Preventing Cisplatin-Induced Neuropathy and Related Emotional Disorders with the Coadministration of Duloxetine and Hydrogen-Rich Water in Male and Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
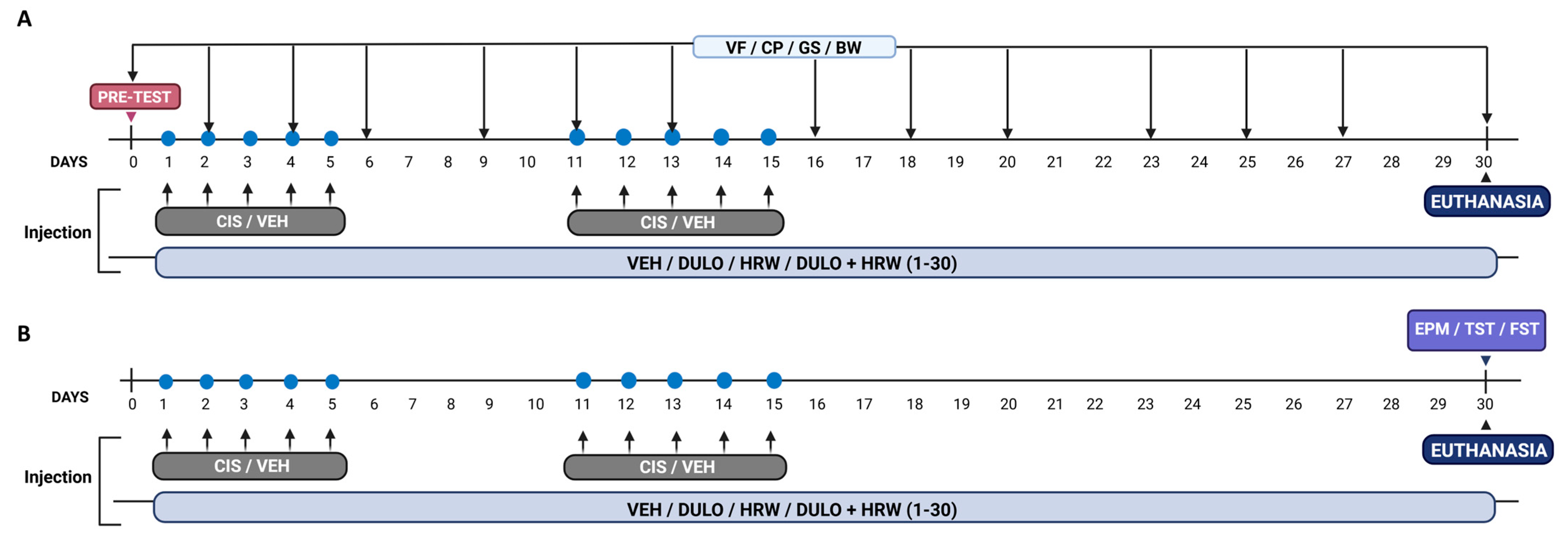
2.2. Experimental Design
2.3. Administration of Drugs
2.4. Allodynia
2.5. Grip Strength Test
2.6. Anxiety- and Depressive-like Comportments
2.7. Western Blot
2.8. Statistical Analyses
3. Results
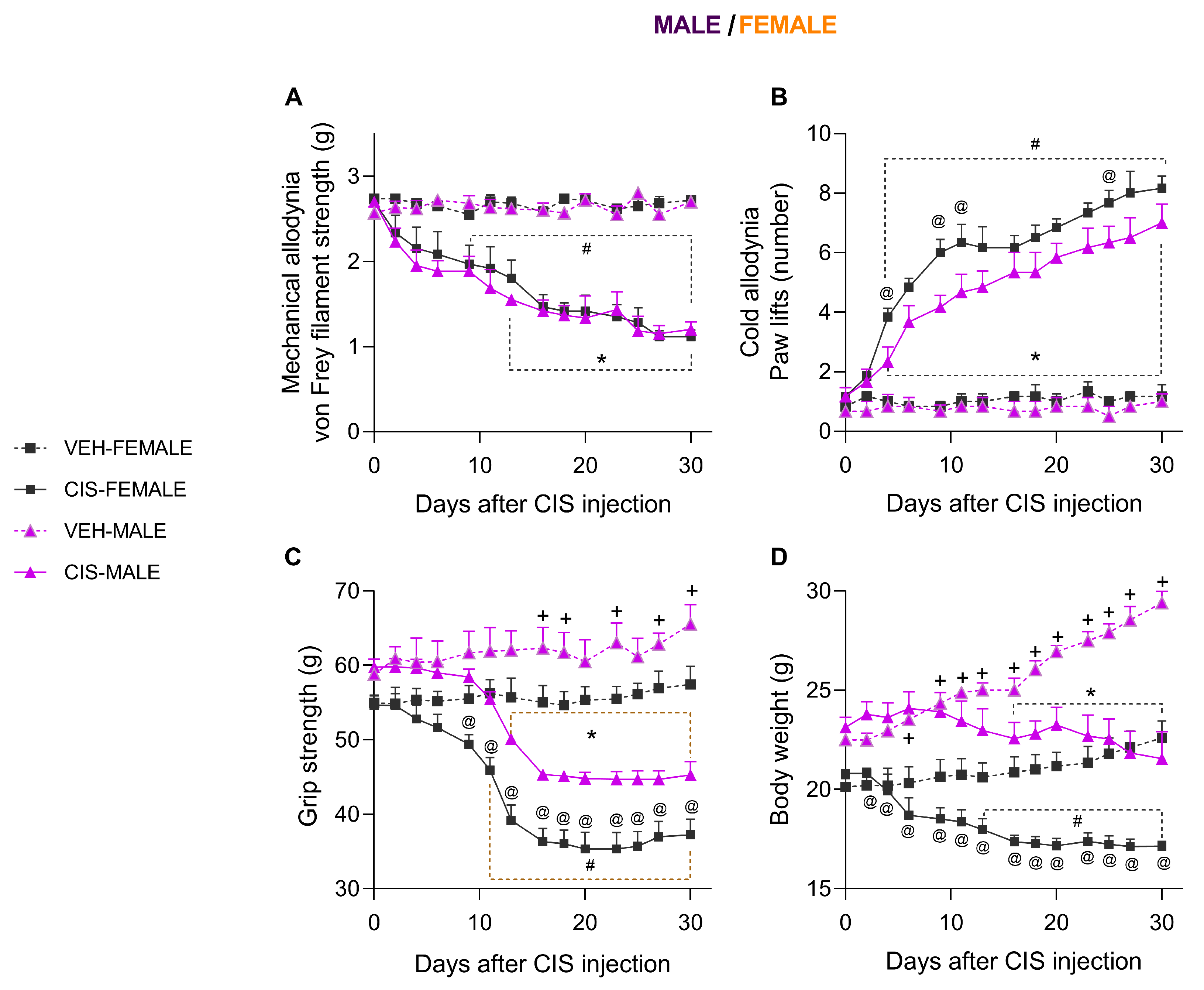
3.1. The Impact of Sex on the Mechanical and Cold Allodynia and the Grip Strength and Body Weight Loss Caused by CIS in Mice
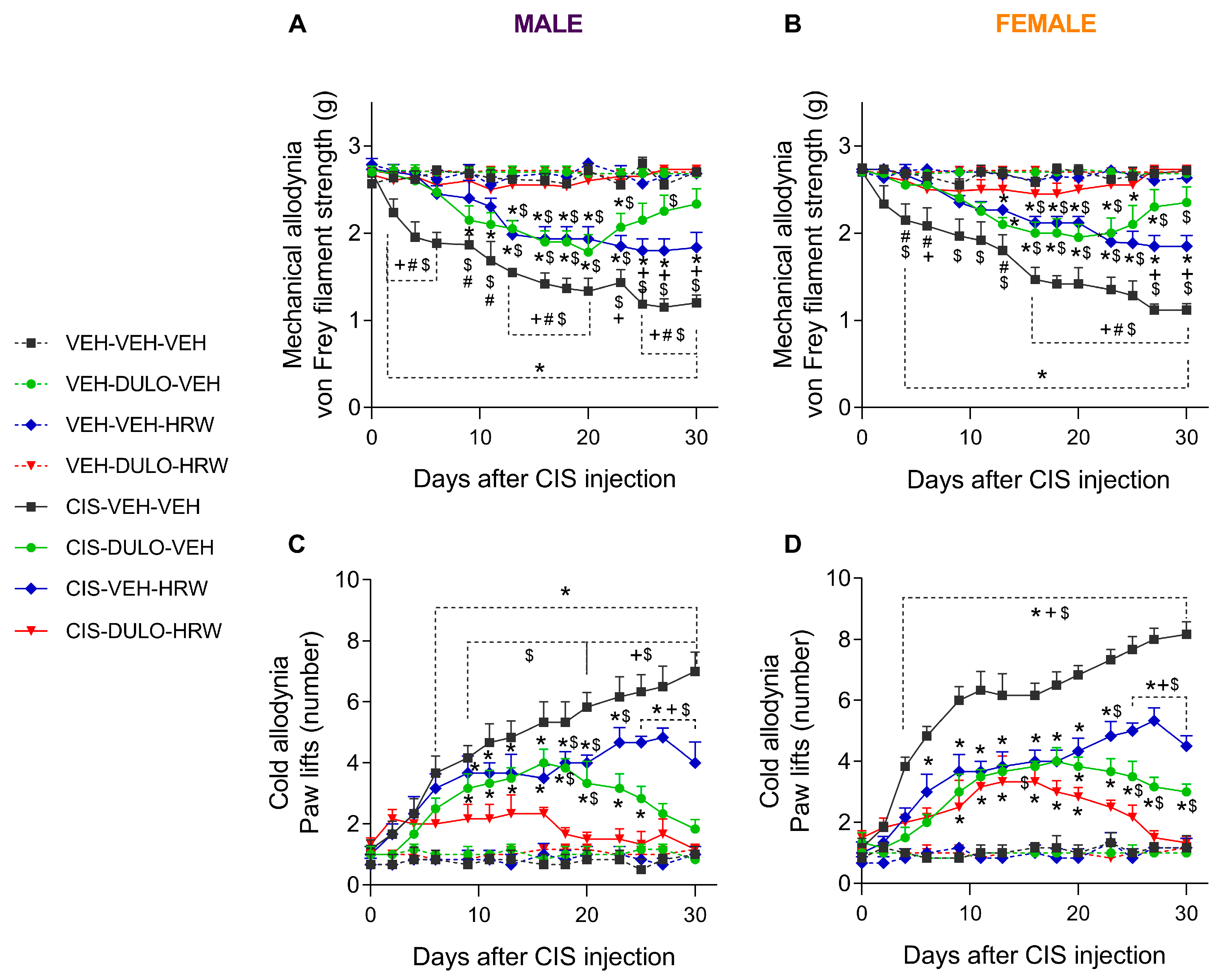
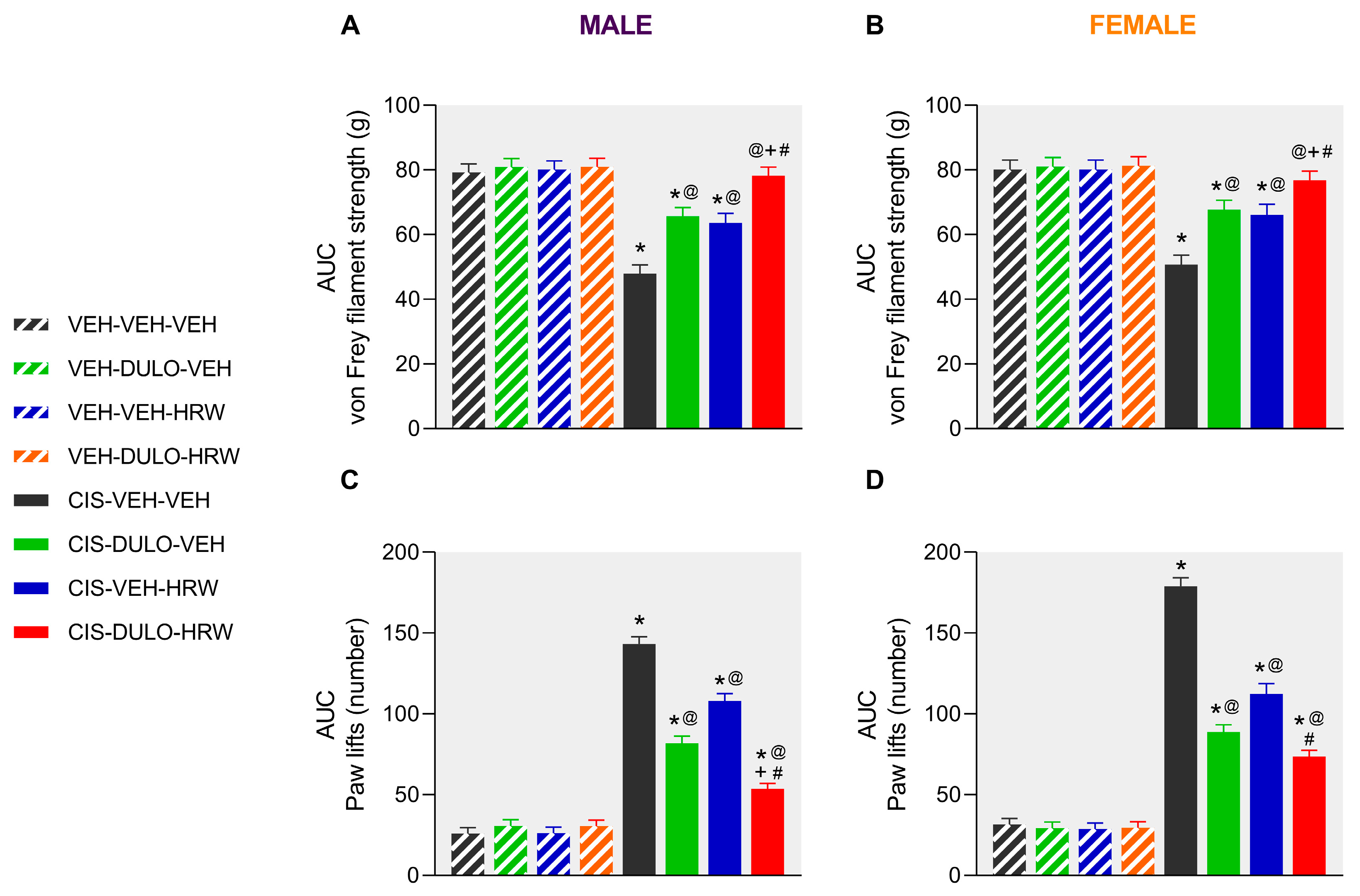
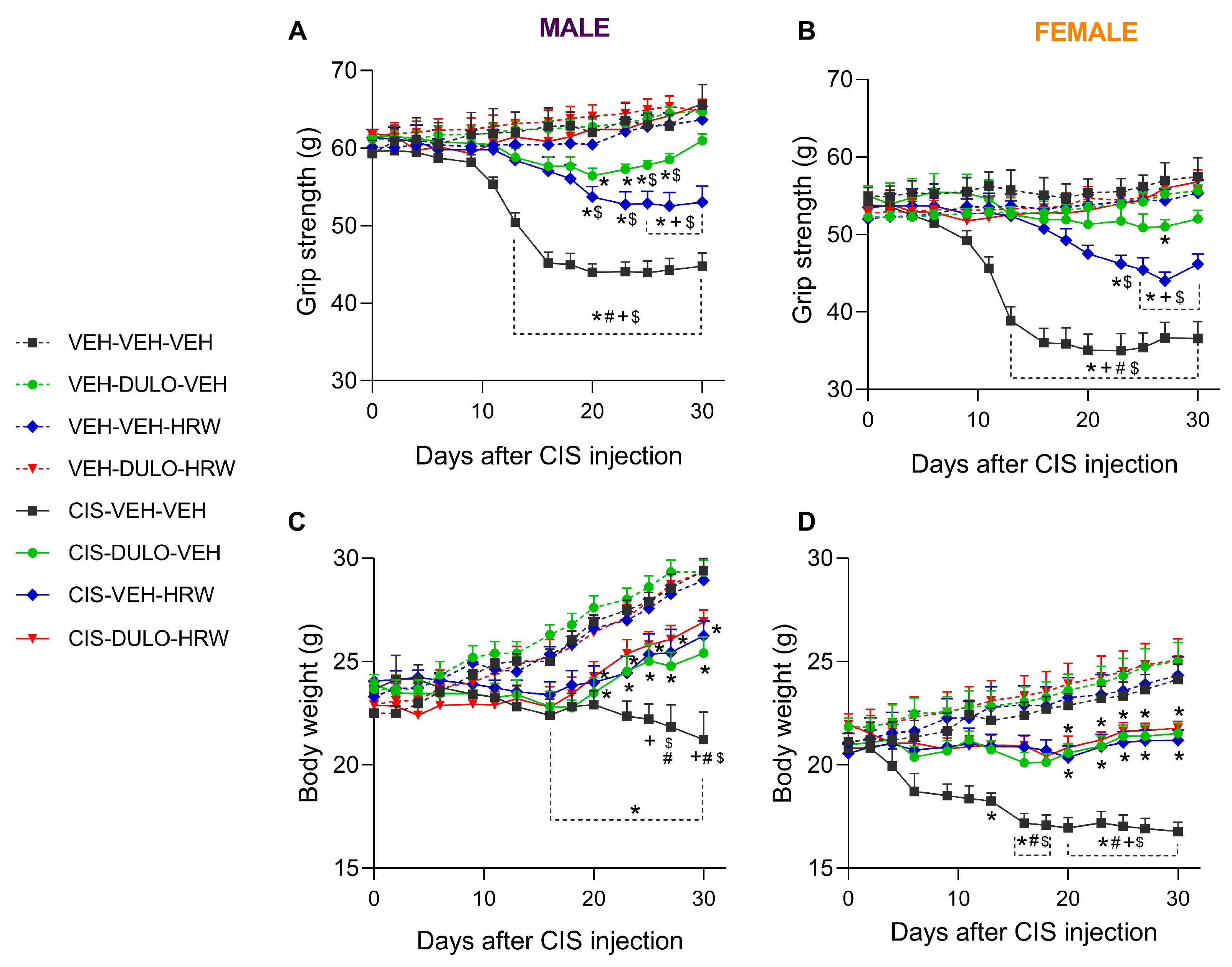
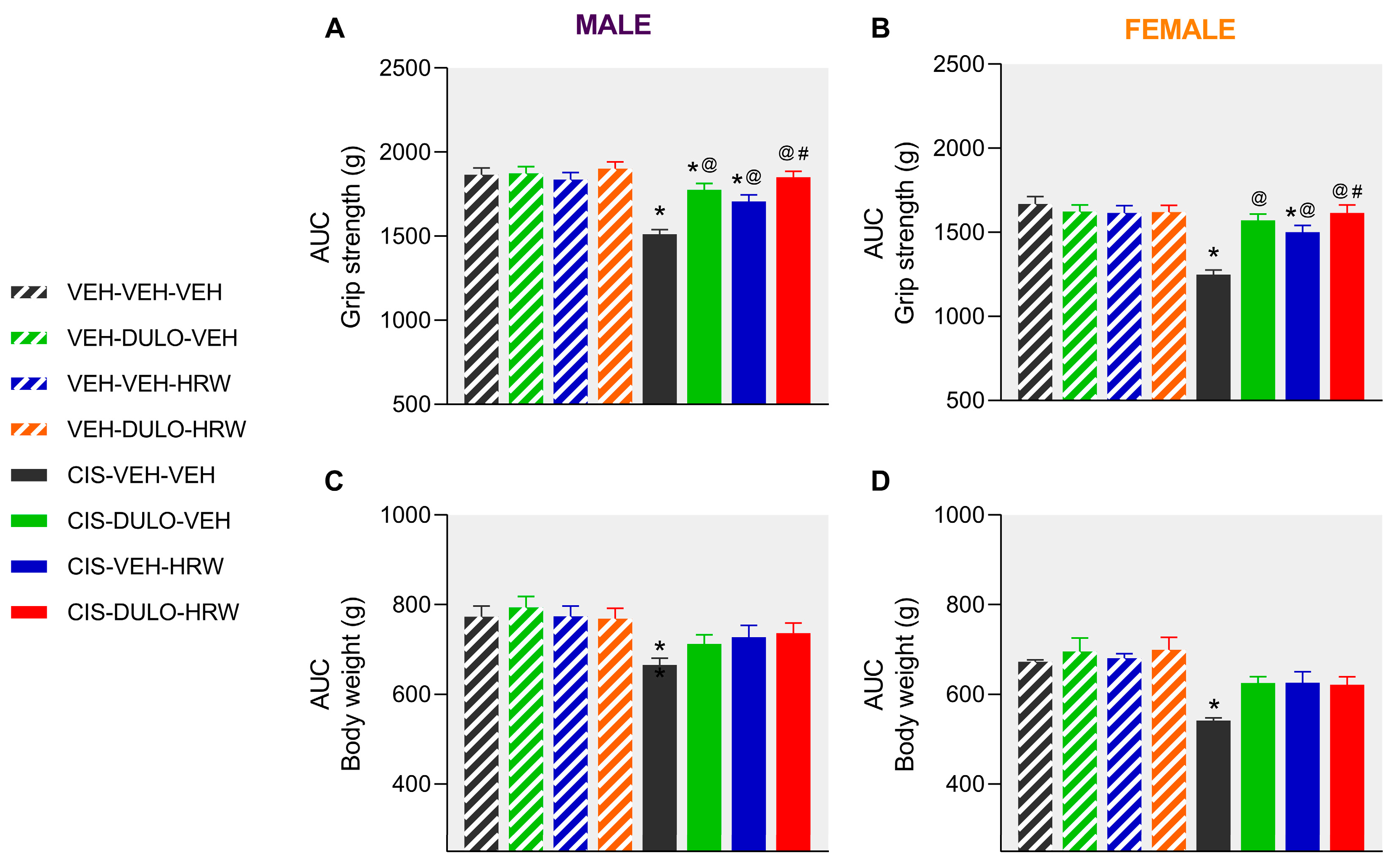
3.2. The Effects of DULO Combined with HRW on the Mechanical and Cold Allodynia and the Grip Strength and Weight Loss Instigated by CIS in Male and Female Mice
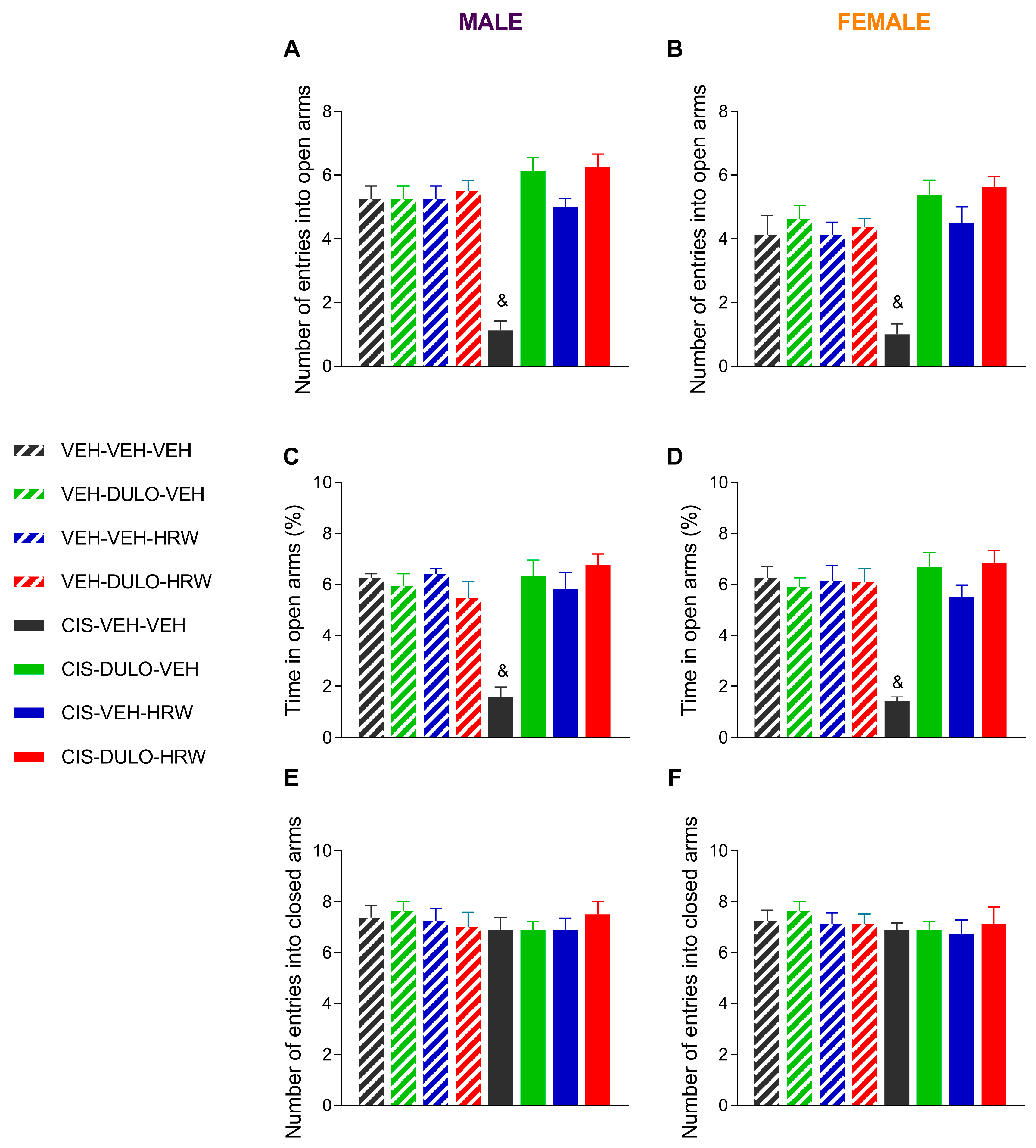
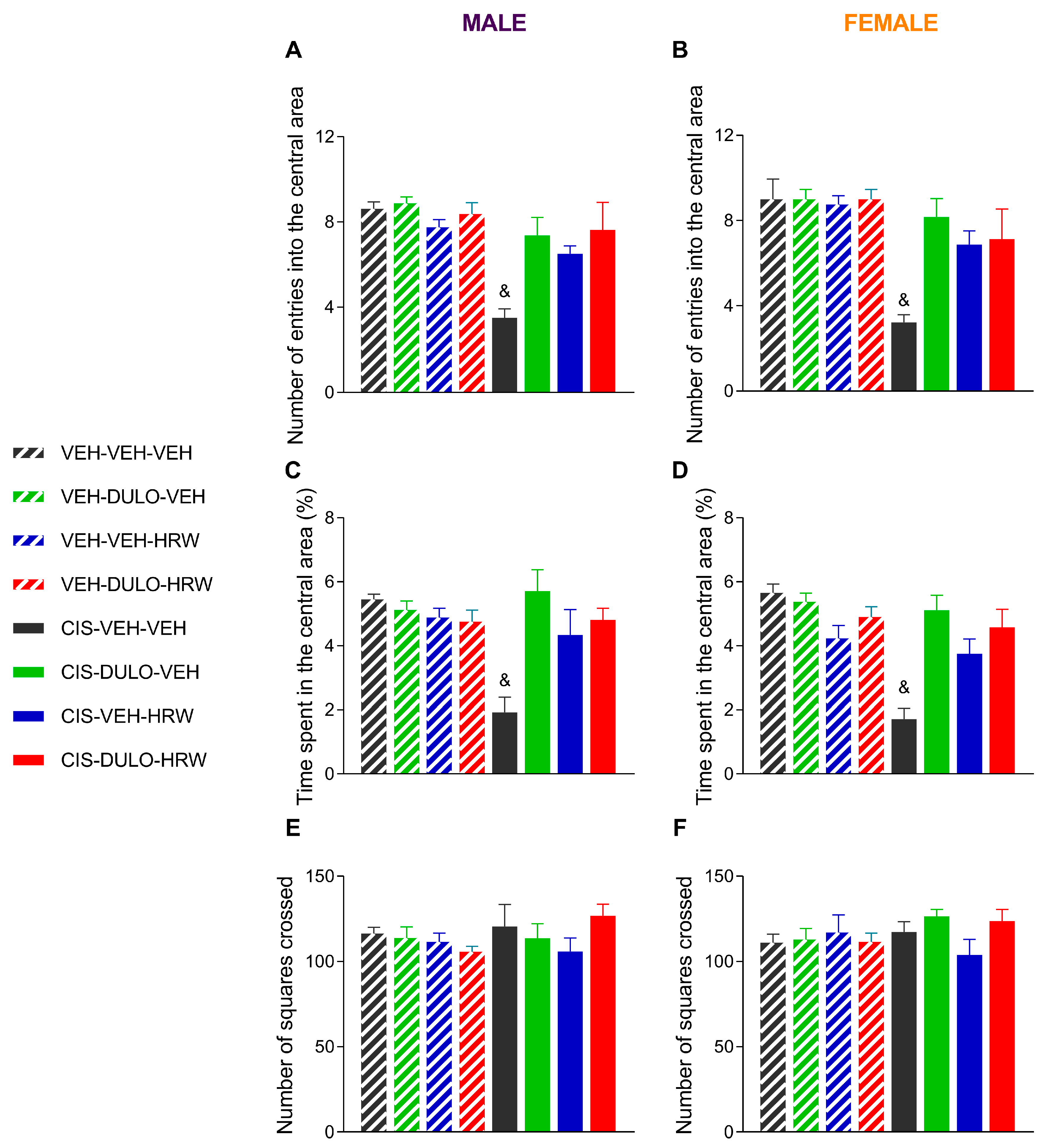
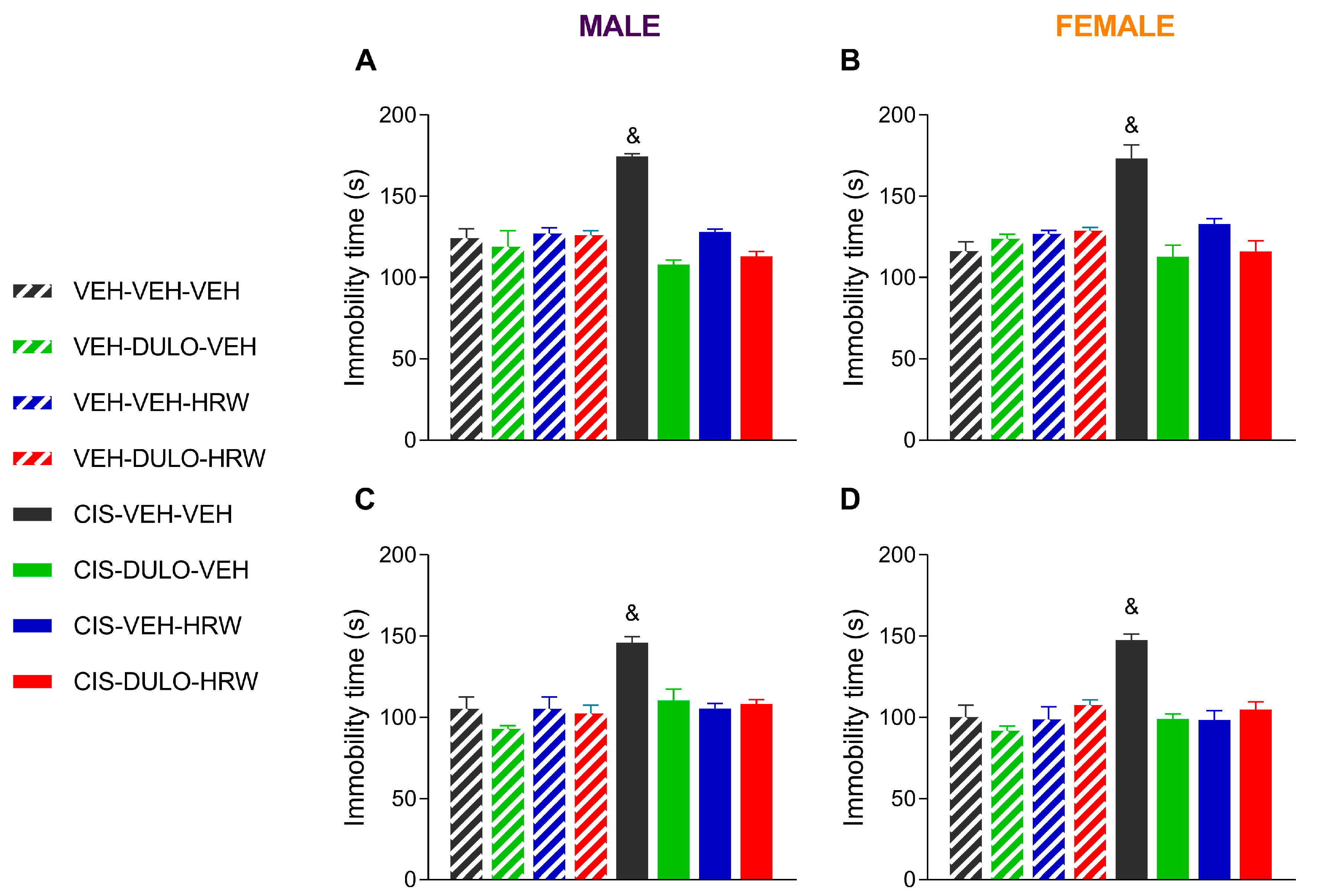
3.3. Treatment with DULO and HRW Alone and Combined Avoided the Development of Emotional Disorders Associated with CIS-Induced Chemotherapy
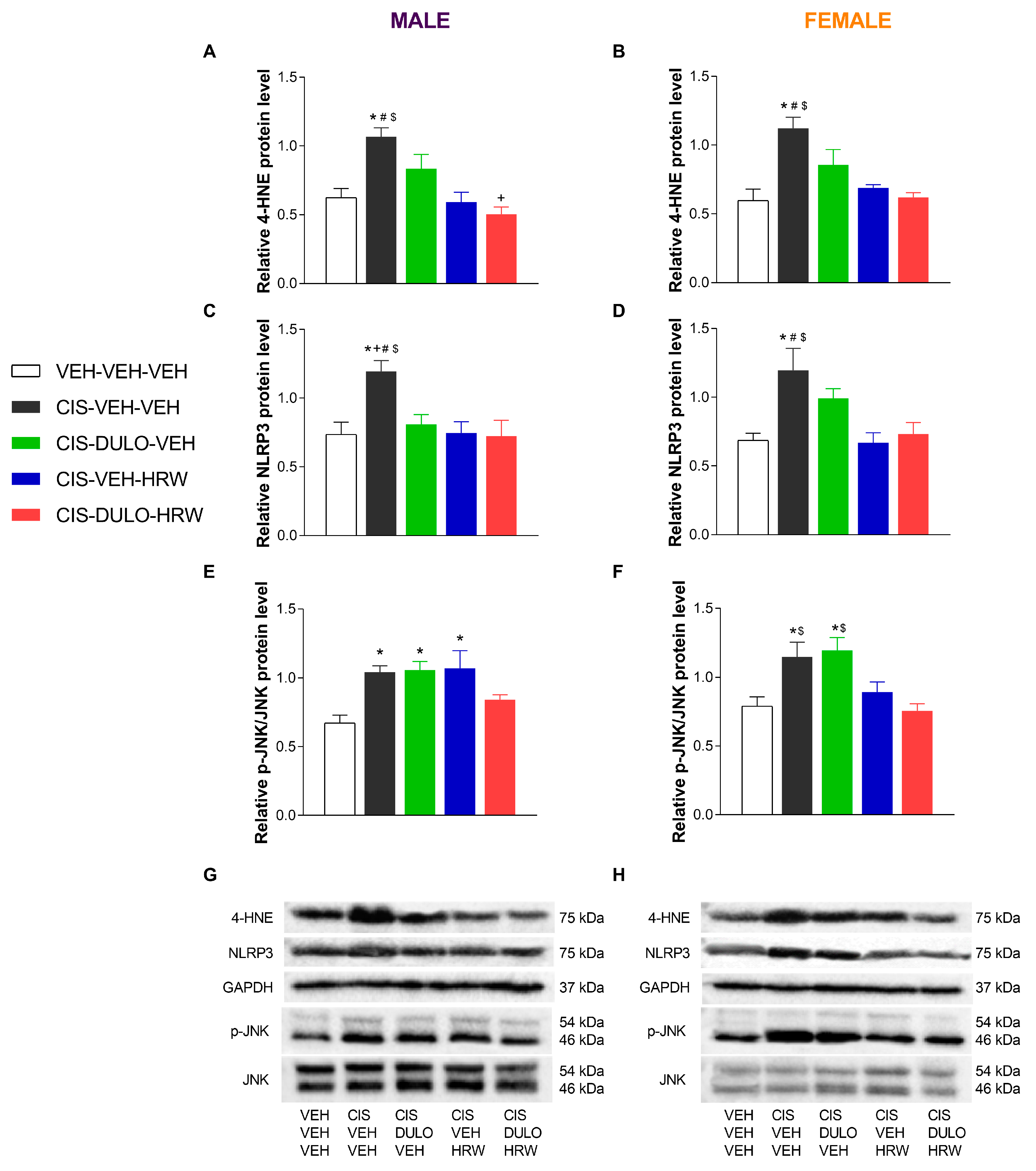
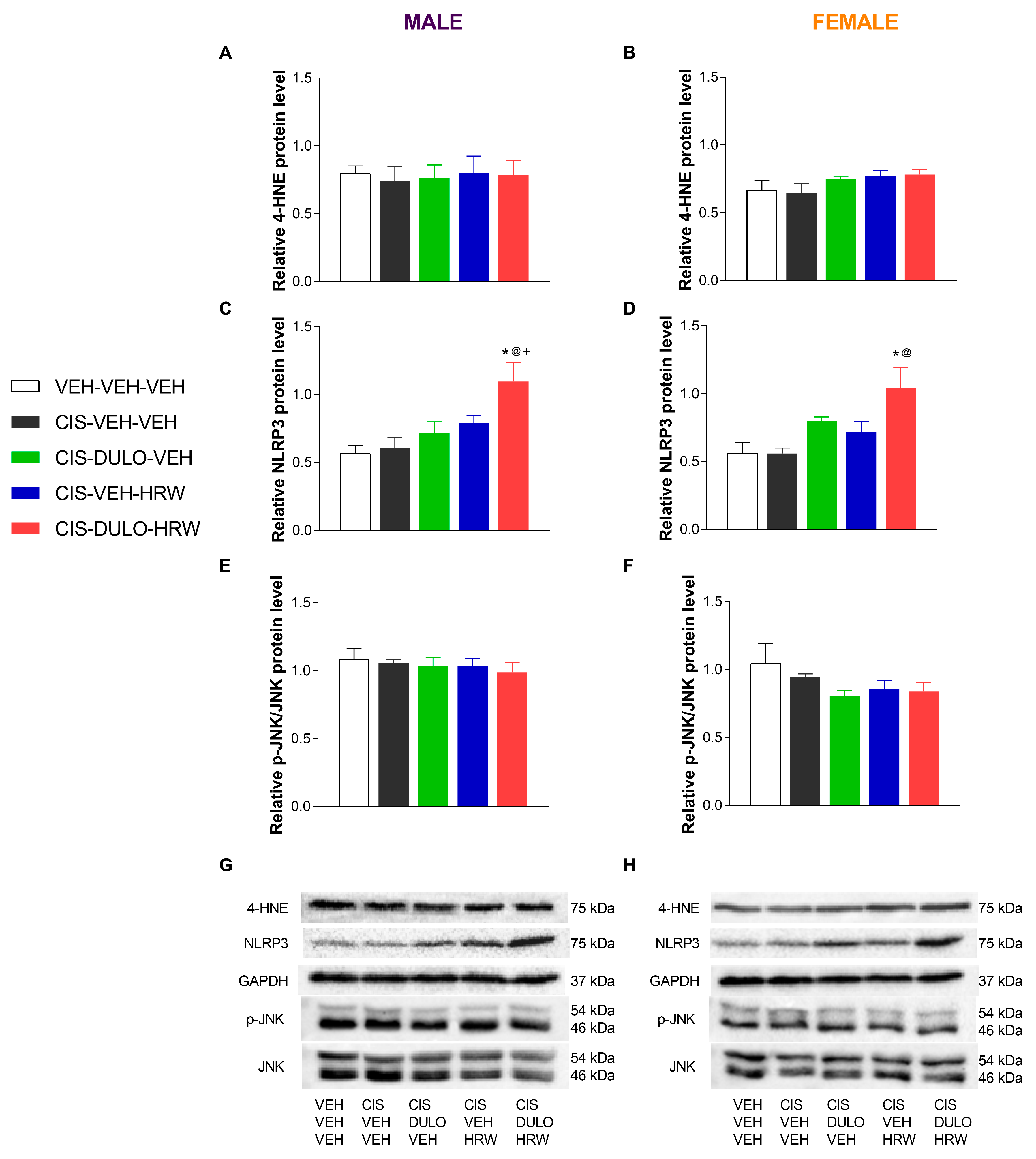
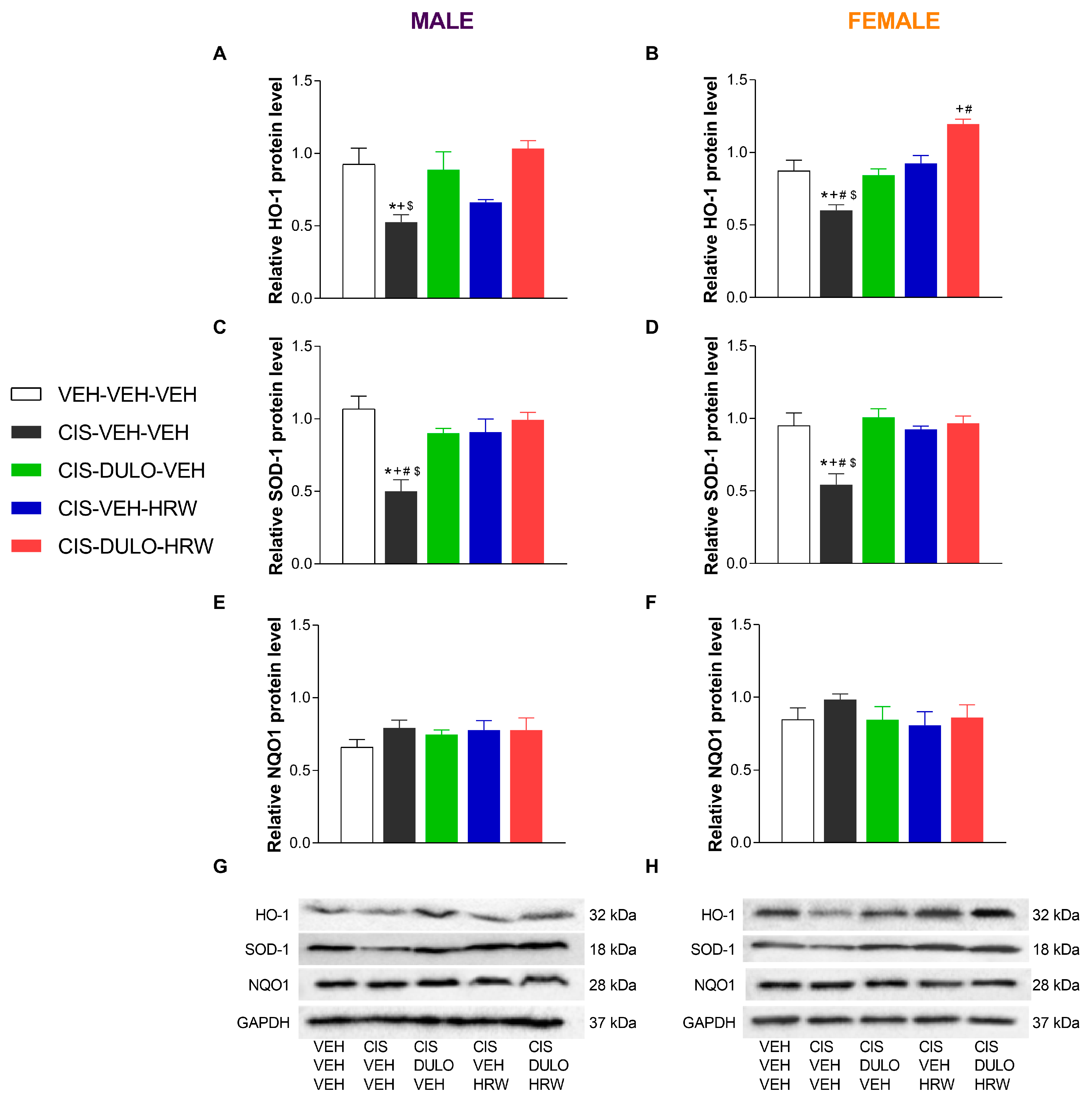
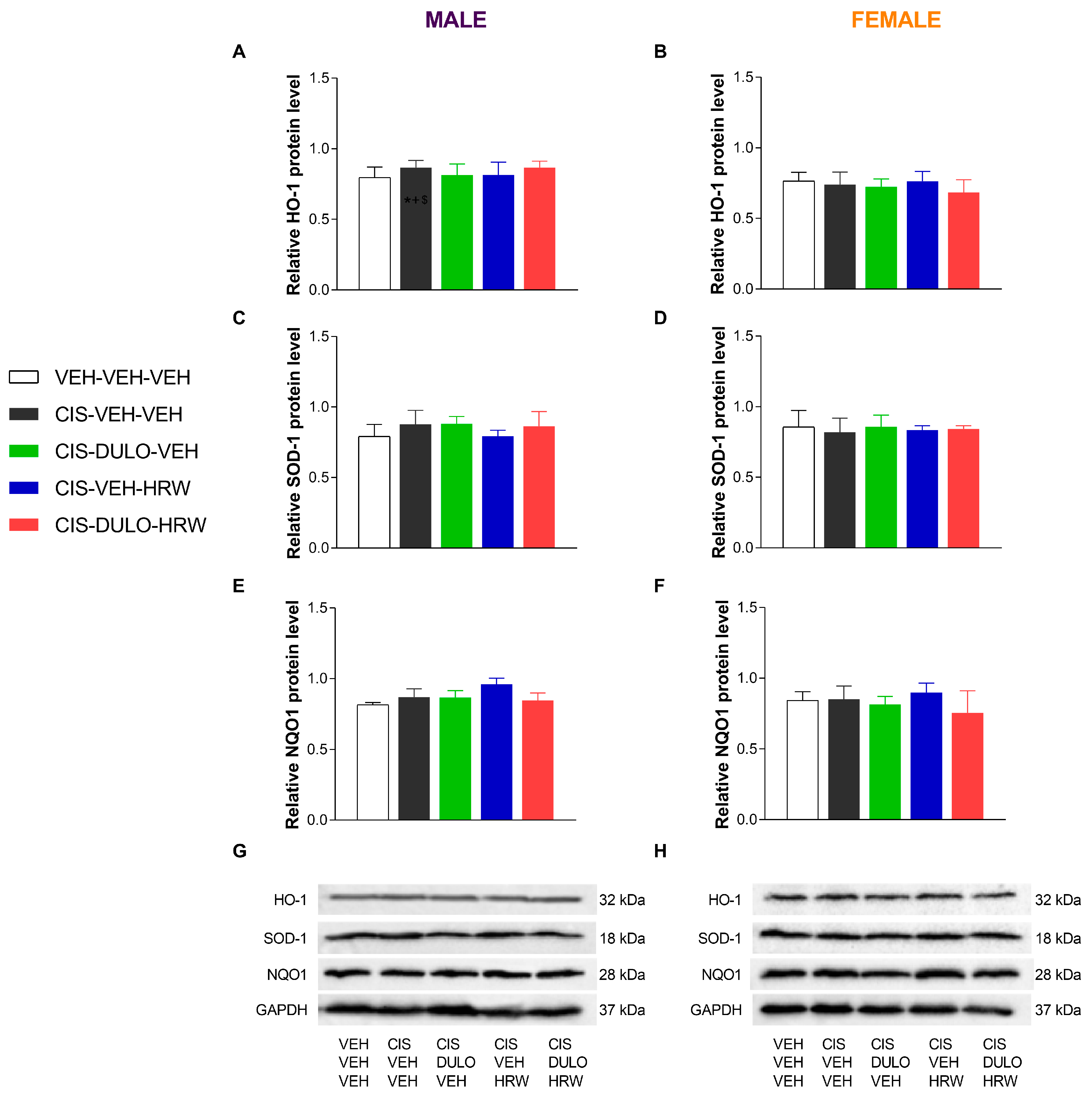
3.4. The Impact of Treatment with DULO and HRW Alone and Combined on the Oxidative Stress, Inflammatory Responses, and Plasticity Changes Triggered by CIS in the DRG and AMG of Male and Female Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, A.; Corradini, L.; Nicholson, J.R.; Smith, M.T. Assessment of the Anti-Allodynic and Anti-Hyperalgesic Efficacy of a Glycine Transporter 2 Inhibitor Relative to Pregabalin, Duloxetine and Indomethacin in a Rat Model of Cisplatin-Induced Peripheral Neuropathy. Biomolecules 2021, 11, 940. [Google Scholar] [CrossRef]
- Hung, H.W.; Liu, C.Y.; Chen, H.F.; Chang, C.C.; Chen, S.C. Impact of Chemotherapy-Induced Peripheral Neuropathy on Quality of Life in Patients with Advanced Lung Cancer Receiving Platinum-Based Chemotherapy. Int. J. Environ. Res. Public Health. 2021, 18, 5677. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar] [PubMed]
- Becker, G.; Atuati, S.F.; Oliveira, S.M. G Protein-Coupled Receptors and Ion Channels Involvement in Cisplatin-Induced Peripheral Neuropathy: A Review of Preclinical Studies. Cancers 2024, 16, 580. [Google Scholar] [CrossRef]
- Jesus Palma, A.C.; Antunes Júnior, C.R.; Barreto, E.S.R.; Alencar, V.B.; Souza, A.K.D.N.; Mathias, C.M.C.; Lins-Kusterer, L.E.F.; Azi, L.M.T.A.; Kraychete, D.C. Pharmacological Treatment of Chemotherapy-Induced Neuropathy: A Systematic Review of Randomized Clinical Trials. Pain Manag. Nurs. 2025, 26, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Aleid, A.M.; Alshehri, F.; Alasiri, N.; Alhomoud, F.; Alsaegh, S.; Alrasheed, M.; Aljaddua, S.; Alasiri, A.; Boukhari, A.; Alhussain, A.A.; et al. Efficacy of Duloxetine for Postspine Surgery Pain: A Systematic Review and Meta-Analysis. Brain Behav. 2025, 15, e70217. [Google Scholar] [CrossRef]
- Sałat, K.; Furgała-Wojas, A.; Sałat, R. The Microglial Activation Inhibitor Minocycline, Used Alone and in Combination with Duloxetine, Attenuates Pain Caused by Oxaliplatin in Mice. Molecules 2021, 26, 3577. [Google Scholar] [CrossRef]
- Katsuyama, S.; Aso, H.; Otowa, A.; Yagi, T.; Kishikawa, Y.; Komatsu, T.; Sakurada, T.; Nakamura, H. Antinociceptive Effects of the Serotonin and Noradrenaline Reuptake Inhibitors Milnacipran and Duloxetine on Vincristine-Induced Neuropathic Pain Model in Mice. ISRN Pain 2014, 2014, 915464. [Google Scholar] [CrossRef]
- Lobos, N.; Lux, S.; Zepeda, R.J.; Pelissier, T.; Marcos, J.L.; Bustos-Quevedo, G.; Hernández, A.; Constandil, L. Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice. Int. J. Mol. Sci. 2023, 24, 8359. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective Effects of Molecular Hydrogen: A Critical Review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef]
- Martínez-Serrat, M.; Martínez-Martel, I.; Coral-Pérez, S.; Bai, X.; Batallé, G.; Pol, O. Hydrogen-Rich Water as a Novel Therapeutic Strategy for the Affective Disorders Linked with Chronic Neuropathic Pain in Mice. Antioxidants 2022, 11, 1826. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Song, S.; Wan, Y.; Zhang, R.; Tai, M.; Liu, C. Effect of hydrogen-rich water on acute peritonitis of rat models. Int. Immunopharmacol. 2014, 21, 94–101. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J.A. New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharmacol. 2019, 97, 287–292. [Google Scholar] [CrossRef]
- Martínez-Martel, I.; Bai, X.; Batallé, G.; Pol, O. New Treatment for the Cognitive and Emotional Deficits Linked with Paclitaxel-Induced Peripheral Neuropathy in Mice. Antioxidants 2022, 11, 2387. [Google Scholar] [CrossRef]
- Chen, C.; Smith, M.T. The NLRP3 inflammasome: Role in the pathobiology of chronic pain. Inflammopharmacology 2023, 31, 1589–1603. [Google Scholar] [CrossRef]
- Gupta, P.; Makkar, T.K.; Goel, L.; Pahuja, M. Role of inflammation and oxidative stress in chemotherapy-induced neurotoxicity. Immunol. Res. 2022, 70, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Khasabov, S.G.; Olson, J.K.; Uhelski, M.L.; Kim, A.H.; Albi-no-Ramírez, A.M.; Wagner, C.L.; Seybold, V.S.; Simone, D.A. Pioglitazone, a PPARγ agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain 2019, 160, 688–701. [Google Scholar] [CrossRef]
- Meng, J.; Qiu, S.; Zhang, L.; You, M.; Xing, H.; Zhu, J. Berberine Alleviate Cisplatin-Induced Peripheral Neuropathy by Modulating Inflammation Signal via TRPV1. Front. Pharmacol. 2022, 12, 774795. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Ma, J.; Golonzhka, O.; Laumet, G.O.; Gutti, T.; van Duzer, J.H.; Mazitschek, R.; Jarpe, M.B.; Heijnen, C.J.; Kavelaars, A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 2017, 158, 1126–1137. [Google Scholar] [CrossRef]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci. Rep. 2014, 4, 6370. [Google Scholar] [CrossRef]
- Coral-Pérez, S.; Martínez-Martel, I.; Martínez-Serrat, M.; Batallé, G.; Bai, X.; Leite-Panissi, C.R.A.; Pol, O. Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants 2022, 11, 2153. [Google Scholar] [CrossRef]
- Martínez-Martel, I.; Pol, O. A Novel Therapy for Cisplatin-Induced Allodynia and Dysfunctional and Emotional Impairments in Male and Female Mice. Antioxidants 2023, 12, 2063. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Batallé, G.; Bai, X.; Pol, O. The Interaction between Carbon Monoxide and Hydrogen Sulfide during Chronic Joint Pain in Young Female Mice. Antioxidants 2022, 11, 1271. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Naji-Esfahani, H.; Vaseghi, G.; Safaeian, L.; Pilehvarian, A.A.; Abed, A.; Rafieian-Kopaei, M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. 2016, 50, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Junigan, J.M.; Trinh, R.; Kavelaars, A.; Heijnen, C.J.; Grace, P.M. HDAC6 Inhibition Reverses Cisplatin-Induced Mechanical Hypersensitivity via Tonic Delta Opioid Receptor Signaling. J. Neurosci. 2022, 42, 7862–7874. [Google Scholar] [CrossRef]
- Singh, A.K.; Mahalingam, R.; Squillace, S.; Jacobson, K.A.; Tosh, D.K.; Dharmaraj, S.; Farr, S.A.; Kavelaars, A.; Salvemini, D.; Heijnen, C.J. Targeting the A3 adenosine receptor to prevent and reverse chemotherapy-induced neurotoxicities in mice. Acta Neuropathol. Commun. 2022, 10, 11. [Google Scholar] [CrossRef]
- Nakamura, H.; Kawashiri, T.; Kobayashi, D.; Uchida, M.; Egashira, N.; Shimazoe, T. Analgesic Effects of Sokeikakketsuto on Chemotherapy-Induced Mechanical Allodynia and Cold Hyperalgesia in Rats. Biol. Pharm. Bull. 2021, 44, 271–274. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. American Society of Clinical Oncology Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int. J. Mol. Sci. 2023, 24, 1866. [Google Scholar] [CrossRef] [PubMed]
- Nastić, K.; Pecikoza, U.; Labudović-Borović, M.; Kotur-Stevuljević, J.; Micov, A.; Jovanović, A.; Tomić, M.; Stepanović-Petrović, R. The antidepressant drugs vortioxetine and duloxetine differentially and sex-dependently affect animal well-being, cognitive performance, cardiac redox status and histology in a model of osteoarthritis. Biomed. Pharmacother. 2023, 166, 115360. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J.; Yan, Y.; Shen, Z.; Sun, J.; Zheng, Y. Activating Striatal Parvalbumin Interneurons to Alleviate Chemotherapy-Induced Muscle Atrophy. J. Cachexia Sarcopenia Muscle 2025, 16, e13782. [Google Scholar] [CrossRef]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality burden of pre-treatment weight loss in patients with non-small-cell lung cancer: A systematic literature review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pais, A.; Ferreira, R.; Gil da Costa, R. Platinum-induced muscle wasting in cancer chemotherapy: Mechanisms and potential targets for therapeutic intervention. Life Sci. 2018, 208, 1–9. [Google Scholar] [CrossRef]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.H.; Hankerd, K.M.; Kim, H.K.; Chung, J.M.; La, J.H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15, 1744806919840098. [Google Scholar] [CrossRef]
- Chen, J.A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.P.; Garcia, J.M. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef]
- Saadati, H.; Noroozzadeh, S.; Esmaeili, H.; Amirshahrokhi, K.; Shadman, J.; Niapour, A. The Neuroprotective Effect of Mesna on Cisplatin-Induced Neurotoxicity: Behavioral, Electrophysiological, and Molecular Studies. Neurotox. Res. 2021, 39, 826–840. [Google Scholar] [CrossRef]
- Chen, J.M.; Yang, T.T.; Cheng, T.S.; Hsiao, T.F.; Chang, P.M.; Leu, J.Y.; Wang, F.S.; Hsu, S.L.; Huang, C.F.; Lai, J.M. Modified Sijunzi decoction can alleviate cisplatin-induced toxicity and prolong the survival time of cachectic mice by recovering muscle atrophy. J. Ethnopharmacol. 2019, 233, 47–55. [Google Scholar] [CrossRef]
- Huang, K.C.; Chiang, Y.F.; Huang, T.C.; Chen, H.Y.; Lin, P.H.; Ali, M.; Hsia, S.M. Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo. J. Cachexia Sarcopenia Muscle 2023, 14, 182–197. [Google Scholar] [CrossRef]
- Fardell, J.E.; Irwin, C.M.; Vardy, J.L.; Bell, M.L. Anxiety, depression, and concentration in cancer survivors: National Health and Nutrition Examination Survey results. Support. Care Cancer 2023, 31, 272. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Li, X. Adverse reactions of antidepressant drugs and their application in patients with cardiovascular diseases. Med. Sci. 2020, 45, 1228–1233. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Saad, M.A.; Abdelsalam, R.M. Neuroprotective effect of nebivolol against cisplatin-associated depressive-like behavior in rats. J. Neurochem. 2017, 141, 449–460. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.; Chen, J.; Xue, J.; Zhang, X.; Zhao, M.; Jia, X.; Wang, Y.; Qin, S. Protective Effect of Molecular Hydrogen Following Different Routes of Administration on D-Galactose-Induced Aging Mice. J. Inflamm Res. 2021, 14, 5541–5550. [Google Scholar] [CrossRef]
- Rose, O.; Croonenberg, T.; Clemens, S.; Hinteregger, T.; Eppacher, S.; Huber-Cantonati, P.; Garcia-Miralles, M.; Liuni, R.; Dossena, S. Cisplatin-Induced Hearing Loss, Oxidative Stress, and Antioxidants as a Therapeutic Strategy-A State-of-the-Art Review. Antioxidants 2024, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Al-Aqil, F.; Alqahtani, F.; Alanazi, M.; Nadeem, A.; Ahmad, S.F.; Lapresa, R.; Alharbi, M.; Alshammari, A.; Alotaibi, M.; et al. Alleviation of cisplatin-induced neuropathic pain, neuronal apoptosis, and systemic inflammation in mice by rapamycin. Front. Aging Neurosci. 2022, 14, 891593. [Google Scholar] [CrossRef]
- Vukovic, R.; Selakovic, D.; Stankovic, J.S.K.; Kumburovic, I.; Jovicic, N.; Rosic, G. Alteration of Oxidative stress and apoptotic markers alterations in the rat prefrontal cortex influence behavioral response induced by cisplatin and N-acetylcysteine in the tail suspension test. J. Integr. Neurosci. 2021, 20, 711–718. [Google Scholar] [CrossRef]
- Kumburovic, I.; Selakovic, D.; Juric, T.; Jovicic, N.; Mihailovic, V.; Stankovic, J.K.; Sreckovic, N.; Kumburovic, D.; Jakovljevic, V.; Rosic, G. Antioxidant Effects of Satureja hortensis L. Attenuate the Anxiogenic Effect of Cisplatin in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 8307196. [Google Scholar] [CrossRef]
- Martínez-Martel, I.; Bai, X.; Kordikowski, R.; Leite-Panissi, C.R.A.; Pol, O. The Combination of Molecular Hydrogen and Heme Oxygenase 1 Effectively Inhibits Neuropathy Caused by Paclitaxel in Mice. Antioxidants 2024, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Holland, R.A.; Zheng, H.; Rinaman, L.; Grill, H.J.; De Jonghe, B.C. Excitatory Hindbrain-Forebrain Communication Is Required for Cisplatin-Induced Anorexia and Weight Loss. J. Neurosci. 2017, 37, 362–370. [Google Scholar] [CrossRef]
- Elbaset, M.A.; Afifi, S.M.; Esatbeyoglu, T.; Abdelrahman, S.S.; Saleh, D.O. Neuroprotective Effects of Trimetazidine against Cisplatin-Induced Peripheral Neuropathy: Involvement of AMPK-Mediated PI3K/mTOR, Nrf2, and NF-κB Signaling Axes. Oxid. Med. Cell. Longev. 2024, 2024, 6612009. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Albin, B.; Qubbaj, K.; Adhikari, P.; Yang, I.H. Phytic Acid Maintains Peripheral Neuron Integrity and Enhances Survivability against Platinum-Induced Degeneration via Reducing Reactive Oxygen Species and Enhancing Mitochondrial Membrane Potential. ACS Chem. Neurosci. 2024, 15, 1157–1168. [Google Scholar] [CrossRef]
- Chen, R.; Yin, C.; Fang, J.; Liu, B. The NLRP3 inflammasome: An emerging therapeutic target for chronic pain. J. Neuroinflammation 2021, 18, 84. [Google Scholar] [CrossRef]
- Starobova, H.; Nadar, E.I.; Vetter, I. The NLRP3 Inflammasome: Role and Therapeutic Potential in Pain Treatment. Front Physiol. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Starobova, H.; Monteleone, M.; Adolphe, C.; Batoon, L.; Sandrock, C.J.; Tay, B.; Deuis, J.R.; Smith, A.V.; Mueller, A.; Nadar, E.I.; et al. Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release. J. Exp. Med. 2021, 218, e20201452. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, L.; Luo, H.; Li, X.; Ruan, Y.; Fan, R.; Si, Z.; Chen, Y.; Li, L.; Liu, Y. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022, 12, 1057. [Google Scholar] [CrossRef]
- Hua, T.; Wang, H.; Fan, X.; An, N.; Li, J.; Song, H.; Kong, E.; Li, Y.; Yuan, H. BRD4 Inhibition Attenuates Inflammatory Pain by Ameliorating NLRP3 Inflammasome-Induced Pyroptosis. Front Immunol. 2022, 13, 837977. [Google Scholar] [CrossRef]
- Mokhtari, T.; Uludag, K. Role of NLRP3 Inflammasome in Post-Spinal-Cord-Injury Anxiety and Depression: Molecular Mechanisms and Therapeutic Implications. ACS Chem. Neurosci. 2024, 15, 56–70. [Google Scholar] [CrossRef]
- Nakatani, Y.; Yaguchi, M.; Ogino, K.; Noguchi, R.; Yamamoto, N.; Amano, T. Duloxetine ameliorates lipopolysaccharide-induced microglial activation by suppressing iNOS expression in BV-2 microglial cells. Psychopharmacology 2022, 239, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, W.; Kang, X.; Lin, Z.; Pan, J.; Cheng, S.; Zhang, J. Hydrogen (H2) Alleviates Osteoarthritis by Inhibiting Apoptosis and Inflammation via the JNK Signaling Pathway. J. Inflamm. Res. 2021, 14, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ruan, S.; Jia, J.; Li, Q.; Jia, R.; Wan, L.; Yang, X.; Teng, P.; Peng, Q.; Shi, Y.D.; et al. Hydrogen attenuates postoperative pain through Trx1/ASK1/MMP9 signaling pathway. J. Neuroinflamm. 2023, 20, 22. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Molecular Weight (kDa) | Dilution | Commercial Supply |
|---|---|---|---|
| 4-HNE | 75 | 1:100 | Abcam, Cambridge, UK |
| NLRP3 | 75 | 1:200 | Adipogen Life Sciences, Epalinges, Switzerland |
| p-JNK | 54/46 | 1:250 | Cell Signaling Technology, Danvers, MA, USA |
| JNK | 54/46 | 1:250 | Cell Signaling Technology, Danvers, MA, USA |
| HO-1 | 32 | 1:200 | Abclonal Technology, Woburn, MA, USA |
| SOD-1 | 18 | 1:150 | Novus Biologic, Littleton, CO, USA |
| NQO1 | 28 | 1:250 | Merck, Billerica, MA, USA |
| GAPDH | 37 | 1:5000 | Merck, Billerica, MA, USA |
| Mechanical Allodynia | Thermal Allodynia | Grip Strength | Body Weight | |
|---|---|---|---|---|
| Injection | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.009 |
| Sex | p < 0.328 | p < 0.048 | p < 0.028 | p < 0.002 |
| Time | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Injection × Sex | p < 0.729 | p < 0.080 | p < 0.321 | p < 0.767 |
| Injection × Time | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Sex × Time | p < 0.988 | p < 0.945 | p < 0.126 | p < 0.001 |
| Injection × Sex × Time | p < 0.968 | p < 0.864 | p < 0.761 | p < 0.001 |
| Mechanical Allodynia | Thermal Allodynia | Grip Strength | Body Weight | |
|---|---|---|---|---|
| Treatment | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Sex | p < 0.122 | p < 0.015 | p < 0.002 | p < 0.001 |
| Time | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Treatment × Sex | p < 0.813 | p < 0.016 | p < 0.848 | p < 0.353 |
| Treatment × Time | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Sex × Time | p < 0.984 | p < 0.410 | p < 0.330 | p < 0.001 |
| Treatment × Sex × Time | p < 0.999 | p < 0.999 | p < 0.921 | p < 0.001 |
| Number of Entries into Open Arms | Time in Open Arms | Number of Entries into Closed Arms | |
|---|---|---|---|
| Injection | p < 0.032 | p < 0.001 | p < 0.158 |
| Sex | p < 0.523 | p < 0.884 | p < 0.736 |
| Treatment | p < 0.001 | p < 0.001 | p < 0.879 |
| Injection × Sex | p < 0.216 | p < 0.830 | p < 0.840 |
| Injection × Treatment | p < 0.001 | p < 0.001 | p < 0.484 |
| Sex × Treatment | p < 0.986 | p < 0.781 | p < 0.997 |
| Injection × Sex × Treatment | p < 0.867 | p < 0.905 | p < 0.965 |
| Number Entries into Central Area | Time in Central Area | Number Squares Crossed | |
|---|---|---|---|
| Injection | p < 0.001 | p < 0.001 | p < 0.188 |
| Sex | p < 0.384 | p < 0.338 | p < 0.740 |
| Treatment | p < 0.001 | p < 0.001 | p < 0.397 |
| Injection × Sex | p < 0.548 | p < 0.368 | p < 0.982 |
| Injection × Treatment | p < 0.001 | p < 0.001 | p < 0.182 |
| Sex × Treatment | p < 0.902 | p < 0.748 | p < 0.796 |
| Injection × Sex × Treatment | p < 0.846 | p < 0.910 | p < 0.671 |
| Immobility Time TST | Immobility Time FST | |
|---|---|---|
| Injection | p < 0.001 | p < 0.001 |
| Sex | p < 0.581 | p < 0.172 |
| Treatment | p < 0.001 | p < 0.001 |
| Injection × Sex | p < 0.552 | p < 0.522 |
| Injection × Treatment | p < 0.001 | p < 0.001 |
| Sex × Treatment | p < 0.549 | p < 0.668 |
| Injection × Sex × Treatment | p < 0.947 | p < 0.624 |
| DRG | AMG | |||||
|---|---|---|---|---|---|---|
| 4-HNE | NLRP3 | p-JNK | 4-HNE | NLRP3 | p-JNK | |
| Sex | p < 0.365 | p < 0.757 | p < 0.519 | p < 0.468 | p < 0.759 | p < 0.062 |
| Treatment | p < 0.004 | p < 0.039 | p < 0.002 | p < 0.677 | p < 0.001 | p < 0.147 |
| Sex × Treatment | p < 0.924 | p < 0.675 | p < 0.456 | p < 0.834 | p < 0.805 | p < 0.614 |
| DRG | AMG | |||||
|---|---|---|---|---|---|---|
| HO-1 | SOD-1 | NQO1 | HO-1 | SOD-1 | NQO1 | |
| Sex | p < 0.088 | p < 0.827 | p < 0.909 | p < 0.054 | p < 0.995 | p < 0.359 |
| Treatment | p < 0.001 | p < 0.048 | p < 0.118 | p < 0.900 | p < 0.907 | p < 0.424 |
| Sex × Treatment | p < 0.227 | p < 0.856 | p < 0.758 | p < 0.892 | p < 0.975 | p < 0.895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Martel, I.; Negrini-Ferrari, S.E.; Pol, O. Preventing Cisplatin-Induced Neuropathy and Related Emotional Disorders with the Coadministration of Duloxetine and Hydrogen-Rich Water in Male and Female Mice. Antioxidants 2025, 14, 1004. https://doi.org/10.3390/antiox14081004
Martínez-Martel I, Negrini-Ferrari SE, Pol O. Preventing Cisplatin-Induced Neuropathy and Related Emotional Disorders with the Coadministration of Duloxetine and Hydrogen-Rich Water in Male and Female Mice. Antioxidants. 2025; 14(8):1004. https://doi.org/10.3390/antiox14081004
Chicago/Turabian StyleMartínez-Martel, Ignacio, Sylmara Esther Negrini-Ferrari, and Olga Pol. 2025. "Preventing Cisplatin-Induced Neuropathy and Related Emotional Disorders with the Coadministration of Duloxetine and Hydrogen-Rich Water in Male and Female Mice" Antioxidants 14, no. 8: 1004. https://doi.org/10.3390/antiox14081004
APA StyleMartínez-Martel, I., Negrini-Ferrari, S. E., & Pol, O. (2025). Preventing Cisplatin-Induced Neuropathy and Related Emotional Disorders with the Coadministration of Duloxetine and Hydrogen-Rich Water in Male and Female Mice. Antioxidants, 14(8), 1004. https://doi.org/10.3390/antiox14081004







