Role of Endoplasmic Reticulum Stress-Associated Genes in Septic Neonatal Foals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Acquisition
2.2. Total RNA Extraction and Sequencing
2.3. Transcriptional Analysis of Genes Involved in ER Stress
2.4. Statistical Analysis of the Enrichment of Differentially Expressed Genes (DEGs)
2.5. Assessment of Oxidative Stress Markers, Activities of Antioxidant Defense Enzymes, and Histological Alterations
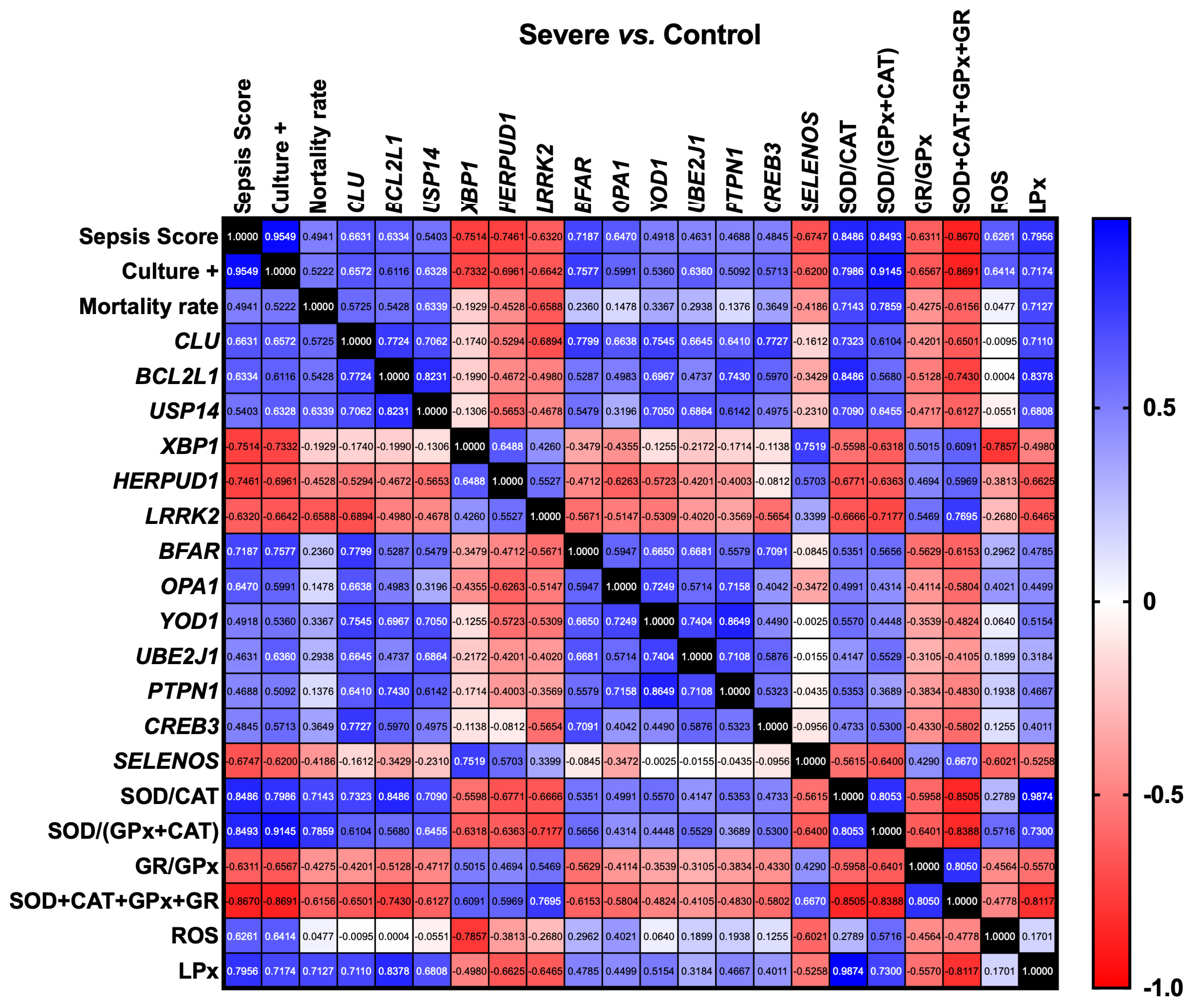
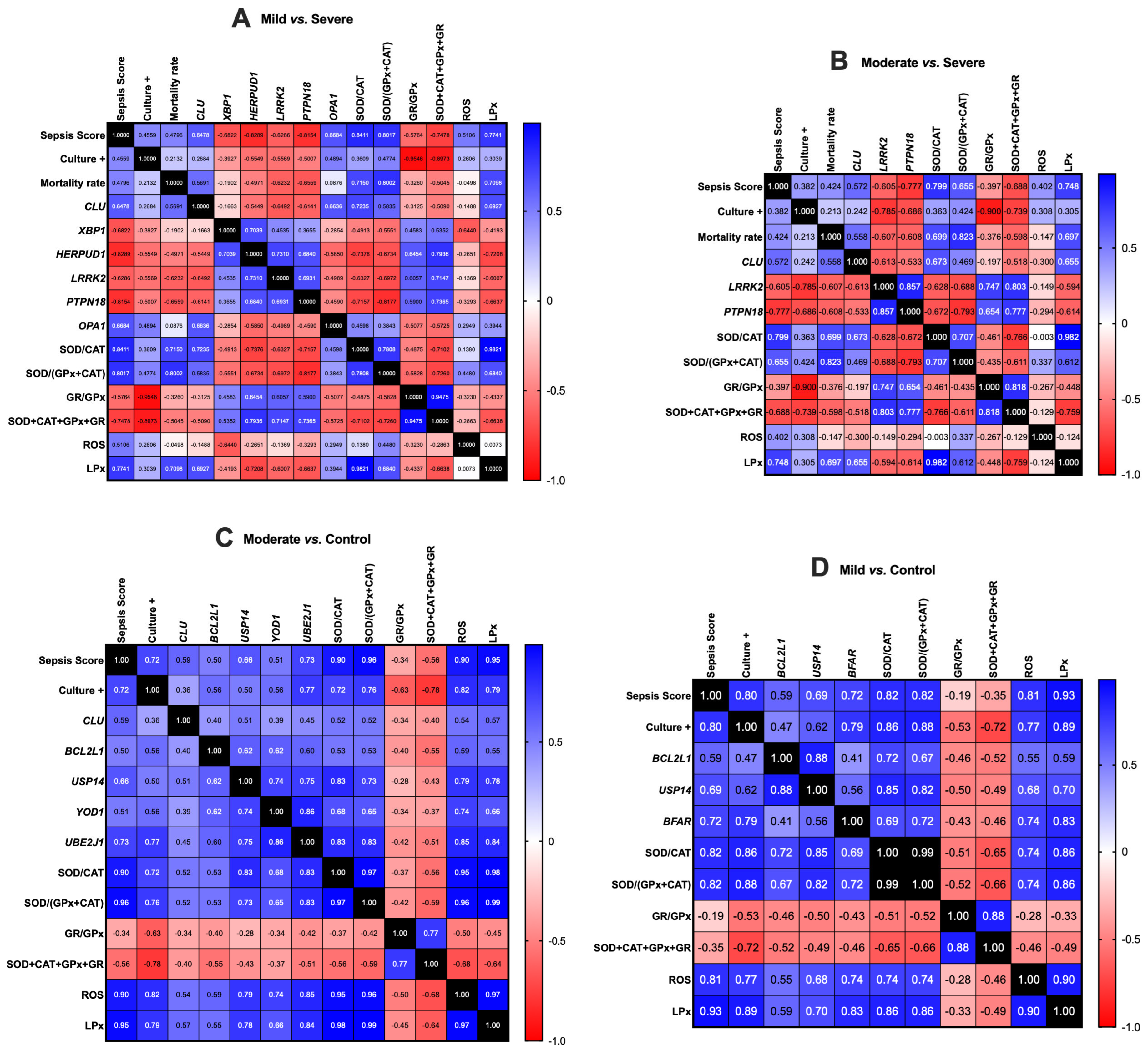
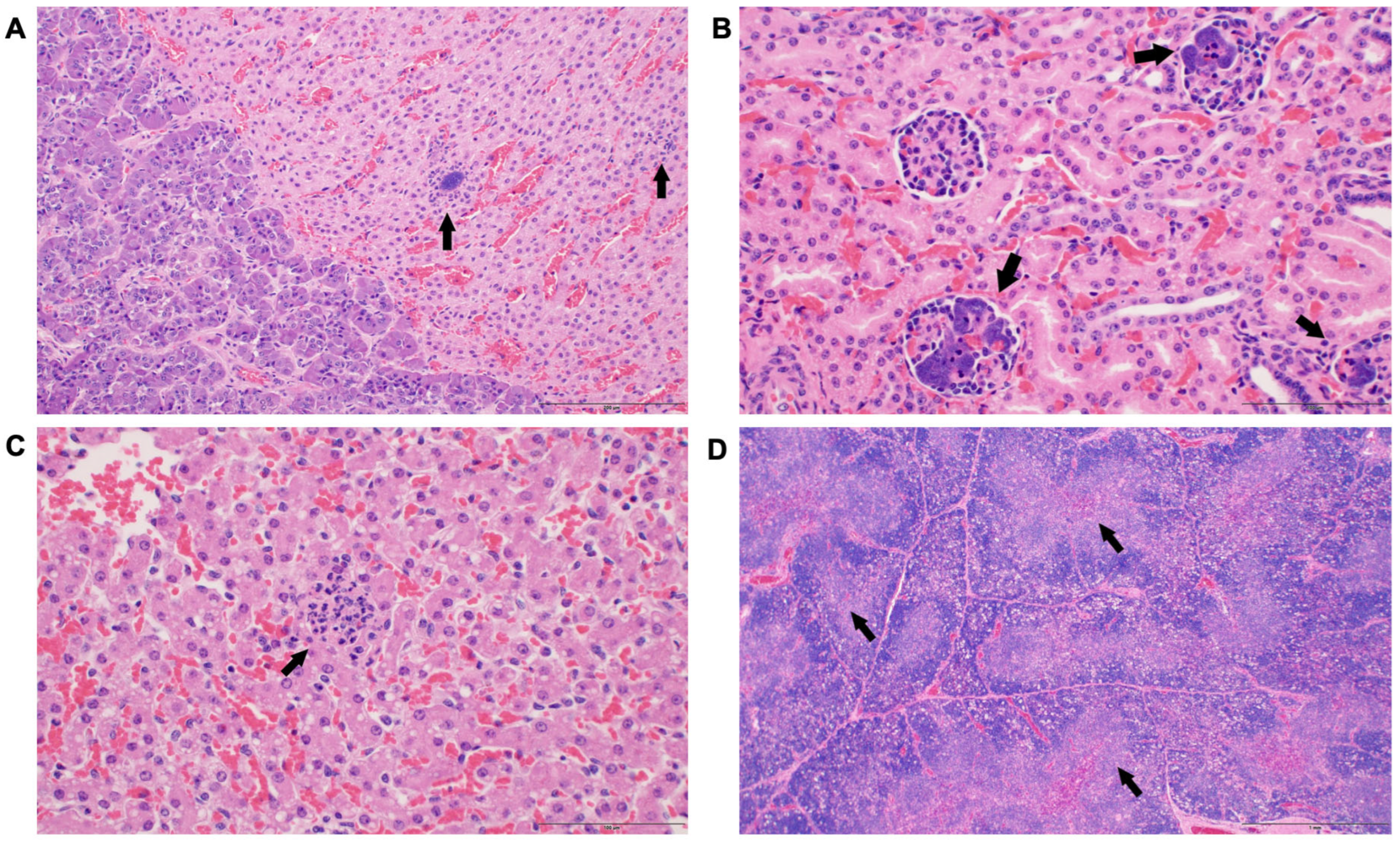
3. Results
3.1. Gene Ontology (GO) Functional Annotation of DEGs
3.2. Gene Expression Correlation Analysis
3.3. Histological Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Sepsis. Available online: https://www.who.int/news-room/fact-sheets/detail/sepsis (accessed on 18 March 2025).
- Peek, S.F.; Semrad, S.; McGuirk, S.M.; Riseberg, A.; Slack, J.A.; Marques, F.; Coombs, D.; Lien, L.; Keuler, N.; Darien, B.J. Prognostic Value of Clinicopathologic Variables Obtained at Admission and Effect of Antiendotoxin Plasma on Survival in Septic and Critically Ill Foals. J. Vet. Intern. Med. 2006, 20, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S. A Review of Equine Sepsis. Equine Vet. Educ. 2015, 27, 99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.D. Causes of and Farm Management Factors Associated with Disease and Death in Foals. J. Am. Vet. Med. Assoc. 1994, 204, 1644–1651. [Google Scholar] [CrossRef]
- Giguère, S.; Weber, E.J.; Sanchez, L.C. Factors Associated with Outcome and Gradual Improvement in Survival over Time in 1065 Equine Neonates Admitted to an Intensive Care Unit. Equine Vet. J. 2017, 49, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Sahoo, D.K.; Faivre, C.; Kopper, J.; Dersh, K.; Beachler, T.; Esser, M. Oxidative Stress in Critically Ill Neonatal Foals. J. Vet. Intern. Med. 2025, 39, e17297. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Wong, D.; Patani, A.; Paital, B.; Yadav, V.K.; Patel, A.; Jergens, A.E. Exploring the Role of Antioxidants in Sepsis-Associated Oxidative Stress: A Comprehensive Review. Front. Cell. Infect. Microbiol. 2024, 14, 1348713. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative Stress, Hormones, and Effects of Natural Antioxidants on Intestinal Inflammation in Inflammatory Bowel Disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Chainy, G.B.N. Hormone-Linked Redox Status and Its Modulation by Antioxidants. Vitam. Horm. 2023, 121, 197–246. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and Oxidative Stress: An Overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. The Inflammatory Response in Sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Bertozzi, G.; Ferrara, M.; Di Fazio, A.; Maiese, A.; Delogu, G.; Di Fazio, N.; Tortorella, V.; La Russa, R.; Fineschi, V. Oxidative Stress in Sepsis: A Focus on Cardiac Pathology. Int. J. Mol. Sci. 2024, 25, 2912. [Google Scholar] [CrossRef]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef]
- Ong, G.; Logue, S.E. Unfolding the Interactions between Endoplasmic Reticulum Stress and Oxidative Stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A. Compromised Rat Testicular Antioxidant Defence System by Hypothyroidism before Puberty. Int. J. Endocrinol. 2012, 2012, 637825. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Chainy, G.B.N. Thyroid Dysfunction and Testicular Redox Status: An Intriguing Association. In Oxidants, Antioxidants, and Impact of the Oxidative Status in Male Reproduction; Academic Press: Cambridge, MA, USA, 2019; pp. 149–170. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A.; Chainy, G.B.N. Rat Testicular Mitochondrial Antioxidant Defence System and Its Modulation by Aging. Acta Biol. Hung. 2008, 59, 413–424. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A.; Bhanja, S.; Chainy, G.B.N. Experimental Hyperthyroidism-Induced Oxidative Stress and Impairment of Antioxidant Defence System in Rat Testis. Indian J. Exp. Biol. 2005, 43, 1058–1067. [Google Scholar] [PubMed]
- Sahoo, D.K.; Roy, A.; Chattopadhyay, S.; Chainy, G.B.N. Effect of T3 Treatment on Glutathione Redox Pool and Its Metabolizing Enzymes in Mitochondrial and Post-Mitochondrial Fractions of Adult Rat Testes. Indian J. Exp. Biol. 2007, 45, 338–346. [Google Scholar] [PubMed]
- Sahoo, D.K.; Roy, A.; Bhanja, S.; Chainy, G.B.N. Hypothyroidism Impairs Antioxidant Defence System and Testicular Physiology during Development and Maturation. Gen. Comp. Endocrinol. 2008, 156, 63–70. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sahoo, D.K.; Roy, A.; Samanta, L.; Chainy, G.B.N. Thiol Redox Status Critically Influences Mitochondrial Response to Thyroid Hormone-Induced Hepatic Oxidative Injury: A Temporal Analysis. Cell Biochem. Funct. 2010, 28, 126–134. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The Aftermath of the Interplay between the Endoplasmic Reticulum Stress Response and Redox Signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Wong, D.M.; Ruby, R.E.; Dembek, K.A.; Barr, B.S.; Reuss, S.M.; Magdesian, K.G.; Olsen, E.; Burns, T.; Slovis, N.M.; Wilkins, P.A. Evaluation of Updated Sepsis Scoring Systems and Systemic Inflammatory Response Syndrome Criteria and Their Association with Sepsis in Equine Neonates. J. Vet. Intern. Med. 2018, 32, 1185–1193. [Google Scholar] [CrossRef]
- Correia, C.N.; McLoughlin, K.E.; Nalpas, N.C.; Magee, D.A.; Browne, J.A.; Rue-Albrecht, K.; Gordon, S.V.; MacHugh, D.E. RNA Sequencing (RNA-Seq) Reveals Extremely Low Levels of Reticulocyte-Derived Globin Gene Transcripts in Peripheral Blood from Horses (Equus caballus) and Cattle (Bos taurus). Front. Genet. 2018, 9, 380776. [Google Scholar] [CrossRef] [PubMed]
- Equus caballus Genome Assembly EquCab3.0—NCBI—NLM. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_002863925.1/ (accessed on 7 April 2025).
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Suwada, K.; Blomme, E.A.G.; Kowalkowski, K.; Liguori, M.J.; Mahalingaiah, P.K.; Mittelstadt, S.; Peterson, R.; Rendino, L.; Vo, A.; et al. Preclinical Species Gene Expression Database: Development and Meta-Analysis. Front. Genet. 2023, 13, 1078050. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A Web Server for Annotation and Identification of Enriched Pathways and Diseases. Nucleic Acids Res. 2011, 39, W316. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING Database in 2025: Protein Networks with Directionality of Regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers 2022, 14, 3525. [Google Scholar] [CrossRef]
- Khan, M.M.; Yang, W.L.; Wang, P. Endoplasmic Reticulum Stress in Sepsis. Shock 2015, 44, 294. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Han, L.; Gao, Y.; Li, L.; Shang, X.; Hu, W.; Xue, C. The Endoplasmic Reticulum Stress-Mediated Apoptosis Signal Pathway Is Involved in Sepsis-Induced Abnormal Lymphocyte Apoptosis. Eur. Surg. Res. 2008, 41, 219–225. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell Death and Endoplasmic Reticulum Stress: Disease Relevance and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeong, J.S.; Kim, S.R.; Park, S.Y.; Chae, H.J.; Lee, Y.C. Inhibition of Endoplasmic Reticulum Stress Alleviates Lipopolysaccharide-Induced Lung Inflammation through Modulation of NF-ΚB/HIF-1α Signaling Pathway. Sci. Rep. 2013, 3, srep01142. [Google Scholar] [CrossRef]
- Ferlito, M.; Wang, Q.; Fulton, W.B.; Colombani, P.M.; Marchionni, L.; Fox-Talbot, K.; Paolocci, N.; Steenbergen, C. Hydrogen Sulfide [Corrected] Increases Survival during Sepsis: Protective Effect of CHOP Inhibition. J. Immunol. 2014, 192, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, T.; Orihashi, K.; Herlambang, B.; Takahashi, S.; Hamaishi, M.; Okada, K.; Sueda, T. Sodium 4-Phenylbutyrate Protects against Spinal Cord Ischemia by Inhibition of Endoplasmic Reticulum Stress. J. Vasc. Surg. 2010, 52, 1580–1586. [Google Scholar] [CrossRef]
- Begum, G.; Yan, H.Q.; Li, L.; Singh, A.; Edward Dixon, C.; Sun, D. Docosahexaenoic Acid Reduces ER Stress and Abnormal Protein Accumulation and Improves Neuronal Function Following Traumatic Brain Injury. J. Neurosci. 2014, 34, 3743–3755. [Google Scholar] [CrossRef]
- Osabe, T.; Shimizu, K.; Kadota, K. Differential Expression Analysis Using a Model-Based Gene Clustering Algorithm for RNA-Seq Data. BMC Bioinform. 2021, 22, 511. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Stewart, T.; Lindgreen, E.M.; Patel, B.; Patel, A.; Trivedi, J.N.; Parker, V.; Rudinsky, A.J.; Winston, J.A.; Bourgois-Mochel, A.; et al. Restorative Effects of Synbiotics on Colonic Ultrastructure and Oxidative Stress in Dogs with Chronic Enteropathy. Antioxidants 2025, 14, 727. [Google Scholar] [CrossRef]
- Everaert, C.; Luypaert, M.; Maag, J.L.V.; Cheng, Q.X.; DInger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-Sequencing Analysis Workflows Using Whole-Transcriptome RT-QPCR Expression Data. Sci. Rep. 2017, 7, 1559. [Google Scholar] [CrossRef]
- Bizoń, A.; Chojdak-Łukasiewicz, J.; Budrewicz, S.; Pokryszko-Dragan, A.; Piwowar, A. Exploring the Relationship between Antioxidant Enzymes, Oxidative Stress Markers, and Clinical Profile in Relapsing–Remitting Multiple Sclerosis. Antioxidants 2023, 12, 1638. [Google Scholar] [CrossRef]
- Torres, L.L.; Quaglio, N.B.; De Souza, G.T.; Garcia, R.T.; Dati, L.M.M.; Moreira, W.L.; De Melo Loureiro, A.P.; De Souza-Talarico, J.N.; Smid, J.; Porto, C.S.; et al. Peripheral Oxidative Stress Biomarkers in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Lourda, M.; Agiostratidou, G.; Kletsas, D.; Gonos, E.S. Differential Effects of Clusterin/Apolipoprotein J on Cellular Growth and Survival. Free Radic. Biol. Med. 2005, 38, 436–449. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Gonos, E.S. Regulation of Clusterin/Apolipoprotein J, a Functional Homologue to the Small Heat Shock Proteins, by Oxidative Stress in Ageing and Age-Related Diseases. Free Radic. Res. 2006, 40, 1324–1334. [Google Scholar] [CrossRef]
- Debure, L.; Vayssière, J.L.; Rincheval, V.; Loison, F.; Le Drŕean, Y.; Michel, D. Intracellular Clusterin Causes Juxtanuclear Aggregate Formation and Mitochondrial Alteration. J. Cell Sci. 2003, 116, 3109–3121. [Google Scholar] [CrossRef] [PubMed]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 Family Proteins as Regulators of Oxidative Stress. Semin. Cancer Biol. 2008, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Lee, M.J.; Park, S.; Oh, D.-C.; Elsasser, S.; Chen, P.-C.; Gartner, C.; Dimova, N.; Hanna, J.; Gygi, S.P.; et al. Enhancement of Proteasome Activity by a Small-Molecule Inhibitor of USP14. Nature 2010, 467, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, E.; Abu Jhaisha, S.; Buendgens, L.; Sapundzhieva, N.; Brozat, J.F.; Hohlstein, P.; Pollmanns, M.R.; Koek, G.H.; Weiskirchen, R.; Trautwein, C.; et al. Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation. Diagnostics 2022, 12, 3010. [Google Scholar] [CrossRef]
- Lonze, B.E.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Yamamoto, A.; Murase, R.; Hirata, Y. Comparative Analysis of CREB3 and CREB3L2 Protein Expression in HEK293 Cells. Int. J. Mol. Sci. 2021, 22, 2767. [Google Scholar] [CrossRef]
- Sheng, Z.; Li, L.; Zhu, L.J.; Smith, T.W.; Demers, A.; Ross, A.H.; Moser, R.P.; Green, M.R. A Genome-Wide RNA Interference Screen Reveals an Essential CREB3L2/ATF5/MCL1 Survival Pathway in Malignant Glioma with Therapeutic Implications. Nat. Med. 2010, 16, 671. [Google Scholar] [CrossRef]
- Liang, G.; Audas, T.E.; Li, Y.; Cockram, G.P.; Dean, J.D.; Martyn, A.C.; Kokame, K.; Lu, R. Luman/CREB3 Induces Transcription of the Endoplasmic Reticulum (ER) Stress Response Protein Herp through an ER Stress Response Element. Mol. Cell. Biol. 2006, 26, 7999–8010. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Prywes, R. Dependence of Site-2 Protease Cleavage of ATF6 on Prior Site-1 Protease Digestion Is Determined by the Size of the Luminal Domain of ATF6. J. Biol. Chem. 2004, 279, 43046–43051. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yoshida, H. TFE3, HSP47, and CREB3 Pathways of the Mammalian Golgi Stress Response. Cell Struct. Funct. 2017, 42, 27–36. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Krajewski, S.; Krajewska, M.; Xie, Z.; Fuess, S.; Kitada, S.; Pawłowski, K.; Godzik, A.; Reed, J.C. BAR: An Apoptosis Regulator at the Intersection of Caspases and Bcl-2 Family Proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 2597–2602. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, C.; Oberholzer, A.; Clare-Salzler, M.; Moldawer, L.L. Apoptosis in Sepsis: A New Target for Therapeutic Exploration. FASEB J. 2001, 15, 879–892. [Google Scholar] [CrossRef]
- Yang, C.S.; Coopersmith, C.M.; Lyons, J.D. Cell Death Proteins in Sepsis: Key Players and Modern Therapeutic Approaches. Front. Immunol. 2023, 14, 1347401. [Google Scholar] [CrossRef] [PubMed]
- Reece, M.D.; Song, C.; Hancock, S.C.; Pereira Ribeiro, S.; Kulpa, D.A.; Gavegnano, C. Repurposing BCL-2 and Jak 1/2 Inhibitors: Cure and Treatment of HIV-1 and Other Viral Infections. Front. Immunol. 2022, 13, 1033672. [Google Scholar] [CrossRef]
- Park, S.S.; Baek, K.H. Synergistic Effect of YOD1 and USP21 on the Hippo Signaling Pathway. Cancer Cell Int. 2023, 23, 209. [Google Scholar] [CrossRef]
- Xu, F.; Ma, Y.; Huang, W.; Gao, J.; Guo, M.; Li, J.; Kong, L.; Liang, G.; Du, R.; Xu, Q.; et al. Typically Inhibiting USP14 Promotes Autophagy in M1-like Macrophages and Alleviates CLP-Induced Sepsis. Cell Death Dis. 2020, 11, 666. [Google Scholar] [CrossRef]
- Gong, X.; Jia, L.; Zhou, L.; Hu, T. USP14 Predicts Poorer Survival Outcomes and Promotes Tumor Progression in Endometrial Carcinoma by Activating NF-ΚB Signaling. Aging 2023, 15, 12120–12135. [Google Scholar] [CrossRef]
- Sasset, L.; Petris, G.; Cesaratto, F.; Burrone, O.R. The VCP/P97 and YOD1 Proteins Have Different Substratedependent Activities in Endoplasmic Reticulum-Associated Degradation (ERAD). J. Biol. Chem. 2015, 290, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Lu, Y.; Gao, H.; Hu, C.; Zhang, J.; Zhao, Q.; Sun, Z.; Peng, Z. Identification of Potential Diagnostic and Prognostic Biomarkers for Sepsis Based on Machine Learning. Comput. Struct. Biotechnol. J. 2023, 21, 2316–2331. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tang, Y.; Liang, F.; Liu, L.; Liang, N.; Yang, X.; Zhang, N.; Yi, Z.; Zhong, Y.; Wang, W.; et al. NLRP3 Inflammasome Contributes to Endotoxin-Induced Coagulation. Thromb. Res. 2022, 214, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, C.; Liu, J.; Zhang, S.; Tang, H.; Liu, Y.; Zhang, S.; Wu, Q.; Zhang, J.; Qi, Z.; et al. YOD1 Protects against MRSA Sepsis-Induced DIC through Lys33-Linked Deubiquitination of NLRP3. Cell Death Dis. 2024, 15, 360. [Google Scholar] [CrossRef]
- Lyu, J.; Zheng, G.; Chen, Z.; Wang, B.; Tao, S.; Xiang, D.; Xie, M.; Huang, J.; Liu, C.; Zeng, Q. Sepsis-Induced Brain Mitochondrial Dysfunction Is Associated with Altered Mitochondrial Src and PTP1B Levels. Brain Res. 2015, 1620, 130–138. [Google Scholar] [CrossRef]
- Coquerel, D.; Neviere, R.; Delile, E.; Mulder, P.; Marechal, X.; Montaigne, D.; Renet, S.; Remy-Jouet, I.; Gomez, E.; Henry, J.P.; et al. Gene Deletion of Protein Tyrosine Phosphatase 1B Protects against Sepsis-Induced Cardiovascular Dysfunction and Mortality. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1032–1044. [Google Scholar] [CrossRef]
- Sun, M.; Li, Y.; Xu, G.; Zhu, J.; Lu, R.; An, S.; Zeng, Z.; Deng, Z.; Cheng, R.; Zhang, Q.; et al. Sirt3-Mediated Opa1 Deacetylation Protects Against Sepsis-Induced Acute Lung Injury by Inhibiting Alveolar Macrophage Pro-Inflammatory Polarization. Antioxid. Redox Signal. 2024, 41, 1014–1030. [Google Scholar] [CrossRef]
- Koenig, P.A.; Nicholls, P.K.; Schmidt, F.I.; Hagiwara, M.; Maruyama, T.; Frydman, G.H.; Watson, N.; Page, D.C.; Ploegh, H.L. The E2 Ubiquitin-Conjugating Enzyme UBE2J1 Is Required for Spermiogenesis in Mice. J. Biol. Chem. 2014, 289, 34490–34502. [Google Scholar] [CrossRef]
- Feng, T.; Deng, L.; Lu, X.; Pan, W.; Wu, Q.; Dai, J. Ubiquitin-Conjugating Enzyme UBE2J1 Negatively Modulates Interferon Pathway and Promotes RNA Virus Infection. Virol. J. 2018, 15, 132. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Harutyunyan, H.; Harutyunyan, A. A Certain Role of SOD/CAT Imbalance in Pathogenesis of Autism Spectrum Disorders. Free Radic. Biol. Med. 2018, 123, 85–95. [Google Scholar] [CrossRef]
- Liu, Y.; Adachi, M.; Zhao, S.; Hareyama, M.; Koong, A.C.; Luo, D.; Rando, T.A.; Imai, K.; Shinomura, Y. Preventing Oxidative Stress: A New Role for XBP1. Cell Death Differ. 2009, 16, 847. [Google Scholar] [CrossRef]
- Paredes, F.; Parra, V.; Torrealba, N.; Navarro-Marquez, M.; Gatica, D.; Bravo-Sagua, R.; Troncoso, R.; Pennanen, C.; Quiroga, C.; Chiong, M.; et al. HERPUD1 Protects against Oxidative Stress-Induced Apoptosis through Downregulation of the Inositol 1,4,5-Trisphosphate Receptor. Free Radic. Biol. Med. 2015, 90, 206. [Google Scholar] [CrossRef]
- Keeney, M.T.; Rocha, E.M.; Hoffman, E.K.; Farmer, K.; Di Maio, R.; Weir, J.; Wagner, W.G.; Hu, X.; Clark, C.L.; Castro, S.L.; et al. LRRK2 Regulates Production of Reactive Oxygen Species in Cell and Animal Models of Parkinson’s Disease. Sci. Transl. Med. 2024, 16, eadl3438. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, T.; Zhang, X.; Li, P.; Ye, L.; Kuang, J.; Tao, L.; Ni, L.; Zhao, Q.; Zhang, J.; et al. Regorafenib Activates Oxidative Stress by Inhibiting SELENOS and Potentiates Oxaliplatin-Induced Cell Death in Colon Cancer Cells. Eur. J. Pharmacol. 2023, 957, 175986. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.; Landvik, N.E.; Lind, H.; Skaug, V.; Haugen, A.; Zienolddiny, S. A Combination of Functional Polymorphisms in the CASP8, MMP1, IL10 and SEPS1 Genes Affects Risk of Non-Small Cell Lung Cancer. Lung Cancer 2011, 71, 123–129. [Google Scholar] [CrossRef]
- He, L.; Wang, B.; Yao, Y.; Su, M.; Ma, H.; Jia, N. Protective Effects of the SEPS1 Gene on Lipopolysaccharide-Induced Sepsis. Mol. Med. Rep. 2014, 9, 1869–1876. [Google Scholar] [CrossRef]
- Curran, J.E.; Jowett, J.B.M.; Elliott, K.S.; Gao, Y.; Gluschenko, K.; Wang, J.; Azim, D.M.A.; Cai, G.; Mahaney, M.C.; Comuzzie, A.G.; et al. Genetic Variation in Selenoprotein S Influences Inflammatory Response. Nat. Genet. 2005, 37, 1234–1241. [Google Scholar] [CrossRef]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic Reticulum and the Unfolded Protein Response: Dynamics and Metabolic Integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhu, L.; Du, S.; Mao, J.; Wang, Y.; Wang, S.; Bo, Q.; Tu, Y.; Yi, Q.Y. The P300/XBP1s/Herpud1 Axis Promotes Macrophage M2 Polarization and the Development of Choroidal Neovascularization. J. Cell. Mol. Med. 2021, 25, 6709. [Google Scholar] [CrossRef] [PubMed]
- Kokame, K.; Agarwal, K.L.; Kato, H.; Miyata, T. Herp, a New Ubiquitin-like Membrane Protein Induced by Endoplasmic Reticulum Stress. J. Biol. Chem. 2000, 275, 32846–32853. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Gao, W.; Zheng, P.; Sun, X.; Wang, L. Medroxyprogesterone Acetate Causes the Alterations of Endoplasmic Reticulum Related MRNAs and LncRNAs in Endometrial Cancer Cells. BMC Med. Genom. 2019, 12, 163. [Google Scholar] [CrossRef]
- George, A.K.; Behera, J.; Kelly, K.E.; Mondal, N.K.; Richardson, K.P.; Tyagi, N. Exercise Mitigates Alcohol Induced Endoplasmic Reticulum Stress Mediated Cognitive Impairment through ATF6-Herp Signaling. Sci. Rep. 2018, 8, 5158. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Cao, M.; Liang, J.; Wu, D.; Gu, X.; Ke, K. Effects of Homocysteine-Induced Endoplasmic Reticulum Protein on Endoplasmic Reticulum Stress, Autophagy, and Neuronal Apoptosis Following Intracerebral Hemorrhage. IBRO Rep. 2020, 9, 207–217. [Google Scholar] [CrossRef]
- Torres, M.; Akhtar, S.; McKenzie, E.A.; Dickson, A.J.; Torres, M.; Akhtar, S.; McKenzie, E.A.; Dickson, A.J. Temperature Down-Shift Modifies Expression of UPR-/ERAD-Related Genes and Enhances Production of a Chimeric Fusion Protein in CHO Cells. Biotechnol. J. 2021, 16, 2000081. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Martin, D.; Li, Y.; Yang, J.; Wang, G.; Margariti, A.; Jiang, Z.; Yu, H.; Zampetaki, A.; Hu, Y.; Xu, Q.; et al. Unspliced X-Box-Binding Protein 1 (XBP1) Protects Endothelial Cells from Oxidative Stress through Interaction with Histone Deacetylase 3. J. Biol. Chem. 2014, 289, 30625–30634. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Siao, K.; Soon, R.K.; Her, C.; Iwawaki, T.; Kohno, K.; Mattis, A.N.; Maher, J.J. Hepatocyte-Specific Deletion of XBP1 Sensitizes Mice to Liver Injury through Hyperactivation of IRE1α. Cell Death Differ. 2021, 28, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Vivas, W.; Weis, S. Tidy up—The Unfolded Protein Response in Sepsis. Front. Immunol. 2022, 13, 980680. [Google Scholar] [CrossRef] [PubMed]
- Clavier, T.; Grangé, S.; Pressat-Laffouilhere, T.; Besnier, E.; Renet, S.; Fraineau, S.; Thiebaut, P.A.; Richard, V.; Veber, B.; Tamion, F. Gene Expression of Protein Tyrosine Phosphatase 1B and Endoplasmic Reticulum Stress During Septic Shock. Front. Med. 2019, 6, 483652. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 Cause Autosomal-Dominant Parkinsonism with Pleomorphic Pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Hui, K.Y.; Fernandez-Hernandez, H.; Hu, J.; Schaffner, A.; Pankratz, N.; Hsu, N.Y.; Chuang, L.S.; Carmi, S.; Villaverde, N.; Li, X.; et al. Functional Variants in the LRRK2 Gene Confer Shared Effects on Risk for Crohn’s Disease and Parkinson’s Disease. Sci. Transl. Med. 2018, 10, 7795. [Google Scholar] [CrossRef]
- Herbst, S.; Gutierrez, M.G. LRRK2 in Infection: Friend or Foe? ACS Infect. Dis. 2019, 5, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Gardet, A.; Benita, Y.; Li, C.; Sands, B.E.; Ballester, I.; Stevens, C.; Korzenik, J.R.; Rioux, J.D.; Daly, M.J.; Xavier, R.J.; et al. LRRK2 Is Involved in the IFN-γ Response and Host Response to Pathogens. J. Immunol. 2010, 185, 5577–5585. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, X.; Li, Y.; Zhao, J.; Liu, Z.; Hu, Z.; Wang, Y.; Yao, Y.; Miller, A.W.; Su, B.; et al. LRRK2 Promotes the Activation of NLRC4 Inflammasome during Salmonella Typhimurium Infection. J. Exp. Med. 2017, 214, 3051–3066. [Google Scholar] [CrossRef]
- Härtlova, A.; Herbst, S.; Peltier, J.; Rodgers, A.; Bilkei-Gorzo, O.; Fearns, A.; Dill, B.D.; Lee, H.; Flynn, R.; Cowley, S.A.; et al. LRRK2 Is a Negative Regulator of Mycobacterium Tuberculosis Phagosome Maturation in Macrophages. EMBO J. 2018, 37, e98694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Liu, B.; Zhang, Y.; Li, Y.; Li, Y.; Wang, Y.; Zhou, H. Regulatory Mechanisms of the CAMP-Responsive Element Binding Protein 3 (CREB3) Family in Cancers. Biomed. Pharmacother. 2023, 166, 115335. [Google Scholar] [CrossRef]
- Kuo, M.L.; Sy, A.J.; Xue, L.; Chi, M.; Michelle, M.T.; Yen, T.; Chiang, M.I.; Chang, L.; Chu, P.; Yen, Y. RRM2B Suppresses Activation of the Oxidative Stress Pathway and Is Up-Regulated by P53 during Senescence. Sci. Rep. 2012, 2, srep00822. [Google Scholar] [CrossRef]
- Ihrie, R.A.; Reczek, E.; Horner, J.S.; Khachatrian, L.; Sage, J.; Jacks, T.; Attardi, L.D. Perp Is a Mediator of P53-Dependent Apoptosis in Diverse Cell Types. Curr. Biol. 2003, 13, 1985–1990. [Google Scholar] [CrossRef]
- Sumner, S.E.; Markley, R.L.; Kirimanjeswara, G.S. Role of Selenoproteins in Bacterial Pathogenesis. Biol. Trace Elem. Res. 2019, 192, 69. [Google Scholar] [CrossRef]
- Ebstein, F.; Küry, S.; Most, V.; Rosenfelt, C.; Scott-Boyer, M.P.; van Woerden, G.M.; Besnard, T.; Papendorf, J.J.; Studencka-Turski, M.; Wang, T.; et al. PSMC3 Proteasome Subunit Variants Are Associated with Neurodevelopmental Delay and Type I Interferon Production. Sci. Transl. Med. 2023, 15, eabo3189. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; He, F. Blood Long Non-Coding RNA Intersectin 1–2 Is Highly Expressed and Links with Increased Th17 Cells, Inflammation, Multiple Organ Dysfunction, and Mortality Risk in Sepsis Patients. J. Clin. Lab. Anal. 2022, 36, e24330. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, M.; Zhang, H.; Gong, L.; Hu, J.; Luo, H.; Zhou, X. Network Analysis Identifies a Gene Biomarker Panel for Sepsis-Induced Acute Respiratory Distress Syndrome. BMC Med. Genom. 2023, 16, 165. [Google Scholar] [CrossRef]
- Zheng, Q.; Xing, J.; Li, X.; Tang, X.; Zhang, D. PRDM16 Suppresses Ferroptosis to Protect against Sepsis-Associated Acute Kidney Injury by Targeting the NRF2/GPX4 Axis. Redox Biol. 2024, 78, 103417. [Google Scholar] [CrossRef]
- Gadelha, R.B.; Machado, C.B.; de Pinho Pessoa, F.M.C.; da Costa Pantoja, L.; Barreto, I.V.; Ribeiro, R.M.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Khayat, A.S.; Moreira-Nunes, C.A. The Role of WRAP53 in Cell Homeostasis and Carcinogenesis Onset. Curr. Issues Mol. Biol. 2022, 44, 5498. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Gerashchenko, M.V.; Delaney, J.R.; Kaya, A.; Kennedy, B.K.; Kaeberlein, M.; Gladyshev, V.N. Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response. PLoS Genet. 2014, 10, e1004019. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Benito, V.; Verhoef, L.G.G.C.; Masucci, M.G.; Dantuma, N.P. Endoplasmic Reticulum Stress Compromises the Ubiquitin–Proteasome System. Hum. Mol. Genet. 2005, 14, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Klaude, M.; Fredriksson, K.; Tjäder, I.; Hammarqvist, F.; Ahlman, B.; Rooyackers, O.; Wernerman, J. Proteasome Proteolytic Activity in Skeletal Muscle Is Increased in Patients with Sepsis. Clin. Sci. 2007, 112, 499–506. [Google Scholar] [CrossRef]
- Otero, A.; Betancor, M.; Eraña, H.; Borges, N.F.; Lucas, J.J.; Badiola, J.J.; Castilla, J.; Bolea, R. Prion-Associated Neurodegeneration Causes Both Endoplasmic Reticulum Stress and Proteasome Impairment in a Murine Model of Spontaneous Disease. Int. J. Mol. Sci. 2021, 22, 465. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Kaneko, M.; Okuma, Y.; Matsubara, K.; Nomura, Y. Endoplasmic Reticulum Stress and Parkinson’s Disease: The Role of HRD1 in Averting Apoptosis in Neurodegenerative Disease. Oxid. Med. Cell. Longev. 2013, 2013, 239854. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Y.; Rahaman, A.; Kumar, V. Towards Understanding the Relationship Between ER Stress and Unfolded Protein Response in Amyotrophic Lateral Sclerosis. Front. Aging Neurosci. 2022, 14, 892518. [Google Scholar] [CrossRef]
- Shacham, T.; Sharma, N.; Lederkremer, G.Z. Protein Misfolding and ER Stress in Huntington’s Disease. Front. Mol. Biosci. 2019, 6, 20. [Google Scholar] [CrossRef]
- Kanuka, M.; Ouchi, F.; Kato, N.; Katsuki, R.; Ito, S.; Miura, K.; Hikida, M.; Tamura, T. Endoplasmic Reticulum Associated Degradation of Spinocerebellar Ataxia-Related CD10 Cysteine Mutant. Int. J. Mol. Sci. 2020, 21, 4237. [Google Scholar] [CrossRef]
- Guo, M.L.; Liao, K.; Periyasamy, P.; Yang, L.; Cai, Y.; Callen, S.E.; Buch, S. Cocaine-Mediated Microglial Activation Involves the ER Stress-Autophagy Axis. Autophagy 2015, 11, 995–1009. [Google Scholar] [CrossRef]
- Yamada, S.; Umeya, T. Case of Acute Onset Ataxia Caused by Klebsiella Pneumoniae Sepsis with the Appearance of Anti-GD1b Antibody. BMJ Case Rep. 2021, 14, e242396. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Wirdefeldt, K.; Jacks, A.; Kamel, F.; Ye, W.; Chen, H. CNS Infections, Sepsis and Risk of Parkinson’s Disease. Int. J. Epidemiol. 2012, 41, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.S.; Chen, H.J.; Liang, J.A.; Kao, C.H. Risk of Sepsis in Patients with Amyotrophic Lateral Sclerosis: A Population-Based Retrospective Cohort Study in Taiwan. BMJ Open 2017, 7, e013761. [Google Scholar] [CrossRef] [PubMed]
- Shirley, C.M.; Kalu, N.; Shamay, M.; Ambinder, R.F. Epstein-Barr Virus Lytic Gene Expression Is Tightly Linked to ER Stress but Not Cytotoxicity with Bortezomib or Nelfinavir. Infect. Agents Cancer 2012, 7, P32. [Google Scholar] [CrossRef]
- Goh, C.; Burnham, K.L.; Ansari, M.A.; de Cesare, M.; Golubchik, T.; Hutton, P.; Overend, L.E.; Davenport, E.E.; Hinds, C.J.; Bowden, R.; et al. Epstein-Barr Virus Reactivation in Sepsis Due to Community-Acquired Pneumonia Is Associated with Increased Morbidity and an Immunosuppressed Host Transcriptomic Endotype. Sci. Rep. 2020, 10, 9838. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Hodai, Y.; Naito, S.; Kondo, Y.; Tsumura, T.; Miyata, T.; Kobayashi, T.; Sugiyama, M.; Onoue, T.; Iwama, S.; et al. Reticulon 4 Reflects Endoplasmic Reticulum Stress in Arginine Vasopressin Neurons. Neuroreport 2025, 36, 540–546. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Müller, B.; Struck, J.; Bergmann, A.; Redl, H.; Christ-Crain, M. Copeptin, a Stable Peptide of the Arginine Vasopressin Precursor, Is Elevated in Hemorrhagic and Septic Shock. Shock 2007, 28, 219–226. [Google Scholar] [CrossRef] [PubMed]


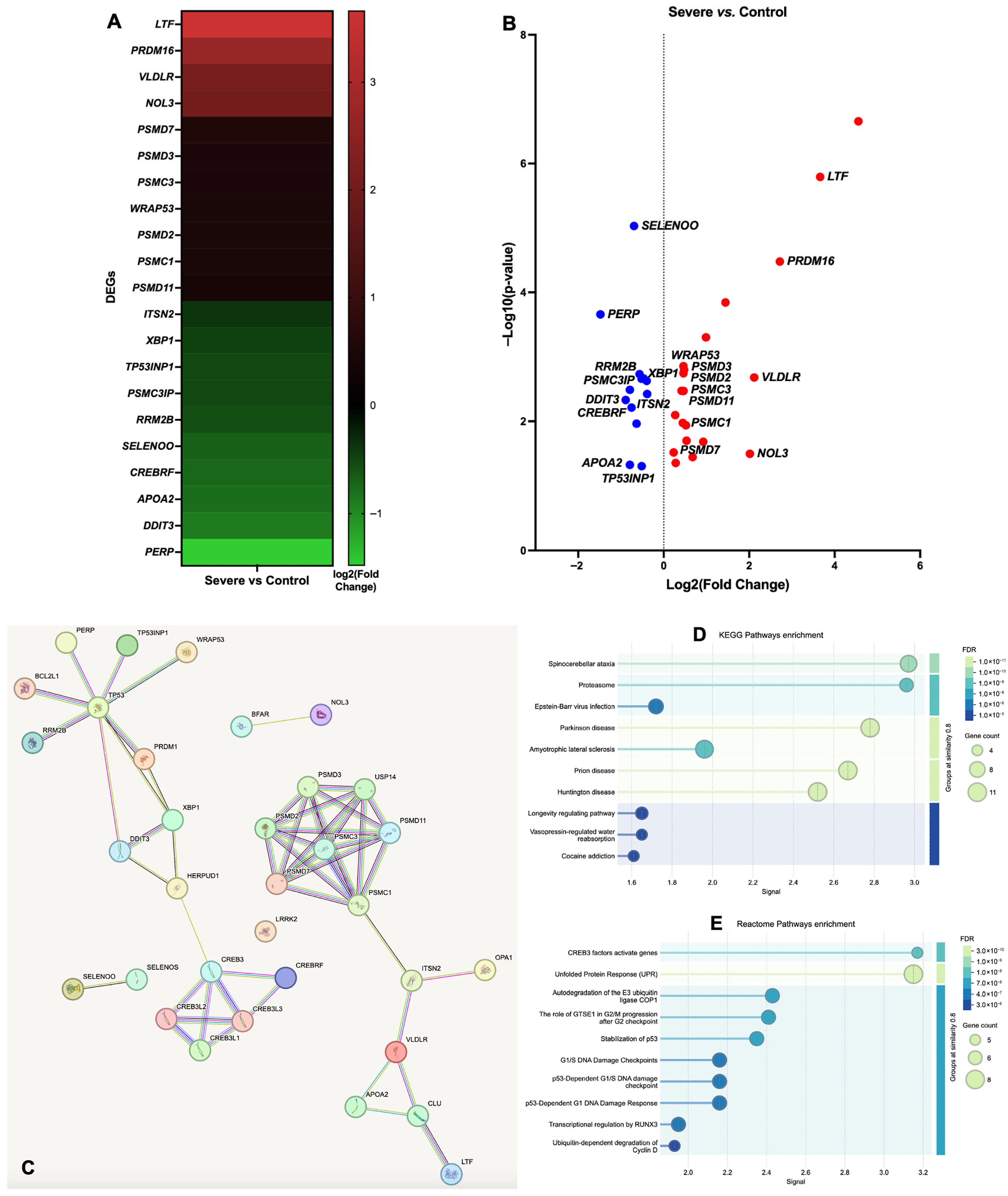

| Group Comparisons | Gene ID | Gene Name | Chromosome | Gene Start Position | Gene End Position | Gene Description | log2 (Fold Change) | p-Value | −log10 (p-Value) |
|---|---|---|---|---|---|---|---|---|---|
| Severe vs. Control | ENSECAG00000007010 | CLU | 2 | 56547399 | 56619680 | Equus caballus clusterin, mRNA. [Source: RefSeq mRNA; Acc: NM_001081944] | 4.555 | 2.21 × 10−7 | 6.656 |
| ENSECAG00000017223 | BCL2L1 | 22 | 23334139 | 23376133 | BCL2 like 1 [Source: VGNC Symbol; Acc: VGNC:15791] | 1.445 | 1.44 × 10−4 | 3.843 | |
| ENSECAG00000022985 | USP14 | 8 | 43792907 | 43839139 | Ubiquitin specific peptidase 14 [Source: VGNC Symbol; Acc: VGNC:24832] | 0.989 | 4.99 × 10−4 | 3.302 | |
| ENSECAG00000009722 | YOD1 | 5 | 3273118 | 3275205 | YOD1 deubiquitinase [Source: HGNC Symbol; Acc: HGNC:25035] | 0.925 | 0.021 | 1.683 | |
| ENSECAG00000019772 | PTPN1 | 22 | 39442194 | 39520318 | Protein tyrosine phosphatase, non-receptor type 1 [Source: VGNC Symbol; Acc: VGNC:22010] | 0.675 | 0.036 | 1.445 | |
| ENSECAG00000024248 | OPA1 | 19 | 33025234 | 33104793 | OPA1, mitochondrial dynamin like GTPase [Source: VGNC Symbol; Acc: VGNC:21035] | 0.520 | 0.012 | 1.939 | |
| ENSECAG00000000184 | CREB3 | 25 | 999440 | 1002983 | cAMP Responsive element binding protein 3 [Source: VGNC Symbol; Acc: VGNC:56871] | 0.280 | 0.044 | 1.355 | |
| ENSECAG00000016920 | BFAR | 13 | 31568145 | 31593542 | Bifunctional apoptosis regulator [Source: VGNC Symbol; Acc: VGNC:15818] | 0.271 | 0.008 | 2.097 | |
| ENSECAG00000012492 | UBE2J1 | 10 | 42734584 | 42758609 | Ubiquitin conjugating enzyme E2 J1 [Source: VGNC Symbol; Acc: VGNC:24721] | 0.232 | 0.030 | 1.517 | |
| ENSECAG00000011366 | HERPUD1 | 3 | 9952325 | 9962055 | Homocysteine inducible ER protein with ubiquitin like domain 1 [Source: VGNC Symbol; Acc: VGNC:18755] | −0.398 | 0.002 | 2.627 | |
| ENSECAG00000014780 | XBP1 | 8 | 10108130 | 10114753 | X-box binding protein 1 [Source: HGNC Symbol; Acc: HGNC:12801] | −0.471 | 0.002 | 2.664 | |
| ENSECAG00000012386 | SELENOS | 1 | 107309263 | 107317239 | Selenoprotein S [Source: VGNC Symbol; Acc: VGNC:22803] | −0.636 | 0.011 | 1.963 | |
| ENSECAG00000010288 | LRRK2 | 6 | 60224506 | 60356951 | Leucine-rich repeat kinase 2 [Source: VGNC Symbol; Acc: VGNC:19801] | −0.791 | 0.003 | 2.489 | |
| Mild vs. Severe | ENSECAG00000011090 | PTPN18 | 18 | 211051 | 255397 | Protein tyrosine phosphatase, non-receptor type 18 [Source: VGNC Symbol; Acc: VGNC:22015] | 0.938 | 4.27 × 10−6 | 5.369 |
| ENSECAG00000010288 | LRRK2 | 6 | 60224506 | 60356951 | Leucine-rich repeat kinase 2 [Source: VGNC Symbol; Acc: VGNC:19801] | 0.780 | 0.007 | 2.136 | |
| ENSECAG00000014780 | XBP1 | 8 | 10108130 | 10114753 | X-box binding protein 1 [Source: HGNC Symbol; Acc: HGNC:12801] | 0.512 | 0.005 | 2.271 | |
| ENSECAG00000011366 | HERPUD1 | 3 | 9952325 | 9962055 | Homocysteine inducible ER protein with ubiquitin like domain 1 [Source: VGNC Symbol; Acc: VGNC:18755] | 0.320 | 0.005 | 2.287 | |
| ENSECAG00000024248 | OPA1 | 19 | 33025234 | 33104793 | OPA1, mitochondrial dynamin like GTPase [Source: VGNC Symbol; Acc: VGNC:21035] | −0.397 | 0.038 | 1.421 | |
| ENSECAG00000007010 | CLU | 2 | 56547399 | 56619680 | Equus caballus clusterin (CLU), mRNA. [Source: RefSeq mRNA; Acc: NM_001081944] | −4.031 | 2.352 × 10−8 | 7.629 | |
| Moderate vs. Severe | ENSECAG00000011090 | PTPN18 | 18 | 211051 | 255397 | Protein tyrosine phosphatase, non-receptor type 18 [Source: VGNC Symbol; Acc: VGNC:22015] | 0.939 | 2.444 × 10−5 | 4.612 |
| ENSECAG00000010288 | LRRK2 | 6 | 60224506 | 60356951 | Leucine-rich repeat kinase 2 [Source: VGNC Symbol; Acc: VGNC:19801] | 0.930 | 0.001 | 2.967 | |
| ENSECAG00000007010 | CLU | 2 | 56547399 | 56619680 | Equus caballus clusterin (CLU), mRNA. [Source: RefSeq mRNA; Acc: NM_001081944] | −2.834 | 0.003 | 2.576 | |
| Moderate vs. Control | ENSECAG00000007010 | CLU | 2 | 56547399 | 56619680 | Equus caballus clusterin (CLU), mRNA. [Source: RefSeq mRNA; Acc: NM_001081944] | 1.720 | 0.030 | 1.516 |
| ENSECAG00000017223 | BCL2L1 | 22 | 23334139 | 23376133 | BCL2 like 1 [Source:VGNC Symbol;Acc:VGNC:15791] | 1.162 | 0.005 | 2.293 | |
| ENSECAG00000009722 | YOD1 | 5 | 3273118 | 3275205 | YOD1 deubiquitinase [Source: HGNC Symbol; Acc: HGNC:25035] | 0.757 | 0.041 | 1.384 | |
| ENSECAG00000022985 | USP14 | 8 | 43792907 | 43839139 | Ubiquitin specific peptidase 14 [Source: VGNC Symbol; Acc: VGNC:24832] | 0.639 | 0.038 | 1.416 | |
| ENSECAG00000012492 | UBE2J1 | 10 | 42734584 | 42758609 | Ubiquitin conjugating enzyme E2 J1 [Source: VGNC Symbol; Acc: VGNC:24721] | 0.247 | 0.019 | 1.730 | |
| Mild vs. Moderate | ENSECAG00000013078 | STXBP1 | 25 | 31639631 | 31702301 | Syntaxin binding protein 1 [Source: VGNC Symbol; Acc: VGNC: 23736] | −1.412 | 0.042 | 1.374 |
| Mild vs. Control | ENSECAG00000017223 | BCL2L1 | 22 | 23334139 | 23376133 | BCL2 like 1 [Source: VGNC Symbol; Acc: VGNC:15791] | 0.907 | 0.037 | 1.437 |
| ENSECAG00000017552 | CREB3L4 | 5 | 40245551 | 40249420 | cAMP Responsive element binding protein 3 like 4 [Source: VGNC Symbol; Acc: VGNC:16856] | 0.792 | 0.035 | 1.454 | |
| ENSECAG00000022985 | USP14 | 8 | 43792907 | 43839139 | Ubiquitin specific peptidase 14 [Source:VGNC Symbol; Acc: VGNC:24832] | 0.579 | 0.035 | 1.459 | |
| ENSECAG00000016920 | BFAR | 13 | 31568145 | 31593542 | Bifunctional apoptosis regulator [Source: VGNC Symbol; Acc: VGNC:15818] | 0.234 | 0.015 | 1.823 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, D.K.; Wong, D.; Paital, B.; Ruby, R.E.; Patel, A. Role of Endoplasmic Reticulum Stress-Associated Genes in Septic Neonatal Foals. Antioxidants 2025, 14, 1024. https://doi.org/10.3390/antiox14081024
Sahoo DK, Wong D, Paital B, Ruby RE, Patel A. Role of Endoplasmic Reticulum Stress-Associated Genes in Septic Neonatal Foals. Antioxidants. 2025; 14(8):1024. https://doi.org/10.3390/antiox14081024
Chicago/Turabian StyleSahoo, Dipak Kumar, David Wong, Biswaranjan Paital, Rebecca E. Ruby, and Ashish Patel. 2025. "Role of Endoplasmic Reticulum Stress-Associated Genes in Septic Neonatal Foals" Antioxidants 14, no. 8: 1024. https://doi.org/10.3390/antiox14081024
APA StyleSahoo, D. K., Wong, D., Paital, B., Ruby, R. E., & Patel, A. (2025). Role of Endoplasmic Reticulum Stress-Associated Genes in Septic Neonatal Foals. Antioxidants, 14(8), 1024. https://doi.org/10.3390/antiox14081024








