Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications
Abstract
1. Introduction
2. Prebiotic Oligosaccharides: Chemical Structures and Novel Production Methods
2.1. Human Milk Oligosaccharides (HMOs)
2.2. Galacto-Oligosaccharides (GOSs)
2.3. Fructo-Oligosaccharides (FOSs)
2.4. Gluco-Oligosaccharides (GlcOSs)
2.5. Other Prebiotic Oligosaccharides
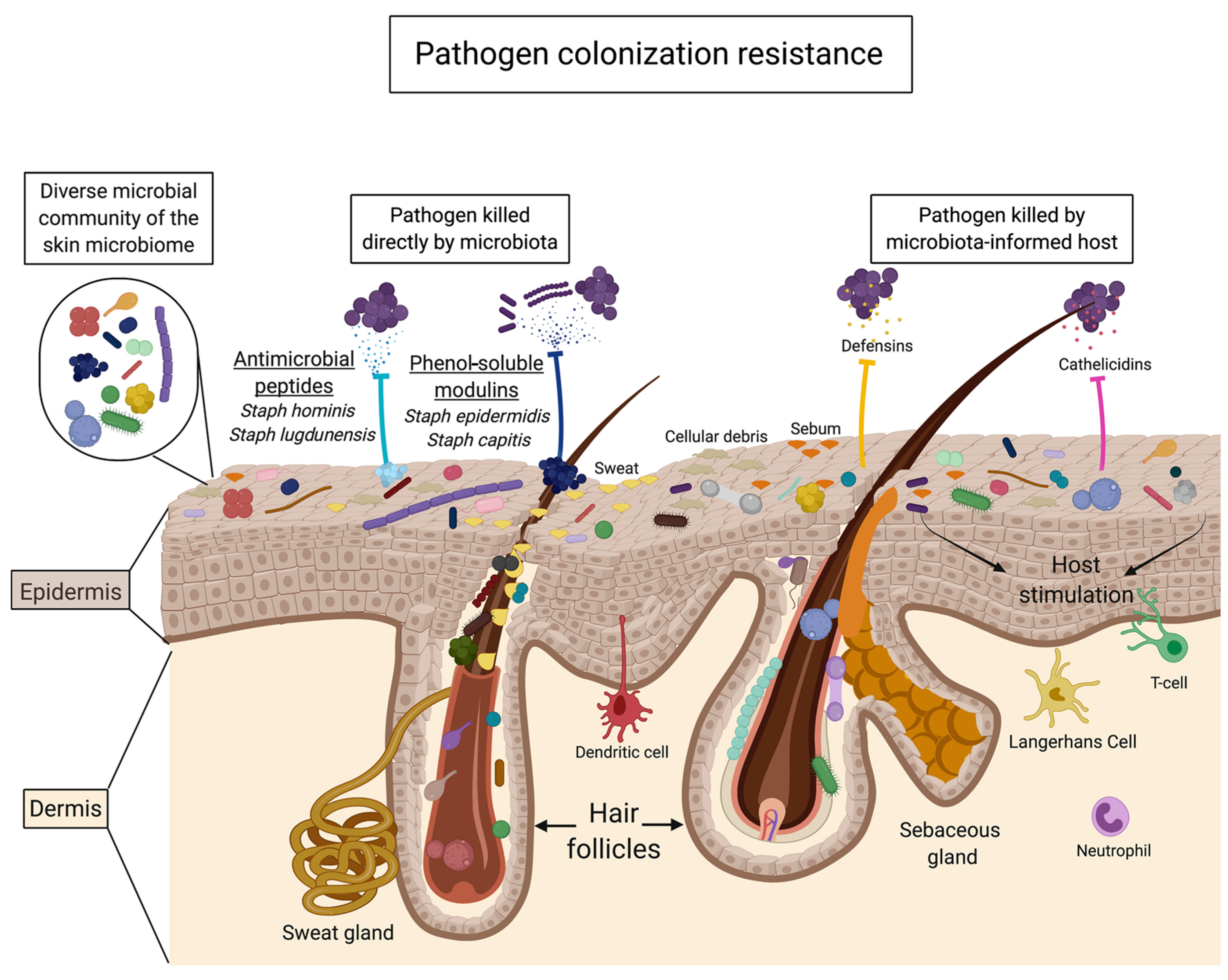
3. In Vitro Studies of Prebiotic Oligosaccharides on the Skin Microbiome and Related Skin Health Effects
3.1. Promotion of Skin Commensals and Inhibition of Pathogens (HMOs, GOSs, FOSs, GlcOSs)
3.2. Anti-Melanogenesis (HMOs, GOSs, AOSs)
3.3. Antioxidant and Anti-Inflammation Activities (AOSs, GlcOSs, FOSs, GOSs, XOSs)
3.4. Wound Healing (GOSs, Thiolated Oligosaccharides)
4. In Vivo Studies of Prebiotic Oligosaccharides on the Skin Microbiome and Related Skin Health Effects
4.1. Alleviation or Prevention of Atopic Dermatitis (HMOs, FOSs, GOSs, AOSs)
4.2. Prevention of Allergies (HMOs, GOSs, FOSs)
4.3. Attenuation of Acne Vulgaris (COSs, FOSs, GOSs)
4.4. Skin Hydration, Anti-Dryness, and Anti-Itching (GlcOSs, GOSs, FOSs, Colloidal Oat)
4.5. Anti-Aging and Photoprotection (GlcOSs, XOSs, GOSs, COSs)
4.6. Mechanism of Action of Oligosaccharides
5. Commercial Cosmetic Compositions Containing Prebiotic Oligosaccharides
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Demessant-Flavigny, A.-L.; Connétable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 3–17. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and atopic dermatitis: A complex and evolving relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef]
- Baquerizo Nole, K.L.; Yim, E.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821. [Google Scholar] [CrossRef]
- Notay, M.; Foolad, N.; Vaughn, A.R.; Sivamani, R.K. Probiotics, prebiotics, and synbiotics for the treatment and prevention of adult dermatological diseases. Am. J. Clin. Dermatol. 2017, 18, 721–732. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, H.S. Skin Deep: The Potential of Microbiome Cosmetics. J. Microbiol. 2024, 62, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Erginer Hasköylü, M.; Toksoy Öner, E. Chapter 16—Fructans in Personal Care Products. In The Book of Fructans; den Ende, W.V., Öner, E.T., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 275–294. [Google Scholar]
- Fitriana, N.; Saksono, B.; Ermawar, R.A.; Wicaksono, M.W. Polysaccharide Applications in Cosmetic. In Biomass-Based Cosmetics: Research Trends and Future Outlook; Arung, E.T., Fatriasari, W., Kusuma, I.W., Kuspradini, H., Shimizu, K., Kim, Y.-u., Azelee, N.I.W., Edis, Z., Eds.; Springer Nature: Singapore, 2024; pp. 273–297. [Google Scholar] [CrossRef]
- Baptista, S.; Freitas, F. Bacterial Polysaccharides: Cosmetic Applications. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–42. [Google Scholar] [CrossRef]
- Rahman, T.; Sarwar, P.F.; Potter, C.; Comstock, S.S.; Klepac-Ceraj, V. Role of human milk oligosaccharide metabolizing bacteria in the development of atopic dermatitis/eczema. Front. Pediatr. 2023, 11, 1090048. [Google Scholar] [CrossRef]
- Tang, L.; Cao, X.; Chen, S.; Jiang, X.; Li, D.; Chen, G. Dietary Galacto-oligosaccharides Ameliorate Atopic Dermatitis-like Skin Inflammation and Behavioral Deficits by Modulating Gut Microbiota–Brain–Skin Axis. J. Agric. Food Chem. 2024, 72, 7954–7968. [Google Scholar] [CrossRef]
- Kim, S.; Kang, B.-G.; Sa, S.; Park, S.Y.; Ryu, K.; Lee, J.; Park, B.; Kwon, M.; Kim, Y.; Kim, J.; et al. Advanced fructo-oligosaccharides improve itching and aberrant epidermal lipid composition in children with atopic dermatitis. Front. Microbiol. 2024, 15, 1383779. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, D.; Liu, Z.; Sun, J.; Mao, X. Advances in agaro-oligosaccharides preparation and bioactivities for revealing the structure-function relationship. Food Res. Int. 2021, 145, 110408. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, S.; Guan, Y.; Wang, D.; Yang, J.; Li, J.; Zhao, W.; Zhang, F. Dual intervention on the gut and skin microbiota attenuates facial cutaneous aging. Food Funct. 2024, 15, 4246–4261. [Google Scholar] [CrossRef]
- Lv, X.; Han, Z.; Huo, H.; Liu, X.; Zhang, J.; Chi, J.; Han, B.; Jiang, Z. Anti-inflammatory and antioxidant succinyl-chitosan oligosaccharide protects human epidermal cell and mouse skin against ultraviolet B-induced photodamage. Carbohydr. Polym. 2025, 351, 123102. [Google Scholar] [CrossRef]
- Shao, L.; Li, T.; Yang, S.; Ma, L.; Cai, B.; Jia, Q.; Jiang, H.; Bai, T.; Li, Y. The prebiotic effects of fructooligosaccharides enhance the growth characteristics of Staphylococcus epidermidis and enhance the inhibition of Staphylococcus aureus biofilm formation. Int. J. Cosmet. Sci. 2025, 47, 155–167. [Google Scholar] [CrossRef]
- Hong, Y.H.; Chang, U.J.; Kim, Y.S.; Jung, E.Y.; Suh, H.J. Dietary galacto-oligosaccharides improve skin health: A randomized double blind clinical trial. Asia Pac. J. Clin. Nutr. 2017, 26, 613–618. [Google Scholar] [PubMed]
- Hong, K.-B.; Hong, Y.H.; Jung, E.Y.; Jo, K.; Suh, H.J. Changes in the Diversity of Human Skin Microbiota to Cosmetic Serum Containing Prebiotics: Results from a Randomized Controlled Trial. J. Pers. Med. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, S.; Kononov, T.; Zahr, A.S. A multi-functional anti-aging moisturizer maintains a diverse and balanced facial skin microbiome. J. Appl. Microbiol. 2022, 133, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Z.; Li, D.-D.; Luo, H.; Li, W.-J.; Huang, Y.-M.; Li, J.-C.; Hu, Z.; Huang, N.; Guo, M.-H.; Chen, Y.; et al. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp. Gerontol. 2018, 103, 27–34. [Google Scholar] [CrossRef]
- Kano, M.; Masuoka, N.; Kaga, C.; Sugimoto, S.; Iizuka, R.; Manabe, K.; Sone, T.; Oeda, K.; Nonaka, C.; Miyazaki, K.; et al. Consecutive intake of fermented milk containing Bifidobacterium breve strain Yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Biosci. Microbiota Food Health 2013, 32, 33–39. [Google Scholar] [CrossRef]
- Suh, M.G.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Inhibitory effect of galactooligosaccharide on skin pigmentation. Prev. Nutr. Food Sci. 2019, 24, 321–326. [Google Scholar] [CrossRef]
- Bergandi, L.; Flutto, T.; Valentini, S.; Thedy, L.; Pramotton, R.; Zenato, S.; Silvagno, F. Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways. Nutrients 2022, 14, 2888. [Google Scholar] [CrossRef]
- Urashima, T.; Ajisaka, K.; Ujihara, T.; Nakazaki, E. Recent advances in the science of human milk oligosaccharides. BBA Adv. 2025, 7, 100136. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Zhang, P.; Li, B. Recent trends in human milk oligosaccharides: New synthesis technology, regulatory effects, and mechanisms of non-intestinal functions. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70147. [Google Scholar] [CrossRef]
- Wei, X.; Leilei, Y.; Jianxin, Z.; Qixiao, Z.; Wei, C.; Tian, F. Human Milk Microbiota and Oligosaccharides: Origin, Structure, Impact Factors, and Benefits for Infant Health. Food Rev. Int. 2025, 41, 1–28. [Google Scholar] [CrossRef]
- Wang, K.; Duan, F.Y.; Sun, T.; Zhang, Y.; Lu, L.L. Galactooligosaccharides: Synthesis, metabolism, bioactivities and food applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6160–6176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mohapatra, S.; Oh, J.-H.; Astmann, T.; van Pijkeren, J.-P.; Pan, X. Novel galacto-oligosaccharides from lactose: Chemical synthesis, structural characterization, and in vitro assessment of prebiotic activity. ACS Sustain. Chem. Eng. 2023, 11, 14031–14045. [Google Scholar] [CrossRef]
- Ambrogi, V.; Francesca, B.; Linqiu, C.; Bas, K.; Margriet, S.; van Sinderen, D. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, M.J.; Soto-Jover, S.; Antolinos, V.; Martinez-Hernandez, G.B.; Lopez-Gomez, A. Manufacturing of Short-Chain Fructooligosaccharides: From Laboratory to Industrial Scale. Food Eng. Rev. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Costa, G.T.; Vasconcelos, Q.; Aragao, G.F. Fructooligosaccharides on inflammation, immunomodulation, oxidative stress, and gut immune response: A systematic review. Nutr. Rev. 2022, 80, 709–722. [Google Scholar] [CrossRef]
- Nobre, C.; Simões, L.S.; Gonçalves, D.A.; Berni, P.; Teixeira, J.A. 5—Fructooligosaccharides production and the health benefits of prebiotics. In Current Developments in Biotechnology and Bioengineering; Rai, A.K., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–138. [Google Scholar]
- Zeng, M.; van Pijkeren, J.-P.; Pan, X. Gluco-oligosaccharides as potential prebiotics: Synthesis, purification, structural characterization, and evaluation of prebiotic effect. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2611–2651. [Google Scholar] [CrossRef]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Qiu, X.; Tang, R.; Zhang, Z.; Ma, Q. Structure-Tunable Glucan Oligomer Production from Glucose and Disaccharide Glycosylation in an Unacidified LiBr·3.2H2O Molten Salt Hydrate. ACS Sustain. Chem. Eng. 2024, 12, 4743–4753. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Xie, X.; Fan, D.; Ouyang, X.; Fan, W.; Qiu, X. Enhanced production and separation of short-chain glucan oligomers from corn stover in an unacidified LiBr molten salt hydrate via pre-extraction of hemicellulose. Green. Chem. 2022, 24, 8812–8819. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, J.; Guan, M.; Liang, H.; Liu, Q. Enhanced cello-oligosaccharides production from cellulose hydrolysis in molten salt hydrate over lignin-based hyper-cross-linked polymer (LHCP) adsorption. Appl. Catal. A Gen. 2022, 644, 118808. [Google Scholar] [CrossRef]
- Xie, X.; Wang, X.; Ouyang, X.; Liu, Q.; Qiu, X. Cascade fractionation of birch into xylose, glucan oligomers, and noncondensed lignin improved using microwave assistance and molten salt hydrates. Green. Chem. 2023, 25, 9272–9281. [Google Scholar] [CrossRef]
- Kumar, R.; Næss, G.; Sørensen, M. Xylooligosaccharides from lignocellulosic biomass and their applications as nutraceuticals: A review on their production, purification, and characterization. J. Sci. Food Agric. 2024, 104, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Li, B.; Cui, J.; Xu, T.; Xu, Y.; Long, M.; Li, J.; Liu, M.; Yang, T.; Du, Y.; Xu, Q. Advances in the preparation, characterization, and biological functions of chitosan oligosaccharide derivatives: A review. Carbohydr. Polym. 2024, 332, 121914. [Google Scholar] [CrossRef]
- Long, J.; Ye, Z.; Li, X.; Tian, Y.; Bai, Y.; Chen, L.; Qiu, C.; Xie, Z.; Jin, Z.; Svensson, B. Enzymatic preparation and potential applications of agar oligosaccharides: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 5818–5834. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Wellness 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Duman, H.; Bechelany, M.; Karav, S. Human Milk Oligosaccharides: Decoding Their Structural Variability, Health Benefits, and the Evolution of Infant Nutrition. Nutrients 2024, 17, 118. [Google Scholar] [CrossRef]
- Lordan, C.; Roche, A.K.; Delsing, D.; Nauta, A.; Groeneveld, A.; MacSharry, J.; Cotter, P.D.; Sinderen, D.v. Linking human milk oligosaccharide metabolism and early life gut microbiota: Bifidobacteria and beyond. Microbiol. Mol. Biol. Rev. 2024, 88, e0009423. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of galactooligosaccharides in milk and whey: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Logtenberg, M.J.; Donners, K.M.; Vink, J.C.; van Leeuwen, S.S.; de Waard, P.; de Vos, P.; Schols, H.A.J.J.o.a. Touching the high complexity of prebiotic vivinal galacto-oligosaccharides using porous graphitic carbon ultra-high-performance liquid chromatography coupled to mass spectrometry. J. Agric. Food Chem. 2020, 68, 7800–7808. [Google Scholar] [CrossRef]
- Miao, M.; Li, S.; Yang, S.; Yan, Q.; Xiang, Z.; Jiang, Z. In Situ Galacto-Oligosaccharides Synthesis in Whey Powder Fortified Milk by a Modified β-Galactosidase and Its Effect on the Techno-Functional Characteristics of Yogurt. J. Agric. Food Chem. 2024, 72, 26431–26440. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, J.; Zhang, Z.; Yan, X. Investigation of dietary fructooligosaccharides from different production methods: Interpreting the impact of compositions on probiotic metabolism and growth. J. Funct. Foods 2020, 69, 103955. [Google Scholar] [CrossRef]
- Rawat, H.K.; Suresh, N.; Isha, S.; Kango, N. Recent developments in the production of prebiotic fructooligosaccharides using fungal fructosyltransferases. Mycology 2024, 15, 564–584. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.P.J.; Wijeyesekera, A.; Rastall, R.A. Inulin-type fructans and short-chain fructooligosaccharides—Their role within the food industry as fat and sugar replacers and texture modifiers—What needs to be considered! Food Sci. Nutr. 2023, 11, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. The prebiotic effect: Review of experimental and human data. In Handbook of Prebiotics; Taylor & Francis: Boca Raton, FL, USA, 2008; pp. 87–110. [Google Scholar] [CrossRef]
- Palaniappan, A.; Emmambux, M.N. The challenges in production technology, health-associated functions, physico-chemical properties and food applications of isomaltooligosaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 3821–3837. [Google Scholar] [CrossRef]
- Liang, X.; Tao, Z.; Xia, W.; Chen, S.; Wu, J. Gentiooligosaccharides. In Biomanufacture of Functional Carbohydrates; Wu, J., Su, L., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Qu, T.; Kraft, J.; Oh, J.-H.; van Pijkeren, J.-P.; Huber, G.W.; Pan, X. High-yield synthesis of glucooligosaccharides (GlOS) as potential prebiotics from glucose via non-enzymatic glycosylation. Green. Chem. 2019, 21, 2686–2698. [Google Scholar] [CrossRef]
- Zeng, M.; Li, N.; Astmann, T.; Oh, J.-H.; van Pijkeren, J.-P.; Pan, X. Facile and efficient chemical synthesis of gluco-oligosaccharides (GlcOS) with diverse glycosidic linkages as potential prebiotics to promote the growth of probiotic bacteria. Food Res. Int. 2023, 165, 112436. [Google Scholar] [CrossRef]
- Liang, H.; Ye, S.; Liu, Q.; Fan, W.; Ma, Q. Confined synthesis of glucan oligomers from glucose in zeolites. Green. Chem. 2025, 27, 1714–1722. [Google Scholar] [CrossRef]
- Chen, P.; Shrotri, A.; Fukuoka, A. Synthesis of cello-oligosaccharides by depolymerization of cellulose: A review. Appl. Catal. A Gen. 2021, 621, 118177. [Google Scholar] [CrossRef]
- Zeng, M.; Pan, X. Insights into solid acid catalysts for efficient cellulose hydrolysis to glucose: Progress, challenges, and future opportunities. Catal. Rev. 2022, 64, 445–490. [Google Scholar] [CrossRef]
- Pang, Z.; Li, N.; Dong, C.; Ji, H.; Liao, Y.; Yang, G.; Pan, X. Insights into the dissolution of cellulose in lithium bromide solution. Carbohydr. Polym. Technol. Appl. 2024, 7, 100522. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Q.; Sabnis, S.; Zheng, W.; Vlachos, D.G.; Fan, W.; Li, W.; Ma, L. Production of high-yield short-chain oligomers from cellulose via selective hydrolysis in molten salt hydrates and separation. Green. Chem. 2019, 21, 5030–5038. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, S.; Fan, W.; Ouyang, X.; Qiu, X. Separation of short-chain glucan oligomers from molten salt hydrate and hydrolysis to glucose. Green. Chem. 2021, 23, 4114–4124. [Google Scholar] [CrossRef]
- Zeng, M.; Pan, X. Purification and fractionation of cellooligosaccharides synthesized from controlled cellulose hydrolysis by sulfuric acid using nanofiltration. Sep. Purif. Technol. 2024, 348, 127800. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Fan, D.; Guan, M.; Ma, Q.; Li, S.; Ouyang, X.; Qiu, X.; Fan, W. Adsorption-Enhanced Glucan Oligomer Production from Cellulose Hydrolysis over Hyper-Cross-Linked Polymer in Molten Salt Hydrate. ACS Appl. Mater. Interfaces 2021, 13, 52082–52091. [Google Scholar] [CrossRef]
- Hirayama, J.; Kobayashi, H.; Fukuoka, A. Amorphization and Semi-Dry Conversion of Crystalline Cellulose to Oligosaccharides by Impregnated Phosphoric Acid. Bull. Chem. Soc. Jpn. 2020, 93, 273–278. [Google Scholar] [CrossRef]
- Xie, X.; Li, L.; Wang, X.; Liu, Q.; Ouyang, X.; Qiu, X. Fractionation of High-Yield Noncondensed Lignin and Glucan Oligomers from Lignocellulose in a Novel Biphasic System. J. Agric. Food Chem. 2025, 73, 2880–2889. [Google Scholar] [CrossRef]
- Xie, X.; Wang, X.; Ouyang, X.; Liu, Q.; Qiu, X. Rapid fractionation of noncondensed lignin from birch via acidified LiCl molten salt hydrates. Ind. Crops Prod. 2024, 207, 117744. [Google Scholar] [CrossRef]
- Amorim, C.; Silverio, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef]
- Santibanez, L.; Henriquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Dejonghe, W.; Vanbroekhoven, K.; Rakotoarivonina, H.; Rémond, C. Enzymatic Production of Xylo-oligosaccharides from Destarched Wheat Bran and the Impact of Their Degree of Polymerization and Substituents on Their Utilization as a Carbon Source by Probiotic Bacteria. J. Agric. Food Chem. 2021, 69, 13217–13226. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Gao, J.; Du, L.; Liu, Y.; Guo, N.; Sun, J.; Dong, H.; Mao, X. Chitinase and Deacetylase-Based Chitin-Degrading Bacteria: One-Pot Cascade Bioconversion of Chitin to Chitooligosaccharides. J. Agric. Food Chem. 2024, 72, 23937–23946. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Yu, J.; Wei, Q.; Ren, X. Study on the deacetylation and mechanism of chitin in natural deep eutectic solvent. Int. J. Biol. Macromol. 2024, 255, 127698. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Nomani, M.; Adcock, I.; Folkerts, G.; Garssen, J. Galactooligosaccharides and 2′-fucosyllactose can directly suppress growth of specific pathogenic microbes and affect phagocytosis of neutrophils. Nutrition 2022, 96, 111601. [Google Scholar] [CrossRef]
- Petrov, A.; Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Pjanović, R.; Bezbradica, D. Prebiotic effect of galacto-oligosaccharides on the skin microbiota and determination of their diffusion properties. Int. J. Cosmet. Sci. 2022, 44, 309–319. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Meunier, C.; Gaio, E.; Murat, V.; Micheletto, M.; Tedesco, E.; Benetti, F. Effects of short chain fructo-oligosaccharides on selected skin bacteria. Sci. Rep. 2022, 12, 9702. [Google Scholar] [CrossRef]
- Akiyama, H.; Oono, T.; Huh, W.K.; Yamasaki, O.; Akagi, Y.; Uemura, H.; Yamada, T.; Iwatsuki, K. Actions of gluco-Oligosaccharide against Staphylococcus aureus. J. Dermatol. 2002, 29, 580–586. [Google Scholar] [CrossRef]
- Liu-Walsh, F.; Tierney, N.K.; Hauschild, J.; Rush, A.K.; Masucci, J.; Leo, G.C.; Capone, K.A. Prebiotic Colloidal Oat Supports the Growth of Cutaneous Commensal Bacteria Including S. epidermidis and Enhances the Production of Lactic Acid. Clin. Cosmet. Investig. Dermatol. 2021, 14, 73–82. [Google Scholar] [CrossRef]
- Kim, S.-H.; Eom, S.-H.; Yu, D.; Lee, M.-S.; Kim, Y.-M. Oligochitosan as a potential anti-acne vulgaris agent: Combined antibacterial effects against Propionibacterium acnes. Food Sci. Biotechnol. 2017, 26, 1029–1036. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Gasparri, F.; Di Campli, E.; Di Fermo, P.; D’Ercole, S.; Cellini, L.; Di Giulio, M. Prebiotic Combinations Effects on the Colonization of Staphylococcal Skin Strains. Microorganisms 2021, 9, 37. [Google Scholar] [CrossRef]
- Fournière, M.; Bedoux, G.; Souak, D.; Bourgougnon, N.; Feuilloley, M.G.J.; Latire, T. Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains. Molecules 2021, 26, 4763. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Han, J.Y.; Heo, H.; Lee, S.M.; Brito, S.; Cha, B.; Lei, L.; Lee, S.H.; Bin, B.-H.; Lee, M.-G.; et al. A Study on Melanin Reduction through Autophagy by 2′-Fucosyllactose. J. Soc. Cosmet. Sci. Korea 2022, 48, 105–112. [Google Scholar] [CrossRef]
- Heo, H.; Cha, B.; Jang, D.; Park, C.; Park, G.; Kwak, B.-M.; Bin, B.-H.; Park, J.-H.; Lee, M.-G. Human milk oligosaccharide 2′-fucosyllactose promotes melanin degradation via the autophagic AMPK–ULK1 signaling axis. Sci. Rep. 2022, 12, 13983. [Google Scholar] [CrossRef]
- Yun, E.J.; Lee, S.; Kim, J.H.; Kim, B.B.; Kim, H.T.; Lee, S.H.; Pelton, J.G.; Kang, N.J.; Choi, I.-G.; Kim, K.H. Enzymatic production of 3,6-anhydro-L-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl. Microbiol. Biotechnol. 2013, 97, 2961–2970. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different levels of skin whitening activity among 3,6-Anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Jo, E.; Gajanayaka, N.D.; Bandara, M.S.; Marasinghe, S.D.; Park, G.-H.; Lee, S.-J.; Oh, C.; Lee, Y. Odd-Numbered Agaro-Oligosaccharides Produced by α-Neoagaro-Oligosaccharide Hydrolase Exert Antioxidant Activity in Human Dermal Fibroblasts. Mar. Drugs 2024, 22, 495. [Google Scholar] [CrossRef]
- Kim, M.; Jang, J.-K.; Park, Y.-S. Production Optimization, Structural Analysis, and Prebiotic- and Anti-Inflammatory Effects of Gluco-Oligosaccharides Produced by Leuconostoc lactis SBC001. Microorganisms 2021, 9, 200. [Google Scholar] [CrossRef]
- Yeung, Y.K.; Kang, Y.-R.; So, B.R.; Jung, S.K.; Chang, Y.H. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. 2021, 118, 106779. [Google Scholar] [CrossRef]
- Hajjar, R.; Oliero, M.; Cuisiniere, T.; Fragoso, G.; Calvé, A.; Djediai, S.; Annabi, B.; Richard, C.S.; Santos, M.M. Improvement of colonic healing and surgical recovery with perioperative supplementation of inulin and galacto-oligosaccharides. Clin. Nutr. 2021, 40, 3842–3851. [Google Scholar] [CrossRef]
- Noreen, S.; Bernkop-Schnürch, A. Thiolated Poly- and Oligosaccharide-Based Hydrogels for Tissue Engineering and Wound Healing. Adv. Funct. Mater. 2024, 34, 2310129. [Google Scholar] [CrossRef]
- Jean-Pierre, H.G.; Lamothe, T.; Yves, G.; Marchenay, B.; Pierre, F.; Monsan, B.; Paul, F.M.B.; Pelenc, V. Cosmetic Compositions Containing Oligosaccharides. U.S. Patent 5518733A, 21 May 1996. [Google Scholar]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Kamilijiang, M.; Abudukelimu, N.; Bakri, M.; Zang, D.; Xu, N.; Zhao, J.; Aisa, H.A. Anti-melanogenic effect of a novel oligosaccharide derived from almond on forskolin-stimulated melanogenesis in B16F10 melanoma cells. Food Biosci. 2024, 62, 105013. [Google Scholar] [CrossRef]
- Vieira, T.F.; Corrêa, R.C.G.; Peralta, R.A.; Peralta-Muniz-Moreira, R.F.; Bracht, A.; Peralta, R.M. An Overview of Structural Aspects and Health Beneficial Effects of Antioxidant Oligosaccharides. Curr. Pharm. Des. 2020, 26, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar] [CrossRef]
- Cao, R.; Wang, B.; Bai, T.; Zhu, Y.; Cheng, J.; Zhang, J. Structural and functional impacts of glycosylation-induced modifications in rabbit myofibrillar proteins. Int. J. Biol. Macromol. 2024, 283, 137583. [Google Scholar] [CrossRef]
- Van den Ende, W.; Peshev, D.; De Gara, L. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends Food Sci. Technol. 2011, 22, 689–697. [Google Scholar] [CrossRef]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as functional ingredients: Focus on Mediterranean fish aquaculture. Rev. Aquac. 2018, 10, 800–832. [Google Scholar] [CrossRef]
- Lee, Y.H.; Verma, N.K.; Thanabalu, T. Prebiotics in atopic dermatitis prevention and management. J. Funct. Foods 2021, 78, 104352. [Google Scholar] [CrossRef]
- Kim, J.-H.; Baek, J.; Sa, S.; Park, J.; Kih, M.; Kim, W. Kestose-enriched fructo-oligosaccharide alleviates atopic dermatitis by modulating the gut microbiome and immune response. J. Funct. Foods 2021, 85, 104650. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Wang, X.; Ren, F. Alginate oligosaccharide-mediated butyrate-HIF-1α axis improves skin aging in mice. J. Pharm. Anal. 2024, 14, 100911. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Milani, M.; Micali, G. Effects of oral supplementation with FOS and GOS prebiotics in women with adult acne: The “S.O. Sweet” study: A proof-of-concept pilot trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.G.; Ferreira, V.F.; da Silva, F.d.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Jenkins, G. A novel topical ingredient derived from seaweed significantly reduces symptoms of acne vulgaris: A general literature review. J. Cosmet. Sci. 2013, 64, 219–226. [Google Scholar] [PubMed]
- Hong, K.-B.; Jeong, M.; Han, K.S.; Hwan Kim, J.; Park, Y.; Suh, H.J. Photoprotective effects of galacto-oligosaccharide and/or Bifidobacterium longum supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Int. J. Food Sci. Nutr. 2015, 66, 923–930. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masuoka, N.; Kano, M.; Iizuka, R. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes 2014, 5, 121–128. [Google Scholar] [CrossRef]
- Berardesca, E.; Abril, E.; Serio, M.; Cameli, N. Effects of topical gluco-oligosaccharide and collagen tripeptide F in the treatment of sensitive atopic skin. Int. J. Cosmet. Sci. 2009, 31, 271–277. [Google Scholar] [CrossRef]
- Michelle Garay, M.; Judith Nebus, M.; Menas Kizoulis, B. Antiinflammatory activities of colloidal oatmeal (Avena sativa) contribute to the effectiveness of oats in treatment of itch associated with dry, irritated skin. J. Drugs Dermatol. 2015, 14, 43–48. [Google Scholar]
- Suh, M.G.; Bae, G.Y.; Jo, K.; Kim, J.M.; Hong, K.-B.; Suh, H.J. Photoprotective Effect of Dietary Galacto-Oligosaccharide (GOS) in Hairless Mice via Regulation of the MAPK Signaling Pathway. Molecules 2020, 25, 1679. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.R.; Kim, N.R.; Park, S.-J.; Lee, M.; Kim, O.-K. Combination of Bifidobacterium longum and Galacto-Oligosaccharide Protects the Skin from Photoaging. J. Med. Food 2021, 24, 606–616. [Google Scholar] [CrossRef]
- Han, K.; Hong, K.-B.; Ahn, Y.; Jo, K.; Jung, J.; Suh, H.J. Effects of Collagen-Tripeptide and Galacto-oligosaccharide Mixture on Skin Photoaging Inhibition in UVB-exposed Hairless Mice. Photochem. Photobiol. 2022, 98, 1172–1181. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Jing, C.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Protective effect of oligosaccharides isolated from Panax ginseng C. A. Meyer against UVB-induced skin barrier damage in BALB/c hairless mice and human keratinocytes. J. Ethnopharmacol. 2022, 283, 114677. [Google Scholar] [CrossRef] [PubMed]
- Deleuran, M.; Dézfoulian, B.; Elberling, J.; Knutar, I.; Lapeere, H.; Lossius, A.H.; Schuttelaar, M.L.A.; Stockman, A.; Wikström, E.; Bradley, M.; et al. Systemic anti-inflammatory treatment of atopic dermatitis during conception, pregnancy and breastfeeding: Interdisciplinary expert consensus in Northern Europe. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ordnung, M.; Mank, M.; Stahl, B.; Kurz, D.; Marosvölgyi, T.; Decsi, T.; Rothenbacher, D.; Genuneit, J.; Siziba, L.P. Potential sex differences in human milk fatty acids and their association with atopic dermatitis: Results of the Ulm SPATZ health study. Pediatr. Allergy Immunol. 2023, 34, e13992. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Binia, A.; Godfrey, K.M.; El-Heis, S.; Cutfield, W.S. Do human milk oligosaccharides protect against infant atopic disorders and food allergy? Nutrients 2020, 12, 3212. [Google Scholar] [CrossRef]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; van’t Land, B.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and Preventive Effects in Allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef]
- Niedźwiedzka, A.; Micallef, M.P.; Biazzo, M.; Podrini, C. The Role of the Skin Microbiome in Acne: Challenges and Future Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 11422. [Google Scholar] [CrossRef]
- Podwojniak, A.; Tan, I.J.; Sauer, J.; Neubauer, Z.; Rothenberg, H.; Ghani, H.; Parikh, A.K.; Cohen, B. Acne and the cutaneous microbiome: A systematic review of mechanisms and implications for treatments. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 793–805. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Sugawara, T.; Kikuchi, K.; Tagami, H.; Aiba, S.; Sakai, S. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J. Dermatol. Sci. 2012, 66, 154–159. [Google Scholar] [CrossRef]
- Ecoskin®. Pre/Postbiotic to Reveal the Radiance of the Complexion. Available online: https://www.solabia.com/cosmetics/fr/produit/ecoskin/ (accessed on 1 January 2022).
- Stout, H.S.; Murphy, K.A. Skin Rejuvenation and Defense Systems and Methods. U.S. Patent 10485752B2, 26 November 2019. [Google Scholar]
- Bioecolia®. Prebiotic Protector of the Microbiota. Available online: https://www.solabia.com/cosmetics/fr/produit/bioecolia/ (accessed on 1 January 2022).
- Hillion, M. Interactions Peau/Microbiote Cutané: Étude du Microbiote Cutané Cultivable et Influence de Produits Cosmétiques sur la Virulence Bactérienne. Apports de la Technique de Spectrométrie de Masse MALDI-TOF. Ph.D. Thesis, Université de Rouen, Évreux, France, 2013. [Google Scholar]
- Benita, G.; Cornu, P. Compositions Based on Prebioitcs and Extracts of Orchioid Curculigo and Uses Thereof. France Patent FR3009962A1, 28 August 2013. [Google Scholar]
- Glycolift®. Anti-Aging Tensor. Available online: https://www.solabia.com/cosmetics/product/glycolift/ (accessed on 1 January 2022).
- Ellen, S.; Kurtz, W.W. Colloidal oatmeal: History, chemistry and clinical properties. J. Drugs Dermatol. 2007, 6, 167–170. [Google Scholar]

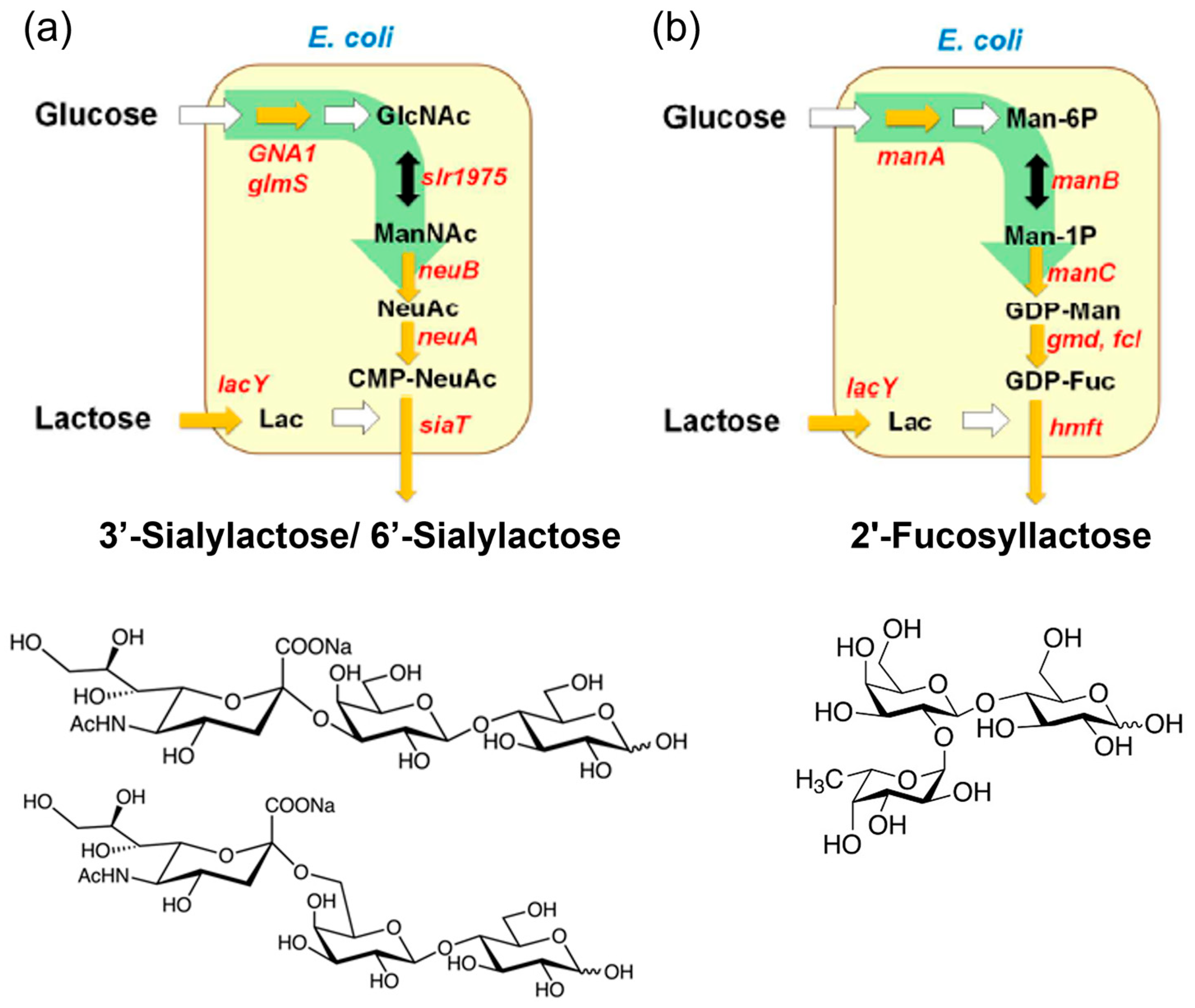
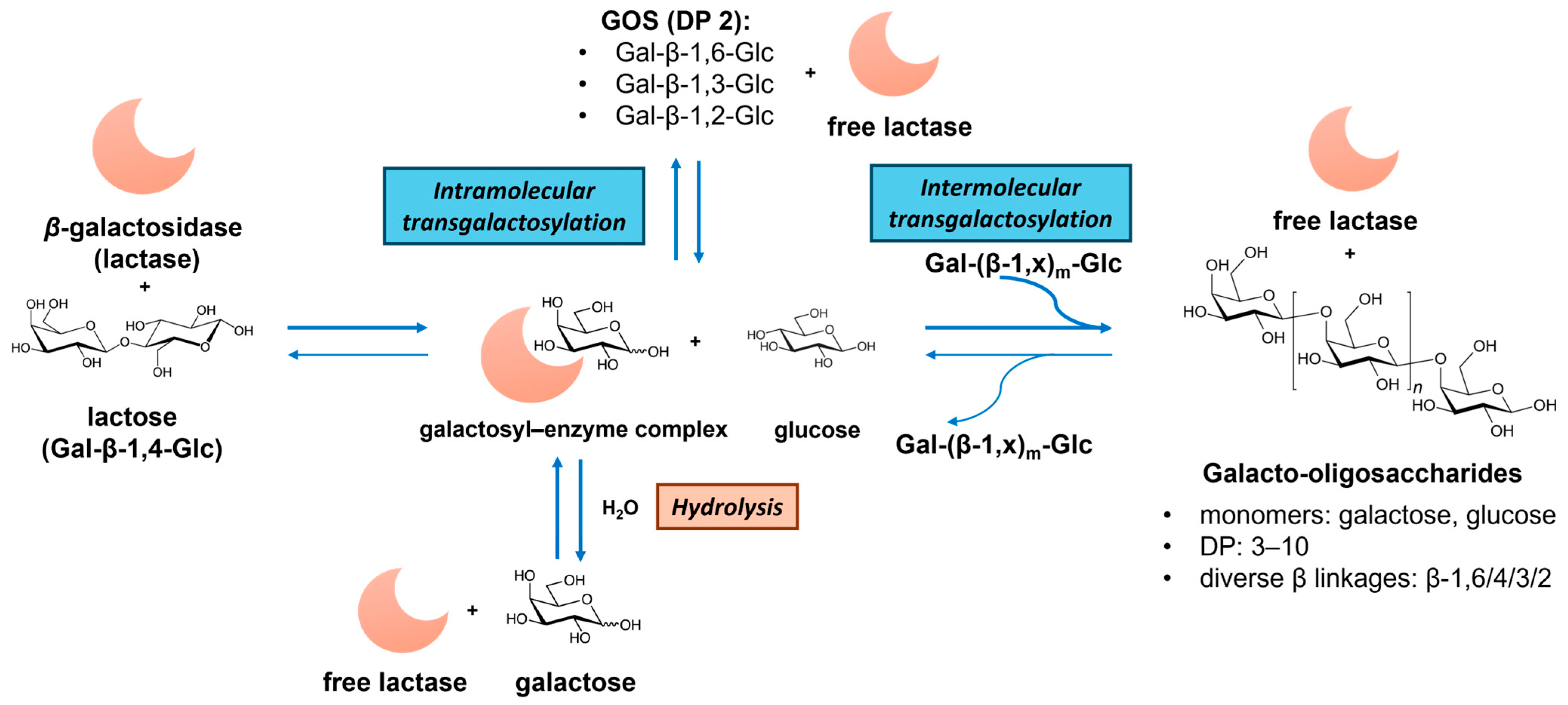
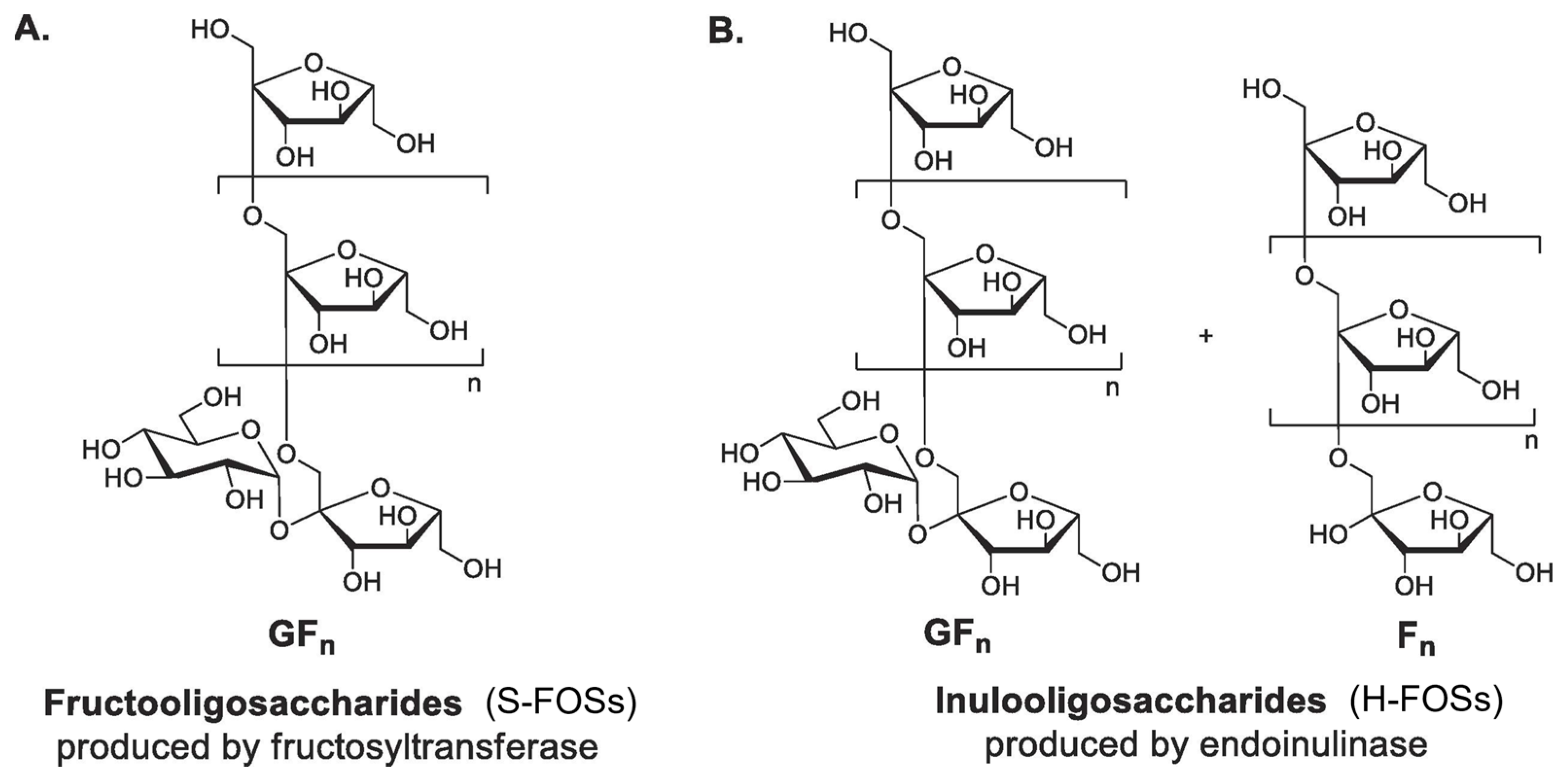

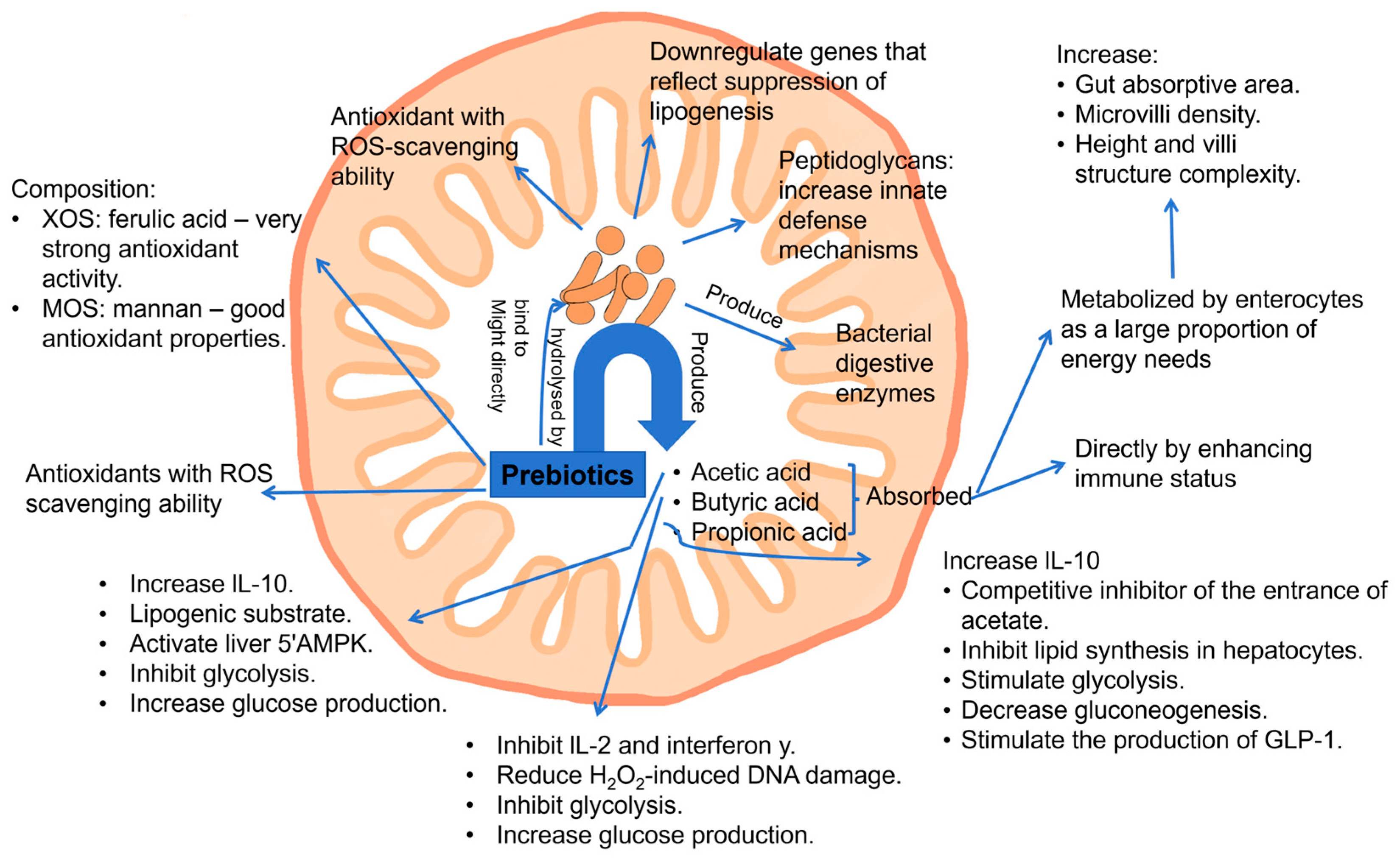

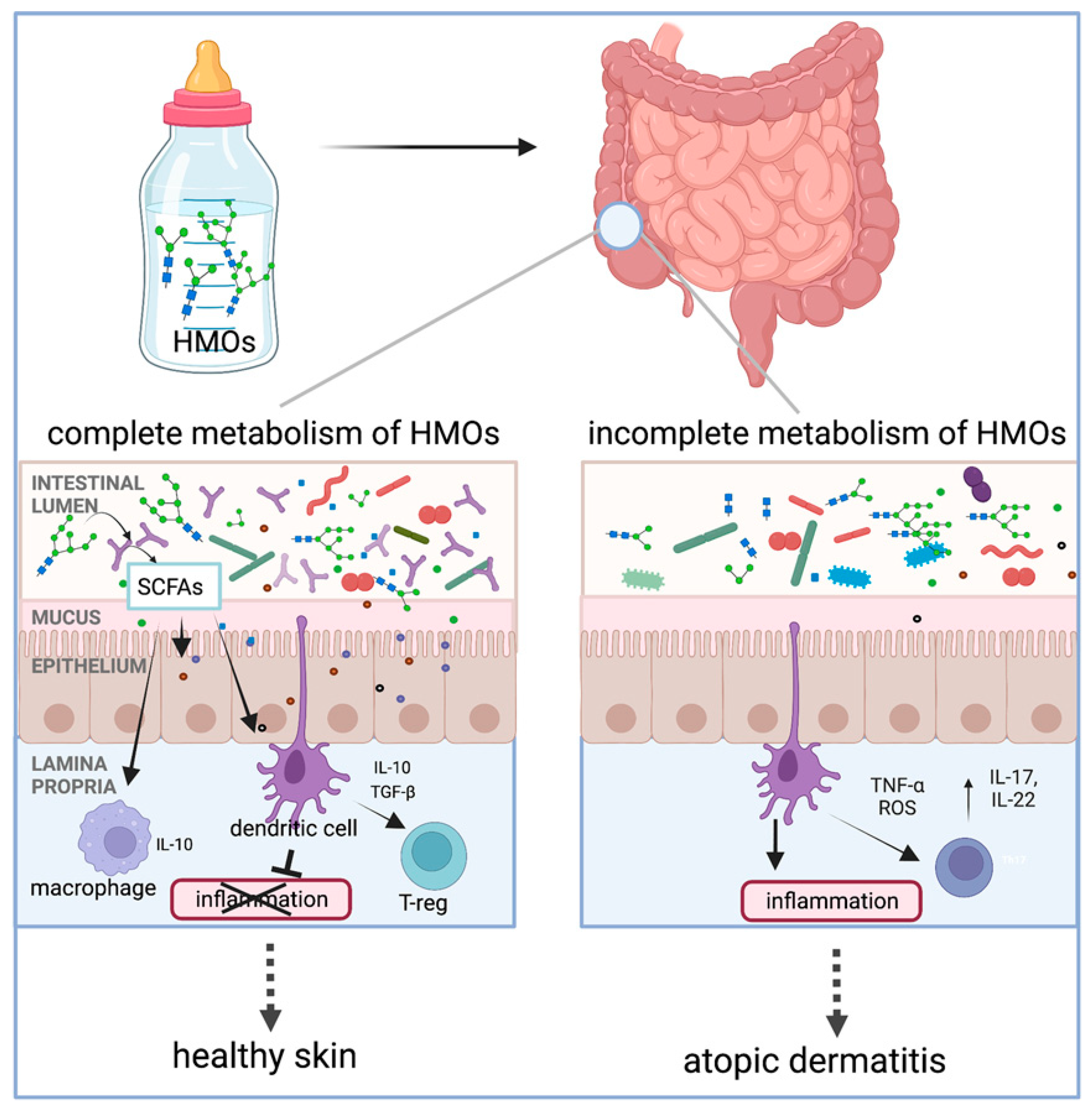
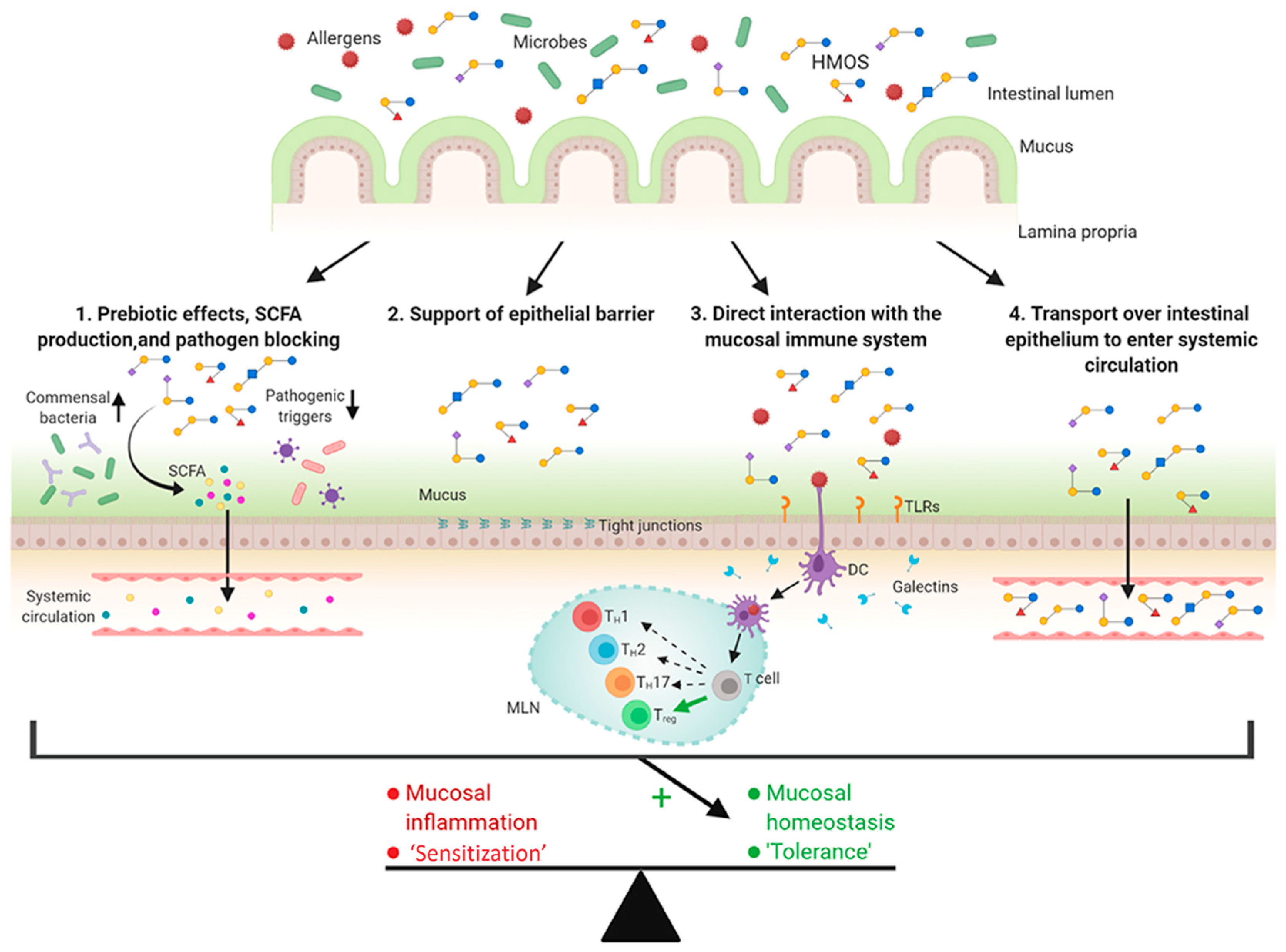
| Trivial Names | Abbr. | Monomer Unit(s) | Glycosidic Linkage(s) | DP | Production Methods | Ref. | |
|---|---|---|---|---|---|---|---|
| human milk oligosaccharides | HMOs | Neu5Ac, fucose, galactose, GlcNAc, acetylglucosamine, glucose | β-(1→4), β-(1→6), β-(1→3), α-(1→2), α-(1→3), α-(1→6) | 2–10 | Glycosyl transfer reaction of sugar nucleotides (e.g., UDP-GlcNAc, GDP-Fuc, and CMP-Neu5Ac) and lactose by glycosyl transferase expressed by modified microorganisms | [31,32,33] | |
| galacto-oligosaccharides | GOSs | galactose, glucose | β-(1→4), β-(1→6), β-(1→3), β-(1→2), β-(1→1) terminal β-(1→4) | 2–10 | Enzymatic transgalactosylation of lactose by β-galactosidase | [34,35,36] | |
| fructo-oligosaccharides | FOSs | fructose, glucose | β-(2→1), terminal α-(1→2) | 2–20 or 2–5 | Enzymatic depolymerization of inulin by inulinase; enzymatic transfructosylation from sucrose by β-fructosidase | [37,38,39] | |
| gluco-oligosaccharides | α-glucan oligosaccharides | α-GlcOSs | glucose | α-(1→6), α-(1→4), α-(1→2), α-(1→3), α-(1→1); | 2–20 | Enzymatic hydrolysis of starch followed by transglycosylation by α-transglucosidase; enzymatic transglycosylation from sucrose by dextransucrase | [40,41] |
| β-gluco-oligosaccharides | β-GlcOSs | glucose | β-(1→4) | 2–20 | Enzymatic/chemical hydrolysis of cellulose or lignocellulosic biomass | [42,43,44,45] | |
| xylo-oligosaccharides | XOSs | xylose | β-(1→4) | 2–10 | Enzymatic/chemical depolymerization of hemicellulose xylan by β-xylanases | [46] | |
| Chitosan oligosaccharides | COSs | glucosamine | β-(1→4) | 2–20 | Enzymatic/chemical depolymerization of chitin followed by deacetylation | [47,48] | |
| agaro-oligosaccharides/neoagaro-oligosaccharides | AOSs/NAOSs | alternating 3,6-anhydrogalactose and galactose | α-(1→3), β-(1→4) | 2–10 | Enzymatic/chemical hydrolysis of agarose (extracted from red algae) | [49,50] | |
| Health Benefits | Oligosaccharides | Effect on Skin Microbiota | Ref. |
|---|---|---|---|
| Promotion of beneficial skin commensals and inhibition of skin pathogens | 2′-fucosyllactose (2′-FL, in HMO); GOSs | GOSs (10%, 5%) and 2.5% 2′-FL significantly decreased Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) bacterial growth/CFUs (p ≤ 0.05); 1.5% 2′-FL significantly suppressed the lag time of SA growth (p ≤ 0.05) and was effective against SA and PA at 2.5% (p ≤ 0.01). | [80] |
| GOSs | A study found that 5% (w/v) GOS stimulated S. epidermidis DSM 20044 and inhibited S. aureus ATCC 25923 both in nutrient broth and cosmetic formulations; higher release rates of GOS oil-in-water (O/W) gel emulsions, with controlled release of GOSs (DP 3) in the hydrogel. | [81] | |
| FOSs | FOSs (DP 3–5) promoted the growth of beneficial S. epidermidis ATCC 12228 while inhibiting both pathogenic C. acnes CCUG 1794T and S. aureus ATCC 6538 in an in vitro human epithelium model. | [82] | |
| GlcOSs | A study found that 5% GlcOS inhibited the glycocalyx production by S. aureus cells and suppressed S. aureus colonization on the horny cells of atopic dermatitis lesions | [83] | |
| β-glucan (in colloidal oat) | A study found that 1% colloidal oat (containing 5% β-glucan) promoted the growth rate and metabolism of S. epidermidis ATCC 12228 and increased the production of lactic acid (a natural moisturizing factor). | [84] | |
| Oligochitosan | A study found that 10 kDa oligochitosan showed the highest antimicrobial effect on C. acnes with a minimum inhibitory concentration of 32–64 μg/mL; it also worked synergistically with antibiotics (tetracycline or erythromycin) against C. acnes. | [85] | |
| FOSs, GOSs, IMOs, inulin | FOSs, GOSs, IMOs, and inulin significantly promoted the in vitro growth of S. epidermidis CCSM0287 and increased SCFA production; the addition of 2% FOSs post-fermentation supernatant significantly inhibited S. aureus CCSM0424 biofilm formation. | [23] | |
| FOSs, GOSs, IMOs, LAG | A study found that 1% GOS/FOS/IMO/LAG with 1% xylitol showed species-specific antibacterial/antibiofilm against pathogenic S. aureus strains while not affecting S. epidermidis strains. | [86] | |
| Oligosaccharides derived from Ulva sp. (green alga) | Oligosaccharides derived from Ulva sp. (1.5 kDa, 8 kDa) at 1000 μg/mL did not significantly modify the cytotoxicity of S. aureus MFP03 and S. epidermidis MFP04 towards HaCaT keratinocytes in vitro, but they could modify the bacterial biofilm structures; the oligosaccharides induced a decreased inflammatory potential of both acne and non-acne C. acnes strains on keratinocytes of up to 39.8%. | [87] | |
| Anti-melanogenesis | 2′-fucosyllactose | An MTT assay performed on MNT-1 cells and human-derived melanocytes showed a 40% reduction in melanin production from 2′-FL treatment (no cytotoxicity at 20 g/L or less), which was mediated through autophagy activation and inhibited the expression of melanogenesis proteins TYR and TYRP1. | [88,89] |
| GOSs | GOSs (74.9% purity) at 70 mg/mL or lower concentrations showed no cytotoxicity in B16F10 melanoma cells in vitro; melanin accumulation was inhibited at 14 mg/mL GOS or higher; 14–35 mg/mL GOS showed a protective effect on HaCaT keratinocytes upon UVB irradiation. | [29] | |
| AOSs, NAOSs | 3,6-anyhydro-L-galactose (AHG, prepared from enzymatic hydrolysis of agarose) at a concentration of 100 μg/mL showed significantly lower melanin production compared to arbutin in an in vitro skin whitening assay; 100/200 μg/mL AHG showed strong anti-inflammatory activity in suppressing nitrite production. | [90] | |
| AOSs, NAOSs | AOSs, NAOSs, and AHG at 50 μg/mL did not show significant cytotoxicity in murine B16 melanoma cells (B16F10) and human epidermal melanocytes (HEMs) in vitro; AHG and NAOSs (DP 4, DP 6) at 50 μg/mL both significantly inhibited the α-melanocyte-stimulating hormone (α-MSH)-induced melanin production in B16F10 cells and HEMs (AHG highest), while AOSs of DP 3, DP 5, and DP 7 (50 μg/mL) did not; AHG is the monomer unit responsible for the anti-melanogenic effect of agar-derived oligosaccharides. | [91] | |
| Antioxidant and anti-inflammation | AOSs, NAOSs | A mixture of AHG and AOSs (DP 3) showed significantly higher reactive oxygen species (ROS)-scavenging activity than NAOSs (DP 2 and DP 4) in H2O2-induced human dermal fibroblasts (HDFs) in vitro, exerting ability in protecting skin from oxidative stress (antioxidant activity); AHG and AOSs (DP 3) exhibited no cytotoxicity in HDFs and promoted cell proliferation. | [92] |
| α-gluco-oligosaccharides (α-GlcOSs) | α-GlcOSs (average Mw 1137 Da) with 24% α-(1→4) and 76% α-(1→6) linkages exhibited a prebiotic effect on six bacterial and yeast strains and anti-inflammatory activity towards RAW 265.7 macrophage cells by suppressing the expression of nitric oxide synthase, tumor necrosis factor-α, IL-1β, IL-6, and IL-10 and inhibiting the nuclear factor-kappa B (NF-κB) signaling pathways. | [93] | |
| pectic oligosaccharides (POSs) | POSs derived from the Fenton hydrolysis of okra pectin showed improved antioxidant and anti-inflammatory activities compared with okra pectin; POSs of 1.79 kDa (lowest Mw) showed the highest antioxidant activity by scavenging DPPH and ABTS radicals and also the highest anti-inflammatory activity by inhibiting LPS-induced NO production, iNOS expression, and expression of IL-1β and IL-6 mRNAs by suppressing NF-κB signaling pathways in RAW 264.7 cells. | [94] | |
| Wound healing | GOSs | GOSs (prepared from whey permeate via fermentation by Lactobacillus delbrueckii ssp.) promoted wound healing and skin health by its direct activity on keratinocyte functions, including stimulation on the beneficial reversible inflammatory response, mitochondrial respiration, cell migration, and differentiation in HaCaT cells in vitro. | [30] |
| GOSs, inulin | A diet supplemented with GOSs or inulin improved the general health of mice undergoing surgical colonic anastomosis, indicated by increased body weight and induced thickening of the colonic wall; increased butyrate production and better anastomotic healing was observed, with enhanced mucosal continuity. | [95] | |
| Thiolated chitosan oligosaccharides/hyaluronic acid oligosaccharide-based hydrogels | Thiolated poly- and oligosaccharide-based hydrogels were found to have good biocompatibility, biodegradability, and nontoxicity, and favorable for wound healing processes, such as in situ gelling, cell adhesion (bioadhesion), drug release controlling, enzyme inhibitory, and metal binding properties (mitigate toxic effects). | [96] |
| Functions | Treatment | Effect on Skin–Gut Microbiota and Skin Health | Trial Type | Ref. |
|---|---|---|---|---|
| Alleviation/prevention of atopic dermatitis and allergies | Oral HMOs | HMO metabolism in infants helps establish the infant gut microbiota, modulate the immune system, and prevent against atopic dermatitis (AD)/eczema development or reduce the severity through the regulation of the immune response (e.g., IL-10) | human trial | [17,105] |
| Oral GOSs | Dietary GOS treatment (0.5 or 1.5 g/kg) on DNFB-induced AD mice (N = 40) markedly relieved skin inflammation by decreasing IgE, IL-4, IL-13, IFN-γ, and TNF-α production and regulating PPAR-γ/NF-κB signaling; GOS treatment also significantly improved anxiety- and depressive-like symptoms by normalizing the neurotransmitter levels in the brain | mice studies | [18] | |
| Oral FOSs | Oral intake of kestose-rich FOSs (>85% kestose, 7.3 mg/day) alleviated atopic dermatitis in ovalbumin-sensitized Balb/c mice (N = 42) by modulating the gut–skin axis (altered the intestinal microbiome and significantly increased butyric and propionic acid levels in mice feces) and immune response (markedly decreased IgE levels and regulatory T cell-mediated T helper cell type 2, mast cells, and eosinophils) | mice studies | [106] | |
| Oral alginate oligosaccharides (AOSs) | Oral gavage (10 mg/kg body weight) of AOSs in natural aging mice caused modulation of the colonic butyrate-HIF-1α axis homeostasis, which promoted entry of butyrate into the skin and upregulated the mitophagy level, thus improving skin aging via the HDAC3/PHDHIF-1α/mitophagy loop in the skin of mice | mice studies | [107] | |
| Attenuation of acne vulgaris | Oral FOSs and GOSs | Consecutive intake (3 months) of FOSs (100 mg) and GOSs (500 mg) in female adults with acne improved glycemic and lipid metabolism | human trials (N = 12) | [108] |
| Oligochitosan (chitosan oligosaccharides) | A study found that 10 kDa oligochitosan showed the highest antimicrobial effect, with minimum inhibitory concentration values of 32–64 μg/mL on C. acnes; combination of tetracycline– or erythromycine–10 kDa oligochitosan resulted in a median Σ fractional inhibitory concentration (FIC) range of 0.02–0.56 | in vitro and in vitro | [85,109] | |
| Seaweed oligosaccharides with zinc complex (SOZC) | A study found that 5.6% SOZC reduced C. acnes counts by 74% in vitro, and SOZC was found to have soothing effects, indicated by increased levels of interleukin 1 alpha by 11.1%; a gel containing 5% SOZC significantly improved acne vulgaris symptoms by 14 days with reduced sensitivity and improved skin healing | double-blind human trials (N = 12) | [110] | |
| Skin hydration, anti-dryness | Topical GOSs | GOS treatment (in a cosmetic serum) on healthy females (40–60 yrs) significantly improved skin water-holding capacity (positively correlated with Enhydrobacter), reduced transepidermal water loss (TEWL, negatively correlated with Enterobacteriaceae), erythema index, wrinkle depth, and S. aureus populations | double-blind human trials (N = 60) | [24,25] |
| Oral GOSs | Oral administration of GOSs (100 mg, 12 weeks) on hairless mice increased the water-holding capacity of the skin, prevented transepidermal water loss, and reduced erythema formation (16.8%) | mice studies | [111] | |
| Oral GOSs with B. breve | Daily intake of fermented milk containing prebiotic GOSs and probiotic B. breve (YIT 12272, Yakult) for 4 weeks was found to benefit skin condition through a reduction in serum total phenol levels; it also prevented skin dryness (increased hydration level of stratum corneum) and disrupted keratinization in healthy adult women (N = 40) | a double-blind, placebo-controlled, randomized human trial | [28,112] | |
| Topical α-gluco-oligosaccharides (α-GlcOSs) | Topical application of α-GlcOSs (in a lotion, 4 weeks) and collagen tripeptide F in females with sensitive atopic skin (N = 40, 30–59 yrs) significantly reduced TEWL and improved skin hydration, roughness, desquamation, and skin irritability | a double-blind, placebo-controlled, randomized human trial | [113] | |
| Anti-itching | Oral FOSs | Oral take of FOSs (4.25 g 1-kestose) for 12 weeks alleviated itching and sleep disturbance symptoms in children (N = 48, 2–17 yrs) with atopic dermatitis while inducing specific alterations in the skin microbiome (decreased Lachnospiraceae abundance; increased Prevotella and Rothia abundance) and epidermal lipid profiles | a randomized, double-blind, placebo-controlled human trial | [19] |
| Topical colloidal oat (starch and β-glucans) | Topical application (6-week, twice a day) of a moisture containing 1% colloidal oat on female subjects with dry skin significantly increased the concentration of lactic acid (a natural moisturizing factor for stratum corneum); 1% colloidal oat altered the gene expression profile of S. epidermidis, alleviating skin dryness | in vitro and human trials | [84] | |
| Topical colloidal oatmeal (containing 5% β-glucans, 5% fiber) | Two-week application (twice a day) of a colloidal oatmeal skin protectant lotion on healthy females (N = 29) with itchy dry skin on their lower legs showed significant improvement in skin dryness, scaling, roughness, and itch intensity, potentially attributed to the antioxidant and anti-inflammatory activities of colloidal oatmeal | a blind human trial | [114] | |
| Anti-aging and photoprotection | Oral GOSs | Oral intake of GOSs (200 mg/kg) increased TEWL, decreased water-holding capacity, and significantly reduced the wrinkle area and mean wrinkle length in hairless mice exposed to UVB; inflammatory cytokines (IL-6, IL-12, TNF-α) induced by UVB were significantly reduced; UVB-induced MAPK phosphorylation of kinases (JNK, p38, ERK) contributing to skin aging was significantly inhibited | mice studies | [115] |
| Oral GOSs with B. longum | Oral treatment of GOSs with B. longum protected the skin of hairless mice against UVB-induced photo-aging and showed anti-inflammatory and antioxidative effects | mice studies | [111,116] | |
| Oral GOSs with collagen tripeptide (CTP) | Oral administration of CTP and GOS mixtures (different ratios) in UVB-irradiated hairless mice (8 weeks) showed an inhibitory effect on photo-aging, including a reduction in photoaged physical parameters (TEWL, wrinkle area) and serum levels of pro-inflammatory cytokines; the treatment also reversed the increasing abundance of Firmicutes induced by UVB and increased the Verrucomicrobiota abundance | mice studies | [117] | |
| Topical α-glucan oligosaccharides | Treatment (28 days) with a moisturizer formulated with α-glucan oligosaccharides and postbiotics (Pseudoalteromonas ferment extract) positively influenced skin microbiome diversity and balance and provided anti-aging benefits to females (N = 25, 35–65 yrs) with Fitzpatrick skin types I–VI, moderate crow’s feet wrinkles, and global face photodamage | a human trial followed EU Scientific Committee on Consumer Safety (SCCS) protocol | [26] | |
| Oral and/or topical xylo-oligosaccharides (XOSs) | Oral and/or topical application of XOSs regulated facial cutaneous aging in females (N = 77) by inhibiting Cutibacterium abundance and the enrichment of intestinal Bifidobacterium | a double-blind placebo-controlled human trial | [21] | |
| Topical chitosan oligosaccharides (COSs) | Topical application (10 weeks) of COSs (average Mw ≤ 1000 Da, 50/100/200 mg/mL) on hairless mice (N = 12) contributed to the prevention of UV-induced skin dryness, epidermal hyperplasia, wrinkle formation by increasing activities of antioxidative enzymes, and suppressing the production of pro-inflammatory cytokines | mice studies | [27] | |
| Topical succinyl-chitosan oligosaccharides (SU-COSs) | SU-COSs (6400 Da, substitution degree 69.26%) exhibited good biocompatibility and promoted the migration of HaCaT cells in vitro; in vitro treatment of SU-COSs exerted a protective effect on UVB-damaged HaCaT cells by preserving cytoskeletal morphology, regulating cell cycle, scavenging intracellular reactive oxygen species, and inhibiting apoptosis; in vivo treatment of SU-COSs repaired UVB-damaged mice skin by reducing skin erythema and water loss, relieving crusting, and maintaining skin epidermis thickness the stability of collagen fibers | in vitro and mice studies | [22] | |
| Topical ginseng oligosaccharide extract (GSOs) | GSO (1000 Da, β-linkages) treatment (0.2/1.0/2.0 mg/cm2/day) on the dorsal skin of BALB/c hairless mice (N = 25) attenuated UVB-induced epidermal thickening and moisture loss; GSOs improved the expression of skin barrier proteins (FLG, IVL, AQP3) related to skin dryness and the enzymes involved in stratum corneum exfoliation (increased DSG1, decreased KLK7) | mice studies | [118] |
| Pathway | Oligosaccharides | Administration Route | Mechanism of Action | Skin Health Benefits |
|---|---|---|---|---|
| AMPK–ULK1 | HMOs (2′-FL) | oral | Activates AMPK, phosphorylates ULK1, induces autophagy, and downregulates melanogenic enzymes (TYR, TYRP1). | Anti-melanogenesis |
| MAPK | GOSs | oral | Inhibits UVB-induced MAPK (JNK/p38/ERK), reducing wrinkle formation and inflammation. | Anti-aging, photoprotection, anti-wrinkle |
| COSs | topical | Suppresses MAPK signaling, scavenges ROS, and protects against UV damage. | Photoprotection, anti-aging | |
| XOSs | oral, topical | Modulates Cutibacterium abundance and lipid synthesis via MAPK. | Anti-aging, microbiome balance | |
| NF-κB | GOSs | oral | Downregulates NF-κB, reducing pro-inflammatory cytokines (IL-6, TNF-α). | Anti-atopic dermatitis, anti-inflammation |
| FOSs | oral | Enhances SCFA production, indirectly suppressing NF-κB in the gut–skin axis. | Skin hydration, itch relief | |
| AOSs (alginate) | oral | Upregulates the butyrate–HIF-1α axis, inhibiting NF-κB-driven inflammation. | Anti-aging, skin repair | |
| GlcOSs | topical | Blocks NF-κB activation in keratinocytes, reducing oxidative stress. | Anti-inflammatory, sensitive skin relief | |
| SCFAs | HMOs | oral | Gut microbiota metabolize HMOs to SCFAs (butyrate), promoting Treg cells and IL-10. | Prevents infant atopic dermatitis |
| GOSs | oral | Increases fecal SCFAs (acetate/butyrate), modulating the gut–skin immune response. | Anti-eczema, anxiolytic effects | |
| FOSs | oral | Boosts Lactobacillus-derived SCFAs, improving skin barrier lipids. | Reduces transepidermal water loss (TEWL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Li, Y.; Cheng, J.; Wang, J.; Liu, Q. Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications. Antioxidants 2025, 14, 754. https://doi.org/10.3390/antiox14060754
Zeng M, Li Y, Cheng J, Wang J, Liu Q. Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications. Antioxidants. 2025; 14(6):754. https://doi.org/10.3390/antiox14060754
Chicago/Turabian StyleZeng, Meijun, Yang Li, Jie Cheng, Jingyu Wang, and Qiyu Liu. 2025. "Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications" Antioxidants 14, no. 6: 754. https://doi.org/10.3390/antiox14060754
APA StyleZeng, M., Li, Y., Cheng, J., Wang, J., & Liu, Q. (2025). Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications. Antioxidants, 14(6), 754. https://doi.org/10.3390/antiox14060754






