Galangin Regulates Oxidative Stress Levels in Porcine Embryos Through Interaction with the Neh1 Domain of Nrf2
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and In Vitro Maturation (IVM)
2.2. Parthenogenetic Activation and IVC
2.3. Treatment with GAL
2.4. Embryo Microinjection
2.5. Measurement of Intracellular ROS and Glutathione GSH Levels
2.6. Measurement of the Mitochondrial Membrane Potential
2.7. Cell Proliferation and Apoptosis Assays
2.8. Counting the Number of Blastocyst Cells
2.9. Quantitative Real-Time PCR (qRT–PCR)
2.10. Identification of GAL Target Genes and Antioxidant-Related Targets
2.11. GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
2.12. Construction of Protein–Protein Interaction (PPI) Networks
2.13. Molecular Docking Analysis
2.14. Cellular Thermal Shift Assay (CETSA)
2.15. Drug Affinity Responsive Target Stability (DARTS) Assay
2.16. Expression of Recombinant Nrf2
2.17. Western Blotting
2.18. Immunofluorescence
2.19. Statistical Analysis
3. Results
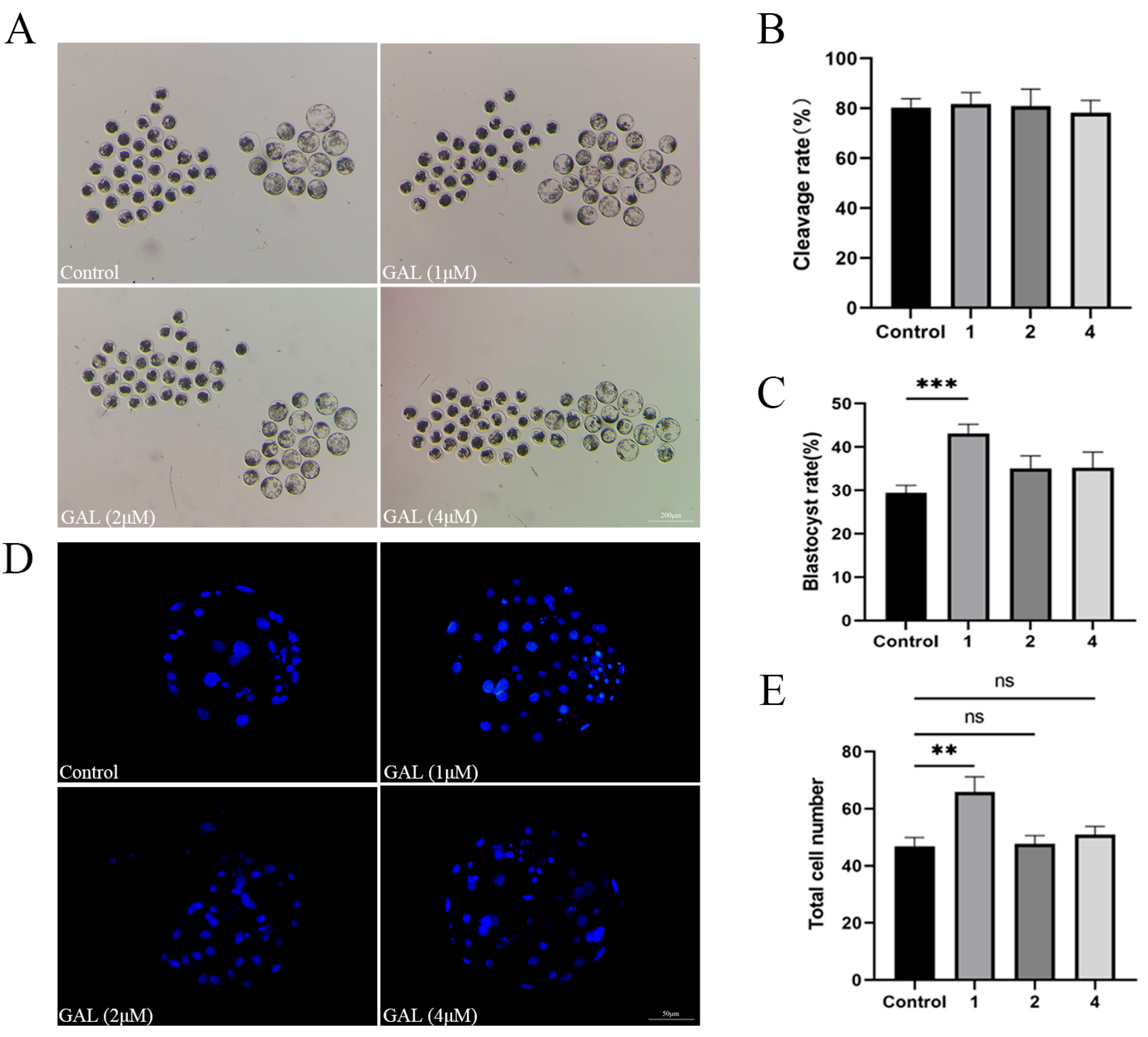
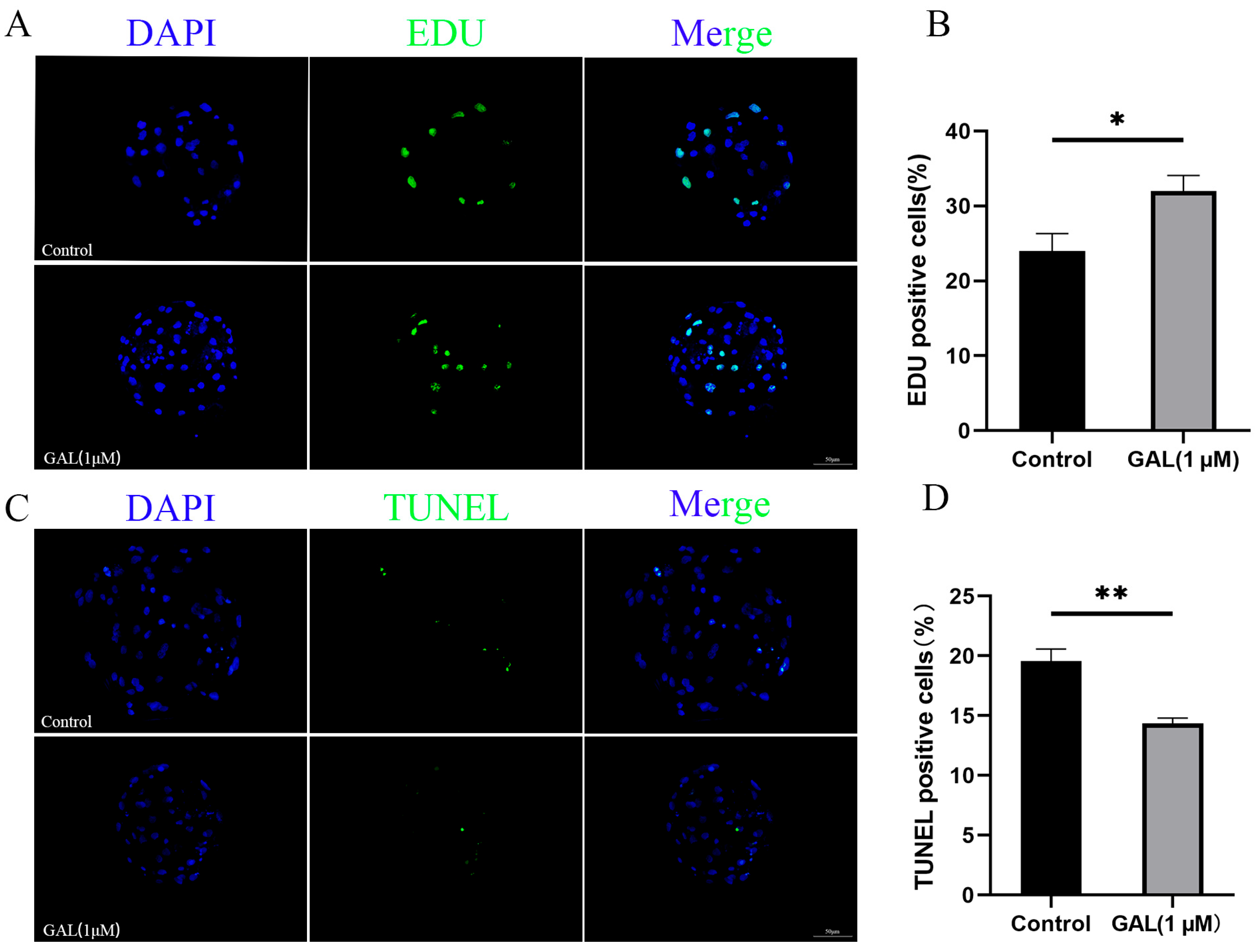
3.1. GAL Improves the Development of Porcine Parthenogenetic Embryos
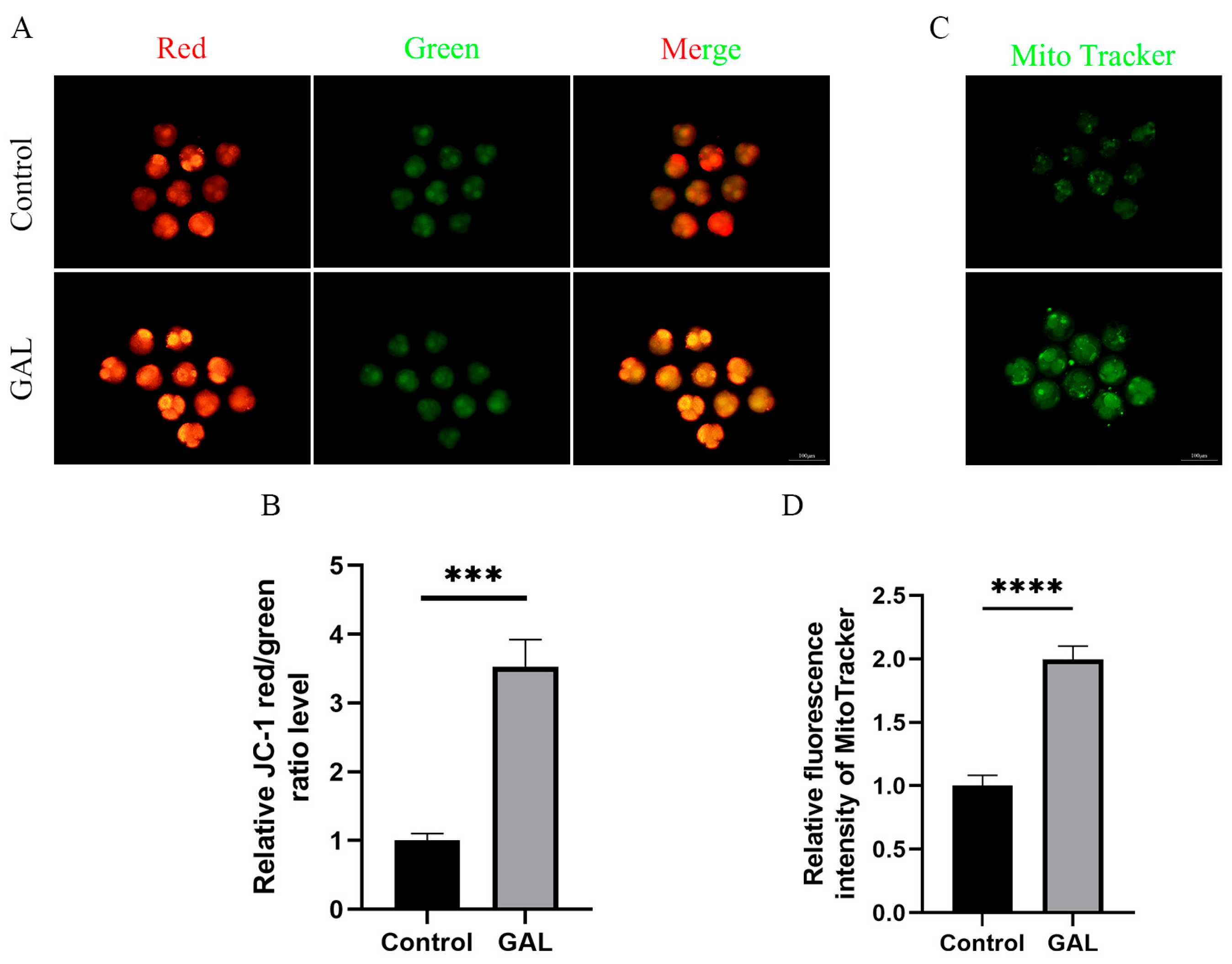
3.2. GAL Optimizes Mitochondrial Function
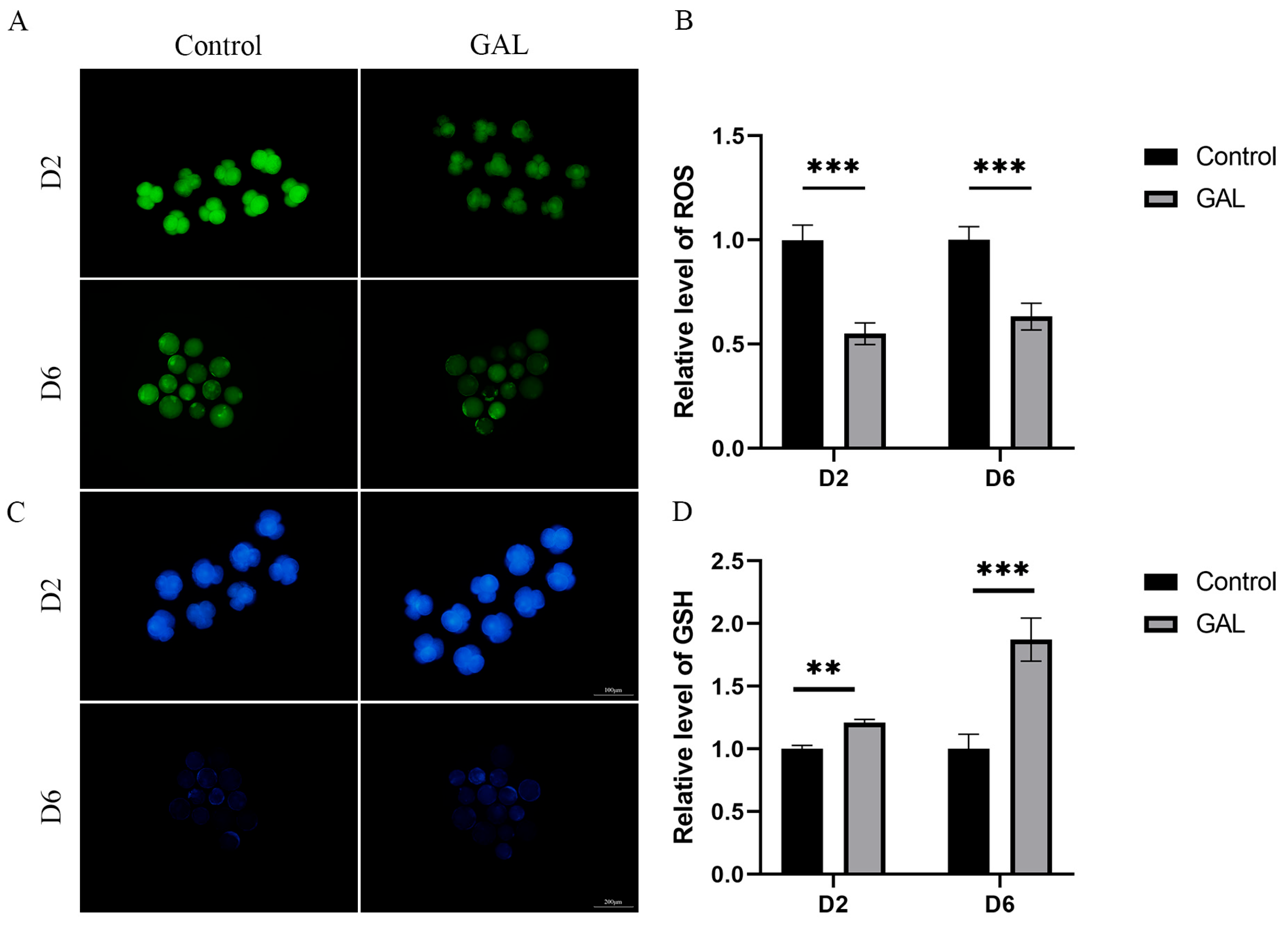
3.3. GAL Increases the Antioxidant Capacity of Early Porcine Embryos
3.4. Predicting Potential Targets Through Which GAL Exerts Antioxidant Effects
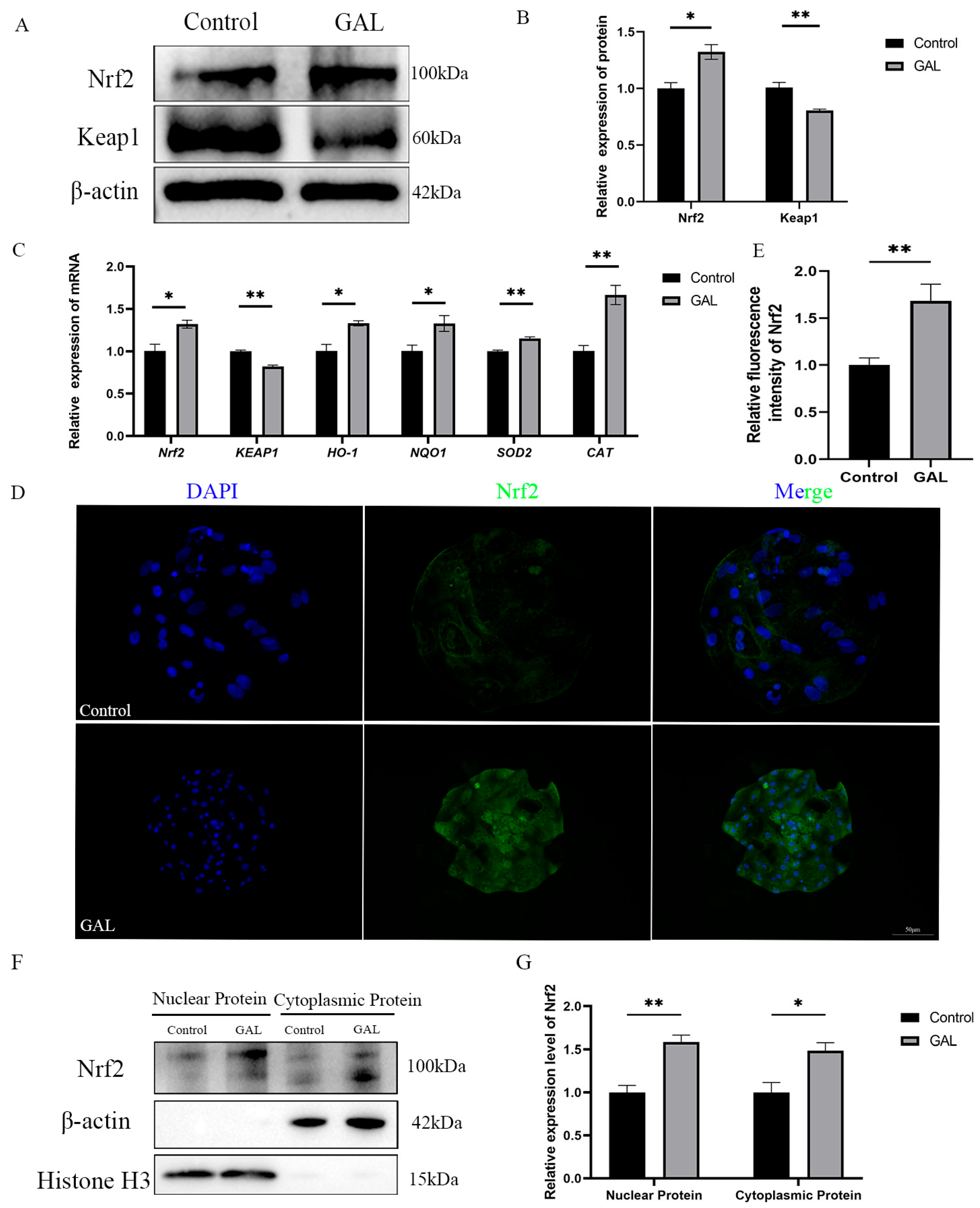
3.5. GAL Exerts Antioxidant Effects Through the Nrf2/Keap1 Signaling Pathway
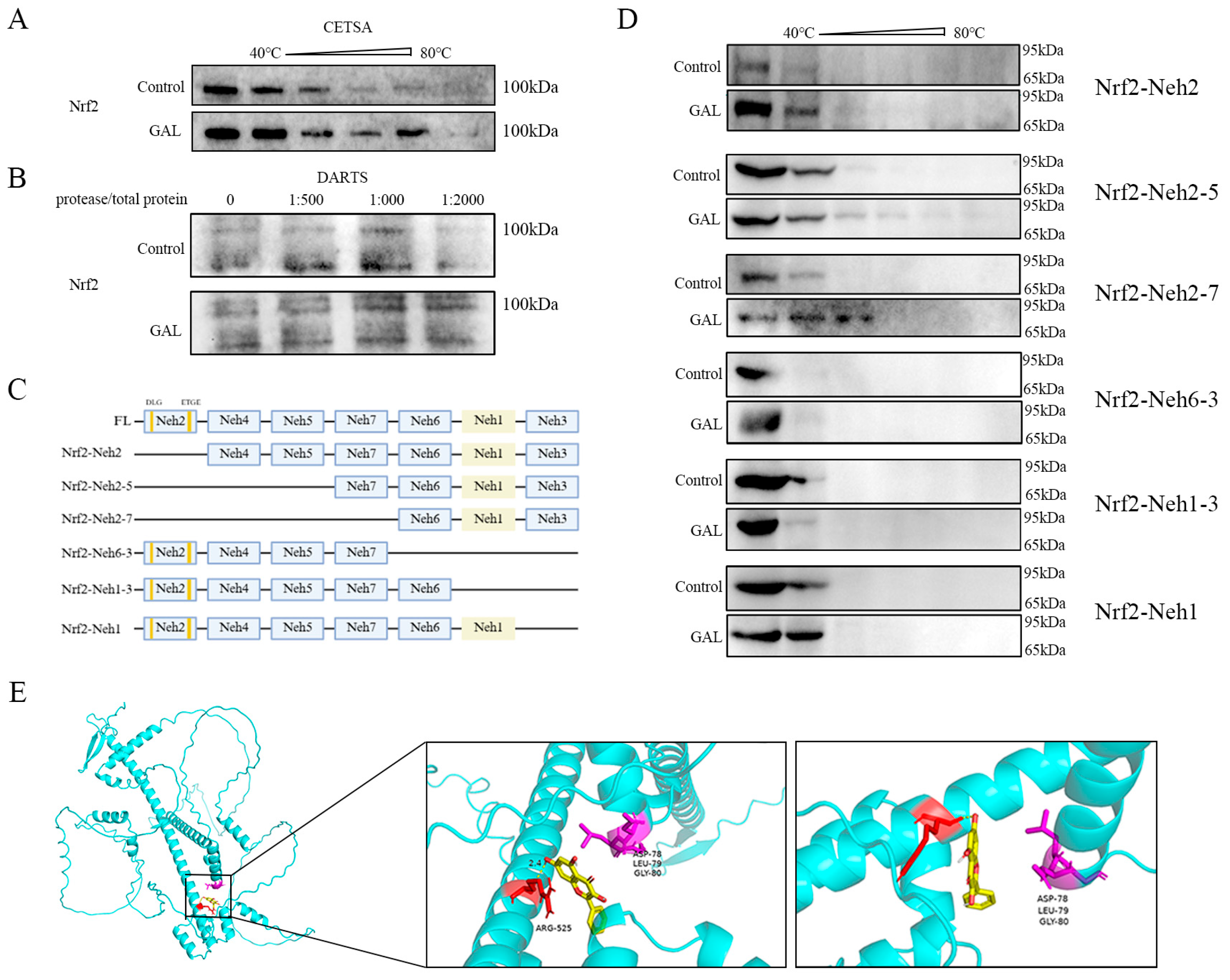
3.6. GAL Enhances Protein Stability by Interacting with the Nrf2-Neh1 Domain
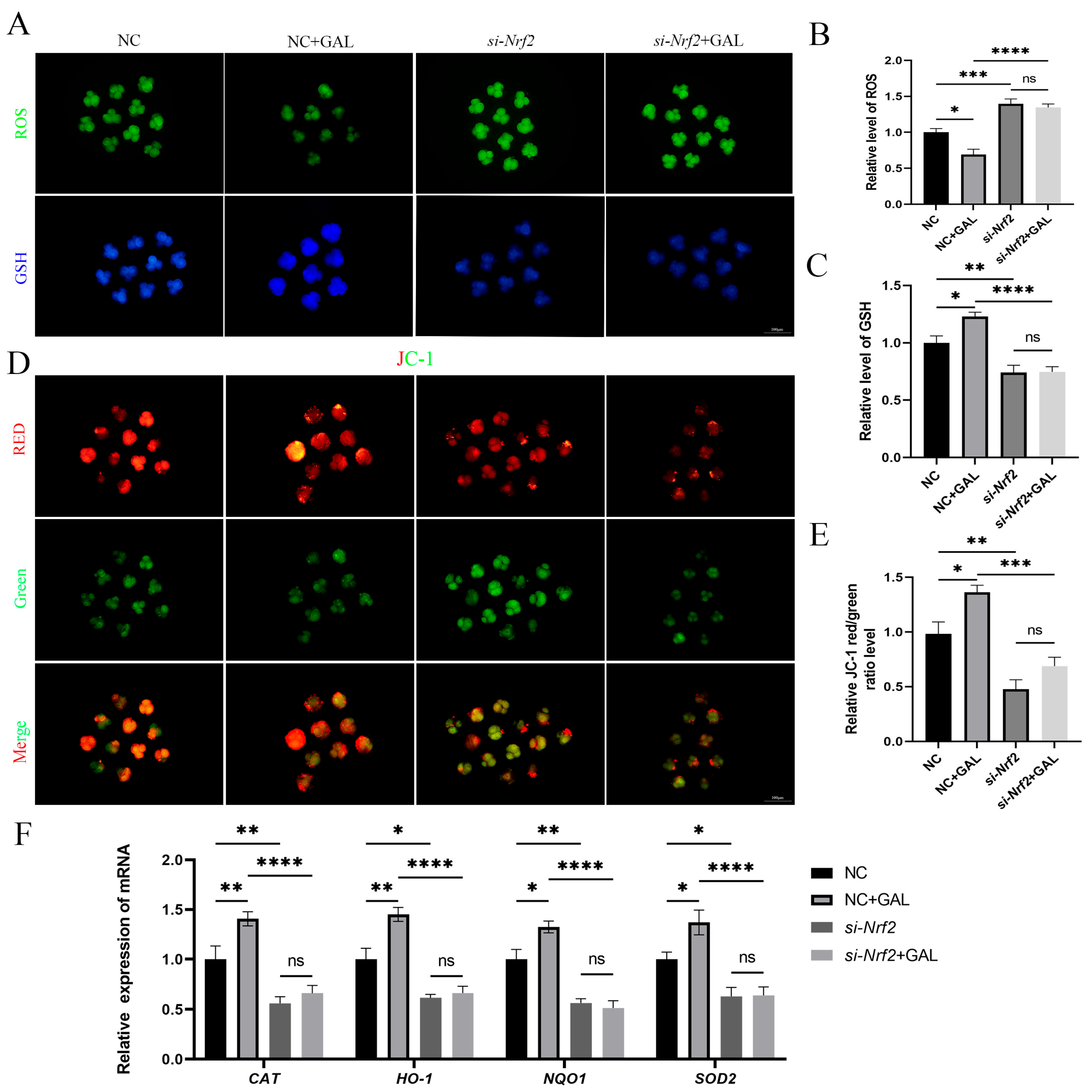
3.7. The Antioxidant Effects of GAL Are Abolished After Nrf2 Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goto, K.; Kajihara, Y.; Kosaka, S.; Koba, M.; Nakanishi, Y.; Ogawa, K. Pregnancies after co-culture of cumulus cells with bovine embryos derived from in-vitro fertilization of in-vitro matured follicular oocytes. J. Reprod. Fertil. 1988, 83, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, M.; Zeyland, J.; Slomski, R.; Lipinski, D. Genetically Modified Pigs as Organ Donors for Xenotransplantation. Mol. Biotechnol. 2017, 59, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.A.; Alminana, C.; Cuello, C.; Parrilla, I.; Roca, J.; Vazquez, J.M.; Martinez, E.A. Brief coincubation of gametes in porcine in vitro fertilization: Role of sperm:oocyte ratio and post-coincubation medium. Theriogenology 2007, 67, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Onishi, A.; Kashiwazaki, N.; Iwamoto, M.; Noguchi, J.; Kaneko, H.; Akita, T.; Nagai, T. Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol. Reprod. 2002, 66, 1033–1041. [Google Scholar] [CrossRef]
- Bauer, B.K.; Isom, S.C.; Spate, L.D.; Whitworth, K.M.; Spollen, W.G.; Blake, S.M.; Springer, G.K.; Murphy, C.N.; Prather, R.S. Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos. Biol. Reprod. 2010, 83, 791–798. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol. Reprod. 2012, 87, 24. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Romek, M.; Gajda, B.; Krzysztofowicz, E.; Smorag, Z. Changes of lipid composition in non-cultured and cultured porcine embryos. Theriogenology 2010, 74, 265–276. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wang, C.; Chaille, J.M.; Machaty, Z. Effect of resveratrol on the development of porcine embryos produced in vitro. J. Reprod. Dev. 2010, 56, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.J.; Zhou, W.; Nie, Z.W.; Shin, K.T.; Cui, X.S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2020, 68, e12627. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cheng, D.; Gao, X.; Bao, J.; Ma, X.; Wang, H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod. Domest. Anim. 2012, 47, 873–879. [Google Scholar] [CrossRef]
- Choi, M.J.; Park, J.S.; Park, J.E.; Kim, H.S.; Kim, H.S. Galangin Suppresses Pro-Inflammatory Gene Expression in Polyinosinic-Polycytidylic Acid-Stimulated Microglial Cells. Biomol. Ther. 2017, 25, 641–647. [Google Scholar] [CrossRef]
- Lee, J.J.; Ng, S.C.; Hsu, J.Y.; Liu, H.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin Reverses H(2)O(2)-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1alpha/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef]
- Tan, K.T.; Li, S.; Panny, L.; Lin, C.C.; Lin, S.C. Galangin ameliorates experimental autoimmune encephalomyelitis in mice via modulation of cellular immunity. J. Immunotoxicol. 2021, 18, 50–60. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, M.Y.; Han, L.; Tai, Y.; Cao, S.; Li, J.; Zhao, H.; Wang, R.; Lv, B.; Shan, Z.; et al. Galangin inhibits programmed cell death-ligand 1 expression by suppressing STAT3 and MYC and enhances T cell tumor-killing activity. Phytomedicine 2023, 116, 154877. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 system in cancers: Stress response and anabolic metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef]
- Stepkowski, T.M.; Kruszewski, M.K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic. Biol. Med. 2011, 50, 1186–1195. [Google Scholar] [CrossRef]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a "tethering" mechanism: A two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006, 281, 24756–24768. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Motohashi, H.; O’Connor, T.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 2002, 294, 1–12. [Google Scholar] [CrossRef]
- He, C.H.; Gong, P.; Hu, B.; Stewart, D.; Choi, M.E.; Choi, A.M.; Alam, J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001, 276, 20858–20865. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Hsiao, Y.P.; Lin, Y.T.; Chen, C.; Lee, C.M.; Liao, W.C.; Tsou, S.C.; Lin, H.W.; Chang, Y.Y. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1alpha-Sirt1 Pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Ma, W.; Guo, W.; Shang, F.; Li, Y.; Li, W.; Liu, J.; Ma, C.; Teng, J. Bakuchiol Alleviates Hyperglycemia-Induced Diabetic Cardiomyopathy by Reducing Myocardial Oxidative Stress via Activating the SIRT1/Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 3732718. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Iida, K.; Kang, M.I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Morado, S.; Cetica, P.; Beconi, M.; Thompson, J.G.; Dalvit, G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction 2013, 145, 471–478. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.M.; Johnson, M.H. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991, 113, 551–560. [Google Scholar] [CrossRef]
- Ozkaya, M.O.; Naziroglu, M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil. Steril. 2010, 94, 2465–2466. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Shih, Y.F.; Lee, T.H.; Liu, C.H.; Tsao, H.M.; Huang, C.C.; Lee, M.S. Effects of reactive oxygen species levels in prepared culture media on embryo development: A comparison of two media. Taiwan J. Obstet. Gynecol. 2014, 53, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Saeki, K.; Hoshi, M.; Kainuma, H. Effects of oxygen concentration and oviductal epithelial tissue on the development of in vitro matured and fertilized bovine oocytes cultured in protein-free medium. Theriogenology 1994, 41, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Reggio, B.C.; Godke, R.A.; Hansel, W. Development of in-vitro-derived bovine embryos cultured in 5% CO2 in air or in 5% O2, 5% CO2 and 90% N2. Hum. Reprod. 1999, 14, 458–464. [Google Scholar] [CrossRef]
- Campugan, C.A.; Lim, M.; Chow, D.J.X.; Tan, T.C.Y.; Li, T.; Saini, A.A.; Orth, A.; Reineck, P.; Schartner, E.P.; Thompson, J.G.; et al. The effect of discrete wavelengths of visible light on the developing murine embryo. J. Assist. Reprod. Genet. 2022, 39, 1825–1837. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Oh, S.J.; Gong, S.P.; Lee, S.T.; Lee, E.J.; Lim, J.M. Light intensity and wavelength during embryo manipulation are important factors for maintaining viability of preimplantation embryos in vitro. Fertil. Steril. 2007, 88, 1150–1157. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef]
- Gutierrez-Anez, J.C.; Henning, H.; Lucas-Hahn, A.; Baulain, U.; Aldag, P.; Sieg, B.; Hensel, V.; Herrmann, D.; Niemann, H. Melatonin improves rate of monospermic fertilization and early embryo development in a bovine IVF system. PLoS ONE 2021, 16, e0256701. [Google Scholar] [CrossRef]
- Carrillo-Gonzalez, D.F.; Hernandez-Herrera, D.Y.; Maldonado-Estrada, J.G. The role of L-carnitine in bovine embryo metabolism. A review of the effect of supplementation with a metabolic modulator on in vitro embryo production. Anim. Biotechnol. 2023, 34, 413–423. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed. Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Khodr, H.; Hider, R.C.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330 Pt 3, 1173–1178. [Google Scholar] [CrossRef]
- Chiu, D.T. Oxidative stress in biology and medicine. Biomed. J. 2014, 37, 97–98. [Google Scholar] [CrossRef]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin, a natural flavonoid reduces mitochondrial oxidative damage in streptozotocin-induced diabetic rats. Redox Rep. 2018, 23, 29–34. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, M.A.; Cho, H.J.; Oh, S.K.; Lee, I.K.; Kim, U.K.; Lee, K.Y. Galangin prevents aminoglycoside-induced ototoxicity by decreasing mitochondrial production of reactive oxygen species in mouse cochlear cultures. Toxicol. Lett. 2016, 245, 78–85. [Google Scholar] [CrossRef]
- Wen, S.Y.; Chen, J.Y.; Weng, Y.S.; Aneja, R.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin suppresses H2O2-induced aging in human dermal fibroblasts. Environ. Toxicol. 2017, 32, 2419–2427. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, X.; Ouyang, H.; Lu, B.; Yu, Z.; Wang, Z.; Ji, L. Natural flavonoid galangin alleviates microglia-trigged blood-retinal barrier dysfunction during the development of diabetic retinopathy. J. Nutr. Biochem. 2019, 65, 1–14. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Alsawaf, S.; Mathew, S.; Nasar, N.A.; Hussain, M.I.; Cherkaoui, N.M.; Ayyub, M.; Alsaid, S.Y.; Thomas, J.G.; Cyril, A.C.; et al. Pharmacodynamics and safety in relation to dose and response of plant flavonoids in treatment of cancers. Inflammopharmacology 2025, 33, 11–47. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Kuramoto, T.; Tanaka, M.; Mitzumoto, S.; Montag, M.; Yoshida, A. Prospective randomized multicentre comparison on sibling oocytes comparing G-Series media system with antioxidants versus standard G-Series media system. Reprod. Biomed. Online 2020, 40, 637–644. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Hawkes, H.J.; Karlenius, T.C.; Tonissen, K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta 2014, 1840, 303–314. [Google Scholar] [CrossRef]
- Higgins, L.G.; Kelleher, M.O.; Eggleston, I.M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharmacol. 2009, 237, 267–280. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Madduma Hewage, S.R.K.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Fernando, P.; Oh, M.C.; Park, J.E.; Shilnikova, K.; Moon, Y.J.; Shin, D.O.; et al. Galangin Activates the ERK/AKT-Driven Nrf2 Signaling Pathway to Increase the Level of Reduced Glutathione in Human Keratinocytes. Biomol. Ther. 2017, 25, 427–433. [Google Scholar] [CrossRef]
- Chaihongsa, N.; Maneesai, P.; Sangartit, W.; Rattanakanokchai, S.; Potue, P.; Khamseekaew, J.; Bunbupha, S.; Pakdeechote, P. Cardiorenal dysfunction and hypertrophy induced by renal artery occlusion are normalized by galangin treatment in rats. Biomed. Pharmacother. 2022, 152, 113231. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liao, J.; Pei, R.; Yu, W.; Han, Q.; Li, Y.; Guo, J.; Hu, L.; Pan, J.; Tang, Z. Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 2018, 204, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhao, H.; Han, Y.; Li, L.; Xiong, S.; Zeng, L.; Xiao, Y.; Wei, L.; Xiong, X.; Gao, P.; et al. HIF-1alpha ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020, 53, e12909. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056–6063. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- Amoutzias, G.D.; Veron, A.S.; Weiner, J., 3rd; Robinson-Rechavi, M.; Bornberg-Bauer, E.; Oliver, S.G.; Robertson, D.L. One billion years of bZIP transcription factor evolution: Conservation and change in dimerization and DNA-binding site specificity. Mol. Biol. Evol. 2007, 24, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Bailey, T.; Bhattarai, S.; Subedi, U.; Miller, C.; Ara, H.; Kidambi, S.; Sun, H.; Panchatcharam, M.; et al. Electrophilic Aldehyde 4-Hydroxy-2-Nonenal Mediated Signaling and Mitochondrial Dysfunction. Biomolecules 2022, 12, 1555. [Google Scholar] [CrossRef]
- Hu, F.; Liu, F. Mitochondrial stress: A bridge between mitochondrial dysfunction and metabolic diseases? Cell. Signal. 2011, 23, 1528–1533. [Google Scholar] [CrossRef]
- Lee, J.J.; Ng, S.C.; Ni, Y.T.; Liu, J.S.; Chen, C.J.; Padma, V.V.; Huang, C.Y.; Kuo, W.W. Protective effects of galangin against H2O2/UVB-induced dermal fibroblast collagen degradation via hsa-microRNA-4535-mediated TGFbeta/Smad signaling. Aging 2021, 13, 25342–25364. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Pestana, C.R.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant activity of flavonoids in isolated mitochondria. Phytother. Res. 2008, 22, 1213–1218. [Google Scholar] [CrossRef]
- Sato, D.; Itami, N.; Tasaki, H.; Takeo, S.; Kuwayama, T.; Iwata, H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS ONE 2014, 9, e94488. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tanga, B.M.; Bang, S.; Seong, G.; Saadeldin, I.M.; Qamar, A.Y.; Shim, J.; Choi, K.; Lee, S.; Cho, J. Vitamin C enhances porcine cloned embryo development and improves the derivation of embryonic stem-like cells. Reprod. Biol. 2022, 22, 100632. [Google Scholar] [CrossRef]

| Gene Name | Sequence | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| Nrf2 | F: ATCCAGCGGATTGCTCGTAG R: TCAAATCCATGTCCTTGGCG | 155 | XM_013984303.2 |
| KEAP1 | F: ATGGCGGGGCCTCTGA R: CTCAGGGGCAGAAATTGGGT | 114 | NM_001114671.1 |
| HO-1 | F: GGCTGAGAATGCCGAGTTCA R: GTGGTACAAGGACGCCATCA | 88 | NM_001004027.1 |
| NQO1 | F: GTATAAAGTAGCCGGGCGCT R: AGTGCTTTTCTGACCGCCAT | 162 | NM_001159613.1 |
| SOD2 | F: AGGCGCTGAAAAAGGGTGAT R: AAGTCGCGTTTGATGGCTTC | 163 | NM_214127.2 |
| CAT | F: GCTGAGTCCGAAGTCGTCTA R: GTCAGGATATCAGGTTTCTGCG | 173 | NM_214301.2 |
| β-actin | F: TTCTAGGCGGACTTGCAGC R: GCTTCTCAGCAGACAGGAGG | 128 | XM_021086047.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Z.-C.; Shi, S.-M.; Liu, L.-Y.; Qu, L.-Y.; Li, J.-H.; Jia, G.-L.; He, Y.-Y.; Li, L.-X.; Jin, Y.-X.; Zhang, M.-J.; et al. Galangin Regulates Oxidative Stress Levels in Porcine Embryos Through Interaction with the Neh1 Domain of Nrf2. Antioxidants 2025, 14, 822. https://doi.org/10.3390/antiox14070822
Chi Z-C, Shi S-M, Liu L-Y, Qu L-Y, Li J-H, Jia G-L, He Y-Y, Li L-X, Jin Y-X, Zhang M-J, et al. Galangin Regulates Oxidative Stress Levels in Porcine Embryos Through Interaction with the Neh1 Domain of Nrf2. Antioxidants. 2025; 14(7):822. https://doi.org/10.3390/antiox14070822
Chicago/Turabian StyleChi, Zhi-Chao, Shu-Ming Shi, Li-Ying Liu, Lin-Yi Qu, Jing-Hang Li, Guan-Lin Jia, Yu-Yan He, Lin-Xuan Li, Yong-Xun Jin, Ming-Jun Zhang, and et al. 2025. "Galangin Regulates Oxidative Stress Levels in Porcine Embryos Through Interaction with the Neh1 Domain of Nrf2" Antioxidants 14, no. 7: 822. https://doi.org/10.3390/antiox14070822
APA StyleChi, Z.-C., Shi, S.-M., Liu, L.-Y., Qu, L.-Y., Li, J.-H., Jia, G.-L., He, Y.-Y., Li, L.-X., Jin, Y.-X., Zhang, M.-J., & Yu, X.-F. (2025). Galangin Regulates Oxidative Stress Levels in Porcine Embryos Through Interaction with the Neh1 Domain of Nrf2. Antioxidants, 14(7), 822. https://doi.org/10.3390/antiox14070822







