Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Morris Water Maze (MWM) Assay
2.3. Novel Object Recognition (NOR) Assay
2.4. Brain Tissue Lipid Content Measurement
2.5. Cell Culture and Treatments
2.6. LD Staining
2.7. Immunofluorescence Staining
2.8. RNA Extraction and Quantitative PCR (q-PCR)
2.9. Protein Extraction and Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Sustained Cognitive Dysfunction and Glial Activation in Mice a Week Post-Recovery from Acute HH Exposure
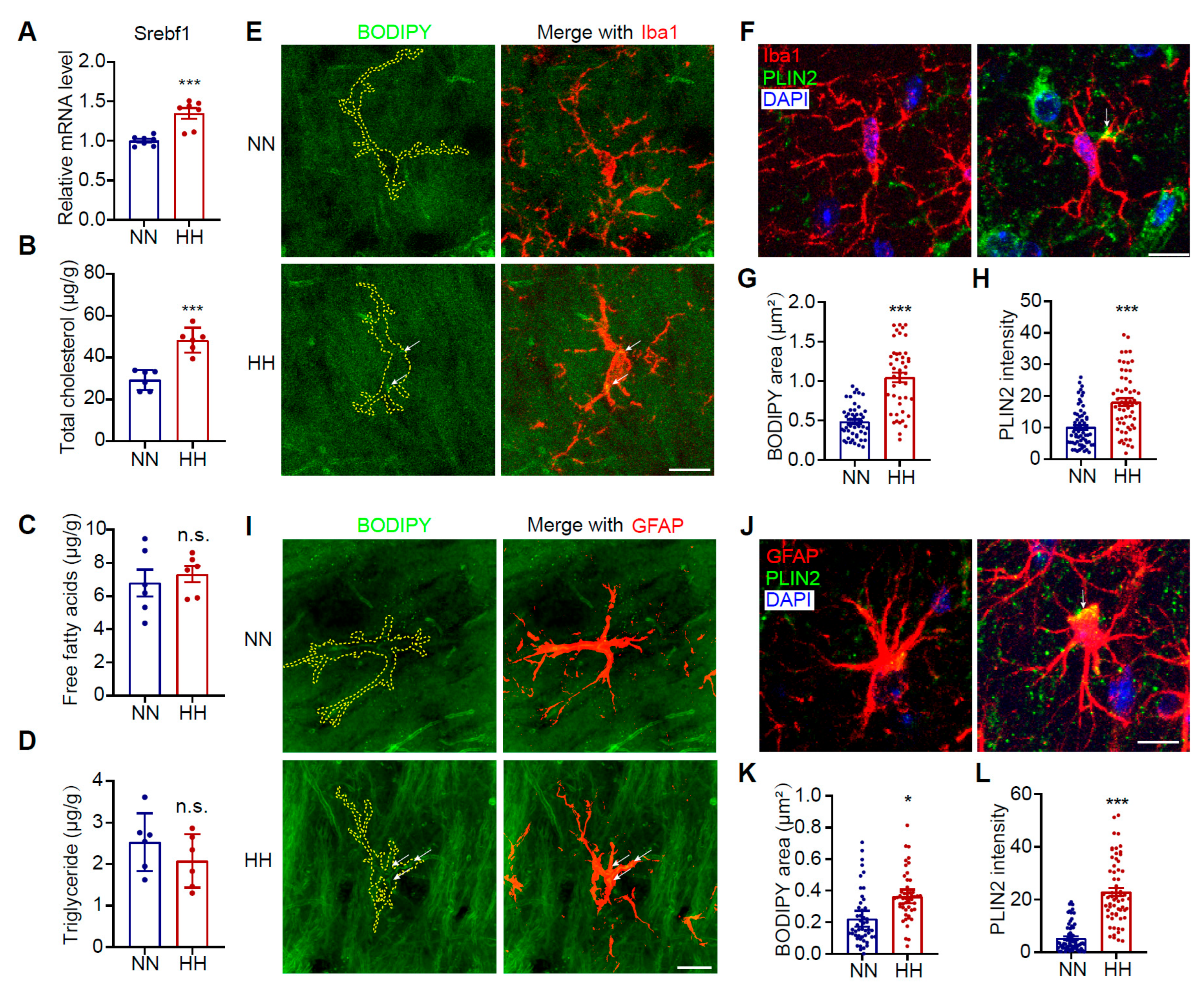
3.2. HH Exposure Induces LD Accumulation in Microglia and Astrocytes in the Mouse Brain
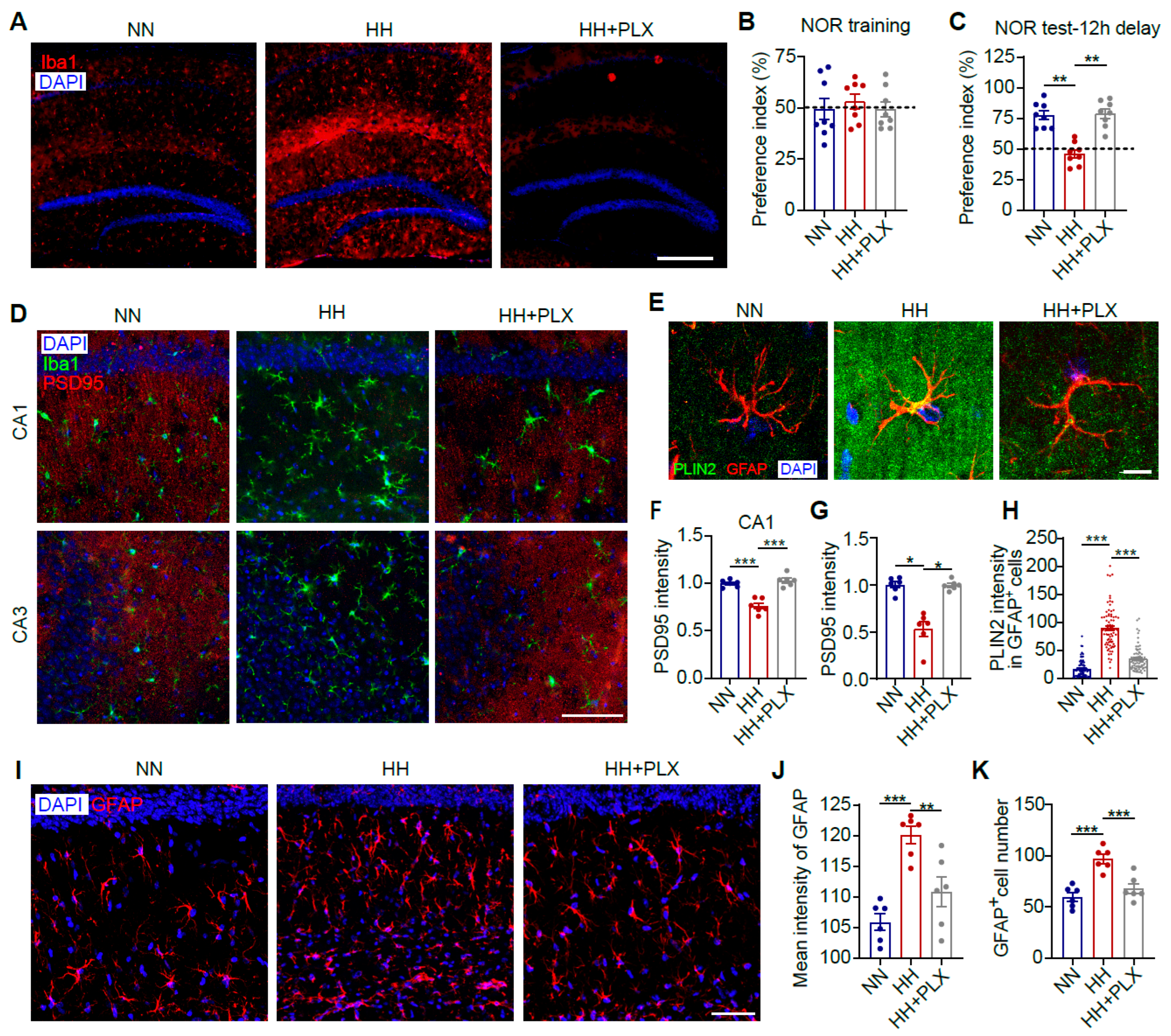
3.3. Microglia Depletion Attenuates HACI and Astrocytic LD Accumulation
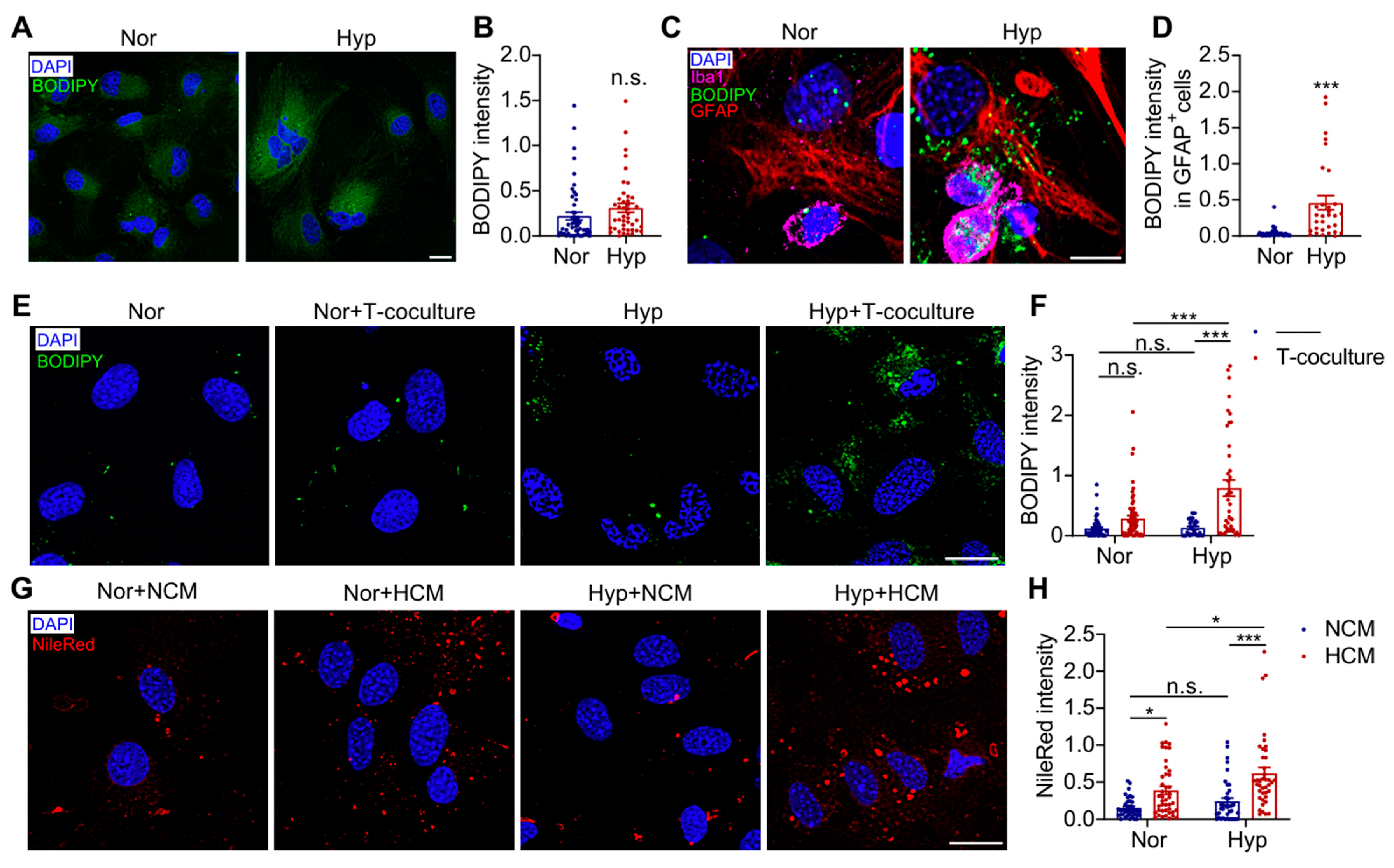
3.4. Hypoxia-Induced Astrocyte LD Accumulation Depends on Microglia-Derived Cytokines
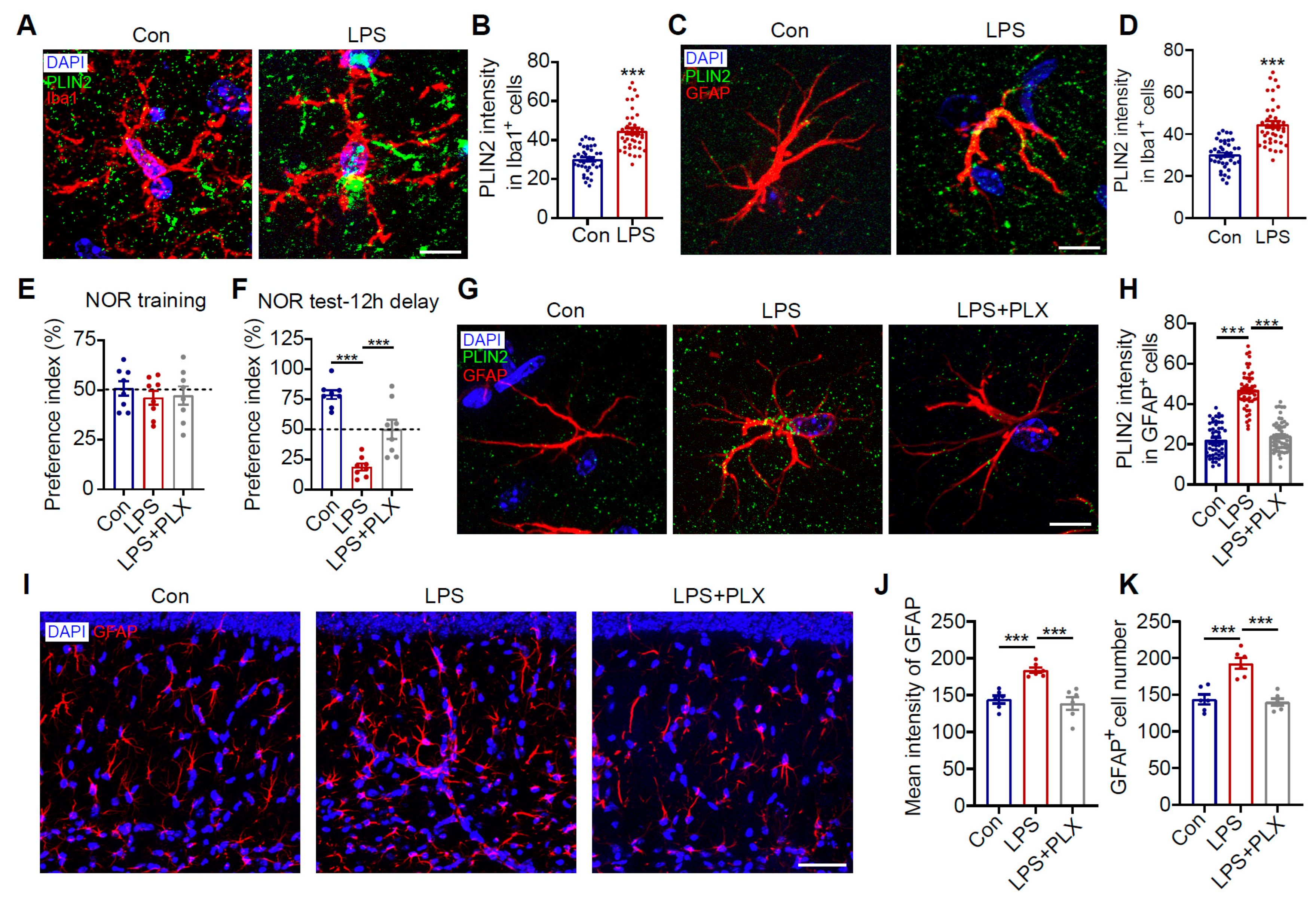
3.5. LPS-Activated Pro-Inflammatory Microglia Secrete IL-1β to Trigger Astrocyte LD Accumulation
3.6. Microglia Drive Inflammation-Induced Astrocytic LD Accumulation and Cognitive Deficits
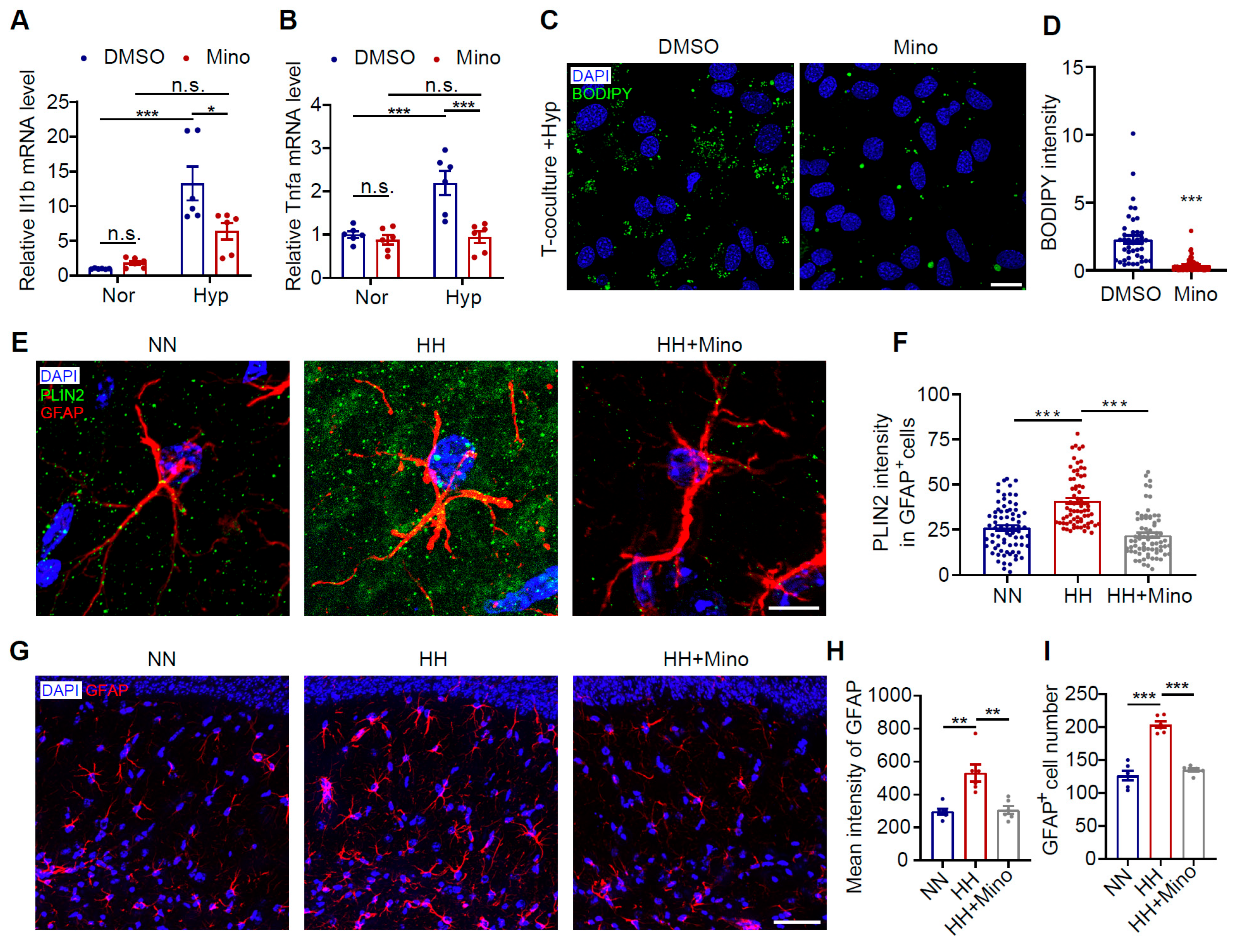
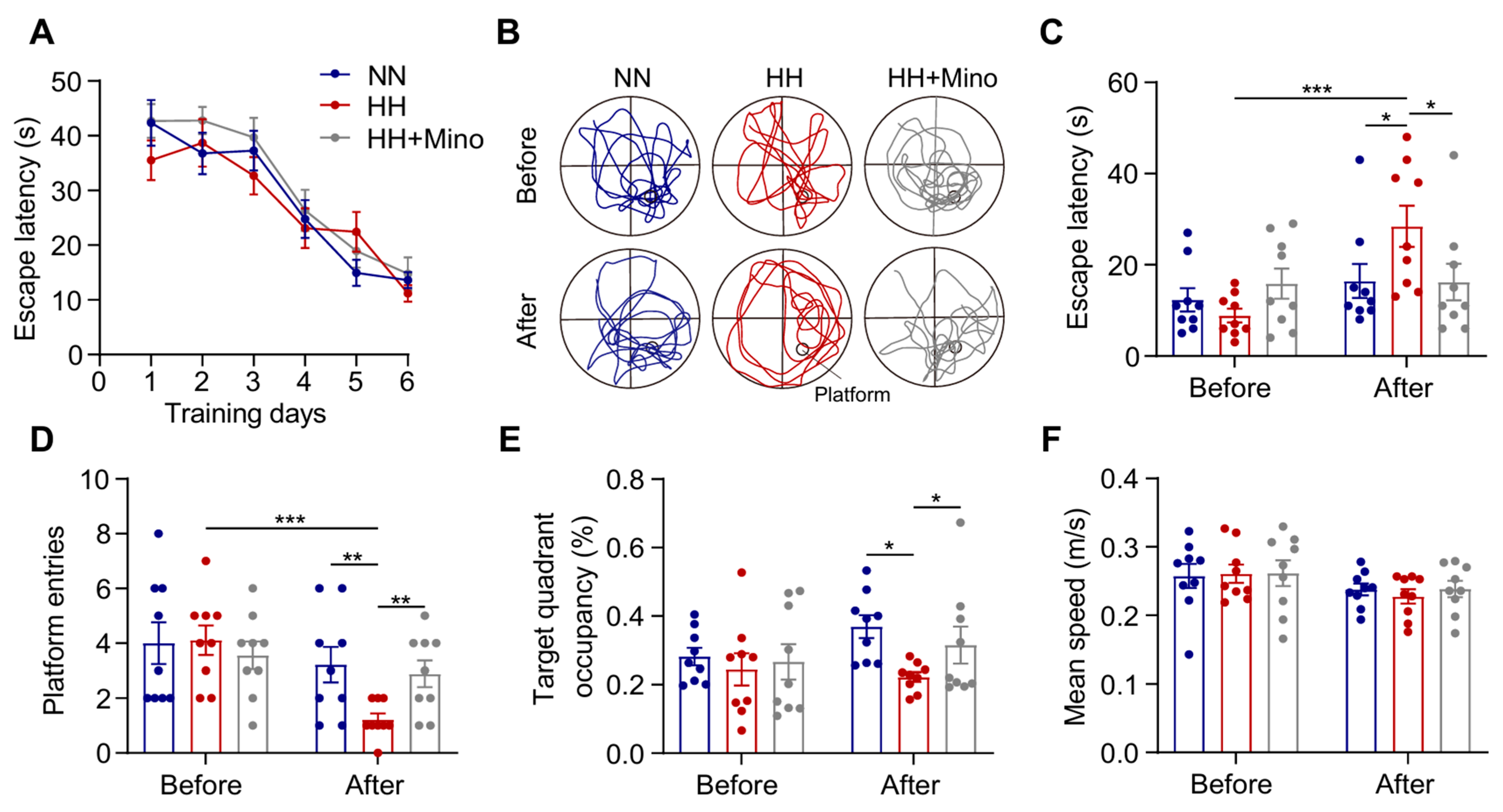
3.7. Minocycline Reduces Astrocytic LDs and Improves Cognitive Impairment in HH-Exposed Mice by Inhibiting Microglial Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garrido, E.; Botella de Maglia, J.; Castillo, O. Acute, subacute and chronic mountain sickness. Rev. Clin. Esp. 2021, 221, 481–490. [Google Scholar] [CrossRef]
- Koester-Hegmann, C.; Bengoetxea, H.; Kosenkov, D.; Thiersch, M.; Haider, T.; Gassmann, M.; Schneider Gasser, E.M. High-Altitude Cognitive Impairment Is Prevented by Enriched Environment Including Exercise via VEGF Signaling. Front. Cell Neurosci. 2018, 12, 532. [Google Scholar] [CrossRef]
- Davis, J.E.; Wagner, D.R.; Garvin, N.; Moilanen, D.; Thorington, J.; Schall, C. Cognitive and psychomotor responses to high-altitude exposure in sea level and high-altitude residents of Ecuador. J. Physiol. Anthr. 2015, 34, 2. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.; Lu, C.; Wu, H.; Ding, J.; Li, J.; Ding, J.; Gao, Y.; Wang, G.; Luo, Q. Decreased cold-inducible RNA-binding protein (CIRP) binding to GluRl on neuronal membranes mediates memory impairment resulting from prolonged hypobaric hypoxia exposure. CNS Neurosci. Ther. 2024, 30, e70059. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Lin, Y.; Li, Y.; Wang, H.; Wang, Z.; Liu, H.; Hu, Y.; Liu, L. Mechanism, prevention and treatment of cognitive impairment caused by high altitude exposure. Front. Physiol. 2023, 14, 1191058. [Google Scholar] [CrossRef]
- Turner, R.E.F.; Gatterer, H.; Falla, M.; Lawley, J.S. High-altitude cerebral edema: Its own entity or end-stage acute mountain sickness? J. Appl. Physiol. 2021, 131, 313–325. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, H.; Liu, Y.; Zhao, Z.; Zhang, Q.; Xue, C.; Zou, Y.; Cao, Z.; Luo, W. Cirbp-PSD95 axis protects against hypobaric hypoxia-induced aberrant morphology of hippocampal dendritic spines and cognitive deficits. Mol. Brain 2021, 14, 129. [Google Scholar] [CrossRef]
- Simka, M.; Latacz, P.; Czaja, J. Possible Role of Glymphatic System of the Brain in the Pathogenesis of High-Altitude Cerebral Edema. High. Alt. Med. Biol. 2018, 19, 394–397. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Luo, T.; Yue, T.; Xiao, W.; Yang, L.; Zhang, Z.; Han, F.; Long, P.; Hu, Y. Progress in the Treatment of High Altitude Cerebral Edema: Targeting REDOX Homeostasis. J. Inflamm. Res. 2023, 16, 2645–2660. [Google Scholar] [CrossRef]
- Xie, N.; Fan, F.; Jiang, S.; Hou, Y.; Zhang, Y.; Cairang, N.; Wang, X.; Meng, X. Rhodiola crenulate alleviates hypobaric hypoxia-induced brain injury via adjusting NF-kappaB/NLRP3-mediated inflammation. Phytomedicine 2022, 103, 154240. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Qin, Y.; Wang, L.; Zheng, J.; Gao, F. Protective effects of epigallocatechin-3-gallate counteracting the chronic hypobaric hypoxia-induced myocardial injury in plain-grown rats at high altitude. Cell Stress Chaperones 2023, 28, 921–933. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain lipidomics: From functional landscape to clinical significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef]
- Wei, W.; Lattau, S.S.J.; Xin, W.; Pan, Y.; Tatenhorst, L.; Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Hao, Z.; et al. Dynamic Brain Lipid Profiles Modulate Microglial Lipid Droplet Accumulation and Inflammation Under Ischemic Conditions in Mice. Adv. Sci. 2024, 11, e2306863. [Google Scholar] [CrossRef]
- Yang, X.; Yao, K.; Zhang, M.; Zhang, W.; Zu, H. New insight into the role of altered brain cholesterol metabolism in the pathogenesis of AD: A unifying cholesterol hypothesis and new therapeutic approach for AD. Brain Res. Bull. 2025, 224, 111321. [Google Scholar] [CrossRef]
- Taskin, E.; Ozcan, K.; Canacankatan, N.; Satar, M.; Yapicioglu, H.Y.; Erdogan, S. The effects of indomethacin on caspases, glutathione level and lipid peroxidation in the newborn rats with hypoxic-ischemic cerebral injury. Brain Res. 2009, 1289, 118–123. [Google Scholar] [CrossRef]
- Vacek, L.; Dvorak, A.; Bechynska, K.; Kosek, V.; Elkalaf, M.; Trinh, M.D.; Fiserova, I.; Pospisilova, K.; Slovakova, L.; Vitek, L.; et al. Hypoxia Induces Saturated Fatty Acids Accumulation and Reduces Unsaturated Fatty Acids Independently of Reverse Tricarboxylic Acid Cycle in L6 Myotubes. Front. Endocrinol. 2022, 13, 663625. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Atkinson, R.A.; Richardson, L.; Koulman, A.; Murray, A.J.; Harridge, S.D.R.; Martin, D.S.; Levett, D.Z.H.; Mitchell, K.; Mythen, M.G.; et al. Metabolomic and lipidomic plasma profile changes in human participants ascending to Everest Base Camp. Sci. Rep. 2019, 9, 2297. [Google Scholar] [CrossRef]
- Liang, H.; Yan, J.; Song, K. Comprehensive lipidomic analysis reveals regulation of glyceride metabolism in rat visceral adipose tissue by high-altitude chronic hypoxia. PLoS ONE 2022, 17, e0267513. [Google Scholar] [CrossRef]
- Roberts, A.C.; Butterfield, G.E.; Cymerman, A.; Reeves, J.T.; Wolfel, E.E.; Brooks, G.A. Acclimatization to 4,300-m altitude decreases reliance on fat as a substrate. J. Appl. Physiol. 1996, 81, 1762–1771. [Google Scholar] [CrossRef]
- van Deijk, A.F.; Camargo, N.; Timmerman, J.; Heistek, T.; Brouwers, J.F.; Mogavero, F.; Mansvelder, H.D.; Smit, A.B.; Verheijen, M.H. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia 2017, 65, 670–682. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Liu, Y. Navigating the metabolic maze: Anomalies in fatty acid and cholesterol processes in Alzheimer’s astrocytes. Alzheimers Res. Ther. 2024, 16, 63. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Weber-Boyvat, M.; Kroll, J.; Trimbuch, T.; Olkkonen, V.M.; Rosenmund, C. The lipid transporter ORP2 regulates synaptic neurotransmitter release via two distinct mechanisms. Cell Rep. 2022, 41, 111882. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kim, J.; Kim, C.S.; Tu, T.H.; Kim, M.S.; Suk, K.; Kim, D.H.; Lee, B.J.; Choi, H.S.; Park, T.; et al. Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 2017, 591, 1742–1751. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, A.; Acaz-Fonseca, E.; Boya, P.; Arevalo, M.A.; Garcia-Segura, L.M. Lipotoxic Effects of Palmitic Acid on Astrocytes Are Associated with Autophagy Impairment. Mol. Neurobiol. 2019, 56, 1665–1680. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Liu, J.; Pan, Y.; Zhang, M.; Chen, L. Senescent Phenotype of Astrocytes Leads to Activation of BV2 Microglia and N2a Neuronal Cells Death. Molecules 2022, 27, 5925. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Van Hove, H.; De Feo, D.; Greter, M.; Becher, B. Central Nervous System Macrophages in Health and Disease. Annu. Rev. Immunol. 2025, 43, 589–613. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Niu, Y.; Wan, B.; Lu, Y.; Luo, Q.; Zhu, L. CX3CL1/CX3CR1 signal mediates M1-type microglia and accelerates high-altitude-induced forgetting. Front. Cell Neurosci. 2023, 17, 1189348. [Google Scholar] [CrossRef]
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; He, S.; Chen, J.; Wang, C. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 2018, 193, 270–281. [Google Scholar] [CrossRef]
- Spangenberg, E.; Severson, P.L.; Hohsfield, L.A.; Crapser, J.; Zhang, J.; Burton, E.A.; Zhang, Y.; Spevak, W.; Lin, J.; Phan, N.Y.; et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 3758. [Google Scholar] [CrossRef]
- Hong, X.Y.; Qiu, S.H.; Wu, X.; Chen, S.Z.; Chen, X.X.; Zhang, B.; He, A.Q.; Xu, Y.; Wang, J.Q.; Gao, Y.C.; et al. Efficacy and Safety of Anlotinib in Overall and Disease-Specific Advanced Gynecological Cancer: A Real-World Study. Drug Des. Dev. Ther. 2023, 17, 2025–2033. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Chen, G.; Lu, Y.; Wang, D.; Zhu, L. Intermittent hypoxia therapy ameliorates beta-amyloid pathology via TFEB-mediated autophagy in murine Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 240. [Google Scholar] [CrossRef]
- Yao, D.P.; Li, T.; Yu, L.; Hu, M.X.; He, Y.; Zhang, R.M.; Wu, J.J.; Li, S.Y.; Kuang, W.H.; Yang, X.F.; et al. Selective degradation of hyperphosphorylated tau by proteolysis-targeting chimeras ameliorates cognitive function in Alzheimer’s disease model mice. Front. Pharmacol. 2024, 15, 1351792. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Wan, B.; Dong, Z.; Xue, Y.; Luo, Q.; Wang, D.; Lu, Y.; Zhu, L. NRF1-mediated microglial activation triggers high-altitude cerebral edema. J. Mol. Cell Biol. 2022, 14, majc036. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Fan, X.; Wu, X.; Wang, D.; Zhu, L. Intermittent hypoxia training enhances Abeta endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 121. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Hofwimmer, K.; de Paula Souza, J.; Subramanian, N.; Vujicic, M.; Rachid, L.; Mereau, H.; Zhao, C.; Dror, E.; Barreby, E.; Bjorkstrom, N.K.; et al. IL-1beta promotes adipogenesis by directly targeting adipocyte precursors. Nat. Commun. 2024, 15, 7957. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, X.; Zhang, J.; Zhou, H.; Sun, B.; Feng, J. DNA binding protein HMGB1 secreted by activated microglia promotes the apoptosis of hippocampal neurons in diabetes complicated with OSA. Brain Behav. Immun. 2018, 73, 482–492. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, P.; Ding, W.; Bian, J.; Wang, D.; Wang, X.; Luo, Q.; Wu, X.; Zhu, L. Pharmacological inhibition of mitochondrial division attenuates simulated high-altitude exposure-induced cerebral edema in mice: Involvement of inhibition of the NF-kappaB signaling pathway in glial cells. Eur. J. Pharmacol. 2022, 929, 175137. [Google Scholar] [CrossRef]
- Aboouf, M.A.; Thiersch, M.; Soliz, J.; Gassmann, M.; Schneider Gasser, E.M. The Brain at High Altitude: From Molecular Signaling to Cognitive Performance. Int. J. Mol. Sci. 2023, 24, 10179. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Guo, H.; Li, Q.; Yan, C.; Li, Y.; He, S.; Wang, N.; Wang, Q. Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of TREM1 in diabetes-associated cognitive impairment. Autophagy 2023, 19, 2639–2656. [Google Scholar] [CrossRef]
- Xin, W.; Pan, Y.; Wei, W.; Gerner, S.T.; Huber, S.; Juenemann, M.; Butz, M.; Bähr, M.; Huttner, H.B.; Doeppner, T.R. TGF-β1 Decreases Microglia-Mediated Neuroinflammation and Lipid Droplet Accumulation in an In Vitro Stroke Model. Int. J. Mol. Sci. 2023, 24, 17329. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Park, J.H.; Cha, B.Y.; Hoe, H.S. Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling. J. Neuroinflamm. 2022, 19, 187. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, M.; Li, Y.; Li, Y.; Hua, Y.; Fan, Y. Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways. Biochem. Pharmacol. 2021, 186, 114464. [Google Scholar] [CrossRef]
- Huang, C.; Ye, T.; Chen, B.; Chen, Z.; Ye, Y.; Liu, H. Intranasal administration of lipopolysaccharide reverses chronic stress-induced depression-like behavior in mice by microglial stimulation. Int. Immunopharmacol. 2023, 120, 110347. [Google Scholar] [CrossRef]
- Moreno-Rodriguez, M.; Perez, S.E.; Martinez-Gardeazabal, J.; Manuel, I.; Malek-Ahmadi, M.; Rodriguez-Puertas, R.; Mufson, E.J. Frontal Cortex Lipid Alterations During the Onset of Alzheimer’s Disease. J. Alzheimers Dis. 2024, 98, 1515–1532. [Google Scholar] [CrossRef]
- Li, D.; Xu, N.; Hou, Y.; Ren, W.; Zhang, N.; Wang, X.; Sun, Y.; Lu, W.; Qu, G.; Yu, Y.; et al. Abnormal lipid droplets accumulation induced cognitive deficits in obstructive sleep apnea syndrome mice via JNK/SREBP/ACC pathway but not through PDP1/PDC pathway. Mol. Med. 2022, 28, 3. [Google Scholar] [CrossRef]
- Ozerskaia, I.V.; Khachatryan, L.G.; Kolosova, N.G.; Polyanskaya, A.V.; Kasanave, E.V. The role of omega-3 polyunsaturated fatty acids in child development. Vopr. Pitan. 2024, 93, 6–18. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Zhang, X.; Liu, L.; Zhou, B.; Mo, R.; Li, Y.; Li, H.; Li, F.; Tao, Y.; et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4(-/-): A double-blind, randomized, placebo-controlled crossover trial. Clin. Nutr. 2020, 39, 2092–2105. [Google Scholar] [CrossRef]
- Lee, J.A.; Hall, B.; Allsop, J.; Alqarni, R.; Allen, S.P. Lipid metabolism in astrocytic structure and function. Semin. Cell Dev. Biol. 2021, 112, 123–136. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Ungerer, N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011, 50, 357–371. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Pang, M.Z.; Li, H.X.; Dai, X.Q.; Wang, X.B.; Liu, J.Y.; Shen, Y.; Xu, X.; Zhong, Z.M.; Wang, H.; Liu, C.F.; et al. Melatonin Ameliorates Abnormal Sleep-Wake Behavior via Facilitating Lipid Metabolism in a Zebrafish Model of Parkinson’s Disease. Neurosci. Bull. 2024, 40, 1901–1914. [Google Scholar] [CrossRef]
- Xin, W.; Pan, Y.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.; Kilic, E.; Hermann, D.M.; et al. Preconditioned extracellular vesicles from hypoxic microglia reduce poststroke AQP4 depolarization, disturbed cerebrospinal fluid flow, astrogliosis, and neuroinflammation. Theranostics 2023, 13, 4197–4216. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Chen, H.; Qin, Y.; Cheng, J.; He, B.; Wan, Y.; Zhu, D.; Gao, F. Epigallocatechin-3-Gallate Ameliorated Iron Accumulation and Apoptosis and Promoted Neuronal Regeneration and Memory/Cognitive Functions in the Hippocampus Induced by Exposure to a Chronic High-Altitude Hypoxia Environment. Neurochem. Res. 2022, 47, 2254–2262. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, F.; Xie, N.; Zhang, Y.; Wang, X.; Meng, X. Rhodiola crenulata alleviates hypobaric hypoxia-induced brain injury by maintaining BBB integrity and balancing energy metabolism dysfunction. Phytomedicine 2024, 128, 155529. [Google Scholar] [CrossRef]
- Ma, B.; Li, Q.; Li, M.; Wang, J.; Fan, N.; Yang, S.; Shi, W.; Wang, R.; Yin, D. Effect of butylphthalide on prevention and treatment of high altitude cerebral edema in rats. Heliyon 2024, 10, e27833. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Bahrami, B.F.; Badar, S.; Morris, D.L. Minocycline attenuates hypoxia-inducible factor-1alpha expression correlated with modulation of p53 and AKT/mTOR/p70S6K/4E-BP1 pathway in ovarian cancer: In vitro and in vivo studies. Am. J. Cancer Res. 2015, 5, 575–588. [Google Scholar]
- Hu, X.; Wu, B.; Wang, X.; Xu, C.; He, B.; Cui, B.; Lu, Z.; Jiang, H. Minocycline attenuates ischemia-induced ventricular arrhythmias in rats. Eur. J. Pharmacol. 2011, 654, 274–279. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Shi, Y.; Zou, Y.; Yang, Z.; Zhi, W.; Zhao, Z.; Shen, W.; Chen, L.; Wu, Y.; et al. Acute exposure of microwave impairs attention process by activating microglial inflammation. Cell Biosci. 2024, 14, 2. [Google Scholar] [CrossRef]
- Zhan, F.; Dong, Y.; Zhou, L.; Li, X.; Zhou, Z.; Xu, G. Minocycline alleviates LPS-induced cognitive dysfunction in mice by inhibiting the NLRP3/caspase-1 pathway. Aging 2024, 16, 2989–3006. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Qiao, S.; Zou, H.; Yu, X.J.; Yang, Y.; Li, Z.; Wang, J.; Chen, M.S.; Xu, J.; et al. Lipid droplet accumulation mediates macrophage survival and Treg recruitment via the CCL20/CCR6 axis in human hepatocellular carcinoma. Cell Mol. Immunol. 2024, 21, 1120–1130. [Google Scholar] [CrossRef]
- Ding, Y.; Gong, W.; Zhang, S.; Shen, J.; Liu, X.; Wang, Y.; Chen, Y.; Meng, G. Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem. Pharmacol. 2021, 192, 114665. [Google Scholar] [CrossRef]
- Governa, V.; de Oliveira, K.G.; Bang-Rudenstam, A.; Offer, S.; Cerezo-Magana, M.; Li, J.; Beyer, S.; Johansson, M.C.; Mansson, A.S.; Edvardsson, C.; et al. Protumoral lipid droplet-loaded macrophages are enriched in human glioblastoma and can be therapeutically targeted. Sci. Transl. Med. 2024, 16, eadk1168. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidant 2024, 13, 484. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- MacDonald, K.P.; Palmer, J.S.; Cronau, S.; Seppanen, E.; Olver, S.; Raffelt, N.C.; Kuns, R.; Pettit, A.R.; Clouston, A.; Wainwright, B.; et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 2010, 116, 3955–3963. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Marmol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, L.; Xu, Z.; Jiang, L.; Zhai, Y.; Liu, D.; Wu, Q. U-shaped relationship between non-high-density lipoprotein cholesterol and cognitive impairment in Chinese middle-aged and elderly: A cross-sectional study. BMC Public Health 2024, 24, 1624. [Google Scholar] [CrossRef]
- Hua, R.; Ma, Y.; Li, C.; Zhong, B.; Xie, W. Low levels of low-density lipoprotein cholesterol and cognitive decline. Sci. Bull. 2021, 66, 1684–1690. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Zhao, T.; Qiao, M.; Zhao, X.; Zhao, M.; Xu, L.; Zhao, Y.; Wu, L.; Wu, K.; et al. Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice. Brain Behav. Immun. 2017, 64, 266–275. [Google Scholar] [CrossRef]
- Song, T.T.; Bi, Y.H.; Gao, Y.Q.; Huang, R.; Hao, K.; Xu, G.; Tang, J.W.; Ma, Z.Q.; Kong, F.P.; Coote, J.H.; et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J. Neuroinflamm. 2016, 13, 63. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Cao, S.; Fang, Y.; Zhu, L.; Wang, X. Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes. Antioxidants 2025, 14, 918. https://doi.org/10.3390/antiox14080918
Fan X, Cao S, Fang Y, Zhu L, Wang X. Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes. Antioxidants. 2025; 14(8):918. https://doi.org/10.3390/antiox14080918
Chicago/Turabian StyleFan, Xiaoyang, Sitong Cao, Yujie Fang, Li Zhu, and Xueting Wang. 2025. "Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes" Antioxidants 14, no. 8: 918. https://doi.org/10.3390/antiox14080918
APA StyleFan, X., Cao, S., Fang, Y., Zhu, L., & Wang, X. (2025). Pro-Inflammatory Microglia Exacerbate High-Altitude-Induced Cognitive Impairment by Driving Lipid Droplet Accumulation in Astrocytes. Antioxidants, 14(8), 918. https://doi.org/10.3390/antiox14080918







