Herbal Cuscutae Semen Contributes to Oxidative Stress Tolerance and Extends Lifespan via Sirtuin1 in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CS Extract Preparation
2.3. C. Elegans Strains and Maintenance
2.4. Food Clearance Assay
2.5. Lifespan Assay
2.6. Body Bending Assay
2.7. Pharyngeal Pumping Assay
2.8. Lipofuscin Assay
2.9. Fecundity Assay
2.10. Oxidative or Heat Stress Resistance Assay
2.11. Quantification of Malondialdehyde (MDA)
2.12. Quantification of Antioxidant Enzyme Activity
2.13. ATP Determination
2.14. ROS Quantification
2.15. Immunofluorescence Staining
2.16. RT-Quantitative Polymerase Chain Reaction (qPCR) Assay
2.17. Western Blotting
2.18. Cell Culture and Treatment
2.19. Dual-Luciferase Reporter Gene Analyses of SIRT1 Transcriptional Activity
2.20. Ultra-High Performance Liquid Chromatography-Q Exactive-Mass Spectrometry (UHPLC-QE-MS) for the Identification of CS
2.21. Statistical Analysis
3. Result
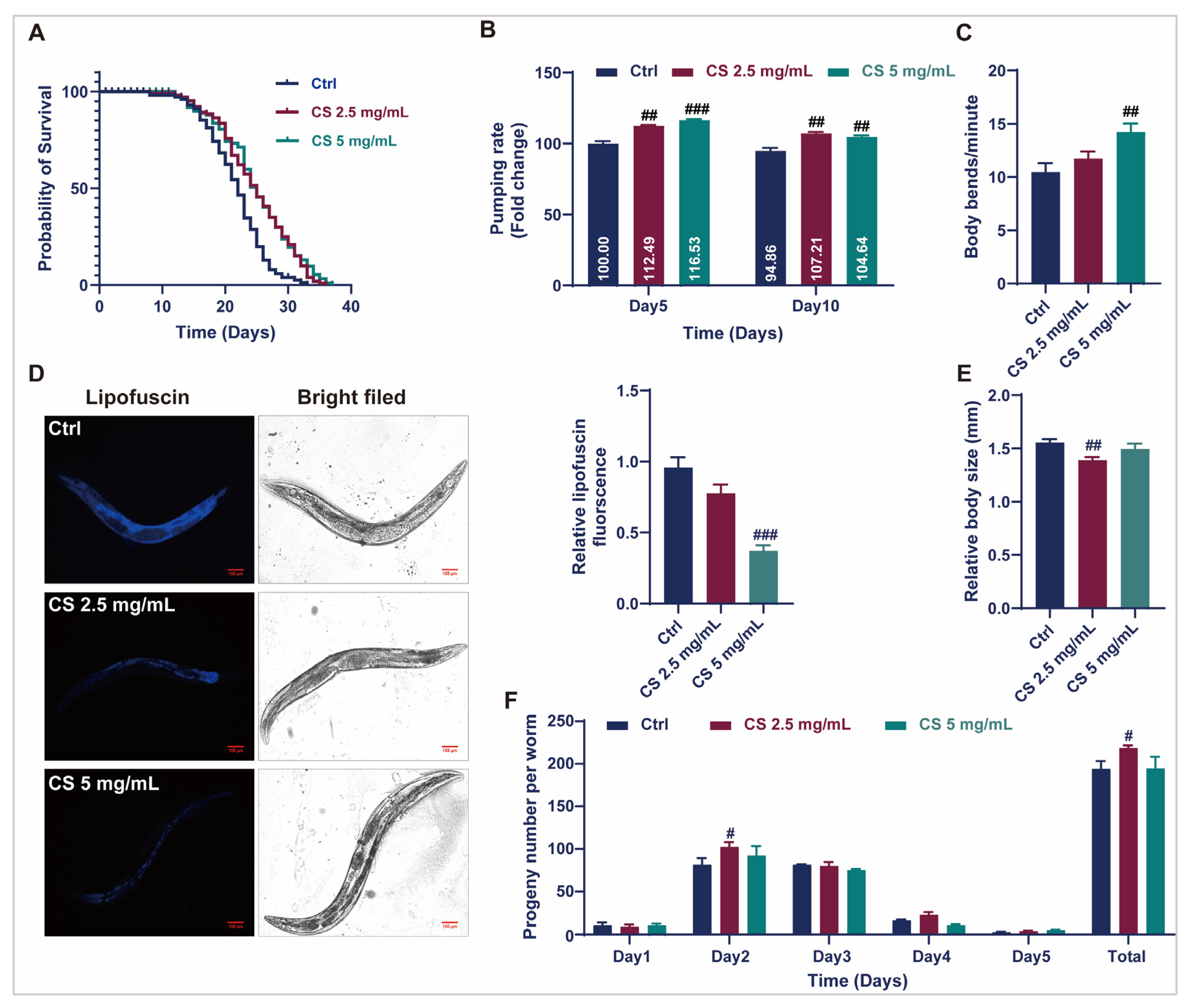
3.1. CS Extended the Lifespan and Improved the Health Status of C. elegans
3.2. CS Protected Against Oxidative Stress in C. elegans
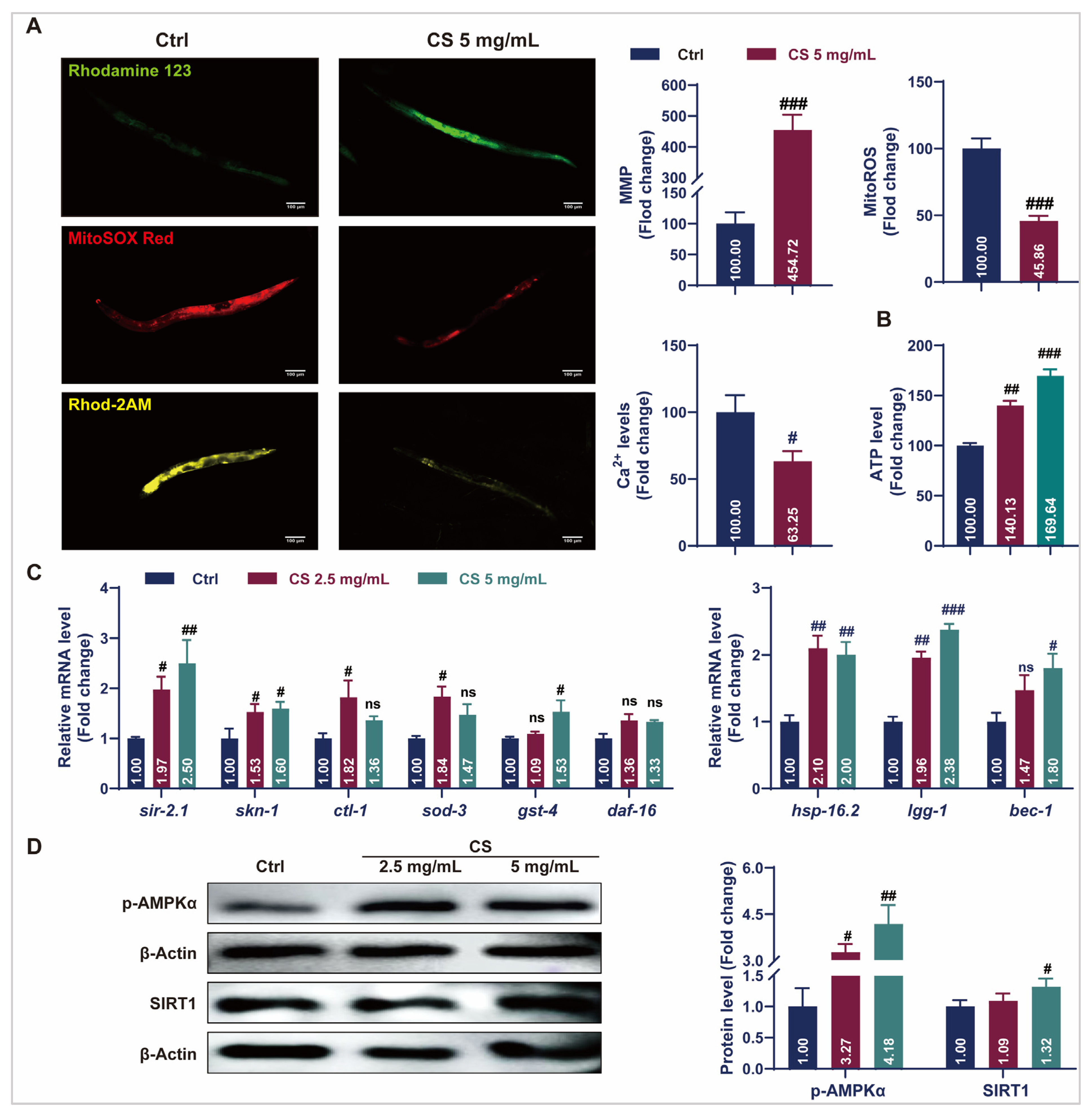
3.3. CS Maintained Mitochondrial Homeostasis and Activated Stress Response Pathways in C. elegans
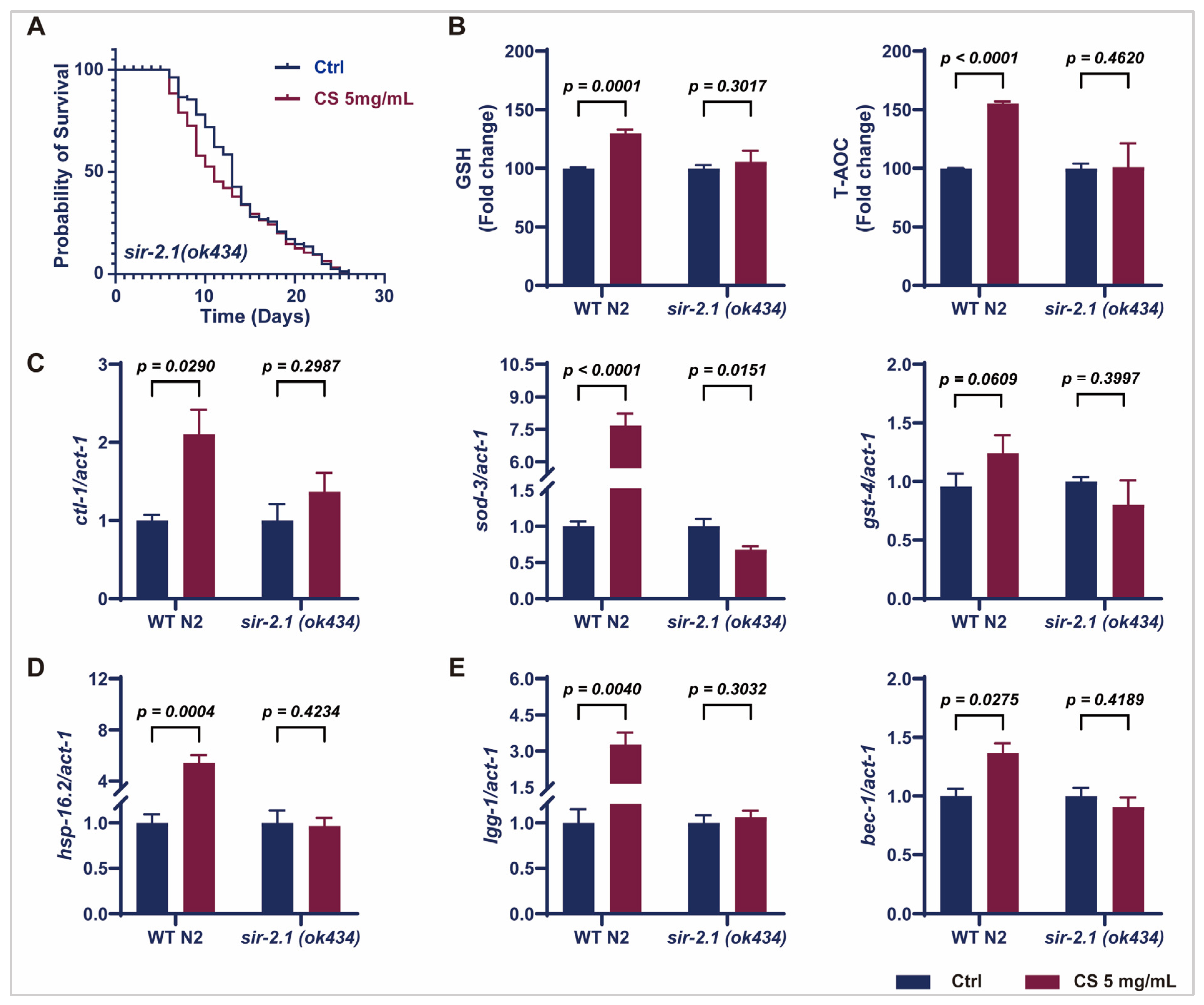
3.4. Sir-2.1 Is Essential for the CS-Induced Extension of Lifespan and Enhancement of Oxidative Stress Resistance in C. elegans
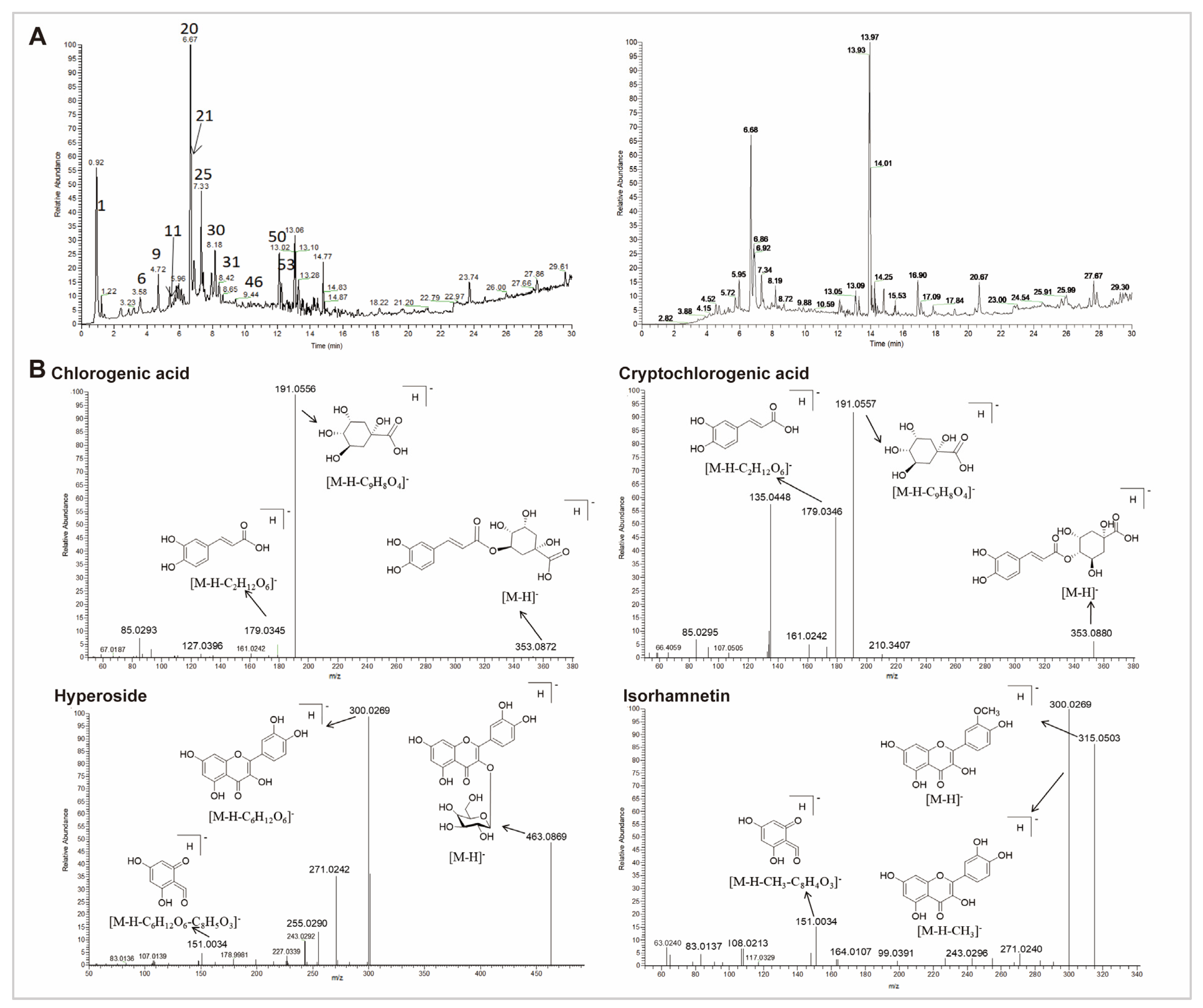
3.5. Chemical Characterization of CS
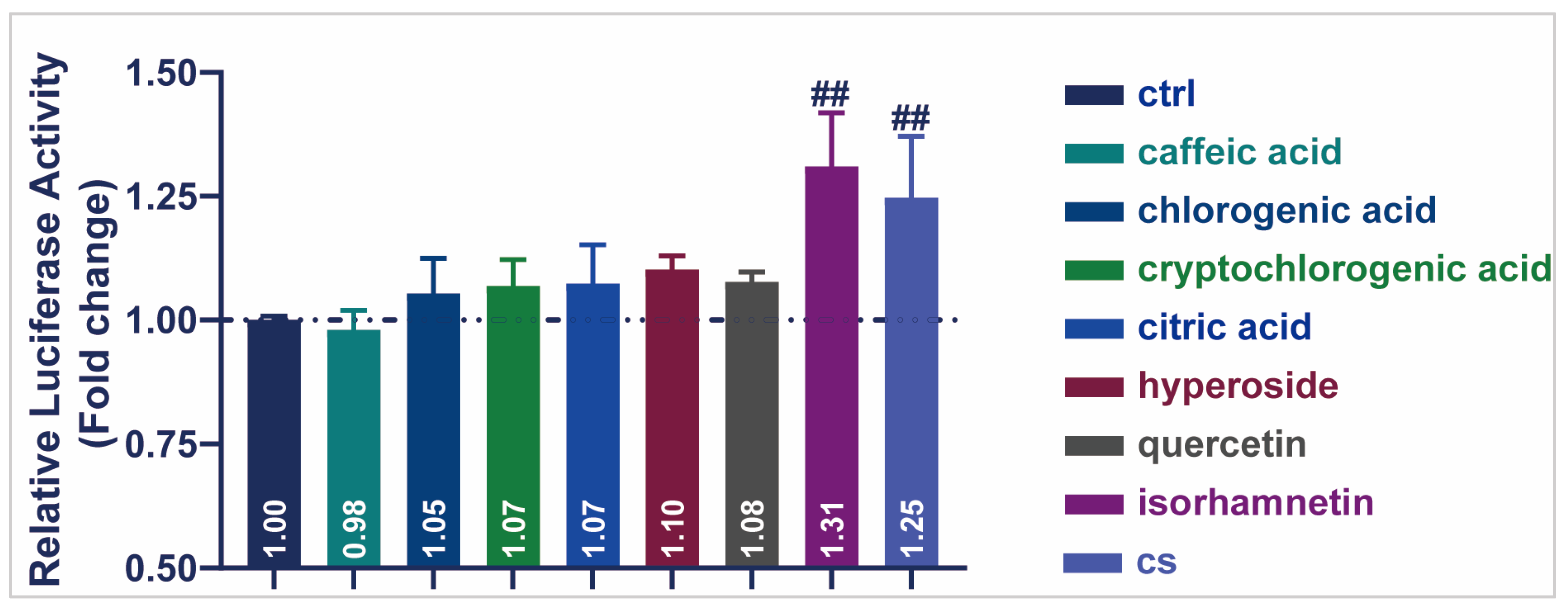
3.6. Active Compound of CS Enhanced the Transcriptional Activity of SIRT1 in HEK293T Cells
4. Discussion
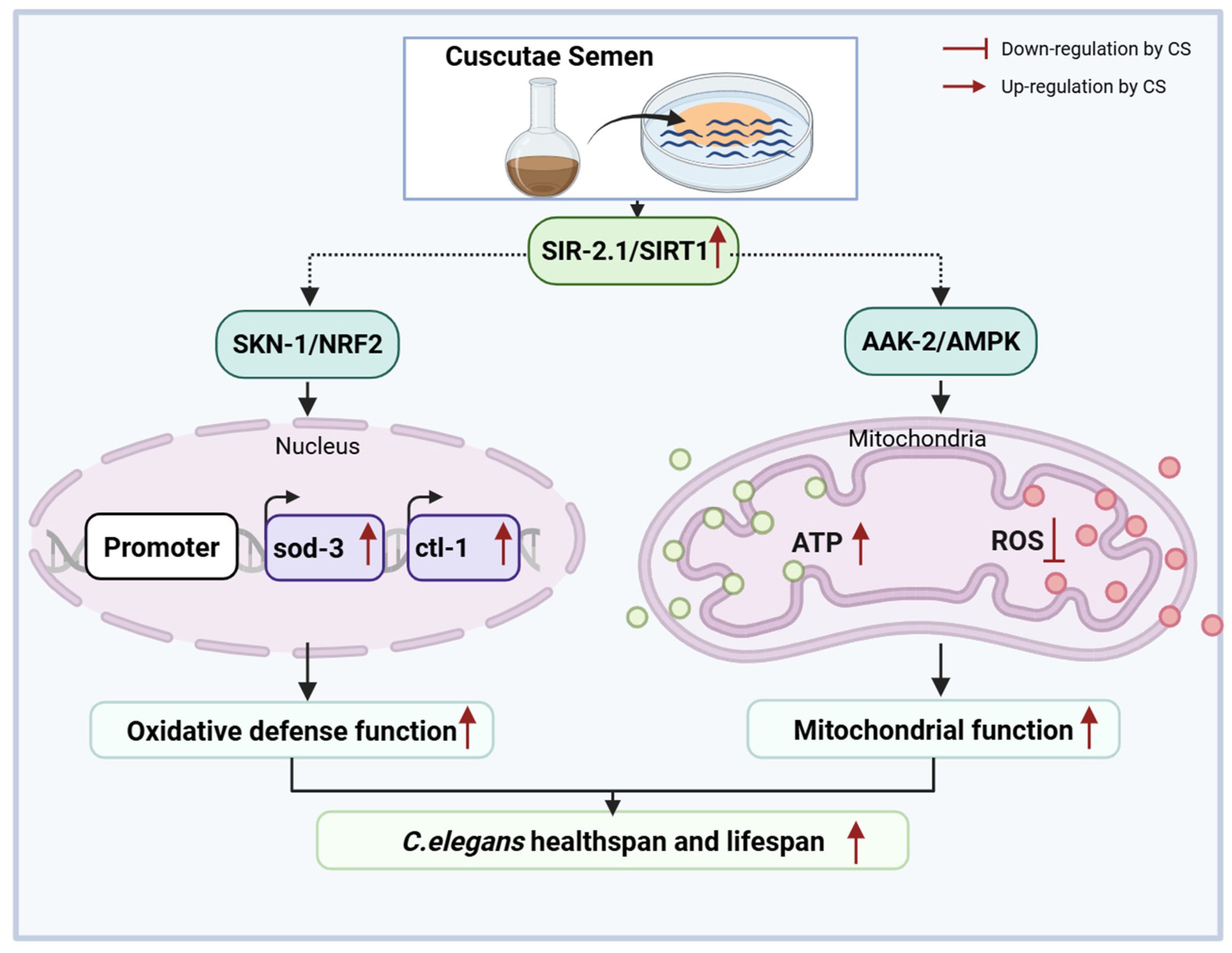
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Cuscutae Semen |
| C. elegans | Caenorhabditis elegans |
| MMP | Mitochondrial membrane potential |
| ATP | Adenosine triphosphate |
| NGM | Nematode growth medium |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| T-AOC | Total antioxidant capacity |
| SIRT1 | Silent information regulator sirtuin 1 |
| p-AMPKα | Phosphorylated AMP-activated protein kinase α subunit |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| FOXO | Fork-head box |
| ESI | Electrospray ionization |
| UPLC | Ultra-performance liquid chromatography |
| MS | Mass spectrometry |
| TIC | Total ion chromatogram |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Luan, Y.; Zhu, X.F.; Jiao, Y.X.; Liu, H.; Huang, Z.; Pei, J.Y.; Xu, Y.W.; Yang, Y.; Ren, K.D. Cardiac cell senescence: Molecular mechanisms, key proteins and therapeutic targets. Cell Death Discov. 2024, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Hiden, U.; Krstic, J.; Panzitt, K.; Wagner, M.; Enzinger, C.; Khalil, M.; Abdellatif, M.; Malle, E.; Madl, T.; et al. Targeting organ-specific mitochondrial dysfunction to improve biological aging. Pharmacol. Ther. 2024, 262, 108710. [Google Scholar] [CrossRef]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging—Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, K.M.; Truttmann, M.C. Caenorhabditis elegans as a model system for studying aging-associated neurodegenerative diseases. Transl. Med. Aging 2020, 4, 60–72. [Google Scholar] [CrossRef]
- Ye, Y.L.; Gu, Q.Y.; Sun, X.L. Potential of Caenorhabditis elegansas an antiaging evaluation model for dietary phytochemicals: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3084–3105. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Ni, Y.j.; Liu, Y.q. Hesperetin Increases Lifespan and Antioxidant Ability Correlating with IIS, HSP, mtUPR, and JNK Pathways of Chronic Oxidative Stress in Caenorhabditis elegans. Int. J. Mol. Sci. 2024, 25, 13148. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Sun, X.J.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 5, 548. [Google Scholar] [CrossRef]
- Bi, S.J.; Jiang, X.Y.; Ji, Q.Z.; Wang, Z.H.; Ren, J.; Wang, S.; Yu, Y.; Wang, R.Q.; Liu, Z.P.; Liu, J.H.; et al. The sirtuin-associated human senescence program converges on the activation of placenta-specific gene PAPPA. Dev. Cell 2024, 59, 991–1009.e1012. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Guarente, L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 2011, 477, E1–E2. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Izrayelit, Y.; Park, D.; Malik, R.U.; Zimmermann, A.; Mahanti, P.; Fox, B.W.; Bethke, A.; Doering, F.; Riddle, D.L.; et al. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc. Natl. Acad. Sci. USA 2013, 110, 5522–5527. [Google Scholar] [CrossRef] [PubMed]
- Han, L.M.; Tong, T.J.; Yun, D.; He, X.D.; Hu, K.X.; Cao, J.Z.; Zhao, L.J. Sirtuins and their biological relevance in aging and age-related diseases. Aging Dis. 2020, 11, 927. [Google Scholar]
- Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Punica granatum L. leaf extract enhances stress tolerance and promotes healthy longevity through HLH-30/TFEB, DAF16/FOXO, and SKN1/NRF2 crosstalk in Caenorhabditis elegans. Phytomedicine 2024, 134, 155971. [Google Scholar] [CrossRef]
- Blackwell Keith, T.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Denzel, M.S.; Lapierre, L.R.; Mack, H.I.D. Emerging topics in C. elegans aging research: Transcriptional regulation, stress response and epigenetics. Mech. Ageing Dev. 2019, 177, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.A.; Lamitina, T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 2012, 12, 112–120. [Google Scholar] [CrossRef]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.H.; Donkor, P.O.; Gao, X.M.; Chang, Y.X. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef]
- Commission, C.P. Chinese Pharmacopeia; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Chen, L.; Hu, Y.; Xin, G.; Chen, Y.; Zong, Z.; Song, H.; Sun, Y.; Xu, B.; Zhang, W.; Wang, H.; et al. Research progress on chemical constituents, pharmacological effects of Cuscutae Semen and its quality marker prediction. Chin. Tradit. Herb. Drugs 2024, 55, 5298–5314. [Google Scholar]
- The Website of the Central People’s Government of the PRC. Notice of the Ministry of Health on Further Regulating the Management of Health Food Raw Materials: Health Law Supervision 2002 no. 51 [M/OL]. Available online: http://www.nhc.gov.cn/sps/s3593/200810/bc239ea3d226449b86379f645dfd881d.shtml (accessed on 28 April 2025).
- Zhong, H.; Tang, Z.-Q.; Li, Y.-F.; Wang, M.; Sun, W.-Y.; He, R.-R. The evolution and significance of medicine and food homology. Acupunct. Herb. Med. 2024, 4, 19–35. [Google Scholar] [CrossRef]
- Lan, H.; Du, S.M. Study on the anti-aging action of the extracts of Cuscuta chinensis in natural aging mice. China Pharm. 2015, 21, 3667–3669. [Google Scholar]
- Zhang, Y.; Wu, Y.L.; Li, B.; Tian, J. Phloretin prolongs lifespan of Caenorhabditis elegans via inhibition of NDUFS1 and NDUFS6 at mitochondrial complex I. Free Radic. Biol. Med. 2024, 221, 283–295. [Google Scholar] [CrossRef]
- He, Z.J.; Shi, M.; Liu, H. YAnti-aging effect of cuscuta extract on D-galactose induced aging mice. Chin. J. Gerontol. 2015, 35, 5444–5446. [Google Scholar]
- Dong, Y.; Ge, W. Research progress on anti-aging effect of Cuscutae Semen. Pract. Geriatr. 2024, 38, 219–222. [Google Scholar]
- Shi, W.; Liu, M.; Wang, Z.C.; Wu, Y.K.; Wu, K.M. Xin Jia Congrong Tusizi Decoction Improves Mitochondrial Biogenesis of Ovarian Granulosa Cells Injury via SlRT1/PGC-1a Signaling Pathway. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2024, 26, 1269–1278. [Google Scholar]
- Shi, W.; Yin, X.M.; Wang, Z.C.; Wu, Y.K.; Wu, K.M. Effects of modified Congrong Tusizi Decoction on mitochondrial functionin rats with diminished ovarian reserve via regulating AMPK/SIRT1signaling pathway. Chin. Tradit. Pat. Med. 2023, 45, 3221–3227. [Google Scholar]
- Shi, W.; Liu, M.; Wang, Z.C.; Wu, Y.K.; Wu, K.M. Effect of medicated serum containing Xinjia Congrong Tusizi Decoction on mitochondrial energy metabolism, oxidative stress and dynamics of human ovarian granulosa cells induced by triptolide. J. Chin. Med. Mater. 2023, 46, 2573–2578. [Google Scholar]
- Xiao, Y.; Zhang, L.; Liu, Y. Protocol for assessing the healthspan of Caenorhabditis elegans after potential anti-aging drug treatment. STAR Protoc. 2023, 4, 102285. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, L.; Liu, X.M.; Zhang, D.Q.; Liu, Y.; Li, Y.H. 23-O-acetylshengmanol-3-O-α-L-arabinoside alleviates lipopolysaccharide-induced acute lung injury through inhibiting IκB/NF-κB and MAPK/AP-1 signaling pathways. J. Ethnopharmacol. 2023, 300, 115725. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Barardo, D.; Thornton, D.; Thoppil, H.; Walsh, M.; Sharifi, S.; Ferreira, S.; Anžič, A.; Fernandes, M.; Monteiro, P.; Grum, T.; et al. The Drug Age database of aging-related drugs. Aging Cell 2017, 16, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Gubert, P.; Puntel, B.; Lehmen, T.; Bornhorst, J.; Avila, D.S.; Aschner, M.; Soares, F.A.A. Reversible reprotoxic effects of manganese through DAF-16 transcription factor activation and vitellogenin downregulation in Caenorhabditis elegans. Life Sci. 2016, 15, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Gil-Sánchez, I.; Moreno-Arribas, M.V.; Bartolomé, B.; Sanz-Buenhombre, M.; Guadarrama, A.; Santos-Buelga, C.; González-Paramás, A.M. Antioxidant characterization and biological effects of grape pomace extracts supplementation in Caenorhabditis elegans. Foods 2019, 8, 75. [Google Scholar] [CrossRef]
- Cong, W.S.; Wang, P.; Qu, Y.; Tang, J.L.; Bai, R.; Zhao, Y.L.; Chen, C.Y.; Bi, X.L. Evaluation of the influence of fullerenol on aging and stress resistance using Caenorhabditis elegans. Biomaterials 2015, 42, 78–86. [Google Scholar] [CrossRef]
- Guo, X.H.; Xin, Q.C.; Wei, P.; Hua, Y.T.; Zhang, Y.C.; Su, Z.Y.Q.; She, G.M.; Yuan, R.J. Antioxidant and anti-aging activities of Longan crude and purified polysaccharide (LP-A) in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 267, 131634. [Google Scholar] [CrossRef]
- Cai, X.G.; Zhang, Z.M.; Xu, A.X.; Meng, Y.; Ge, B.; Song, X.R.; Lu, X.A. Experimental study on the synergistic effect of Ligustrum ligustrum polysaccharide and dodder polysaccharide on scavenging oxygen free radicals and anti-aging. J. Med. Res. 2007, 8, 74–75. [Google Scholar]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.H.; Li, M.Y.; Zhang, G.H.; Kong, J.M. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Sudevan, S.; Takiura, M.; Kubota, Y.; Higashitani, N.; Cooke, M.; Ellwood, R.A.; Etheridge, T.; Szewczyk, N.J.; Higashitani, A. Mitochondrial dysfunction causes Ca2+ overload and ECM degradation–mediated muscle damage in C. elegans. FASEB J. 2019, 33, 9540–9550. [Google Scholar] [CrossRef]
- Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Nurturing longevity through natural compounds: Where do we stand, and where do we go? Food Front. 2024, 5, 267–310. [Google Scholar] [CrossRef]
- Tyshkovskiy, A.; Ma, S.; Shindyapina, A.V.; Tikhonov, S.; Lee, S.-G.; Bozaykut, P.; Castro, J.P.; Seluanov, A.; Schork, N.J.; Gorbunova, V.; et al. Distinct longevity mechanisms across and within species and their association with aging. Cell 2023, 186, 2929–2949.e2920. [Google Scholar] [CrossRef]
- Sun, M.L.; Wei, C.M.; Gao, Y.H.; Chen, X.Y.; Zhong, K.X.; Li, Y.Z.; Yang, Z.; Gao, Y.H.; Wang, H.B. TSG Extends the Longevity of Caenorhabditis elegans by Targeting the DAF-16/SKN-1/SIR-2.1-Mediated Mitochondrial Quality Control Process. Antioxidants 2024, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Chen, S.M.; Lang, J.; Luo, J.; Chen, J.H.; Zhang, L.P.; Sun, Z.J.; Dong, D.L. The clinical antiprotozoal drug nitazoxanide and its metabolite tizoxanide extend Caenorhabditis elegans lifespan and healthspan. Acta Pharm. Sin. B 2024, 14, 3266–3280. [Google Scholar] [CrossRef]
- Wu, Z.M.; Qu, J.; Zhang, W.Q.; Liu, G.H. Stress, epigenetics, and aging: Unraveling the intricate crosstalk. Mol. Cell 2024, 84, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, A.; Viswanathan, M.; Robert Horvitz, H.; Guarente, L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tissenbaum, H.A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006, 127, 48–56. [Google Scholar] [CrossRef]
- Lapierre, L.R.; De Magalhaes Filho, C.D.; McQuary, P.R.; Chu, C.-C.; Visvikis, O.; Chang, J.T.; Gelino, S.; Ong, B.; Davis, A.E.; Irazoqui, J.E.; et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat. Commun. 2013, 4, 2267. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Impaired Autophagic Flux in Glucose-Deprived Cells: An Outcome of Lysosomal Acidification Failure Exacerbated by Mitophagy Dysfunction. Mol. Cells 2023, 46, 655–663. [Google Scholar] [CrossRef]
- Raynes, R.; Leckey, B.D.; Nguyen, K.; Westerheide, S.D. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J. Biol. Chem. 2012, 287, 29045–29053. [Google Scholar] [CrossRef] [PubMed]
- Păcularu-Burada, B.; Cîrîc, A.-I.; Begea, M. Anti-Aging Effects of Flavonoids from Plant Extracts. Foods 2024, 13, 2441. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Yan, G.; Sun, H.; Yang, L.; Kong, L.; Sun, Y.; Han, Y.; Zhao, Q.; Kang, S.; Wang, X. Version updating of strategy for drug discovery based on effective constituents of traditional Chinese medicines. Acupunct. Herb. Med. 2023, 3, 158–179. [Google Scholar] [CrossRef]
- Boyce, F.M.; Baker, J.M. High-throughput Functional Screening using a Homemade Dual-glow Luciferase Assay. J. Vis. Exp. 2014, 50282. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Wang, Y.; Ding, J.; Wang, R. Isorhamnetin Alleviates High Glucose-Aggravated Inflammatory Response and Apoptosis in Oxygen-Glucose Deprivation and Reoxygenation-Induced HT22 Hippocampal Neurons Through Akt/SIRT1/Nrf2/HO-1 Signaling Pathway. Inflammation 2021, 44, 1993–2005. [Google Scholar] [CrossRef]
- Pubchem. Compound Database [Dataset]. 2025. Available online: https://pubchemncbinlmnihgov (accessed on 28 April 2025).
- Zong, Z.; Hu, Y.; Xu, L.-L.; Xin, G.; Zhang, W.; Chen, Y.; Liu, S.; Sun, X.; Song, H.; Li, W.; et al. Based on UPLC-Q-TOF-MS and network pharmacology, the components and mechanisms of Cuscuta chinensis-Lycium barbarum in the treatment of early-onset ovarian insufficiency were explored. Chin. Tradit. Pat. Med. 2024, 47, 649–658. [Google Scholar]
- Li, W.; Zhang, Y.; Bai, J.; Xiang, Z.; Ding, J.; Ji, Y. Identification of chemical constituents in Cuscuta chinensis using HPLC-ESI/Q-TOF MS/MS. Bio. Technol. Indian J. 2013, 8, 563–567. [Google Scholar]
- Gao, T.-H. Studies on Chemical Constituents of Cuscuta chinensis Lam. Master’s Thesis, Jilin University, Jilin, China, 2009. [Google Scholar]
- Zhang, Y. A Comparative Study on the Chemical Components and Tonifying Kidney Yang Effects of Different Processed Products of Cuscuta chinensis. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. [Google Scholar]
- Ye, M.; Yan, Y.N.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid. Commun. Mass. Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef]
- Anis, E.; Anis, I.; Ahmed, S.; Ghulam, M.; Abdul, M.; Nighat, A.; Syed, M.; Abdul, H.; Syed, S.; Choudhary, M.I. α-glucosidase inhibitory constituents from Cuscuta reflexa. Chem. Pharm. Bull. 2002, 50, 112–114. [Google Scholar] [CrossRef]
- Li, G.-W.; Sun, D.-M.; Hu, Q.-P.; Suo, C.; He, J.; Tong, P.; Chen, X.; Cao, S. Fingerprints and quantitative analysis of Cuscuta australis R. Br. processed products. Cent. South Pharm. 2022, 20, 992–998. [Google Scholar]
- Wang, J.; Tan, D.; Wei, G.; Guo, Y.; Chen, C.; Zhu, H.; Xue, Y.; Yin, C.; Zhang, Y. Studies on the Chemical Constituents of Cuscuta chinensis. Chem Nat Compd 2016, 52, 1133–1136. [Google Scholar] [CrossRef]
- Rho, T.; Yoon, K.D. Application of off-line two-dimensional high-performance countercurrent chromatography on the chloroform-soluble extract of Cuscuta auralis seeds. J. Sep. Sci. 2018, 41, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Du, X.M.; Kohinata, K.; Kawasaki, T.; Guo, Y.; Miyahara, K. Components of the ether-insoluble resin glycoside-like fraction from Cuscuta chinensis. Phytochemistry 1998, 48, 843–850. [Google Scholar] [CrossRef]
- Fan, B.-Y.; Luo, J.-G.; Gu, Y.-C.; Kong, L.-Y. Unusual ether-type resin glycoside dimers from the seeds of Cuscuta chinensis. Tetrahedron 2014, 70, 2003–2014. [Google Scholar] [CrossRef]
- Vijikumar, S.; Ramanathan, K.; Devi, B. Cuscuta reflexa Roxb-A wonderful miracle plant in ethnomedicine. Indian J. Nat. Sci. 2011, 976, 997. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, Y.; Hu, J.; Gu, Y.; Li, W.; Yue, H.; An, S.; Sun, N.; Zhang, P.; Li, N.; et al. Herbal Cuscutae Semen Contributes to Oxidative Stress Tolerance and Extends Lifespan via Sirtuin1 in Caenorhabditis elegans. Antioxidants 2025, 14, 786. https://doi.org/10.3390/antiox14070786
Chen C, Liu Y, Hu J, Gu Y, Li W, Yue H, An S, Sun N, Zhang P, Li N, et al. Herbal Cuscutae Semen Contributes to Oxidative Stress Tolerance and Extends Lifespan via Sirtuin1 in Caenorhabditis elegans. Antioxidants. 2025; 14(7):786. https://doi.org/10.3390/antiox14070786
Chicago/Turabian StyleChen, Chunyan, Yudie Liu, Jing Hu, Yihan Gu, Weiwei Li, Hui Yue, Sijing An, Na Sun, Peng Zhang, Nan Li, and et al. 2025. "Herbal Cuscutae Semen Contributes to Oxidative Stress Tolerance and Extends Lifespan via Sirtuin1 in Caenorhabditis elegans" Antioxidants 14, no. 7: 786. https://doi.org/10.3390/antiox14070786
APA StyleChen, C., Liu, Y., Hu, J., Gu, Y., Li, W., Yue, H., An, S., Sun, N., Zhang, P., Li, N., & Miao, L. (2025). Herbal Cuscutae Semen Contributes to Oxidative Stress Tolerance and Extends Lifespan via Sirtuin1 in Caenorhabditis elegans. Antioxidants, 14(7), 786. https://doi.org/10.3390/antiox14070786





