding, H., & Zhang, X. (2025). CIRBP Stabilizes Slc7a11 mRNA to Sustain the SLC7A11/GPX4 Antioxidant Axis and Limit Ferroptosis in Doxorubicin-Induced Cardiotoxicity. Antioxidants, 14(8), 930. https://doi.org/10.3390/antiox14080930






