Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications
Abstract
1. Introduction
2. The Physicochemical Properties of Puerarin
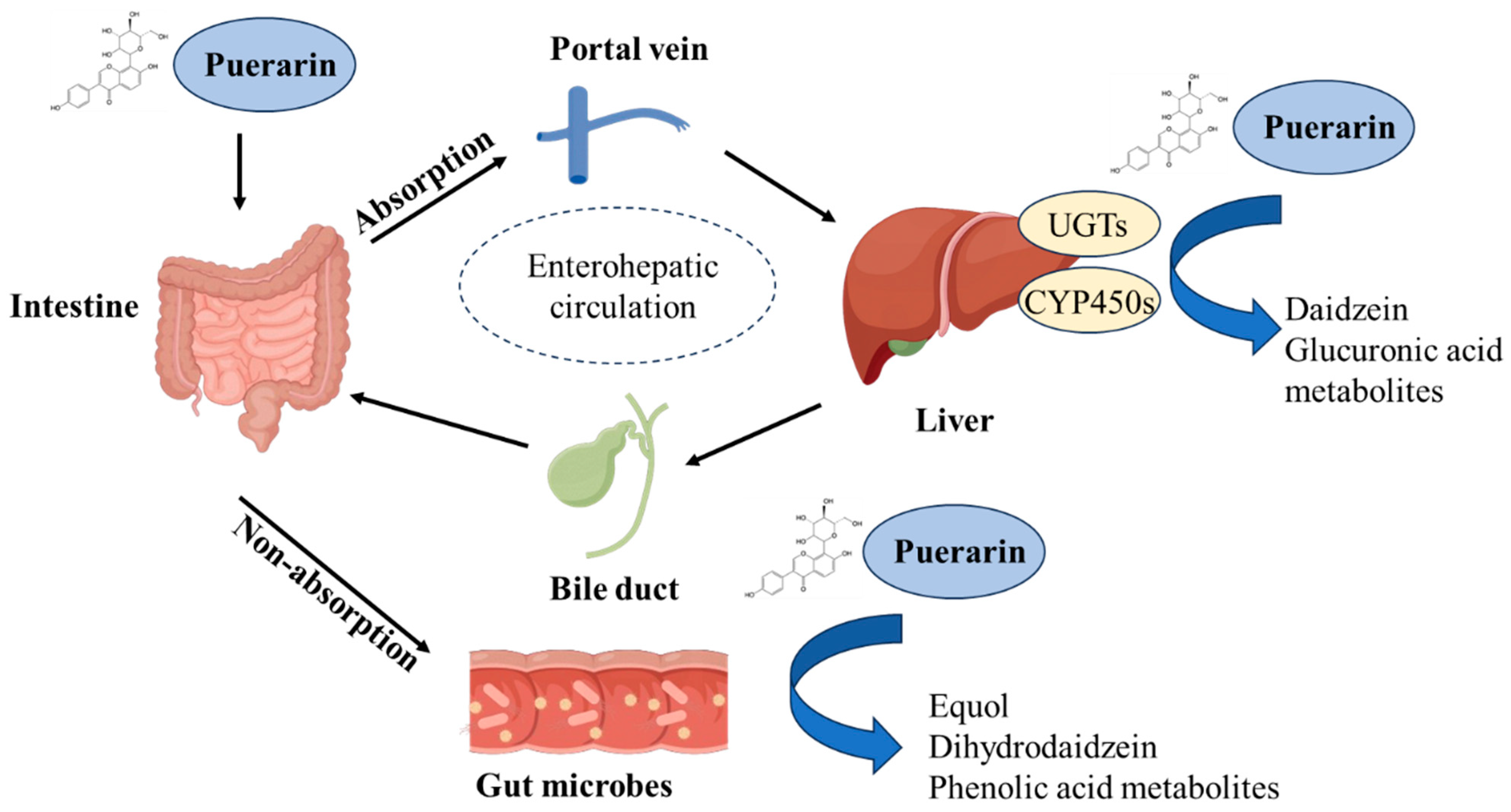
3. Absorption and Metabolism of Puerarin
4. The Regulatory Effect of Puerarin on Intestinal Health of Farm Animals
4.1. Effects of Puerarin on Intestinal Morphology
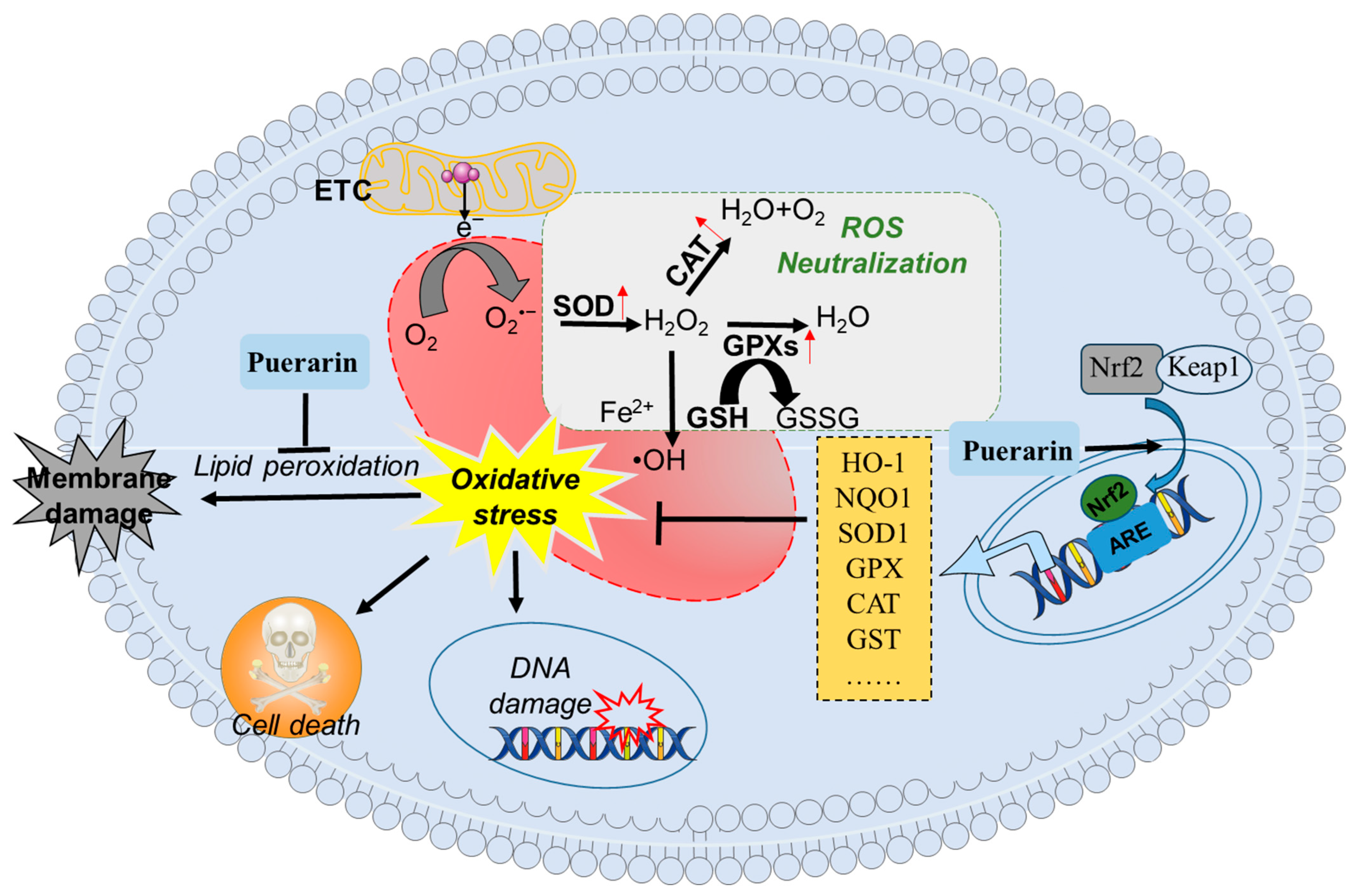
4.2. Effect of Puerarin on Intestinal Antioxidant Capacity
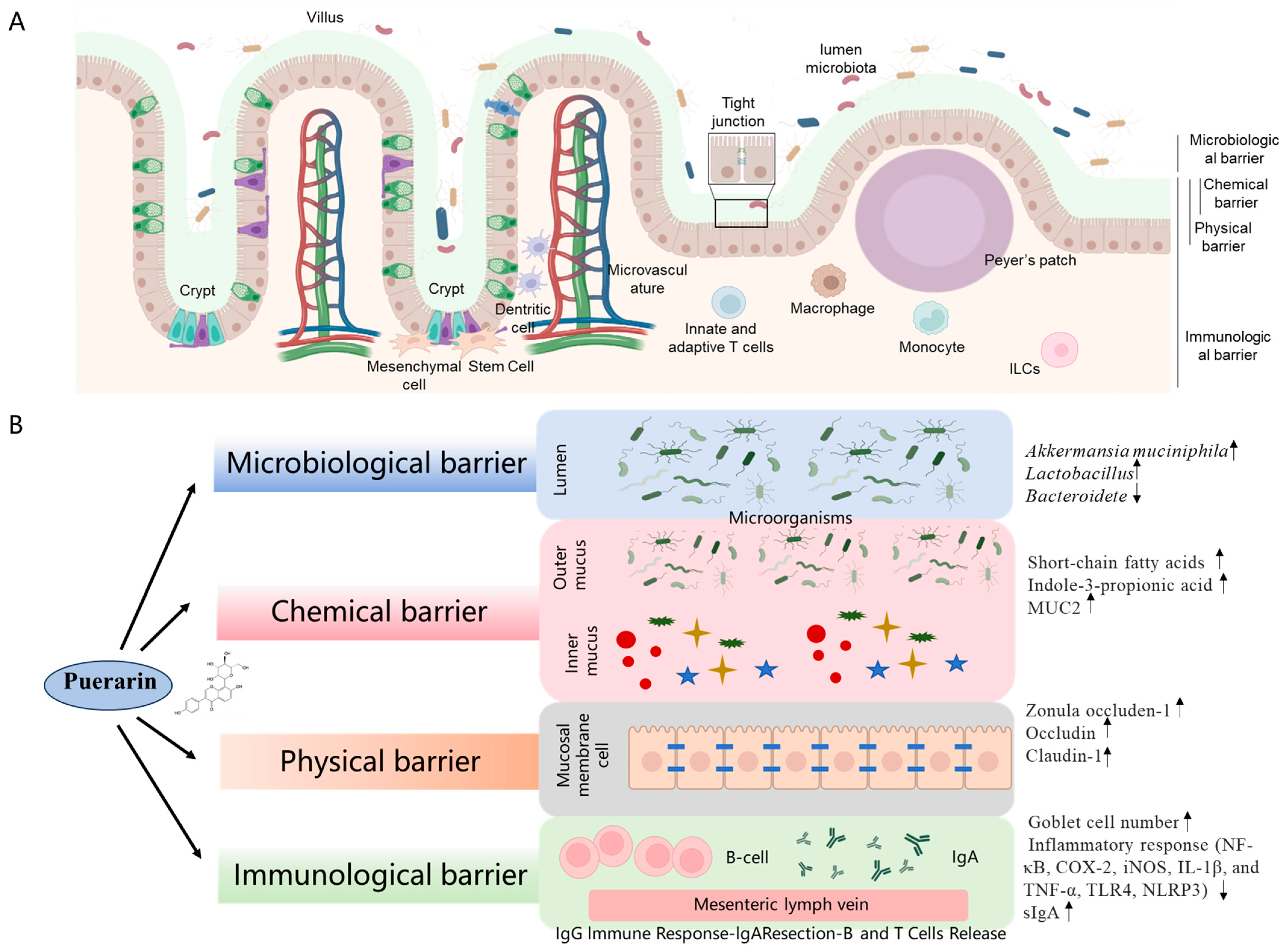
4.3. Effect of Puerarin on Intestinal Barrier Function
4.3.1. Intestinal Microbiological Barrier
4.3.2. Intestinal Chemical Barrier
4.3.3. Intestinal Mechanical Barrier
4.3.4. Intestinal Mucosal Immune Barrier
4.4. Enteric Nervous System
4.5. Others
5. Pharmacological Modulation of Production Performance in Livestock by Puerarin
5.1. Poultry Health
5.2. Porcine Health
5.3. Cattle Health
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Piao, X. Potential Effects of 25-Hydroxycholecalciferol on the Growth Performance, Blood Antioxidant Capacity, Intestinal Barrier Function and Microbiota in Broilers under Lipopolysaccharide Challenge. Antioxidants 2022, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Coffey, A.; O’Mahony, J.M. Alternatives to antibiotics in veterinary medicine: Considerations for the management of Johne’s disease. Anim. Health Res. Rev. 2023, 24, 12–27. [Google Scholar] [CrossRef]
- Zhi, W.; Liu, Y.; Wang, X.; Zhang, H. Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis. J. Ethnopharmacol. 2023, 301, 115749. [Google Scholar] [CrossRef]
- Chen, T.; Lin, Y.; Cao, Z.; Xue, Y.; Wang, W.; Wang, X. Network pharmacology analysis and experimental study strategy reveals the potential mechanism of puerarin against rotavirus. Ann. Transl. Med. 2022, 10, 14. [Google Scholar] [CrossRef]
- Yang, M.; Xia, L.; Song, J.; Hu, H.; Zang, N.; Yang, J.; Zou, Y.; Wang, L.; Zheng, X.; He, Q.; et al. Puerarin ameliorates metabolic dysfunction-associated fatty liver disease by inhibiting ferroptosis and inflammation. Lipids Health Dis. 2023, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef]
- Li, H.T.; Zhong, K.; Xia, Y.F.; Song, J.; Chen, X.Q.; Zhao, W.; Zeng, X.H.; Chen, T.X. Puerarin improves busulfan-induced disruption of spermatogenesis by inhibiting MAPK pathways. Biomed. Pharmacother. 2023, 165, 115231. [Google Scholar] [CrossRef]
- Fang, X.; Lan, X.; Zhu, M.; He, M.; Sun, M.; Cao, Y.; Zhu, D.; Guo, D.; Luo, H. Puerarin Induces Macrophage M2 Polarization to Exert Antinonalcoholic Steatohepatitis Pharmacological Activity via the Activation of Autophagy. J. Agric. Food Chem. 2024, 72, 7187–7202. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Sun, S.; Gong, D.; Liu, R.; Wang, R.; Chen, D.; Yuan, T.; Wang, S.; Xing, C.; Lv, Y.; Du, G.; et al. Puerarin Inhibits NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis via P2X7 Receptor in Cardiomyocytes and Macrophages. Int. J. Mol. Sci. 2023, 24, 13169. [Google Scholar] [CrossRef]
- Lin, S.P.; Zhu, L.; Shi, H.; Ye, S.; Li, Q.; Yin, X.; Xie, Q.; Xu, Q.; Wei, J.X.; Mei, F.; et al. Puerarin prevents sepsis-associated encephalopathy by regulating the AKT1 pathway in microglia. Phytomedicine 2023, 121, 155119. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, D.; Yan, P.; Zheng, F.; Zhu, H.; Yuan, Z.; Yang, X.; Zuo, Y.; Chen, C.; Lu, H.; et al. Puerarin suppresses macrophage M1 polarization to alleviate renal inflammatory injury through antagonizing TLR4/MyD88-mediated NF-κB p65 and JNK/FoxO1 activation. Phytomedicine 2024, 132, 155813. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Suliburska, J.; Sidor, A. Puerarin-an isoflavone with beneficial effects on bone health. Front. Biosci. (Landmark Ed.) 2021, 26, 1653–1667. [Google Scholar] [CrossRef]
- Wu, M.; Yi, D.; Zhang, Q.; Wu, T.; Yu, K.; Peng, M.; Wang, L.; Zhao, D.; Hou, Y.; Wu, G. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus. Sci. Rep. 2021, 11, 6552. [Google Scholar] [CrossRef] [PubMed]
- Shaojun, Z.; Yanyan, X.; Jian, C.; Xia, Z.; Qiang, F.; Saiping, J. Effects of puerarin on lipopolysaccharide-induced myocardial dysfunction in isolated rat hearts. Pak. J. Pharm. Sci. 2017, 30, 1195–1202. [Google Scholar] [PubMed]
- Wang, Y.S.; Li, B.Y.; Xing, Y.F.; Huang, J.C.; Chen, Z.S.; Yue, L.; Zou, Y.G.; Guo, B. Puerarin Ameliorated PCOS through Preventing Mitochondrial Dysfunction Dependent on the Maintenance of Intracellular Calcium Homeostasis. J. Agric. Food Chem. 2024, 72, 2963–2976. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, A.; Liu, C.; Li, Y.; Shi, Y.; Zhou, M.S. Puerarin Improves Vascular Insulin Resistance and Cardiovascular Remodeling in Salt-Sensitive Hypertension. Am. J. Chin. Med. 2017, 45, 1169–1184. [Google Scholar] [CrossRef]
- Wei, H.; Sun, M.; Wang, R.; Zeng, H.; Zhao, B.; Jin, S. Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling. J. Cell. Mol. Med. 2024, 28, e18239. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Zhou, H.; Li, Y.; Wang, A.; Wang, H.; Zhou, M.S. Puerarin protects against endothelial dysfunction and end-organ damage in Ang II-induced hypertension. Clin. Exp. Hypertens. 2017, 39, 58–64. [Google Scholar] [CrossRef]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Y.; Shi, X.; Zou, T.; Yang, L.; OuYang, J.; Bian, Y.; Liu, Y.; Li, G. Dietary puerarin supplementation improves immune function in the small intestines of oxidized oil-challenged broilers. Anim. Sci. J. 2023, 94, e13895. [Google Scholar] [CrossRef]
- Wang, R.; Li, T.; Pan, Z.; Chen, H.; Xu, S.; Lu, X.; Shi, K.; Bian, B.; Wu, G. Effect of Dietary Puerarin Supplementation on Growth Performance, Immune Response, Antioxidant Capacity, and Intestinal Morphology in Domestic Pigeons (Columba livia). J. Poult. Sci. 2024, 61, 2024003. [Google Scholar] [CrossRef]
- Du, X.; Zhao, D.; Pian, H.; Li, Y.; Wu, X.; Liu, F.; Yu, D. Effects of puerarin as a feed additive on the laying performance, egg quality, endocrine hormones, antioxidant capacity, and intestinal morphology of aged laying hens. Poult. Sci. 2024, 103, 103420. [Google Scholar] [CrossRef]
- Li, M.; Yuan, D.; Liu, Y.; Jin, H.; Tan, B. Dietary Puerarin Supplementation Alleviates Oxidative Stress in the Small Intestines of Diquat-Challenged Piglets. Animals 2020, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.K.; Leung, S.W.; Xu, Y.C.; Vanhoutte, P.M.; Man, R.Y. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur. J. Pharmacol. 2006, 552, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Ramzan, R.; Zhang, R.; Zhao, D.; Khalid, N.; Deng, M.; Dong, L.; Aziz, M.; Batool, R.; Zhang, M. Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties. Foods 2022, 11, 3591. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C. Puerarin-A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. Int. J. Mol. Sci. 2024, 25, 5222. [Google Scholar] [CrossRef]
- Quan, D.Q.; Xu, G.X.; Wu, X.G. Studies on preparation and absolute bioavailability of a self-emulsifying system containing puerarin. Chem. Pharm. Bull. 2007, 55, 800–803. [Google Scholar] [CrossRef]
- Kato, E.; Kawabata, J. Glucose uptake enhancing activity of puerarin and the role of C-glucoside suggested from activity of related compounds. Bioorg. Med. Chem. Lett. 2010, 20, 4333–4336. [Google Scholar] [CrossRef]
- Zhang, L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019, 26, 860–869. [Google Scholar] [CrossRef]
- Anukunwithaya, T.; Poo, P.; Hunsakunachai, N.; Rodsiri, R.; Malaivijitnond, S.; Khemawoot, P. Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 2018, 19, 25. [Google Scholar] [CrossRef]
- He, Y.X.; Liu, M.N.; Wu, H.; Lan, Q.; Liu, H.; Mazhar, M.; Xue, J.Y.; Zhou, X.; Chen, H.; Li, Z. Puerarin: A hepatoprotective drug from bench to bedside. Chin. Med. 2024, 19, 139. [Google Scholar] [CrossRef]
- Song, X.; Bai, X.; Liu, S.; Dong, L.; Deng, H.; Wang, C. A Novel Microspheres Formulation of Puerarin: Pharmacokinetics Study and In Vivo Pharmacodynamics Evaluations. Evid. Based Complement. Altern. Med. 2016, 2016, 4016963. [Google Scholar] [CrossRef]
- Ye, Q.; Li, J.; Li, T.; Ruan, J.; Wang, H.; Wang, F.; Zhang, X. Development and evaluation of puerarin-loaded controlled release nanostructured lipid carries by central composite design. Drug Dev. Ind. Pharm. 2021, 47, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
- Cheng, G.; Hu, R.; Ye, L.; Wang, B.; Gui, Y.; Gao, S.; Li, X.; Tang, J. Preparation and In Vitro/In Vivo Evaluation of Puerarin Solid Self-Microemulsifying Drug Delivery System by Spherical Crystallization Technique. AAPS PharmSciTech 2016, 17, 1336–1346. [Google Scholar] [CrossRef]
- Wu, H.; Lu, C.; Zhou, A.; Min, Z.; Zhang, Y. Enhanced oral bioavailability of puerarin using microemulsion vehicle. Drug Dev. Ind. Pharm. 2009, 35, 138–144. [Google Scholar] [CrossRef]
- Tang, T.T.; Hu, X.B.; Liao, D.H.; Liu, X.Y.; Xiang, D.X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: Comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. Int. J. Nanomed. 2013, 8, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Yi, Y.; Wu, W.; Hu, F.; Hu, K.; Feng, J. Effects of particle size on the pharmacokinetics of puerarin nanocrystals and microcrystals after oral administration to rat. Int. J. Pharm. 2013, 458, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Li, Y.J.; Yang, L.; Hu, Y.Y.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Borneol and A-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018, 25, 1858–1864. [Google Scholar] [CrossRef]
- Zhao, L.X.; Liu, A.C.; Yu, S.W.; Wang, Z.X.; Lin, X.Q.; Zhai, G.X.; Zhang, Q.Z. The permeability of puerarin loaded poly(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood-brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol. Pharm. Bull. 2013, 36, 1263–1270. [Google Scholar] [CrossRef]
- Kong, H.; Zhang, G.; Cheng, J.; Shi, R.; Zhang, M.; Cao, P.; Zhao, Y.; Qu, H.; Wang, Q. Distribution kinetics of puerarin in rat hippocampus after acute local cerebral ischemia. J. Pharm. Biomed. Anal. 2019, 164, 196–201. [Google Scholar] [CrossRef]
- Gao, L.; Ji, X.; Song, J.; Liu, P.; Yan, F.; Gong, W.; Dang, S.; Luo, Y. Puerarin protects against ischemic brain injury in a rat model of transient focal ischemia. Neurol. Res. 2009, 31, 402–406. [Google Scholar] [CrossRef]
- Li, Y.; Pan, W.S.; Chen, S.L.; Xu, H.X.; Yang, D.J.; Chan, A.S. Pharmacokinetic, tissue distribution, and excretion of puerarin and puerarin-phospholipid complex in rats. Drug Dev. Ind. Pharm. 2006, 32, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Jing, Q.; Shen, Y.; Ma, H.; Cui, J. Quantitative determination of puerarin in dog plasma by HPLC and study on the relative bioavailability of sustained release tablets. J. Pharm. Biomed. Anal. 2006, 41, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhi, H.; Du, F.; Ye, Z.; Wang, N.; Qin, W.; Li, J. A HPLC-UV method for the determination of puerarin in rat plasma after intravenous administration of PEGylated puerarin conjugate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3297–3302. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Brissie, N.; Moore, R.; Wyss, J.M.; Barnes, S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3708–3712. [Google Scholar] [CrossRef]
- Luo, C.F.; Cai, B.; Hou, N.; Yuan, M.; Liu, S.M.; Ji, H.; Xiong, L.G.; Xiong, W.; Luo, J.D.; Chen, M.S. UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes. Arch. Toxicol. 2012, 86, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.F.; Yuan, M.; Chen, M.S.; Liu, S.M.; Ji, H. Metabolites of puerarin identified by liquid chromatography tandem mass spectrometry: Similar metabolic profiles in liver and intestine of rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 363–370. [Google Scholar] [CrossRef]
- Luo, C.F.; Hou, N.; Tian, J.; Yuan, M.; Liu, S.M.; Xiong, L.G.; Luo, J.D.; Chen, M.S. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 933–940. [Google Scholar] [CrossRef]
- Hou, N.; Cai, B.; Ou, C.W.; Zhang, Z.H.; Liu, X.W.; Yuan, M.; Zhao, G.J.; Liu, S.M.; Xiong, L.G.; Luo, J.D.; et al. Puerarin-7-O-glucuronide, a water-soluble puerarin metabolite, prevents angiotensin II-induced cardiomyocyte hypertrophy by reducing oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 535–545. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, J.; Sun, M.; Ji, Y.; Ji, H. Comparative Pharmacokinetic Profiles of Puerarin in Rat Plasma by UHPLC-MS/MS after Oral Administration of Pueraria lobata Extract and Pure Puerarin. J. Anal. Methods Chem. 2020, 2020, 4258156. [Google Scholar] [CrossRef]
- Adelman, D.C.; Murray, J.; Wu, T.T.; Mäki, M.; Green, P.H.; Kelly, C.P. Measuring Change In Small Intestinal Histology In Patients With Celiac Disease. Am. J. Gastroenterol. 2018, 113, 339–347. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Ruan, Z.; Li, J.; Zhang, L.; Lu, H.; Xu, Z. Puerarin Rebuilding the Mucus Layer and Regulating Mucin-Utilizing Bacteria to Relieve Ulcerative Colitis. J. Agric. Food Chem. 2020, 68, 11402–11411. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y. The Protective Effects of Bacillus Coagulans, Puerarin, and Zinc Oxide on Intestinal Function in Piglets Infected with Escherichia coli. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2020. (In Chinese). [Google Scholar]
- Lu, Y.; Ge, S.; Zhang, H.; Lu, W.; Bao, X.; Pan, S.; Pang, Q. Metabolomic and microbiome analysis of the protective effects of Puerarin against Salmonella Enteritidis Infection in chicks. BMC Vet. Res. 2023, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhang, L.; Lu, Z.; Wang, T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Phoophitphong, D.; Wangnaitham, S.; Srisuwatanasagul, S.; Tummaruk, P. The use of proliferating cell nuclear antigen (PCNA) immuno-staining technique to determine number and type of follicles in the gilt ovary. Livest. Sci. 2012, 150, 425–431. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Kim, I.H.; Yang, Y. Dietary hydrolyzed wheat gluten supplementation ameliorated intestinal barrier dysfunctions of broilers challenged with Escherichia coli O78. Poult. Sci. 2022, 101, 101615. [Google Scholar] [CrossRef]
- Dai, C.; Sharma, G.; Liu, G.; Shen, J.; Shao, B.; Hao, Z. Therapeutic detoxification of quercetin for aflatoxin B1-related toxicity: Roles of oxidative stress, inflammation, and metabolic enzymes. Environ. Pollut. 2024, 345, 123474. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, X.; Sharma, G.; Dai, C. Thymol as a Potential Neuroprotective Agent: Mechanisms, Efficacy, and Future Prospects. J. Agric. Food Chem. 2024, 72, 6803–6814. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, X.; Dong, M. Puerarin suppresses MPP(+)/MPTP-induced oxidative stress through an Nrf2-dependent mechanism. Food Chem. Toxicol. 2020, 144, 111644. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Sun, H.; Hong, H.; Jin, J.; Bei, C.; Lu, Z.; Zhang, X. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021, 12, 2075–2089. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y. Puerarin reduces cell damage from cerebral ischemia-reperfusion by inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 2024, 693, 149324. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wen, S.; Wang, L.; Zhang, K.; Zhang, C.; Zou, H.; Gu, J.; Liu, X.; Bian, J.; et al. Puerarin alleviates cadmium-induced rat neurocyte injury by alleviating Nrf2-mediated oxidative stress and inhibiting mitochondrial unfolded protein response. Ecotoxicol. Environ. Saf. 2022, 247, 114239. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wu, W.; Zhang, L.; Wen, X.; Xie, J.; Zhang, H. Gut microbiota mediates the effects of inulin on enhancing sulfomucin production and mucosal barrier function in a pig model. Food Funct. 2021, 12, 10967–10982. [Google Scholar] [CrossRef]
- Ma, X.; Xu, T.; Qian, M.; Zhang, Y.; Yang, Z.; Han, X. Faecal microbiota transplantation alleviates early-life antibiotic-induced gut microbiota dysbiosis and mucosa injuries in a neonatal piglet model. Microbiol. Res. 2021, 255, 126942. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Li, Y.; Wu, Y.; Wu, T.; Feng, H.; Xu, Z.; Liu, Y.; Ruan, Z.; Zhou, S. Puerarin improves intestinal barrier function through enhancing goblet cells and mucus barrier. J. Funct. Foods 2020, 75, 104246. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Ding, W.; Ruan, Z.; Zhang, L. Enhanced Intestinal Barriers by Puerarin in Combination with Tryptophan. J. Agric. Food Chem. 2021, 69, 15575–15584. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Zhuang, L.; Chen, X.; Min, H.; Song, S.; Liang, Q.; Li, A.D.; Gao, Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS ONE 2019, 14, e0218490. [Google Scholar] [CrossRef]
- Gao, X.; Yue, C.; Tian, R.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. The regulatory effects of specific polyphenols on Akkermansia are dependent on uridine. Food Chem. 2023, 410, 135367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, X.; Liu, L.; Voglmeir, J.; Zhong, X.; Yu, Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020, 51, 34. [Google Scholar] [CrossRef]
- Koupaei, M.; Saderi, H.; Amin Marashi, S.M.; Fathizadeh, H.; Owlia, P. Evaluation of the effect of Saccharomyces cerevisiae on the expression of enterotoxin genes in Escherichia coli O157: H7 (EHEC) and Escherichia coli H10407 (ETEC). Microb. Pathog. 2022, 164, 105450. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Y.; Di, C.; Zeng, Y.; Zhou, Y.; Chen, Y.; Pan, Z.; Li, Z.; Ling, W. Supplementation of Silymarin Alone or in Combination with Salvianolic Acids B and Puerarin Regulates Gut Microbiota and Its Metabolism to Improve High-Fat Diet-Induced NAFLD in Mice. Nutrients 2024, 16, 1169. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Png, C.W.; Crockford, T.L.; Cornall, R.J.; et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008, 5, e54. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Liu, H.; Chang, G.; Wang, W.; Ji, Z.; Cui, J.; Peng, Y. Pharmacokinetics, Prostate Distribution and Metabolic Characteristics of Four Representative Flavones after Oral Administration of the Aerial Part of Glycyrrhiza uralensis in Rats. Molecules 2022, 27, 3245. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Li, H.; Han, R.; Yong, F.; Fan, Y.; Zhao, B.; Hu, X.; Zhang, T.; Che, D. The protective effect of Eleutheroside E against the mechanical barrier dysfunction triggered by lipopolysaccharide in IPEC-J2 cells. Res. Vet. Sci. 2023, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Runzhi, C.; Peng, W.; Zheng, C.; Lan, T.; Ling, Y.; Chuqi, H.; Na, Y.; Jie, Z. The combination of Puerariae Lobatae Radix and Chuanxiong Rhizoma enhanced the absorption and pharmacokinetics of puerarin by modulating the intestinal barrier and influenced gut microbiota. J. Funct. Foods 2018, 47, 72–82. [Google Scholar]
- Li, B.; Liu, M.; Wang, Y.; Gong, S.; Yao, W.; Li, W.; Gao, H.; Wei, M. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed. Pharmacother. 2020, 132, 110923. [Google Scholar] [CrossRef]
- Obata, Y.; Takahashi, D.; Ebisawa, M.; Kakiguchi, K.; Yonemura, S.; Jinnohara, T.; Kanaya, T.; Fujimura, Y.; Ohmae, M.; Hase, K.; et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 2012, 188, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.E.; Levy, G.; Alli, V.; Zheng, X.; Mole, D.J.; Deitch, E.A. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014, 42, 264–270. [Google Scholar] [CrossRef]
- Fenton, T.M.; Jørgensen, P.B.; Niss, K.; Rubin, S.J.S.; Mörbe, U.M.; Riis, L.B.; Da Silva, C.; Plumb, A.; Vandamme, J.; Jakobsen, H.L.; et al. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity 2020, 52, 557–570.e556. [Google Scholar] [CrossRef]
- Jacobse, J.; Li, J.; Rings, E.; Samsom, J.N.; Goettel, J.A. Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease. Front. Immunol. 2021, 12, 716499. [Google Scholar] [CrossRef]
- Ilchmann-Diounou, H.; Menard, S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front. Immunol. 2020, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Niec, R.E.; Rudensky, A.Y.; Fuchs, E. Inflammatory adaptation in barrier tissues. Cell 2021, 184, 3361–3375. [Google Scholar] [CrossRef]
- Liu, C.; Chi, K.; Yang, M.; Guo, N. Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-κB/MAPK Signaling Pathways in Mice. Toxins 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Q.; Yi, D.; Wu, T.; Chen, H.; Guo, S.; Li, S.; Ji, C.; Wang, L.; Zhao, D.; et al. Quantitative Proteomic Analysis Reveals Antiviral and Anti-inflammatory Effects of Puerarin in Piglets Infected With Porcine Epidemic Diarrhea Virus. Front. Immunol. 2020, 11, 169. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Ma, Y.; Li, H.; Ju, X.; Xu, J. Effect of Puerarin, Baicalin and Berberine Hydrochloride on the Regulation of IPEC-J2 Cells Infected with Enterotoxigenic Escherichia coli. Evid. Based Complement. Altern. Med. 2019, 2019, 7438593. [Google Scholar] [CrossRef]
- Jensen, S.B.; Sheikh, M.A.; Akkouh, I.A.; Szabo, A.; O’Connell, K.S.; Lekva, T.; Engh, J.A.; Agartz, I.; Elvsåshagen, T.; Ormerod, M.; et al. Elevated Systemic Levels of Markers Reflecting Intestinal Barrier Dysfunction and Inflammasome Activation Are Correlated in Severe Mental Illness. Schizophr. Bull. 2023, 49, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.T.; Liu, H. Puerarin attenuates LPS-induced inflammatory injury in gastric epithelial cells by repressing NLRP3 inflammasome-mediated apoptosis. Toxicol. Vitr. 2022, 81, 105350. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsunami, E.; Komori, K.; Hayashi, S.; Kadowaki, M. The isoflavone puerarin induces Foxp3(+) regulatory T cells by augmenting retinoic acid production, thereby inducing mucosal immune tolerance in a murine food allergy model. Biochem. Biophys. Res. Commun. 2019, 516, 626–631. [Google Scholar] [CrossRef]
- Abdullah, N.; Defaye, M.; Altier, C. Neural control of gut homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G718–G732. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.H. Disorders of the enteric nervous system—A holistic view. Nat. Reviews Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Pellegrini, C.; Antonioli, L.; Calderone, V.; Colucci, R.; Fornai, M.; Blandizzi, C. Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog. Neurobiol. 2020, 191, 101806. [Google Scholar] [CrossRef] [PubMed]
- Kunze, W.A.; Mao, Y.K.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef]
- Lyu, Q.; Xue, W.; Liu, R.; Ma, Q.; Kasaragod, V.B.; Sun, S.; Li, Q.; Chen, Y.; Yuan, M.; Yang, Y.; et al. A brain-to-gut signal controls intestinal fat absorption. Nature 2024, 634, 936–943. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, S.; Yang, M.; Li, X.; Li, J.; Chen, T.; Tan, B.; Zhang, J. Study on the effect of puerarin on the regulation mechanism of neurotransmitters in intestinal smooth muscle of diarrhea predominant irritable bowel syndrome. Int. Med. Health Her. 2022, 28, 2919–2923. (In Chinese) [Google Scholar] [CrossRef]
- Huang, C. Study on the Regulation of Intestinal Nerve Homeostasis in IBS-D Rats by Puerarin. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2024. (In Chinese). [Google Scholar]
- Lin, Y.; Lu, Y.; Wang, Y.; Lv, C.; Chen, J.; Luo, Y.; Quan, H.; Yu, W.; Chen, L.; Huang, Z.; et al. The Regeneration of Intestinal Stem Cells Is Driven by miR-29-Induced Metabolic Reprogramming. Engineering 2024, 42, 39–58. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, J.; Liang, Q.; Song, S.; Wang, S.; Yao, X.; Gao, Q.; Wang, L. Puerarin alleviates sleep disorders in aged mice related to repairing intestinal mucosal barrier. Nat. Prod. Bioprospect. 2023, 13, 29. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.-H.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration. Appl. Sci. 2021, 11, 9343. [Google Scholar] [CrossRef]
- Mehmood, T.; Pichyangkura, R.; Muanprasat, C. Chitosan Oligosaccharide Promotes Junction Barrier through Modulation of PI3K/AKT and ERK Signaling Intricate Interplay in T84 Cells. Polymers 2023, 15, 1681. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, X.; Xu, F.; Wang, F.; Wu, H.; Bai, Y.; Li, W.; Zhang, G.; Yuan, J.; Pang, Q. Protective effects of puerarin on liver tissue in Salmonella-infected chicks: A proteomic analysis. Poult. Sci. 2024, 103, 103281. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Ahn, J.M.; Cho, J.H.; Kim, J.Y.; Kang, D.K.; Kim, S.W.; Kim, H.B.; Kim, I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, R.; Dong, Y.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Zhang, Y.; Hou, Y. Dietary Supplementation with Puerarin Improves Intestinal Function in Piglets Challenged with Escherichia coli K88. Animals 2023, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, X.; Zhao, D.; Dai, H. Pharmacological Applications and Action Mechanisms of Phytochemicals as Alternatives to Antibiotics in Pig Production. Front. Immunol. 2021, 12, 798553. [Google Scholar] [CrossRef]
- Fróes, R.; Bezerra, L.; Missasse, J.; Castro, D.; Barbosa, A.; Arce-Cordero, J.; Silva, T.; Portela, R.; Cunha, T.; Oliveira, R. Effects of yeast and exogenous fibrolytic enzyme additives on lamb performance and feed efficiency. Trop. Anim. Health Prod. 2024, 56, 235. [Google Scholar] [CrossRef]
- Nie, C.; Shang, X.; Yang, Z.; Chen, H.; Qu, M.; You, J.; Song, X. Effects of Puerarian at Different Levels on Rumen Fermentation Indices and Nutrient Degradability of Beef Cattle in Vitro. Acta Agric. Univ. Jiangxiensis 2021, 43, 1381–1387. [Google Scholar] [CrossRef]
- Lyu, C.; Yuan, B.; Meng, Y.; Cong, S.; Che, H.; Ji, X.; Wang, H.; Chen, C.; Li, X.; Jiang, H.; et al. Puerarin Alleviates H(2)O(2)-Induced Oxidative Stress and Blood-Milk Barrier Impairment in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 7742. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Chen, X.; Li, J.; Comish, P.; Kang, R.; Tang, D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020, 27, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, A.; Hassan, S.; Ahmed, Z.H.T.; Singh, P.; Maqbool, M.; Meenatchi, R.; Pugazhendhi, A.; Sharma, A. The ruminant gut microbiome vs enteric methane emission: The essential microbes may help to mitigate the global methane crisis. Environ. Res. 2024, 261, 119661. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lv, J.; Chen, X.; Li, T.; Shen, J.; Wang, Z.; Dai, C.; Hao, Z. Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications. Antioxidants 2025, 14, 756. https://doi.org/10.3390/antiox14060756
Zhou J, Lv J, Chen X, Li T, Shen J, Wang Z, Dai C, Hao Z. Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications. Antioxidants. 2025; 14(6):756. https://doi.org/10.3390/antiox14060756
Chicago/Turabian StyleZhou, Jiehong, Jianyu Lv, Xin Chen, Tian Li, Jianzhong Shen, Zhanhui Wang, Chongshan Dai, and Zhihui Hao. 2025. "Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications" Antioxidants 14, no. 6: 756. https://doi.org/10.3390/antiox14060756
APA StyleZhou, J., Lv, J., Chen, X., Li, T., Shen, J., Wang, Z., Dai, C., & Hao, Z. (2025). Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications. Antioxidants, 14(6), 756. https://doi.org/10.3390/antiox14060756







