Paternal and/or Maternal Blackberry (Rubus spp.) Polyphenolic Extract Consumption Improved Paternal Fertility and Differentially Affected Female Offspring Antioxidant Capacity and Metabolic Programming in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Blackberry Methanolic Extract Preparation
2.2. Blackberry Fruit and Methanolic Extract Chemical Characterization
2.2.1. Total Phenolic Content
2.2.2. Identification and Quantification of Phenolic Compounds
2.2.3. Total Antioxidant Capacity
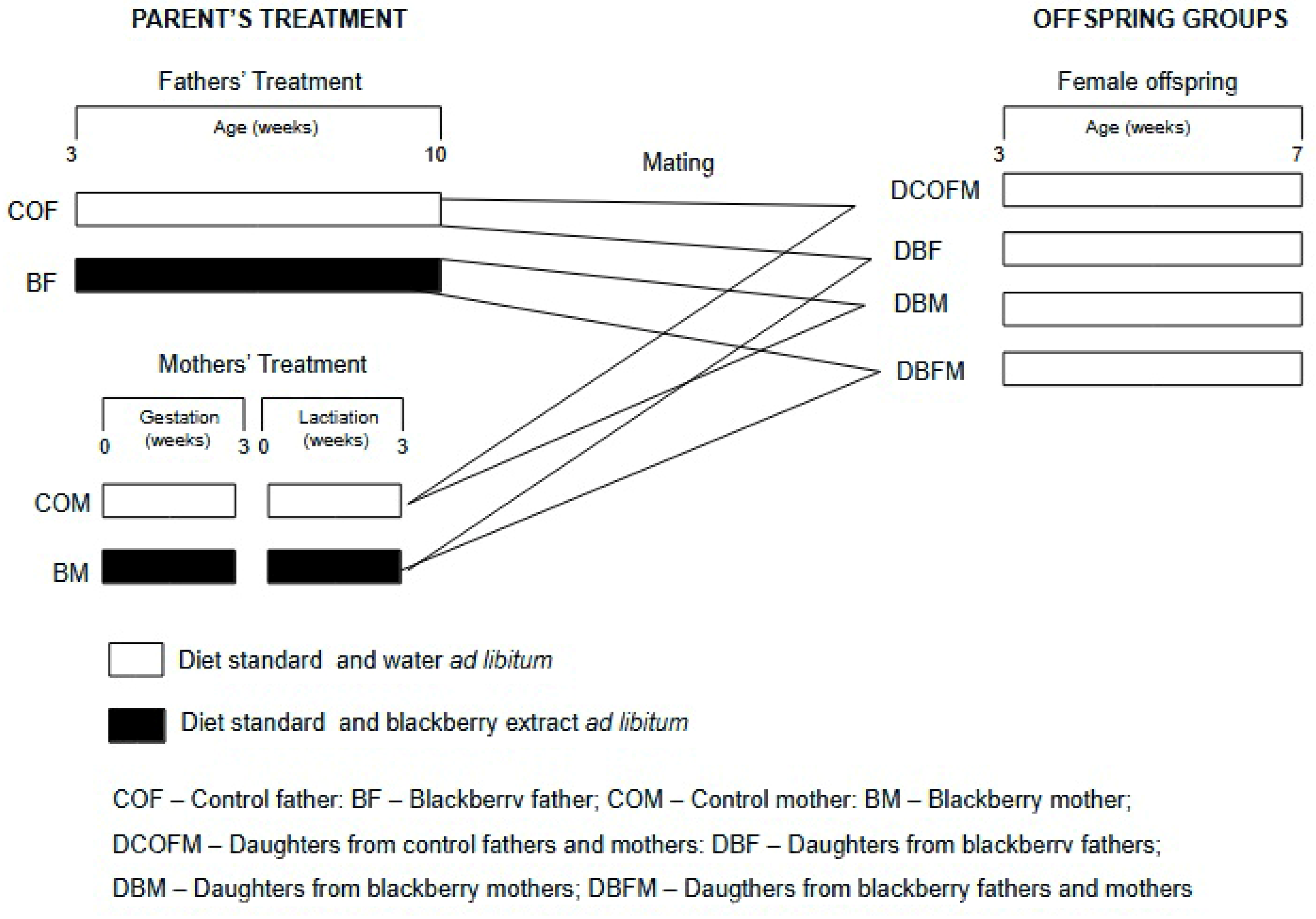
2.3. Experimental Design
2.4. Father’s Reproductive Parameters
2.4.1. Tissue Collection and Preparation
2.4.2. Testicular Histomorphometrical Analysis
2.4.3. Sperm Parameters
2.4.4. Fathers’ Plasma Testosterone Levels
2.5. Gestational Outcomes and Offspring Developmental Parameters
2.6. Fathers, Mothers, and Female Offspring Antioxidant Capacity and Enzyme Activity
2.7. Female Offspring Glucose Tolerance
2.8. Statistical Analysis
3. Results
3.1. Blackberry Fruit and Methanolic Extract Chemical Characterization
3.2. Fathers and Mothers
3.2.1. Body Mass Gain and Organ Weight
3.2.2. Antioxidant Capacity and Enzymatic Activity
3.2.3. Reproductive Parameters and Litter Characteristics
3.3. Female Offspring
3.3.1. Body Weight at Weaning, Body Mass Gain, and Organ Weight
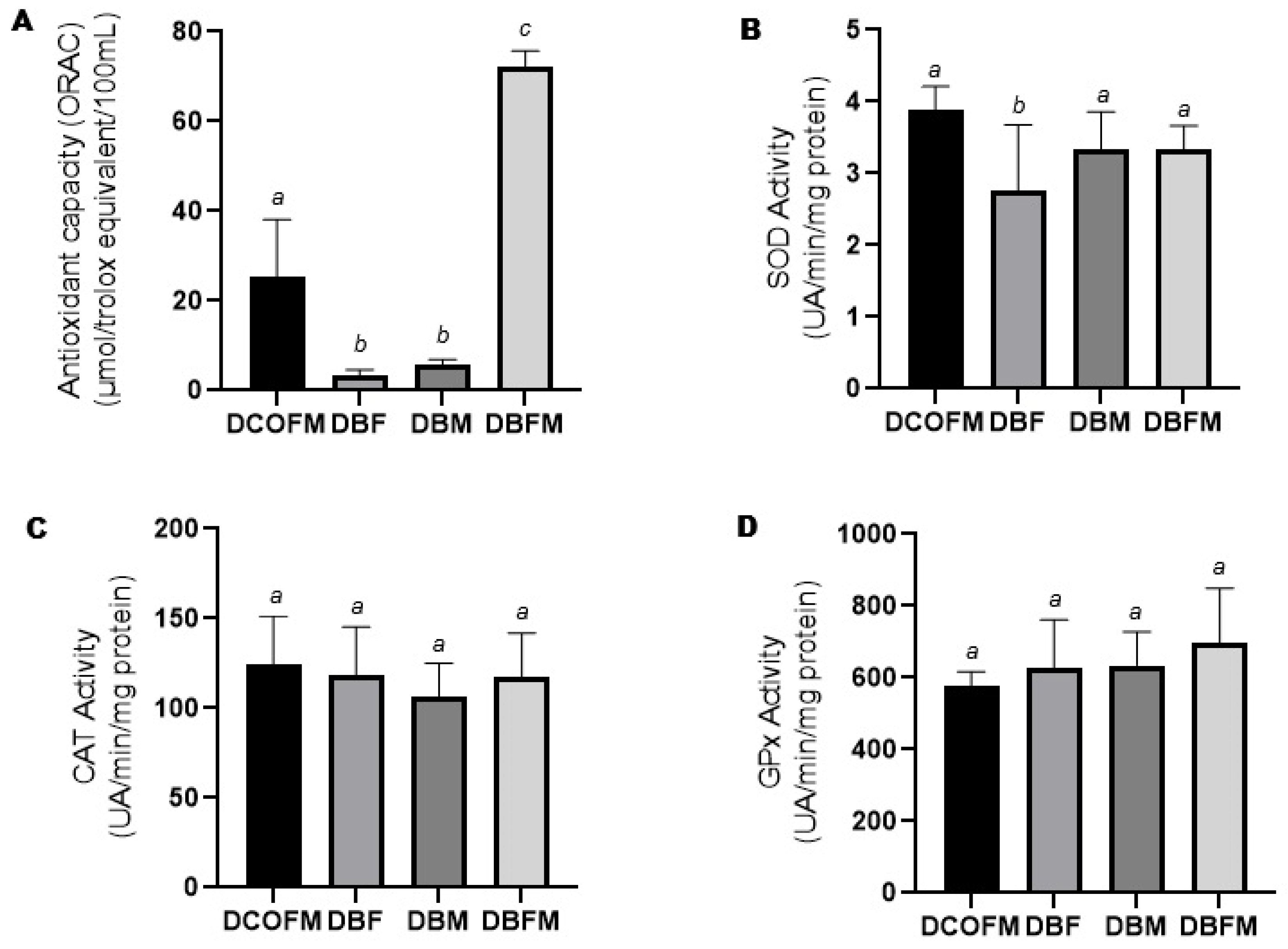
3.3.2. Antioxidant Capacity and Enzymatic Activity
3.3.3. Intraperitoneal Glucose Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassimotto, N.M.; Pinto, M.D.A.S.; Lajolo, F.M. Antioxidant status in humans after consumption of blackberry (Rubus fruticosus L.) juices with and without defatted milk. J. Agric. Food Chem. 2008, 56, 11727–11733. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.S.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Blackberries and Mulberries: Berries with Significant Health-Promoting Properties. Int. J. Mol. Sci. 2023, 24, 12024. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.E.; Lionetto, M.G. Intracellular redox behavior of quercetin and resveratrol singly and in mixtures. Molecules 2023, 28, 4682. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/pro-oxidant actions of polyphenols from grapevine and wine by-products-base for complementary therapy in ischemic heart diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef]
- Silva, L.B.A.R.; Pinheiro-Castro, N.; Novaes, G.M.; Pascoal, G.F.L.; Ong, T.P. Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Res. Int. 2019, 125, 108646. [Google Scholar] [CrossRef]
- Lynch, F.; Lewis, S.; Macciocca, I.; Craig, J.M. Epigenetics and DOHaD: How translation to predictive testing will require a better public understanding. J. Dev. Orig. Health Dis. 2022, 13, 424–430. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, Y.; Yu, J.; Zeng, Y.; Ren, J.; Wu, Y.; Zhang, Q.; Xiao, X. The effect of maternal dietary polyphenol consumption on offspring metabolism. Crit. Rev. Food Sci. Nutr. 2024, 19, 1–18. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Filesi, C.; Masella, R. Management of reproduction and pregnancy complications in maternal obesity: Which role for dietary polyphenols? Biofactors 2014, 40, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Hastings-Tolsma, M.; Stoffel, R.T.; Quintana, A.S.; Kane, R.R.; Turner, J.; Wang, X. Effect of Rubus idaeus L. Consumption during pregnancy on maternal mice and their offspring. J. Med. Food. 2022, 25, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Deckmann, I.; Santos-Terra, J.; Martel, F.; Vieira Carletti, J. Common pregnancy complications and polyphenols intake: An overview. Crit. Rev. Food Sci. Nutr. 2024, 64, 5924–5957. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Oms-Oliu, G.; Crescenti, A.; del Bas, J.M.; Ras, M.R.; Arola, L.; Caimari, A. Distribution of grape seed flavanols and their metabolites in pregnant rats and their fetuses. Mol. Nutr. Food Res. 2013, 57, 1741–1752. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Aitken, R.J.; Lane, M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring. Ann. Nutr. Metab. 2014, 64, 231–238. [Google Scholar] [CrossRef]
- Soubry, A. POHaD: Why we should study future fathers. Environ. Epigenet. 2018, 4, dvy007. [Google Scholar] [CrossRef]
- Pascoal, G.F.L.; Geraldi, M.V.; Maróstica, M.R., Jr.; Ong, T.P. Effect of paternal diet on spermatogenesis and offspring health: Focus on epigenetics and interventions with food bioactive compounds. Nutrients 2022, 14, 2150. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Kang, W.X.; Sandeman, L.Y.; Corbett, M.A.; Owens, J.A.; Lane, M. Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Sci. Rep. 2016, 6, 27010. [Google Scholar] [CrossRef]
- Eslamian, G.; Amirjannati, N.; Rashidkhani, B.; Sadeghi, M.R.; Hekmatdoost, A. Intake of food groups and idiopathic asthenozoospermia: A case-control study. Hum. Reprod. 2012, 27, 3328–3336. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Cui, F.; Kolehmainen, M.; Chen, J.; Zhang, L.; Zarei, I. Exploring the potential protective role of anthocyanins in mitigating micro/nanoplastic-induced reproductive toxicity: A steroid receptor perspective. J. Pharm. Anal. 2025, 15, 101148. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, B.; Bordiga, M.; Li, H.; Travaglia, F.; Bai, S.; Chen, J.; Bai, W. Cyanidin-3-O-glucoside supplement improves sperm quality and spermatogenesis in a mice model of ulcerative colitis. Nutrients 2022, 14, 984. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. Quercetin action on health and female reproduction in mammals. Crit. Rev. Food Sci. Nutr. 2024, 64, 12670–12684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Lu, G.; Gu, J. Pharmacological activity of flavonoid quercetin and its therapeutic potential in testicular injury. Nutrients 2023, 15, 2231. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Zhang, Y.; Bai, S.; Jiang, Y.; Lai, C.; Li, X.; Bai, W. Paternal cyanidin-3-o-glucoside diet improved high-fat, high-fructose diet-induced intergenerational inheritance in male offspring’s susceptibility to high-fat diet-induced testicular and sperm damage. Reprod. Sci. 2025, 32, 1102–1114. [Google Scholar] [CrossRef]
- Ruiz-Martínez, S.M.; Guzmán-Gerónimo, R.I.; Alvarado-Olivarez, M.; Santiago-Roque, I.; Palma-Jacinto, J.A. Effect of blackberry juice consumption by pregnant rats on brain length and cell density of dentate gyrus in male wistar pups. J. Med. Food. 2024, 27, 901–904. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Sestari, I.; Zsögön, A.; Rehder, G.G.; Teixeira, L.L.; Hassimotto, N.M.A.; Purgatto, E.; Benedito, V.A.; Peres, L.E.P. Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv. Micro-Tom) as a tool to produce nutrient-rich fruits. Sci. Hortic. 2014, 175, 111–120. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Predes, F.S.; Diamante, M.A.; Dolder, H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010, 91, 125–131. [Google Scholar] [CrossRef]
- Thayer, K.A.; Ruhlen, R.L.; Howdeshell, K.L.; Buchanan, D.L.; Cooke, P.S.; Preziosi, D.; Welshons, W.V.; Haseman, J.; vom Saal, F.S. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Hum. Reprod. 2001, 16, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Seed, J.; Chapin, R.E.; Clegg, E.D.; Dostal, L.A.; Foote, R.H.; Hurtt, M.E.; Klinefelter, G.R.; Makris, S.L.; Perreault, S.D.; Schrader, S.; et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: A consensus report. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Reprod. Toxicol. 1996, 10, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Adamo, A.M.; Llesuy, S.F.; Pasquini, J.M.; Boveris, A. Brain chemiluminescence and oxidative stress in hyperthyroid rats. Biochem. J. 1989, 263, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Guido, L.N.; Rosim, M.P.; Andrade, F.O.; Jin, L.; Inchauspe, J.; Pires, V.C.; Castro, I.A.; Hilakivi-Clarke, L.; Assis, S.; et al. Paternal programming of breast cancer risk in daughters in a rat model: Opposing effects of animal- and plant-based high-fat diets. Breast Cancer Res. 2016, 18, 71. [Google Scholar] [CrossRef]
- Jordheim, M.; Enerstvedt, K.H.; Andersen, O.M. Identification of cyaniding 3-O-β-(6″-(3-hydroxy-3-methylglutaroyl)glucoside) and other anthocyanins from wild and cultivated blackberries. J. Agric. Food Chem. 2011, 59, 7436–7440. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Lajolo, F.M. Antioxidant status in rats after long-term intake of anthocyanins and ellagitannins from blackberries. J. Sci. Food Agric. 2011, 91, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Puig, E.; Urpí-Sardà, M.; Pérez-Cano, F.J.; Franch, A.; Castellote, C.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Castell, M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J. Agric. Food Chem. 2007, 55, 6431–6438. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; El-Shenawy, N.S.; Ismail, H.A. Protective effects of blackberry and quercetin on sodium fluoride-induced oxidative stress and histological changes in the hepatic, renal, testis and brain tissue of male rat. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 237–251. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and male fertility: The impact of nutrients and antioxidants on sperm energetic metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef]
- Zepeda, A.B.; Calaf, G.M.; Figueroa, C.A.; Farías, J.G. Blueberries prevent the effect of intermittent hypobaric hypoxia in rat epididymis. Andrologia 2014, 46, 766–769. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, C.; Li, X.; Sun, J.; Tian, L.; Bai, W. Cyanidin-3-O-glucoside at low doses protected against 3-chloro-1,2-propanediol induced testis injury and improved spermatogenesis in male rats. J. Agric. Food Chem. 2018, 66, 12675–12684. [Google Scholar] [CrossRef]
- Chen, S.J.; Allam, J.P.; Duan, Y.G.; Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch. Gynecol. Obstet. 2013, 288, 191–199. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Borgmann, H.; Struck, J.P.; Salem, J.; Kuru, T.H. Antioxidant supplementation on male fertility—A systematic review. Antioxidants 2023, 12, 836. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and polyphenols: Concentrations and relation to male infertility and treatment success. Oxid. Med. Cell Longev. 2016, 2016, 9140925. [Google Scholar] [CrossRef]
- Zhu, F.Q.; Hu, J.; Lv, F.H.; Cheng, P.; Gao, S. Effects of oligomeric grape seed proanthocyanidins on L-NAME-induced hypertension in pregnant mice: Role of oxidative stress and endothelial dysfunction. Phytother. Res. 2018, 32, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Crew, J.; Ebersole, J.L.; Kinney, J.W.; Salazar, A.M.; Planinic, P.; Alexander, J.M. Dietary blueberry and soluble fiber improve serum antioxidant and adipokine biomarkers and lipid peroxidation in pregnant women with obesity and at risk for gestational diabetes. Antioxidants 2021, 10, 1318. [Google Scholar] [CrossRef]
- Bertoldo, A.; Pizzol, D.; Yon, D.K.; Callegari, M.; Gobbo, V.; Cuccurese, P.; Butler, L.; Caminada, S.; Stebbing, J.; Richardson, F.; et al. Resveratrol and female fertility: A systematic review. Int. J. Mol. Sci. 2024, 25, 12792. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Agosti, M.; MeNu Group. Nutrition in the first 1000 days: Ten practices to minimize obesity emerging from published science. Int. J. Environ. Res. Public Health 2017, 14, 1491. [Google Scholar] [CrossRef]
- Khazaeel, K.; Hussein, H.A.; Ranjbar, R.; Tabandeh, M.R.; Alahmed, J.A.S. Modulatory effects of quercetin on histological changes, biochemical and oxidative stress of rat placenta induced by inhalation exposure to crude oil vapor. Reprod. Toxicol. 2024, 125, 108560. [Google Scholar] [CrossRef]
- Vanhees, K.; van Schooten, F.J.; van Doorn-Khosrovani, S.B.v.W.; van Helden, S.; Munnia, A.; Peluso, M.; Briedé, J.J.; Haenen, G.R.; Godschalk, R.W. Intrauterine exposure to flavonoids modifies antioxidant status at adulthood and decreases oxidative stress-induced DNA damage. Free Radic. Biol. Med. 2013, 57, 154–161. [Google Scholar] [CrossRef]
- Zielinsky, P.; Martignoni, F.V.; Markoski, M.; Zucatti, K.P.; Dos Santos Marinho, G.; Pozzobon, G.; Magno, P.R.; de Bittencourt Antunes, V.; Sulis, N.M.; Cardoso, A.; et al. Maternal ingestion of cocoa causes constriction of fetal ductus arteriosus in rats. Sci. Rep. 2021, 11, 9929. [Google Scholar] [CrossRef]
- Vanhees, K.; Godschalk, R.W.; Sanders, A.; van Waalwijk van Doorn-Khosrovani, S.B.; van Schooten, F.J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 2011, 290, 350–358. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Xu, H.; Lyv, Y.; Feng, X.; Fang, Y.; Xu, Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014, 53, 1669–1683. [Google Scholar] [CrossRef]
- Gindri Dos Santos, B.; Peres Klein, C.; Scortegagna Crestani, M.; Moura Maurmann, R.; Mateus Hözer, R.; Dos Santos Rodrigues, K.; Maciel August, P.; Matté, C. Naringin supplementation during pregnancy induces sex and region-specific alterations in the offspring’s brain redox status. Int. J. Environ. Res. Public Health 2021, 18, 4805. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The role of dietary polyphenols in pregnancy and pregnancy-related disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Rastegar, M.; Davie, J.R. Epigenetic control. J. Cell. Physiol. 2009, 219, 243–250. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on placental gene expression and fetal antioxidant status, dna-methylation and phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef]
- Ramadan, A.G.; Abdel-Rehim, W.M.; El-Tahan, R.A.; Elblehi, S.S.; Kamel, M.A.; Shaker, S.A. Maternal and paternal obesity differentially reprogram the ovarian mitochondrial biogenesis of F1 female rats. Sci. Rep. 2023, 13, 15480. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Lehrner, A.; Desarnaud, F.; Bader, H.N.; Makotkine, I.; Flory, J.D.; Bierer, L.M.; Meaney, M.J. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 2014, 171, 872–880. [Google Scholar] [CrossRef]
- McPherson, N.O.; Bell, V.G.; Zander-Fox, D.L.; Fullston, T.; Wu, L.L.; Robker, R.L.; Lane, M. When two obese parents are worse than one! Impacts on embryo and fetal development. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E568–E581. [Google Scholar] [CrossRef]
- Ornellas, F.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Combined parental obesity augments single-parent obesity effects on hypothalamus inflammation, leptin signaling (JAK/STAT), hyperphagia, and obesity in the adult mice offspring. Physiol. Behav. 2016, 153, 47–55. [Google Scholar] [CrossRef]
- César, H.; Sertorio, M.N.; de Souza, E.A.; Jamar, G.; Santamarina, A.; Jucá, A.; Casagrande, B.P.; Pisani, L.P. Parental high-fat high-sugar diet programming and hypothalamus adipose tissue axis in male Wistar rats. Eur. J. Nutr. 2022, 61, 523–537. [Google Scholar] [CrossRef]
- Zheng, J.; Alves-Wagner, A.B.; Stanford, K.I.; Prince, N.B.; So, K.; Mul, J.D.; Dirice, E.; Hirshman, M.F.; Kulkarni, R.N.; Goodyear, L.J. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res. Care 2020, 8, e000890. [Google Scholar] [CrossRef]
- Li, S.; Wu, H.; Chen, M.; Tollefsbol, T.O. Paternal combined botanicals contribute to the prevention of estrogen receptor-negative mammary cancer in transgenic mice. J. Nutr. 2023, 153, 1959–1973. [Google Scholar] [CrossRef]
- Rahal, O.M.; Pabona, J.M.; Kelly, T.; Huang, Y.; Hennings, L.J.; Prior, R.L.; Al-Dwairi, A.; Simmen, F.A.; Simmen, R.C. Suppression of Wnt1-induced mammary tumor growth and lower serum insulin in offspring exposed to maternal blueberry diet suggest early dietary influence on developmental programming. Carcinogenesis 2013, 34, 464–474. [Google Scholar] [CrossRef]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 day oral toxicity study of blueberry polyphenols in ovariectomized sprague-dawley rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef]
- Jian, X.; Shi, C.; Xu, T.; Liu, B.; Zhou, L.; Jiang, L.; Liu, K. Efficacy and safety of dietary polyphenol administration as assessed by hormonal, glycolipid metabolism, inflammation and oxidative stress parameters in patients with PCOS: A meta-analysis and systematic review. Crit. Rev. Food Sci. Nutr. 2024, 16, 1–25. [Google Scholar] [CrossRef]
- Hahn, M.; Baierle, M.; Charão, M.F.; Bubols, G.B.; Gravina, F.S.; Zielinsky, P.; Arbo, M.D.; Cristina Garcia, S. Polyphenol-rich food general and on pregnancy effects: A review. Drug Chem. Toxicol. 2017, 40, 368–374. [Google Scholar] [CrossRef]
- Vian, I.; Zielinsky, P.; Zílio, A.M.; Schaun, M.I.; Brum, C.; Lampert, K.V.; De Ávila, N.; Baldissera, G.; Klanovicz, T.M.; Zenki, K.; et al. Increase of prostaglandin E2 in the reversal of fetal ductal constriction after polyphenol restriction. Ultrasound Obstet. Gynecol. 2018, 52, 617–622. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible side effects of polyphenols and their interactions with medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Ros, P.; Díaz, F.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Chowen, J.A. Resveratrol intake during pregnancy and lactation modulates the early metabolic effects of maternal nutrition differently in male and female offspring. Endocrinology 2018, 159, 810–825. [Google Scholar] [CrossRef]

| Blackberry Fruit | Blackberry Methanolic Extract | |
|---|---|---|
| Total phenolic compounds (mg Gallic acid equivalent/100 g or 100 mL) | 157 ± 23 | 154 ± 11 |
| Cyanidin (mg/100 g or 100 mL) | 105 ± 6 | 176 ± 2.4 |
| Quercetin (mg/100 g or 100 mL) | 26 ± 2 | 26 ± 1 |
| Free ellagic acid (mg/100 g or 100 mL) | 6 ± 0.2 | 8 ± 2 |
| Total ellagic acid (ellagitannins) (mg/100 g or 100 mL) | 430 ± 27 | 65 ± 47 |
| Antioxidant capacity (DPPH) (µmoL Trolox equivalent/100 g or 100 mL) | 1525 ± 88 | 80 ± 5 |
| Antioxidant capacity (ORAC) (µmoL Trolox equivalent/100 g or 100 mL) | 2265 ± 612 | 644 ± 770 |
| Variables | Experimental Groups | |
|---|---|---|
| COFs | BFs | |
| Body mass gain (g) | 19.25 ± 2.52 | 18.76 ± 2.45 |
| Testis relative weight (%) | 0.24 ± 0.07 | 0.25 ± 0.09 |
| Epididymis relative weight (%) | 0.08 ± 0.05 | 0.09 ± 0.04 |
| Liver relative weight (%) | 4.04 ± 0.36 | 4.14 ± 0.51 |
| Lung relative weight (%) | 0.76 ± 0.10 | 0.74 ± 0.10 |
| Heart relative weight (%) | 0.60 ± 0.07 | 0.59 ± 0.08 |
| Kidney relative weight (%) | 0.58 ± 0.05 | 0.60 ± 0.07 |
| Abdominal adipose tissue relative weight (%) | 1.85 ± 0.61 | 1.78 ± 0.63 |
| Retroperitoneal adipose tissue relative weight (%) | 0.60 ± 0.68 | 0.51 ± 0.29 |
| Retroepididymal adipose tissue relative weight (%) | 1.58 ± 0.74 | 1.53 ± 0.73 |
| Variables | Experimental Groups | |
|---|---|---|
| COMs | BMs | |
| Body mass gestational gain (g) | 15.73 ± 1.67 | 15.89 ± 2.95 |
| Body mass lactational gain (g) | −1.50 ± 1.61 | −1.25 ± 1.21 |
| Uterus relative weight (with ovary) (%) | 0.46 ± 0.15 | 0.51 ± 0.12 |
| Liver relative weight (%) | 5.61 ± 1.37 | 5.20 ± 0.78 |
| Lung relative weight (%) | 0.97 ± 0.20 | 0.99 ± 0.09 |
| Heart relative weight (%) | 0.68 ± 0.14 | 0.77 ± 0.19 |
| Kidney relative weight (%) | 0.54 ± 0.08 | 0.61 ± 0.07 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| COFs | BFs | COMs | BMs | |
| Plasma antioxidant capacity (ORAC) (µmoL Trolox equivalent/100 mL) | 8.19 ± 3.20 | 58.60 ± 9.64 * | 80.33 ± 20.41 | 80.74 ± 10.06 |
| SOD activity (liver) (UA/min/mg protein) | 2.27 ± 0.23 | 4.76 ± 0.31 * | 3.96 ± 0.93 | 2.88 ± 0.45 * |
| CAT activity (liver) (UA/min/mg protein) | 136.40 ± 14.58 | 151.76 ± 18.03 | 125.69 ± 18.20 | 129.57 ± 26.66 |
| GPx activity (liver) (UA/min/mg protein) | 514.39 ± 56.61 | 586.55 ± 95.28 | 620.86 ± 127.59 | 511.27 ± 106.46 |
| SOD activity (testis) (UA/min/mg protein) | 28.16 ± 0.37 | 17.43 ± 1.16 * | - | - |
| CAT activity (testis) (UA/min/mg protein) | 20.25 ± 4.86 | 9.83 ± 2.36 * | - | - |
| GPx activity (testis) (UA/min/mg protein) | 43.05 ± 5.78 | 44.32 ± 7.89 | - | - |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| COFMs | BFs | BMs | BFMs | |
| Pregnancy rate (%) | 63 a | 79 b | 71 a | 75 a |
| Perinatal mortality (%) | 34 a | 24 b | 19 b | 9 c |
| Number of total pups/litter | 7 ± 1 a | 7 ± 2 a | 7 ± 2 a | 7 ± 2 a |
| Variables | Experimental Groups | |||
|---|---|---|---|---|
| DCOFMs | DBFs | DBMs | DBFMs | |
| Body weight at weaning (g) | 9.05 ± 1.23 a | 8.91 ± 1.83 a | 7.67 ± 1.28 b | 9.02 ± 1.23 a |
| Body mass gain (g) | 7.17 ± 1.51 a | 6.98 ± 1.79 a | 7.63 ± 2.28 a | 7.05 ± 1.58 a |
| Uterus relative weight (with ovary) (%) | 0.66 ± 0.21 a | 0.54 ± 0.22 a | 0.51 ± 0.14 a | 0.68 ± 0.23 a |
| Liver relative weight (%) | 0.66 ± 0.07 a | 0.62 ± 0.08 a | 0.70 ± 0.09 a | 0.69 ± 0.11 a |
| Lung relative weight (%) | 0.84 ± 0.07 a | 0.71 ± 0.11 a | 0.79 ± 0.09 a | 0.78 ± 0.08 a |
| Heart relative weight (%) | 5.77 ± 0.57 a | 5.00 ± 1.10 a | 5.64 ± 0.27 a | 5.23 ± 0.36 a |
| Kidney relative weight (%) | 0.68 ± 0.07 a | 0.63 ± 0.08 a | 0.70 ± 0.06 a | 0.66 ± 0.08 a |
| Retroperitoneal adipose tissue relative weight (%) | 0.67 ± 0.29 a | 0.61 ± 0.28 a | 0.53 ± 0.19 a | 0.52 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, V.C.; Anacleto, S.L.; Matté, C.; Aguiar, O., Jr.; Lajolo, F.M.; Hassimotto, N.M.A.; Ong, T.P. Paternal and/or Maternal Blackberry (Rubus spp.) Polyphenolic Extract Consumption Improved Paternal Fertility and Differentially Affected Female Offspring Antioxidant Capacity and Metabolic Programming in a Mouse Model. Antioxidants 2025, 14, 779. https://doi.org/10.3390/antiox14070779
Pires VC, Anacleto SL, Matté C, Aguiar O Jr., Lajolo FM, Hassimotto NMA, Ong TP. Paternal and/or Maternal Blackberry (Rubus spp.) Polyphenolic Extract Consumption Improved Paternal Fertility and Differentially Affected Female Offspring Antioxidant Capacity and Metabolic Programming in a Mouse Model. Antioxidants. 2025; 14(7):779. https://doi.org/10.3390/antiox14070779
Chicago/Turabian StylePires, Vanessa Cardoso, Sara Lima Anacleto, Cristiane Matté, Odair Aguiar, Jr., Franco Maria Lajolo, Neuza Mariko Aymoto Hassimotto, and Thomas Prates Ong. 2025. "Paternal and/or Maternal Blackberry (Rubus spp.) Polyphenolic Extract Consumption Improved Paternal Fertility and Differentially Affected Female Offspring Antioxidant Capacity and Metabolic Programming in a Mouse Model" Antioxidants 14, no. 7: 779. https://doi.org/10.3390/antiox14070779
APA StylePires, V. C., Anacleto, S. L., Matté, C., Aguiar, O., Jr., Lajolo, F. M., Hassimotto, N. M. A., & Ong, T. P. (2025). Paternal and/or Maternal Blackberry (Rubus spp.) Polyphenolic Extract Consumption Improved Paternal Fertility and Differentially Affected Female Offspring Antioxidant Capacity and Metabolic Programming in a Mouse Model. Antioxidants, 14(7), 779. https://doi.org/10.3390/antiox14070779








