Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia—Focus on the NO System
Abstract
1. Introduction
2. Prenatal Hypoxia and Its Impact on Cardiovascular Development
2.1. Prenatal Hypoxia and Its Consequences
2.2. Causes of Prenatal Hypoxia
2.3. The Role of NO in Heart Regulation
2.4. The Role of NO in the Heart During Fetal Development
2.5. Changes in the Nitric Oxide System in the Heart of Offspring After PH
3. Mechanisms of Cardiovascular Dysfunction After Prenatal Hypoxia
3.1. Disruption of Energy Metabolism in the Myocardium and Mitochondrial Dysfunction in the Offspring After PH
3.2. Nitrosative Stress in the Heart of Offspring After PH
3.3. NO-Dependent Mechanisms of Endothelial Dysfunction After PH
3.4. NO and Cardiomyocyte Apoptosis After PH
4. Molecular Mechanisms and Stress Responses
4.1. The Interaction Between NO and HIFs in the Myocardium After PH
4.2. NO and Inflammation After PH
4.3. NO and HSP70 After PH
4.4. Oxidative Stress in Myocardial Damage After PH
5. Cardioprotection and Therapeutic Approaches
5.1. Cardioprotection After PH
5.2. NO Modulators—Promising Cardioprotectors After PH
5.2.1. Angiolin((S)-2,6-Diaminohexanoic Acid 3-methyl-1,2,4-triazolyl-5-thioacetate)
5.2.2. Tiothiazoline (Morpholine 3-methyl-1,2,4-triazolyl-5-thioacetate; Morpholine Thiazotate)
5.2.3. Mildronate
5.2.4. L-Arginine
5.2.5. Repurposing Pharmacological Agents for Cardiovascular Protection in Prenatal Hypoxia, Comorbid Conditions, and Long-Term Consequences
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patterson, A.J.; Zhang, L. Hypoxia and fetal heart development. Curr. Mol. Med. 2010, 10, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ream, M.; Ray, A.M.; Chandra, R.; Chikaraishi, D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R583–R595. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, T.; Tonon, C.R.; Martins, D.; Fávero, E.L., Jr.; Baumgratz, T.D.; Pereira, F.W.L.; Pinheiro, V.R.; Ballarin, R.S.; Queiroz, D.A.R.; Azevedo, P.S.; et al. Post-Cardiac Arrest: Mechanisms, Management, and Future Perspectives. J. Clin. Med. 2023, 12, 259. [Google Scholar] [CrossRef]
- Zhang, L. Prenatal hypoxia and cardiac programming. J. Soc. Gynecol. Investig. 2005, 12, 2–13. [Google Scholar] [CrossRef]
- Narohan, M.V.; Bazhenova, L.K.; Kapranova, E.I.; Melnikova, E.V.; Belousova, N.A. Posthypoxic Dysfunction of the Cardiovascular System in Newborns. Curr. Issues Pediatr. 2007, 6, 42–46. (In Ukranian) [Google Scholar]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-induced signaling in the cardiovascular system: Pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P. Effects of chronic hypoxia on fetal coronary responses. High Alt. Med. Biol. 2003, 4, 215–224. [Google Scholar] [CrossRef]
- Lucero García Rojas, E.Y.; Villanueva, C.; Bond, R.A. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 709509. [Google Scholar] [CrossRef]
- Chlif, M.; Ammar, M.M.; Said, N.B.; Sergey, L.; Ahmaidi, S.; Alassery, F.; Hamam, H. Mechanism of Dyspnea during Exercise in Children with Corrected Congenital Heart Disease. Int. J. Environ. Res. Public Health 2021, 19, 99. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital Heart Disease: Causes, Diagnosis, Symptoms, and Treatments. Cell Biochem. Biophys. 2015, 72, 857–860. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482316 (accessed on 4 March 2024).
- Okada, Y.; Paton, J.F.R.; López-Barneo, J.; Wilson, R.J.A.; Marina, N.; Pokorski, M. Editorial: Hypoxia and Cardiorespiratory Control. Front. Physiol. 2021, 12, 820815. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Bak, P.G.; Popazova, O.O.; Bukhtiyarova, N.V.; Yadlovsky, O.E. Nitric oxide-dependent mechanism of endothelial dysfunction formation is a promising target link for pharmacological management. Biopolym. Cell 2022, 38, 145–157. [Google Scholar] [CrossRef]
- Belenichev, I.; Kucherenko, L.; Pavlov, S.; Bukhtiyarova, N.; Popazova, O.; Derevianko, N.; Nimenko, G. Therapy of post-COVID-19 syndrome: Improving the efficiency and safety of basic metabolic drug treatment with tiazotic acid (thiotriazoline). Pharmacia 2022, 69, 509–516. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E239–E245. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric oxide enhancement strategies. Future Sci. OA 2015, 1, FSO48. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Napoli, C.; Loscalzo, J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: An overview. Circ. Res. 2002, 90, 21–28. [Google Scholar] [CrossRef]
- Gandoy-Fieiras, N.; Gonzalez-Juanatey, J.R.; Eiras, S. Myocardium Metabolism in Physiological and Pathophysiological States: Implications of Epicardial Adipose Tissue and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 2641. [Google Scholar] [CrossRef]
- Leone, A. Morphology of coronary arteries in relation to ischemic heart disease. J. Cardiol. Curr. Res. 2021, 14, 27–32. [Google Scholar] [CrossRef]
- Peng, X.; Du, J.; Wang, Y. Metabolic signatures in post-myocardial infarction heart failure, including insights into prediction, intervention, and prognosis. Biomed. Pharmacother. 2024, 170, 116079. [Google Scholar] [CrossRef] [PubMed]
- Sutovska, H.; Babarikova, K.; Zeman, M.; Molcan, L. Prenatal Hypoxia Affects Foetal Cardiovascular Regulatory Mechanisms in a Sex- and Circadian-Dependent Manner: A Review. Int. J. Mol. Sci. 2022, 23, 2885. [Google Scholar] [CrossRef] [PubMed]
- Frasch, M.G.; Giussani, D.A. Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development. Cells 2020, 9, 733. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, H.; Liu, J.; Sun, M. Effects of Prenatal Hypoxia on Nervous System Development and Related Diseases. Front. Neurosci. 2021, 15, 755554. [Google Scholar] [CrossRef] [PubMed]
- Hula, N.; Liu, R.; Spaans, F.; Pasha, M.; Quon, A.; Kirschenman, R.; Cooke, C.-L.M.; Davidge, S.T. The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female offspring. Biomedicines 2022, 10, 3019. [Google Scholar] [CrossRef]
- Salameh, A.; Zöbisch, H.; Schröder, B.; Vigelahn, J.; Jahn, M.; Abraham, G.; Seeger, J.; Dähnert, I.; Dhein, S. Effects of Hypoxia and Acidosis on Cardiac Electrophysiology and Hemodynamics. Is NHE-Inhibition by Cariporide Still Advantageous? Front. Physiol. 2020, 11, 224. [Google Scholar] [CrossRef]
- Neubert, E.; Rassler, B.; Hoschke, A.; Raffort, C.; Salameh, A. Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats. Int. J. Mol. Sci. 2023, 24, 11417, Erratum in Int. J. Mol. Sci. 2024, 25, 8408. https://doi.org/10.3390/ijms25158408. [Google Scholar] [CrossRef]
- Takahashi, C.; Hinson, H.E.; Baguley, I.J. Autonomic dysfunction syndromes after acute brain injury. Handb. Clin. Neurol. 2015, 128, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Tjurmina, O.A.; Ajijola, O.A.; Arora, R.; Bolser, D.C.; Chapleau, M.W.; Chen, P.S.; Clancy, C.E.; Delisle, B.P.; Gold, M.R.; et al. Opportunities in Autonomic Neural Mechanisms of Cardiopulmonary Regulation: A Report from the National Heart, Lung, and Blood Institute and the National Institutes of Health office of the Director Workshop. JACC Basic Transl. Sci. 2022, 7, 265–293. [Google Scholar] [CrossRef]
- Grune, J.; Yamazoe, M.; Nahrendorf, M. Electroimmunology and cardiac arrhythmia. Nat. Rev. Cardiol. 2021, 18, 547–564. [Google Scholar] [CrossRef]
- Dwyer, K.D.; Snyder, C.A.; Coulombe, K.L.K. Cardiomyocytes in Hypoxia: Cellular Responses and Implications for Cell-Based Cardiac Regenerative Therapies. Bioengineering 2025, 12, 154. [Google Scholar] [CrossRef]
- Nerheim, P.; Krishnan, S.C.; Olshansky, B.; Shivkumar, K. Apoptosis in the genesis of cardiac rhythm disorders. Cardiol. Clin. 2001, 19, 155–163. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Law, Y.M.; Asante-Korang, A.; Austin, E.D.; Dipchand, A.I.; Everitt, M.D.; Hsu, D.T.; Lin, K.Y.; Price, J.F.; Wilkinson, J.D.; et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e9–e68. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhu, X.; Jiao, Y.; Liu, H.; Huang, Z.; Pei, J.; Xu, Y.; Yang, Y.; Ren, K. Cardiac cell senescence: Molecular mechanisms, key proteins and therapeutic targets. Cell Death Discov. 2024, 10, 78. [Google Scholar] [CrossRef]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef] [PubMed]
- Sisakian, H. Cardiomyopathies: Evolution of pathogenesis concepts and potential for new therapies. World J. Cardiol. 2014, 6, 478–494. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Yamada, T.; Nomura, S. Recent Findings Related to Cardiomyopathy and Genetics. Int. J. Mol. Sci. 2021, 22, 12522. [Google Scholar] [CrossRef]
- Ahluwalia, M.; Kpodonu, J.; Agu, E. Risk Stratification in Hypertrophic Cardiomyopathy: Leveraging Artificial Intelligence to Provide Guidance in the Future. JACC Adv. 2023, 2, 100562. [Google Scholar] [CrossRef]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational Hypoxia and Developmental Plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef] [PubMed]
- McCartan, C.; Mason, R.; Jayasinghe, S.R.; Griffiths, L.R. Cardiomyopathy classification: Ongoing debate in the genomics era. Biochem. Res. Int. 2012, 2012, 796926. [Google Scholar] [CrossRef][Green Version]
- Oh, K.S.; Bender, T.M.; Bowen, A.; Godine, L.; Park, S.C. Transient myocardial ischemia of the newborn infant. Pediatr. Radiol. 1985, 15, 29–33. [Google Scholar] [CrossRef]
- Flores-Nava, G.; Echevarría-Ybargüengoitia, J.L.; Navarro-Barrón, J.L.; García-Alonso, A. Isquemia miocárdica transitoria en el recién nacido con asfixia perinatal (miocardiopatía hipóxica) [Transient myocardial ischemia in newborn babies with perinatal asphyxia (hypoxic cardiomyopathy)]. Bol. Med. Hosp. Infant Mex. 1990, 47, 809–814. (In Spanish) [Google Scholar] [PubMed]
- Posthypoxic myocardial ischemia in newborn: Diagnosis and treatment of severe type. Anesteziol. Reanimatol. 2012, 1, 65–68. (In Russian) [PubMed]
- Ovali, F. Hemodynamic changes and evaluation during hypoxic-ischemic encephalopathy and therapeutic hypothermia. Early Hum. Dev. 2022, 167, 105563. [Google Scholar] [CrossRef] [PubMed]
- Davey, B.; Szwast, A.; Rychik, J. Diagnosis and management of heart failure in the fetus. Minerva Pediatr. 2012, 64, 471–492. [Google Scholar]
- Ding, H.; Luo, Y.; Hu, K.; Huang, H.; Liu, P.; Xiong, M.; Zhu, L.; Yi, J.; Xu, Y. Hypoxia in utero increases the risk of pulmonary hypertension in rat offspring and is associated with vasopressin type-2 receptor upregulation. Mol. Med. Rep. 2020, 22, 4173–4182. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Lewis, C.V.; Hidalgo, D.C.; Posey, J.N.; Jordan, M.; Porfilio, T.E.; Grayck, M.R.; Wright, C.J.; Delaney, C.; Nozik, E.S. A maternal hypoxia mouse model to study the effect of late gestational hypoxia on offspring lung outcomes. Front. Physiol. 2025, 16, 1513703. [Google Scholar] [CrossRef]
- Bhasin, H.; Kohli, C. Myocardial dysfunction as a predictor of the severity and mortality of hypoxic ischaemic encephalopathy in severe perinatal asphyxia: A case-control study. Paediatr. Int. Child Health 2019, 39, 259–264. [Google Scholar] [CrossRef]
- Neary, M.T.; Breckenridge, R.A. Hypoxia at the heart of sudden infant death syndrome? Pediatr. Res. 2013, 74, 375–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joglar, J.A.; Kapa, S.; Saarel, E.V.; Dubin, A.M.; Gorenek, B.; Hameed, A.B.; Lara de Melo, S.; Leal, M.A.; Mondésert, B.; Pacheco, L.D.; et al. HRS expert consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm 2023, 20, e175–e264. [Google Scholar] [CrossRef]
- Waypa, G.B.; Schumacker, P.T. Hypoxia-induced changes in pulmonary and systemic vascular resistance: Where is the O2 sensor? Respir. Physiol. Neurobiol. 2010, 174, 201–211. [Google Scholar] [CrossRef]
- Liang, Y.; Ruan, W.; Jiang, Y.; Smalling, R.; Yuan, X.; Eltzschig, H.K. Interplay of hypoxia-inducible factors and oxygen therapy in cardiovascular medicine. Nat. Rev. Cardiol. 2023, 20, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A. Breath of Life: Heart Disease Link to Developmental Hypoxia. Circulation 2021, 144, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Bo, B.; Li, S.; Zhou, K.; Wei, J. The Regulatory Role of Oxygen Metabolism in Exercise-Induced Cardiomyocyte Regeneration. Front. Cell Dev. Biol. 2021, 9, 664527. [Google Scholar] [CrossRef]
- Hula, N.; Spaans, F.; Vu, J.; Quon, A.; Kirschenman, R.; Cooke, C.M.; Phillips, T.J.; Case, C.P.; Davidge, S.T. Placental treatment improves cardiac tolerance to ischemia/reperfusion insult in adult male and female offspring exposed to prenatal hypoxia. Pharmacol. Res. 2021, 165, 105461. [Google Scholar] [CrossRef]
- Rock, C.R.; Miller, S.L.; Allison, B.J. The Use of Antioxidants for Cardiovascular Protection in Fetal Growth Restriction: A Systematic Review. Antioxidants 2024, 13, 1400. [Google Scholar] [CrossRef]
- Romanowicz, J.; Guerrelli, D.; Dhari, Z.; Mulvany, C.; Reilly, M.; Swift, L.; Vasandani, N.; Ramadan, M.; Leatherbury, L.; Ishibashi, N.; et al. Chronic perinatal hypoxia delays cardiac maturation in a mouse model for cyanotic congenital heart disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1873–H1886. [Google Scholar] [CrossRef]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Zhang, H. Adaptive Cardiac Metabolism Under Chronic Hypoxia: Mechanism and Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 625524. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.M.; McGovern, P.E.; Mejaddam, A.; Rossidis, A.C.; Baumgarten, H.; Kim, A.; Grinspan, J.B.; Licht, D.J.; Didier, R.A.; Vossough, A.; et al. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J. Thorac. Cardiovasc. Surg. 2019, 157, 1982–1991. [Google Scholar] [CrossRef]
- Bhatt, A.B.; Foster, E.; Kuehl, K.; Alpert, J.; Brabeck, S.; Crumb, S.; Davidson, W.R., Jr.; Earing, M.G.; Ghoshhajra, B.B.; Karamlou, T.; et al. American Heart Association Council on Clinical Cardiology. Congenital heart disease in the older adult: A scientific statement from the American Heart Association. Circulation 2015, 131, 1884–1931. [Google Scholar] [CrossRef] [PubMed]
- D’cunha, C.; Sankaran, K. Persistent fetal circulation. Paediatr. Child Health 2001, 6, 744–750. [Google Scholar] [CrossRef]
- Horenstein, M.S.; Diaz-Frias, J.; Guillaume, M. Tetralogy of Fallot; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513288 (accessed on 4 March 2024).
- Niewinski, P.; Tubek, S.; Josiak, K.; Nowak, K.; Ponikowski, P. Cardiac parasympathetic denervation reduces hypoxic tachycardia, baroreflex sensitivity and heart rate variability in humans. Sci. Rep. 2025, 15, 6633. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia—The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Mitrică, M.; Lorusso, L.; Badea, A.-A.; Sîrbu, C.-A.; Pleșa, A.; Stănescu, A.-M.A.; Pleșa, F.C.; Sîrbu, O.M.; Munteanu, A.E. The Hidden Heart: Exploring Cardiac Damage Post-Stroke: A Narrative Review. Medicina 2024, 60, 1699. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of Prenatal Hypoxia in Brain Development, Cognitive Functions, and Neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef]
- Szklarz, M.; Gontarz-Nowak, K.; Matuszewski, W.; Bandurska-Stankiewicz, E. Can Iron Play a Crucial Role in Maintaining Cardiovascular Health in the 21st Century? Int. J. Environ. Res. Public Health 2022, 19, 11990. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Yang, G.; Wang, X.; Liu, F.; Li, Y.; Chen, Y.; Zhou, T.; Xie, D.; Liu, Y.; et al. Energy metabolism: A critical target of cardiovascular injury. Biomed. Pharmacother. 2023, 165, 115271. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.; Fossati, S. Autonomic nervous system and cardiac neuro-signaling pathway modulation in cardiovascular disorders and Alzheimer’s disease. Front. Physiol. 2023, 14, 1060666. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.A.; Hobbins, J.C. Clinical Obstetrics: The Fetus & Mother, 3rd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9780470753293.answ1 (accessed on 1 January 2007).
- Floria, M.; Parteni, N.; Neagu, A.I.; Sascau, R.A.; Statescu, C.; Tanase, D.M. Incomplete right bundle branch block: Challenges in electrocardiogram diagnosis. Anatol. J. Cardiol. 2021, 25, 380–384. [Google Scholar] [CrossRef]
- Doundoulakis, I.; Tsiachris, D.; Kordalis, A.; Soulaidopoulos, S.; Arsenos, P.; Xintarakou, A.; Koliastasis, L.; Vlachakis, P.K.; Tsioufis, K.; Gatzoulis, K.A. Management of Patients with Unexplained Syncope and Bundle Branch Block: Predictive Factors of Recurrent Syncope. Cureus 2023, 15, e35827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, X.M.; Cai, Y.; Shen, S.E.; Dong, L.Y. Post-cardiopulmonary bypass hypoxaemia in paediatric patients undergoing congenital heart disease surgery: Risk factors, features, and postoperative pulmonary complications. BMC Cardiovasc. Disord. 2022, 22, 430. [Google Scholar] [CrossRef]
- Popazova, O.; Belenichev, I.; Abramov, A.; Bukhtiyarova, N.; Chereshniuk, I.; Skoryna, D. Indicators of Bioelectrical Activity of the Rat Heart After Prenatal Hypoxia and Pharmacological Correction. Innov. Biosyst. Bioeng. 2023, 6, 148–160. [Google Scholar] [CrossRef]
- Li, T.; Li, K.; Zhang, S.; Wang, Y.; Xu, Y.; Cronin, S.J.F.; Sun, Y.; Zhang, Y.; Xie, C.; Rodriguez, J.; et al. Overexpression of apoptosis inducing factor aggravates hypoxic-ischemic brain injury in neonatal mice. Cell Death Dis. 2020, 11, 77. [Google Scholar] [CrossRef]
- Liu, M.; Galli, G.; Wang, Y.; Fan, Q.; Wang, Z.; Wang, X.; Xiao, W. Novel Therapeutic Targets for Hypoxia-Related Cardiovascular Diseases: The Role of HIF-1. Front. Physiol. 2020, 11, 774. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkaczenko, H. L-Arginine and Nitric Oxide in Vascular Regulation—Experimental Findings in the Context of Blood Donation. Nutrients 2025, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Boycott, H.E.; Nguyen, M.N.; Vrellaku, B.; Gehmlich, K.; Robinson, P. Nitric Oxide and Mechano-Electrical Transduction in Cardiomyocytes. Front. Physiol. 2020, 11, 606740. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Lei, M. Cardiomyocyte electrophysiology and its modulation: Current views and future prospects. Phil. Trans. R. Soc. B 2023, 378, 20220160. [Google Scholar] [CrossRef]
- Chemin, J.; Girard, C.; Duprat, F.; Lesage, F.; Romey, G.; Lazdunski, M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003, 22, 5403–5411. [Google Scholar] [CrossRef]
- Shi, X.; Qiu, H. Post-Translational S-Nitrosylation of Proteins in Regulating Cardiac Oxidative Stress. Antioxidants 2020, 9, 1051. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, B.; Lee, T.-W.; Lin, Y.-K.; Chen, Y.-C.; Chung, C.-C.; Higa, S.; Chen, Y.-J. Disturbed Cardiac Metabolism Triggers Atrial Arrhythmogenesis in Diabetes Mellitus: Energy Substrate Alternate as a Potential Therapeutic Intervention. Cells 2022, 11, 2915. [Google Scholar] [CrossRef]
- Woo, S.-H.; Kim, J.-C.; Eslenur, N.; Trinh, T.N.; Do, L.N.H. Modulations of Cardiac Functions and Pathogenesis by Reactive Oxygen Species and Natural Antioxidants. Antioxidants 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Fontes, S.K.; Bautista, E.N.; Cheng, Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc. Res. 2022, 118, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.j.; Du, J.m.; Wu, H.h.; Zhou, S.y.; Li, M.; Li, Y.y.; Wang, S.y.; Wang, H.y.; Zheng, Y.; et al. Downregulation of PGAM2 alleviates angiotensin II-induced cardiac hypertrophy by destabilizing HSP90 and inactivating the mTOR/IKKα signaling pathway. Int. J. Biol. Sci. 2025, 21, 1308–1321. [Google Scholar] [CrossRef]
- Guo, B.; Li, Y.; Jin, X.; Liu, S.; Miao, C. Nitric oxide/cyclic GMP pathway mediates the endothelin-1-upregulation of adiponectin expression in rat cardiomyocytes. Biomed. Rep. 2017, 7, 267–271. [Google Scholar] [CrossRef]
- Kayki-Mutlu, G.; Koch, W.J. Nitric Oxide and S-Nitrosylation in Cardiac Regulation: G Protein-Coupled Receptor Kinase-2 and ?-Arrestins as Targets. Int. J. Mol. Sci. 2021, 22, 521. [Google Scholar] [CrossRef] [PubMed]
- Doul, J.; Minaříková, M.; Charvátová, Z.; Maxová, H. Nitric oxide is involved in the cardioprotection of neonatal rat hearts, but not in neonatal ischemic postconditioning. Physiol. Rep. 2024, 12, e16147. [Google Scholar] [CrossRef]
- Sherlock, L.G.; Wright, C.J.; Kinsella, J.P.; Delaney, C. Inhaled nitric oxide use in neonates: Balancing what is evidence-based and what is physiologically sound. Nitric Oxide 2020, 95, 12–16. [Google Scholar] [CrossRef]
- Uray, I.P.; Uray, K. Mechanotransduction at the Plasma Membrane-Cytoskeleton Interface. Int. J. Mol. Sci. 2021, 22, 11566. [Google Scholar] [CrossRef]
- Katoh, K. Signal Transduction Mechanisms of Focal Adhesions: Src and FAK-Mediated Cell Response. Front. Biosci. 2024, 29, 392. [Google Scholar] [CrossRef]
- Ławkowska, K.; Bonowicz, K.; Jerka, D.; Bai, Y.; Gagat, M. Integrins in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Opportunities. Biomolecules 2025, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.V.; Novichkova, M.D. S-Glutathionylation and S-Nitrosylation as Modulators of Redox-Dependent Processes in Cancer Cell. Biochem. Mosc. 2023, 88, 924–943. [Google Scholar] [CrossRef]
- Song, T.; Hui, W.; Huang, M.; Guo, Y.; Yu, M.; Yang, X.; Liu, Y.; Chen, X. Dynamic Changes in Ion Channels during Myocardial Infarction and Therapeutic Challenges. Int. J. Mol. Sci. 2024, 25, 6467. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.; Alwatban, A.Z.; Orfali, R.S.; Lau, L.; Chea, N.; Alotaibi, A.M.; Nam, Y.W.; Zhang, M. Oxidative stress and ion channels in neurodegenerative diseases. Front. Physiol. 2024, 15, 1320086. [Google Scholar] [CrossRef]
- Spiers, J.G.; Steinert, J.R. Nitrergic modulation of ion channel function in regulating neuronal excitability. Channels 2021, 15, 666–679. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Wang, W.L.; Ge, T.Y.; Chen, X.; Mao, Y.; Zhu, Y.Z. Advances in the Protective Mechanism of NO, H2S, and H2 in Myocardial Ischemic Injury. Front. Cardiovasc. Med. 2020, 7, 588206. [Google Scholar] [CrossRef]
- Wei, X.; Yohannan, S.; Richards, J.R. Physiology, Cardiac Repolarization Dispersion and Reserve; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537194 (accessed on 4 March 2024).
- Pappas, G.; Wilkinson, M.L.; Gow, A.J. Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy. Nitric Oxide 2023, 131, 8–17. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Ryzhenko, V.; Pavlov, S.; Suprun, E.; Oksenych, V.; Kamyshnyi, O. Targeting Mitochondrial Dysfunction in Cerebral Ischemia: Advances in Pharmacological Interventions. Antioxidants 2025, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 1042. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Kumar, P.; Veeriah, V.; Muley, A.; Rajendran, S.; Konikkat, S.; Majumder, S.; Mani, K.P.; Chatterjee, S. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. Int. J. Mol. Sci. 2019, 20, 1157. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Patel, N.; Kumar, N.; Rao, S.G.; Angelos, M.G.; Singh, H.; Cai, C.; Khan, M. Human Cardiac Progenitor Cells Enhance Exosome Release and Promote Angiogenesis Under Physoxia. Front. Cell Dev. Biol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Tsaytler, P.; Liu, J.; Blaess, G.; Schifferl, D.; Veenvliet, J.V.; Wittler, L.; Timmermann, B.; Herrmann, B.G.; Koch, F. BMP4 triggers regulatory circuits specifying the cardiac mesoderm lineage. Development 2023, 150, dev201450. [Google Scholar] [CrossRef]
- Kovács, T.; Halasy, V.; Pethő, C.; Szőcs, E.; Soós, Á.; Dóra, D.; de Santa Barbara, P.; Faure, S.; Stavely, R.; Goldstein, A.M.; et al. Essential Role of BMP4 Signaling in the Avian Ceca in Colorectal Enteric Nervous System Development. Int. J. Mol. Sci. 2023, 24, 15664. [Google Scholar] [CrossRef] [PubMed]
- Govier-Cole, A.E.; Wood, R.J.; Fletcher, J.L.; Gonsalvez, D.G.; Merlo, D.; Cate, H.S.; Murray, S.S.; Xiao, J. Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation. eNeuro 2019, 6, ENEURO.0399-18.2019. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Dimri, M. Biochemistry, Guanylate Cyclase; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53715 (accessed on 4 March 2024).
- Bloch, W.; Fleischmann, B.K.; Lorke, D.E.; Andressen, C.; Hops, B.; Hescheler, J.; Addicks, K. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc. Res. 1999, 43, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ghosh, A.; Sundaresan, L.; Kathirvel, P.; Sankaranarayanan, K.; Chatterjee, S. Ectopic release of nitric oxide modulates the onset of cardiac development in avian model. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 593–603. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The molecular mechanisms of cardiac development and related diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef]
- Mensah, I.K.; Gowher, H. Epigenetic Regulation of Mammalian Cardiomyocyte Development. Epigenomes 2024, 8, 25. [Google Scholar] [CrossRef]
- Acauan Filho, B.J.; Pinheiro da Costa, B.E.; Ogando, P.B.; Vieira, M.C.; Antonello, I.C.; Poli-de-Figueiredo, C.E. Serum nitrate and NOx levels in preeclampsia are higher than in normal pregnancy. Hypertens. Pregnancy 2016, 35, 226–233. [Google Scholar] [CrossRef]
- Khetsuriani, T.; Chabashvili, N.; Sanikidze, T. Role of endothelin-1 and nitric oxide level in pathogenesis preeclampsia. Georgian Med. News 2006, 141, 17–21. (In Russian) [Google Scholar] [PubMed]
- Vural, P. Nitric oxide/endothelin-1 in preeclampsia. Clin. Chim. Acta 2002, 317, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Borah, B.; Rimkevicius, T.; Macrander, J.; Piontkivska, H.; Sokolova, I.M.; Beniash, E. The Role of the Vascular Endothelial Growth Factor (VEGF) Signaling in Biomineralization of the Oyster Crassostrea gigas. Front. Mar. Sci. 2018, 5, 309. [Google Scholar] [CrossRef]
- Boldeanu, L.; Dijmărescu, A.L.; Radu, M.; Siloşi, C.A.; Popescu-Drigă, M.V.; Poenariu, I.S.; Siloşi, I.; Boldeanu, M.V.; Novac, M.B.; Novac, L.V. The role of mediating factors involved in angiogenesis during implantation. Rom. J. Morphol. Embryol. 2020, 61, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Groesch, K.A.; Torry, R.J.; Wilber, A.C.; Abrams, R.; Bieniarz, A.; Guilbert, L.J.; Torry, D.S. Nitric oxide generation affects pro- and anti-angiogenic growth factor expression in primary human trophoblast. Placenta 2011, 32, 926–931. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, C.; Liu, C.; Li, H.; Wu, J.; Sun, C. Targeting hypoxia-inducible factor-1alpha: A new strategy for triple-negative breast cancer therapy. Biomed. Pharmacother. 2022, 156, 113861. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Libby, P.; Peng, H.B.; Thannickal, V.J.; Rajavashisth, T.B.; Gimbrone, M.A., Jr.; Shin, W.S.; Liao, J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 1995, 96, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.; van der Vliet, A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 2011, 25, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Rood, K.; Lopez, V.; La Frano, M.R.; Fiehn, O.; Zhang, L.; Blood, A.B.; Wilson, S.M. Gestational Hypoxia and Programing of Lung Metabolism. Front. Physiol. 2019, 10, 1453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kane, A.D.; Kothmann, E.; Giussani, D.A. Detection and response to acute systemic hypoxia. BJA Educ. 2020, 20, 58–64. [Google Scholar] [CrossRef]
- Evans, L.C.; Liu, H.; Pinkas, G.A.; Thompson, L.P. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr. Res. 2012, 71, 25–31. [Google Scholar] [CrossRef]
- Ferreiro, C.R.; Chagas, A.C.; Carvalho, M.H.; Dantas, A.P.; Jatene, M.B.; Bento De Souza, L.C.; Lemos Da Luz, P. Influence of hypoxia on nitric oxide synthase activity and gene expression in children with congenital heart disease: A novel pathophysiological adaptive mechanism. Circulation 2001, 103, 2272–2276. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Góngora, M.C.; Harrison, D.G.; Lambeth, J.D.; Dikalov, S.; Griendling, K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H673–H679. [Google Scholar] [CrossRef]
- Popazova, O.; Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Oksenych, V.; Kamyshnyi, A. Cardioprotective Activity of Pharmacological Agents Affecting NO Production and Bioavailability in the Early Postnatal Period after Intrauterine Hypoxia in Rats. Biomedicines 2023, 11, 2854. [Google Scholar] [CrossRef]
- Dong, Y.; Thompson, L.P. Differential expression of endothelial nitric oxide synthase in coronary and cardiac tissue in hypoxic fetal guinea pig hearts. J. Soc. Gynecol. Investig. 2006, 13, 483–490. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Negash, S.; Longo, L.D.; Raj, J.U. Long-term effects of prenatal hypoxia on endothelium-dependent relaxation responses in pulmonary arteries of adult sheep. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2009, 296, L547–L554. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Kane, A.D.; Lusby, C.M.; Allison, B.J.; Chua, Y.Y.; Kaandorp, J.J.; Nevin-Dolan, R.; Ashmore, T.J.; Blackmore, H.L.; Derks, J.B.; et al. Maternal Allopurinol Prevents Cardiac Dysfunction in Adult Male offspring Programmed by Chronic Hypoxia During Pregnancy. Hypertension 2018, 72, 971–978. [Google Scholar] [CrossRef]
- Hauton, D.; Ousley, V. Prenatal hypoxia induces increased cardiac contractility on a background of decreased capillary density. BMC Cardiovasc. Disord. 2009, 9, 1. [Google Scholar] [CrossRef]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF 1 signaling pathway in hypoxia ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Jung, F.; Palmer, L.A.; Zhou, N.; Johns, R.A. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ. Res. 2000, 86, 319–325. [Google Scholar] [CrossRef]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef]
- Aengwanich, W.; Wandee, J. The effect of increased ambient temperature on Hsp70, superoxide dismutase, nitric oxide, malondialdehyde, and caspase activity in relation to the intrinsic and extrinsic apoptosis pathway of broiler blood cells. J. Therm. Biol. 2022, 105, 103211. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001, 11, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, K.D. Nitrosative stress and transcription. Biol. Chem. 2003, 384, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Alford, J.; Qiu, H. Structural and Functional Remodeling of Mitochondria in Cardiac Diseases. Int. J. Mol. Sci. 2021, 22, 4167. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Kourek, C.; Farmakis, D.; Tsougos, E. Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell. Biomolecules 2024, 14, 1534. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, T.; Hua, J.; Xiong, Z.; Dai, K.; Chen, H.; Li, L.; Peng, J.; Peng, X.; Zheng, Z.; et al. PINK1/Parkin-mediated mitophagy in cardiovascular disease: From pathogenesis to novel therapy. Int. J. Cardiol. 2022, 361, 61–69. [Google Scholar] [CrossRef]
- Parker, A.M.; Tate, M.; Prakoso, D.; Deo, M.; Willis, A.M.; Nash, D.M.; Donner, D.G.; Crawford, S.; Kiriazis, H.; Granata, C.; et al. Characterisation of the Myocardial Mitochondria Structural and Functional Phenotype in a Murine Model of Diabetic Cardiomyopathy. Front. Physiol. 2021, 12, 672252. [Google Scholar] [CrossRef]
- Ravindran, S.; Rau, C.D. The multifaceted role of mitochondria in cardiac function: Insights and approaches. Cell Commun. Signal. 2024, 22, 525. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Chang, X.; Liu, R.; Li, R.; Peng, Y.; Zhu, P.; Zhou, H. Molecular Mechanisms of Mitochondrial Quality Control in Ischemic Cardiomyopathy. Int. J. Biol. Sci. 2023, 19, 426–448. [Google Scholar] [CrossRef] [PubMed]
- McClellan, G.; Weisberg, A.; Winegrad, S. Energy transport from mitochondria to myofibril by a creatine phosphate shuttle in cardiac cells. Am. J. Physiol. 1983, 245 Pt 1, C423–C427. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta. 2006, 1762, 164–180. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, X.; Li, J.; Gao, H.; Li, J.; Hu, W.; Li, H. New Insights into the Role of Mitochondria Quality Control in Ischemic Heart Disease. Front. Cardiovasc. Med. 2021, 8, 774619. [Google Scholar] [CrossRef]
- Chatterjee, P.; Holody, C.D.; Kirschenman, R.; Graton, M.E.; Spaans, F.; Phillips, T.J.; Case, C.P.; Bourque, S.L.; Lemieux, H.; Davidge, S.T. Sex-Specific Effects of Prenatal Hypoxia and a Placental Antioxidant Treatment on Cardiac Mitochondrial Function in the Young Adult offspring. Int. J. Mol. Sci. 2023, 24, 13624. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Chen, L.; Polster, B.M.; Pinkas, G.; Song, H. Prenatal hypoxia impairs cardiac mitochondrial and ventricular function in guinea pig offspring in a sex-related manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1232–R1241. [Google Scholar] [CrossRef]
- Al-Hasan, Y.M.; Pinkas, G.A.; Thompson, L.P. Prenatal Hypoxia Reduces Mitochondrial Protein Levels and Cytochrome c Oxidase Activity in offspring Guinea Pig Hearts. Reprod. Sci. 2014, 21, 883–891. [Google Scholar] [CrossRef]
- Clerc, P.; Rigoulet, M.; Leverve, X.; Fontaine, E. Nitric oxide increases oxidative phosphorylation efficiency. J. Bioenerg. Biomembr. 2007, 39, 158–166. [Google Scholar] [CrossRef]
- Song, H.; Telugu, B.P.; Thompson, L.P. Sexual dimorphism of mitochondrial function in the hypoxic guinea pig placenta. Biol. Reprod. 2019, 100, 208–216. [Google Scholar] [CrossRef]
- Shao, Y.; Li, X.; Wood, J.W.; Ma, J.X. Mitochondrial dysfunctions, endothelial progenitor cells and diabetic retinopathy. J. Diabetes Complicat. 2018, 32, 966–973. [Google Scholar] [CrossRef]
- Hüttemann, M.; Helling, S.; Sanderson, T.H.; Sinkler, C.; Samavati, L.; Mahapatra, G.; Varughese, A.; Lu, G.; Liu, J.; Ramzan, R.; et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta 2012, 1817, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Shemarova, I.; Nesterov, V.; Emelyanova, L.; Korotkov, S. Mitochondrial mechanisms by which gasotransmitters (H2S, NO and CO) protect cardiovascular system against hypoxia. Front. Biosci. 2021, 13, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Ramachandran, A.; Moellering, D.R.; Ceaser, E.; Shiva, S.; Xu, J.; Darley-Usmar, V. Inhibition of mitochondrial protein synthesis results in increased endothelial cell susceptibility to nitric oxide-induced apoptosis. Proc. Natl. Acad. Sci. USA 2002, 99, 6643–6648. [Google Scholar] [CrossRef]
- McCarty, M.F.; Lerner, A. Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3624. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Gasparre, G.; Porcelli, A.M.; Lenaz, G.; Romeo, G. Relevance of mitochondrial genetics and metabolism in cancer development. Cold Spring Harb. Perspect. Biol. 2013, 5, a011411. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Wu, P.; Wei, D. Current Advances of Mitochondrial Dysfunction and Cardiovascular Disease and Promising Therapeutic Strategies. Am. J. Pathol. 2023, 193, 1485–1500. [Google Scholar] [CrossRef]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Janssen, G.M.J.; Kaminski, I.; Geomini, I.; Lemmens, T.; Schiffers, P.M.H.; et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Energy metabolism design of the striated muscle cell. Physiol. Rev. 2021, 101, 1561–1607. [Google Scholar] [CrossRef]
- Tverdokhleb, I.V. Heterogeneity of Mitochondrial Apparatus of Myocardium and Mechanisms of Its Formation in Early Ontogenesis of Rats. Tsitol. Genet. 1998, 32, 8–12. (In Russian) [Google Scholar] [PubMed]
- Leitan, E.B. Pathomorphology and Ultrastructure of Myocardium in Fetuses and Newborns with Asphyxia. Author’s Abstract of Dissertation. Cand. Med. Sci. Novosib. 1981, 16. (In Russian) [Google Scholar]
- Song, H.; Thompson, L.P. Effects of Gestational Hypoxia on PGC1α and Mitochondrial Acetylation in Fetal Guinea Pig Hearts. Reprod. Sci. 2023, 30, 2996–3009. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Summerhill, V.I.; Khotina, V.A.; Popov, M.A.; Uzokov, J.K.; Sukhorukov, V.N. Role of Mitochondria in the Chronification of Inflammation: Focus on Dysfunctional Mitophagy and Mitochondrial DNA Mutations. Gene Expr. 2023, 22, 329–344. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Wang, J.; Tian, J.; Zhang, L.; Wei, Y.; Yang, F.; Xu, Y.; Wang, G. Important Role of Mitochondrial Dysfunction in Immune Triggering and Inflammatory Response in Rheumatoid Arthritis. J. Inflamm. Res. 2024, 17, 11631–11657. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Kang, M.J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 2018, 41, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Schulz, R.; Liaudet, L.; Szabó, C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol. Sci. 2005, 26, 302–310. [Google Scholar] [CrossRef]
- Cai, Z.; Hutchins, J.B.; Rhodes, P.G. Intrauterine hypoxia-ischemia alters nitric oxide synthase expression and activity in fetal and neonatal rat brains. Brain Res. Dev. Brain Res. 1998, 109, 265–269. [Google Scholar] [CrossRef]
- Silvestro, S.; Calcaterra, V.; Pelizzo, G.; Bramanti, P.; Mazzon, E. Prenatal Hypoxia and Placental Oxidative Stress: Insights from Animal Models to Clinical Evidences. Antioxidants 2020, 9, 414. [Google Scholar] [CrossRef]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric Oxide and Reactive Oxygen Species in the Pathogenesis of Preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef]
- Migita, K.; Maeda, Y.; Abiru, S.; Komori, A.; Yokoyama, T.; Takii, Y.; Nakamura, M.; Yatsuhashi, H.; Eguchi, K.; Ishibashi, H. Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett. 2005, 579, 3119–3125. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin; Ali, A. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Arunachalam, G.; Samuel, S.M.; Ding, H.; Triggle, C.R. Peroxynitrite Biology. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bencsik, P.; Kupai, K.; Giricz, Z.; Görbe, A.; Pipis, J.; Murlasits, Z.; Kocsis, G.F.; Varga-Orvos, Z.; Puskás, L.G.; Csonka, C.; et al. Role of iNOS and peroxynitrite-matrix metalloproteinase-2 signaling in myocardial late preconditioning in rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H512–H518. [Google Scholar] [CrossRef]
- Capobianco, E.; White, V.; Sosa, M.; Di Marco, I.; Basualdo, M.N.; Faingold, M.C.; Jawerbaum, A. Regulation of matrix metalloproteinases 2 and 9 activities by peroxynitrites in term placentas from type 2 diabetic patients. Reprod. Sci. 2012, 19, 814–822. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994, 269, 26066–26075. [Google Scholar] [CrossRef] [PubMed]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001, 276, 28799–28805. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Membrino, V.; Di Paolo, A.; Di Crescenzo, T.; Cecati, M.; Alia, S.; Vignini, A. Effects of Animal-Based and Plant-Based Nitrates and Nitrites on Human Health: Beyond Nitric Oxide Production. Biomolecules 2025, 15, 236. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nantaphol, S.; Noppakhunsomboon, K.; Rojsitthisak, P. An update on the current status and prospects of nitrosation pathways and possible root causes of nitrosamine formation in various pharmaceuticals. Saudi Pharm. J. 2023, 31, 295–311. [Google Scholar] [CrossRef]
- Fahrer, J.; Christmann, M. DNA Alkylation Damage by Nitrosamines and Relevant DNA Repair Pathways. Int. J. Mol. Sci. 2023, 24, 4684. [Google Scholar] [CrossRef] [PubMed]
- Grossi, L.; Montevecchi, P.C. S-nitrosocysteine and cystine from reaction of cysteine with nitrous acid. A kinetic investigation. J. Org. Chem. 2002, 67, 8625–8630. [Google Scholar] [CrossRef]
- Abalenikhina, Y.V.; Shchulkin, A.V.; Suchkova, O.N.; Ananyeva, P.D.; Mylnikov, P.Y.; Yakusheva, E.N.; Suchkov, I.A.; Kalinin, R.E. S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity. Biomolecules 2025, 15, 428. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Gorbacheva, S.V.; Demchenko, A.V.; Bukhtiyarova, N.V. The thiol-disulfide balance and the nitric oxide system in the brain tissue of rats subjected to experimental acute impairment of cerebral blood flow: The therapeutic effects of nootropic drugs. Neurochem. J. 2014, 8, 24–27. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef] [PubMed]
- Hogg, N.; Singh, R.J.; Konorev, E.; Joseph, J.; Kalyanaraman, B. S-Nitrosoglutathione as a substrate for gamma-glutamyl transpeptidase. Biochem J. 1997, 323 Pt 2, 477–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Hogg, N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 7891–7896. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Boutin, C.; Clément, C.; Rivoal, J. Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction. Int. J. Mol. Sci. 2024, 25, 9845. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.M.; Holdship, P.; To, T.Q.; Ratcliffe, P.J.; Keeley, T.P. Comparative analysis of N-terminal cysteine dioxygenation and prolyl-hydroxylation as oxygen-sensing pathways in mammalian cells. J. Biol. Chem. 2023, 299, 105156. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, G.; Chhabra, A.; Sethy, N.K.; Jain, N.; Meena, R.N.; Tulsawani, R.; Prasad, D.N.; Kumar, B.; Sharma, M. Cysteine becomes conditionally essential during hypobaric hypoxia and regulates adaptive neuro-physiological responses through CBS/H2S pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165769. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Moncada, S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef]
- Brown, G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 2001, 1504, 46–57. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.-f. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Camp, O.G.; Ramadoss, J.; Chatzicharalampous, C.; Kofinas, G.; Kofinas, J.D. Regulation of nitric oxide generation and consumption. Int. J. Biol. Sci. 2025, 21, 1097–1109. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. Chemistry of peroxynitrite and its relevance to biological systems. Met. Ions Biol. Syst. 1999, 36, 597–619. [Google Scholar] [PubMed]
- Makarov, S.V.; Horváth, A.K.; Makarova, A.S. Reactivity of Small Oxoacids of Sulfur. Molecules 2019, 24, 2768. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Merényi, G. The chemistry of peroxynitrite: Implications for biological activity. Methods Enzymol. 2008, 436, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free. Radic. Biol. Med. 2002, 33, 1451–1464. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Soto, M.E.; Castrejón-Tellez, V.; Rubio-Ruiz, M.E.; Manzano Pech, L.; Guarner-Lans, V. Oxidative, Reductive, and Nitrosative Stress Effects on Epigenetics and on Posttranslational Modification of Enzymes in Cardiometabolic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 8819719. [Google Scholar] [CrossRef]
- Alnajjar, K.S.; Sweasy, J.B. A new perspective on oxidation of DNA repair proteins and cancer. DNA Repair 2019, 76, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, R.; Zharkov, D.O.; Golan, G.; Fernandes, A.S.; Gerchman, S.E.; Matz, E.; Kycia, J.H.; Grollman, A.P.; Shoham, G. Structure of formamidopyrimidine-DNA glycosylase covalently complexed to DNA. J. Biol. Chem. 2002, 277, 19811–19816. [Google Scholar] [CrossRef]
- Jacobs, A.L.; Schär, P. DNA glycosylases: In DNA repair and beyond. Chromosoma 2012, 121, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.P.; Pestana, C.R.; Polizello, A.C.; Pardo-andreu, G.L.; Uyemura, S.A.; Santos, A.C.; Alberici, L.C.; da Silva, R.S.; Curti, C. Release of NO from a nitrosyl ruthenium complex through oxidation of mitochondrial NADH and effects on mitochondria. Nitric Oxide 2012, 26, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Taylor, S.W.; Zhang, B.; Ghosh, S.S.; Capaldi, R.A. Oxidative damage to mitochondrial complex I due to peroxynitrite: Identification of reactive tyrosines by mass spectrometry. J. Biol. Chem. 2003, 278, 37223–37230. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Yadlovskyi, O.; Bukhtiyarova, N.; Ryzhenko, V.; Pavlov, S.; Oksenych, V.; Kamyshnyi, O. Possibility of Using NO Modulators for Pharmacocorrection of Endothelial Dysfunction After Prenatal Hypoxia. Pharmaceuticals 2025, 18, 106. [Google Scholar] [CrossRef]
- Popazova, O.; Belenichev, I.; Yadlovskyi, O.; Oksenych, V.; Kamyshnyi, A. Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Curr. Issues Mol. Biol. 2023, 45, 8704–8715. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Wilkinson, J.D.; Messiah, S.E.; Miller, T.L. Clinical research directions in pediatric cardiology. Curr. Opin. Pediatr. 2009, 21, 585–593. [Google Scholar] [CrossRef]
- Giussani, D.A.; Niu, Y.; Herrera, E.A.; Richter, H.G.; Camm, E.J.; Thakor, A.S.; Kane, A.D.; Hansell, J.A.; Brain, K.L.; Skeffington, K.L.; et al. Heart disease link to fetal hypoxia and oxidative stress. Adv. Exp. Med. Biol. 2014, 814, 77–87. [Google Scholar] [CrossRef]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- llbritton-King, J.D.; García-Cardeña, G. Endothelial cell dysfunction in cardiac disease: Driver or consequence? Front. Cell Dev. Biol. 2023, 11, 1278166. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Yang, Y.; Du, Z.; Fan, Y.; Zhao, Y.; Yuan, S. Oxidative stress on vessels at the maternal-fetal interface for female reproductive system disorders: Update. Front. Endocrinol. 2023, 14, 1118121. [Google Scholar] [CrossRef]
- Paz, A.A.; Jiménez, T.A.; Ibarra-Gonzalez, J.; Astudillo-Maya, C.; Beñaldo, F.A.; Figueroa, E.G.; Llanos, A.J.; Gonzalez-Candia, A.; Herrera, E.A. Gestational hypoxia elicits long-term cardiovascular dysfunction in female guinea pigs. Life Sci. 2025, 361, 123282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lei, J.; Deng, F.; Zhao, C.; Xu, T.; Ji, B.; Fu, M.; Wang, X.; Sun, M.; Zhang, M.; et al. Gestational Hypoxia Impaired Endothelial Nitric Oxide Synthesis Via miR-155-5p/NADPH Oxidase/Reactive Oxygen Species Axis in Male offspring Vessels. J. Am. Heart Assoc. 2024, 13, e032079. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Drummond, G.R.; Sobey, C.G.; De Silva, T.M.; Kemp-Harper, B.K. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol. Res. 2017, 116, 57–69. [Google Scholar] [CrossRef]
- Chandra, S.; Romero, M.J.; Shatanawi, A.; Alkilany, A.M.; Caldwell, R.B.; Caldwell, R.W. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br. J. Pharmacol. 2012, 165, 506–519. [Google Scholar] [CrossRef]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Westenskow, P.D.; Friedlander, M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv. Exp. Med. Biol. 2014, 801, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar] [PubMed]
- Bochenek, M.L.; Gogiraju, R.; Großmann, S.; Krug, J.; Orth, J.; Reyda, S.; Georgiadis, G.S.; Spronk, H.M.; Konstantinides, S.; Münzel, T.; et al. EPCR-PAR1 biased signaling regulates perfusion recovery and neovascularization in peripheral ischemia. JCI Insight 2022, 7, e157701. [Google Scholar] [CrossRef]
- Ireland, H.; Konstantoulas, C.J.; Cooper, J.A.; Hawe, E.; Humphries, S.E.; Mather, H.; Goodall, A.H.; Hogwood, J.; Juhan-Vague, I.; Yudkin, J.S.; et al. EPCR Ser219Gly: Elevated sEPCR, prothrombin F1+2, risk for coronary heart disease, and increased sEPCR shedding in vitro. Atherosclerosis 2005, 183, 283–292. [Google Scholar] [CrossRef]
- Arai, M.; Imai, H.; Koumura, T.; Yoshida, M.; Emoto, K.; Umeda, M.; Chiba, N.; Nakagawa, Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J. Biol. Chem. 1999, 274, 4924–4933. [Google Scholar] [CrossRef]
- Roveri, A.; Casasco, A.; Maiorino, M.; Dalan, P.; Calligaro, A.; Ursini, F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J. Biol. Chem. 1992, 267, 6142–6146. [Google Scholar] [CrossRef]
- Oh, S.-J.; Ikeda, M.; Ide, T.; Hur, K.Y.; Lee, M.-S. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discov. 2022, 8, 414. [Google Scholar] [CrossRef]
- Tian, P.; Xu, Z.; Guo, J.; Zhao, J.; Chen, W.; Huang, W.; Wang, M.; Mi, C.; Zhang, Y.; Yang, Y.; et al. Hypoxia causes trophoblast cell ferroptosis to induce miscarriage through lnc-HZ06/HIF1α-SUMO/NCOA4 axis. Redox Biol. 2024, 70, 103073. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Forgione, M.A.; Weiss, N.; Heydrick, S.; Cap, A.; Klings, E.S.; Bierl, C.; Eberhardt, R.T.; Farber, H.W.; Loscalzo, J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1255–H1261. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Qiao, D.; Zhao, X.; Wang, L.; Zhang, J.; Liu, D.; Zhang, H. The molecular characterizations of Cu/ZnSOD and MnSOD and its responses of mRNA expression and enzyme activity to Aeromonas hydrophila or lipopolysaccharide challenge in Qihe crucian carp Carassius auratus. Fish Shellfish. Immunol. 2017, 67, 429–440. [Google Scholar] [CrossRef]
- Giles, B.L.; Suliman, H.; Mamo, L.B.; Piantadosi, C.A.; Oury, T.D.; Nozik-Grayck, E. Prenatal hypoxia decreases lung extracellular superoxide dismutase expression and activity. Am. J. Physiol Lung Cell. Mol. Physiol. 2002, 283, L549–L554. [Google Scholar] [CrossRef][Green Version]
- Sherlock, L.G.; Trumpie, A.; Hernandez-Lagunas, L.; McKenna, S.; Fisher, S.; Bowler, R.; Wright, C.J.; Delaney, C.; Nozik-Grayck, E. Redistribution of Extracellular Superoxide Dismutase Causes Neonatal Pulmonary Vascular Remodeling and PH but Protects Against Experimental Bronchopulmonary Dysplasia. Antioxidants 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Malhotra, R.; Lin, Z.; Vincenz, C.; Brosius, F.C. 3rd. Hypoxia induces apoptosis via two independent pathways in Jurkat cells: Differential regulation by glucose. Am. J. Physiol. Cell Physiol. 2001, 281, C1596–C1603. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, Y.; Liu, W.; Li, C.; Zhao, R.; Long, X.; Rong, J.; Deng, W.; Shen, C.; Yuan, J.; et al. CircHIPK3 regulates the autophagy and apoptosis of hypoxia/reoxygenation-stimulated cardiomyocytes via the miR-20b-5p/ATG7 axis. Cell Death Discov. 2021, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kholodenko, R.V.; Majouga, A.G.; Yarygin, K.N. Apoptotic MSCs and MSC-Derived Apoptotic Bodies as New Therapeutic Tools. Curr. Issues Mol. Biol. 2022, 44, 5153–5172. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. Pathol. Basis Vet. Dis. 2017, 2–43.e19. [Google Scholar] [CrossRef]
- Geatrell, J.C.; Gan, P.M.; Mansergh, F.C.; Kisiswa, L.; Jarrin, M.; Williams, L.A.; Evans, M.J.; Boulton, M.E.; Wride, M.A. Apoptosis gene profiling reveals spatio-temporal regulated expression of the p53/Mdm2 pathway during lens development. Exp. Eye Res. 2009, 88, 1137–1151. [Google Scholar] [CrossRef][Green Version]
- James, T.N. Normal and abnormal consequences of apoptosis in the human heart: From postnatal morphogenesis to paroxysmal arrhythmias. Trans. Am. Clin. Climatol. Assoc. 1994, 105, 145–177; discussion 177–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, Y.; Abid, R.; Dababneh, S.; Bakr, M.; Aslani, T.; Cook, D.P.; Vanderhyden, B.C.; Park, J.G.; Munshi, N.V.; Hui, C.C.; et al. Transcriptional regulation of the postnatal cardiac conduction system heterogeneity. Nat. Commun. 2024, 15, 6550. [Google Scholar] [CrossRef]
- Torella, D.; Indolfi, C.; Nadal-Ginard, B. Generation of new cardiomyocytes after injury: De novo formation from resident progenitors vs. replication of pre-existing cardiomyocytes. Ann. Transl. Med. 2015, 3 (Suppl. S1), S8. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, Y.; Chen, Z.B.; Rhee, J.-W.; Deng, Y.; Wang, Z.V. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells 2023, 12, 679. [Google Scholar] [CrossRef]
- Yehya, A.; Azar, J.; Al-Fares, M.; Boeuf, H.; Abou-Kheir, W.; Zeineddine, D.; Hadadeh, O. Cardiac differentiation is modulated by anti-apoptotic signals in murine embryonic stem cells. World J. Stem Cells 2024, 16, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R. Apoptosis in the cardiovascular system. Heart 2002, 87, 480–487. [Google Scholar] [CrossRef]
- Van Empel, V.P.M.; Bertrand, A.T.A.; Hofstra, L.; Crijns, H.J.; Doevendans, P.A.; De Windt, L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005, 67, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kang, P.M. Apoptosis in cardiovascular diseases: Mechanism and clinical implications. Korean Circ. J. 2010, 40, 299–305. [Google Scholar] [CrossRef]
- Krijnen, P.A.; Nijmeijer, R.; Meijer, C.J.; Visser, C.A.; Hack, C.E.; Niessen, H.W. Apoptosis in myocardial ischaemia and infarction. J. Clin. Pathol. 2002, 55, 801–811. [Google Scholar] [CrossRef]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef]
- Koehler, R.C.; Yang, Z.J.; Lee, J.K.; Martin, L.J. Perinatal hypoxic-ischemic brain injury in large animal models: Relevance to human neonatal encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 2092–2111. [Google Scholar] [CrossRef]
- Griffith, T.S.; Yu, X.; Herndon, J.M.; Green, D.R.; Ferguson, T.A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 1996, 5, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Le Hingrat, Q.; Sereti, I.; Landay, A.L.; Pandrea, I.; Apetrei, C. The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection. Front. Immunol. 2021, 12, 695674. [Google Scholar] [CrossRef]
- Bae, S.; Xiao, Y.; Li, G.; Casiano, C.A.; Zhang, L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H983–H990. [Google Scholar] [CrossRef]
- Troy, C.M.; Derossi, D.; Prochiantz, A.; Greene, L.A.; Shelanski, M.L. Downregulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxide-peroxynitrite pathway. J. Neurosci. 1996, 16, 253–261. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Crimi, M.; Esposti, M.D. Apoptosis-induced changes in mitochondrial lipids. Biochim. Biophys. Acta 2011, 1813, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Mitochondrial glutathione: Features, regulation and role in disease. Biochim. Biophys. Acta 2013, 1830, 3317–3328. [Google Scholar] [CrossRef]
- Korotkov, S.M. Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria. Int. J. Mol. Sci. 2023, 24, 14459. [Google Scholar] [CrossRef]
- Taimor, G.; Hofstaetter, B.; Piper, H.M. Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischemia. Cardiovasc. Res. 2000, 45, 588–594. [Google Scholar] [CrossRef]
- Botting, K.J.; McMillen, I.C.; Forbes, H.; Nyengaard, J.R.; Morrison, J.L. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J. Am. Heart. Assoc. 2014, 3, e000531. [Google Scholar] [CrossRef]
- Mĕlková, Z.; Lee, S.B.; Rodriguez, D.; Esteban, M. Bcl-2 prevents nitric oxide-mediated apoptosis and poly(ADP-ribose) polymerase cleavage. FEBS Lett. 1997, 403, 273–278. [Google Scholar] [CrossRef]
- Gotoh, Y.; Cooper, J.A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 1998, 273, 17477–17482. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, K.G.; Contois, L.R.; Tevosian, S.G.; Davis, R.J.; Paulson, K.E. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc. Natl. Acad. Sci. USA 1996, 93, 12908–12913. [Google Scholar] [CrossRef]
- Ing, D.J.; Zang, J.; Dzau, V.J.; Webster, K.A.; Bishopric, N.H. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ. Res. 1999, 84, 21–33. [Google Scholar] [CrossRef]
- Yfantis, A.; Mylonis, I.; Chachami, G.; Nikolaidis, M.; Amoutzias, G.D.; Paraskeva, E.; Simos, G. Transcriptional Response to Hypoxia: The Role of HIF-1-Associated Co-Regulators. Cells 2023, 12, 798. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Vranesić-Bender, D. The role of nutraceuticals in anti-aging medicine. Acta. Clin. Croat. 2010, 49, 537–544. [Google Scholar] [PubMed]
- Xu, L.; Song, H.; Qiu, Q.; Jiang, T.; Ge, P.; Su, Z.; Ma, W.; Zhang, R.; Huang, C.; Li, S.; et al. Different Expressions of HIF-1? and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice. Int. J. Mol. Sci. 2021, 22, 6705. [Google Scholar] [CrossRef]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Madai, S.; Kilic, P.; Schmidt, R.M.; Bas-Orth, C.; Korff, T.; Büttner, M.; Klinke, G.; Poschet, G.; Marti, H.H.; Kunze, R. Activation of the hypoxia-inducible factor pathway protects against acute ischemic stroke by reprogramming central carbon metabolism. Theranostics 2024, 14, 2856–2880. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and comprehensive insights into HIF-1 stabilization under normoxic conditions: Implications for cellular adaptation and therapeutic strategies in cancer. Cell. Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.K.; Warfel, N.A. Strange Bedfellows: Nuclear Factor, Erythroid 2-Like 2 (Nrf2) and Hypoxia-Inducible Factor 1 (HIF-1) in Tumor Hypoxia. Antioxidants 2017, 6, 27. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Brocato, J.; Chervona, Y.; Costa, M. Molecular responses to hypoxia-inducible factor 1α and beyond. Mol. Pharmacol. 2014, 85, 651–657. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Q.; Lu, L.; Li, Y.; Chen, P.J.; Duan, C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014, 6, 1110–1121. [Google Scholar] [CrossRef]
- Luo, S.Y.; Wang, J.Q.; Liu, C.; Gao, X.M.; Zhang, Y.B.; Ding, J.; Hou, C.C.; Zhu, J.Q.; Lou, B.; Shen, W.L.; et al. Hif-1α/Hsf1/Hsp70 signaling pathway regulates redox homeostasis and apoptosis in large yellow croaker (Larimichthys crocea) under environmental hypoxia. Zool. Res. 2021, 42, 746–760. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: The prospect of using HSP70 modulators. Front. Cell. Neurosci. 2023, 17, 1131683. [Google Scholar] [CrossRef]
- Agani, F.H.; Puchowicz, M.; Chavez, J.C.; Pichiule, P.; LaManna, J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am. J. Physiol. Cell Physiol. 2002, 283, C178–C186. [Google Scholar] [CrossRef]
- Sandau, K.B.; Fandrey, J.; Brüne, B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood 2001, 97, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakamura, S.; Koyano, K.; Konishi, Y.; Kondo, M.; Kusaka, T. Neonatal asphyxia as an inflammatory disease: Reactive oxygen species and cytokines. Front. Pediatr. 2023, 11, 1070743. [Google Scholar] [CrossRef]
- Serdar, M.; Walther, K.-A.; Gallert, M.; Kempe, K.; Obst, S.; Labusek, N.; Herrmann, R.; Herz, J.; Felderhoff-Müser, U.; Bendix, I. Prenatal inflammation exacerbates hyperoxia-induced neonatal brain injury. J. Neuroinflammation 2025, 22, 57. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Scholz, C.C.; Cavadas, M.A.; Tambuwala, M.M.; Hams, E.; Rodríguez, J.; von Kriegsheim, A.; Cotter, P.; Bruning, U.; Fallon, P.G.; Cheong, A.; et al. Regulation of IL-1β-induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18490–18495. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-?B Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef]
- Baird, N.A.; Turnbull, D.W.; Johnson, E.A. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J. Biol. Chem. 2006, 281, 38675–38681. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Cherniy, V.I.; Nagornaya, E.A.; Bukhtiyarova, N.V.; Kucherenko, V.I. Neuroprotection and neuroplasticity. Kiev Logos 2015, 510. [Google Scholar]
- Belenichev, I.F.; Kolesnik, Y.M.; Pavlov, S.V.; Sokolik, E.P.; Bukhtiyarova, N.V. Disturbance of HSP70 chaperone activity is a possible mechanism of mitochondrial dysfunction. Neurochem. J. 2011, 5, 251–256. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.W.; Yenari, M.A. Heat shock protein signaling in brain ischemia and injury. Neurosci. Lett. 2020, 715, 134642. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Bao, E.; Tang, S. The Protective Role of Heat Shock Proteins against Stresses in Animal Breeding. Int. J. Mol. Sci. 2024, 25, 8208. [Google Scholar] [CrossRef]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology, biochemistry, and physiology. Pharmacol. Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Kang, I. Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. Int. J. Mol. Sci. 2024, 25, 4209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Cheng, S.-Y.; Liu, C.-H.; Tsai, W.-C.; Wu, H.-H.; Huang, M.-D. Exploration of the Truncated Cytosolic Hsp70 In Plants-Unveiling the Diverse T1 Lineage and the Conserved T2 Lineage. Front. Plant Sci. 2023, 14, 1279540. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 Chaperones: Cellular Functions and Molecular Mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Kudryavtseva, S.S.; Leisi, E.V.; Kurochkina, L.P.; Barinova, K.V.; Schmalhausen, E.V. Regulation by Different Types of Chaperones of Amyloid Transformation of Proteins Involved in the Development of Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 2747. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Storey, K.B. Chaperone Proteins: Universal Roles in Surviving Environmental Stress. Cell Stress Chaperones 2023, 28, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villanueva, J.F.; Díaz-Molina, R.; García-González, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of The Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) As A Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Hu, S.; Tan, J.; Qin, L.; Lv, L.; Yan, W.; Zhang, H.; Tang, B.; Wang, C. Molecular Chaperones and Parkinson’s Disease. Neurobiol. Dis. 2021, 160, 105527. [Google Scholar] [CrossRef]
- Serlidaki, D.; Van Waarde, M.A.W.H.; Rohland, L.; Wentink, A.S.; Dekker, S.L.; Kamphuis, M.J.; Boertien, J.M.; Brunsting, J.F.; Nillegoda, N.B.; Bukau, B.; et al. Functional Diversity Between HSP70 Paralogs Caused by Variable Interactions with Specific Co-Chaperones. J. Biol. Chem. 2020, 295, 7301–7316. [Google Scholar] [CrossRef]
- Reddy, R.K.; Mao, C.; Baumeister, P.; Austin, R.C.; Kaufman, R.J.; Lee, A.S. Endoplasmic Reticulum Chaperone Protein GRP78 Protects Cells from Apoptosis Induced by Topoisomerase Inhibitors: Role of ATP Binding Site in Suppression of Caspase-7 Activation. J. Biol. Chem. 2003, 278, 20915–20924. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-Shock Protein 70 Inhibits Apoptosis by Preventing Recruitment of Procaspase-9 to the Apaf-1 Apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T.; Samarghandian, S. Molecular and Cellular Pathways Contributing to Brain Aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Nagai, M.; Kaji, H. Thermal Effect on Heat Shock Protein 70 Family to Prevent Atherosclerotic Cardiovascular Disease. Biomolecules 2023, 13, 867. [Google Scholar] [CrossRef]
- Niinuma, S.A.; Lubbad, L.; Lubbad, W.; Moin, A.S.M.; Butler, A.E. The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 1838. [Google Scholar] [CrossRef] [PubMed]
- Evgen’ev, M.B.; Onikienko, S.B.; Chuvakova, L.N.; Garbuz, D.G.; Zatsepina, O.G. The Role of Hsp70 in Adaptation to Adverse Conditions and Its Possible Medical Application. Front. Biosci. 2023, 28, 25. [Google Scholar] [CrossRef] [PubMed]
- Hulina, A.; Grdić Rajković, M.; Jakšić Despot, D.; Jelić, D.; Dojder, A.; Čepelak, I.; Rumora, L. Extracellular Hsp70 Induces Inflammation and Modulates LPS/LTA-Stimulated Inflammatory Response In THP-1 Cells. Cell Stress Chaperones 2018, 23, 373–384. [Google Scholar] [CrossRef]
- Alberti, G.; Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway De Macario, E.; Campanella, C.; Macario, A.J.L.; Marino Gammazza, A. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Komarova, E.Y.; Meshalkina, D.A.; Bychkova, N.V.; Aksenov, N.D.; Abkin, S.V.; Margulis, B.A.; Guzhova, I.V. Exogenously Delivered Heat Shock Protein 70 Displaces Its Endogenous Analogue and Sensitizes Cancer Cells to Lymphocytes-Mediated Cytotoxicity. Oncotarget 2014, 5, 3101–3114. [Google Scholar] [CrossRef]
- Demyanenko, S.V.; Kalyuzhnaya, Y.N.; Bachurin, S.S.; Khaitin, A.M.; Kunitsyna, A.E.; Batalshchikova, S.A.; Evgen’ev, M.B.; Garbuz, D.G. Exogenous Hsp70 Exerts Neuroprotective Effects in Peripheral Nerve Rupture Model. Exp. Neurol. 2024, 373, 114670. [Google Scholar] [CrossRef]
- Belenichev, I.; Ryzhenko, V.; Popazova, O.; Bukhtiyarova, N.; Gorchakova, N.; Oksenych, V.; Kamyshnyi, O. Optimization of The Search for Neuroprotectors Among Bioflavonoids. Pharmaceuticals 2024, 17, 877. [Google Scholar] [CrossRef]
- Aliyeva, O.; Belenichev, I.; Bukhtiyarova, N.; Semenov, D.; Voloshchuk, S. Comparative Assessment of The Effectiveness of HSP70/HIF-1α System Modulators After Prenatal Hypoxia. Biomed. Pharmacol. J. 2024, 17, 223–233. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Aliyeva, E.G.; Kamyshny, O.M.; Bukhtiyarova, N.V.; Ryzhenko, V.P.; Gorchakova, N.O. Pharmacological Modulation of Endogenous Neuroprotection After Experimental Prenatal Hypoxia. Neurochem. J. 2022, 16, 68–75. [Google Scholar] [CrossRef]
- Cao, J.; Yang, L.; Wang, L.; Zhao, Q.; Wu, D.; Li, M.; Mu, Y. Heat Shock Protein 70 Attenuates Hypoxia Induced Apoptosis of Pulmonary Microvascular Endothelial Cells Isolated from Neonatal Rats. Mol. Med. Rep. 2021, 24, 690. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Tian, J.; Yang, B.; Li, Y.; Li, Q.; Liu, S. Contribution of HIF-1α To Heat Shock Response by Transcriptional Regulation of HSF1/HSP70 Signaling Pathway in Pacific Oyster, Crassostrea Gigas. Mar. Biotechnol. 2023, 25, 691–700. [Google Scholar] [CrossRef]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef]
- Sulzbacher, M.M.; Ludwig, M.S.; Heck, T.G. Oxidative Stress and Decreased Tissue HSP70 Are Involved in the Genesis of Sepsis: HSP70 As a Therapeutic Target. Rev. Bras. Ter. Intensiv. 2020, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell. Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Ahmad, S.; White, C.W.; Chang, L.Y.; Schneider, B.K.; Allen, C.B. Glutamine Protects Mitochondrial Structure and Function in Oxygen Toxicity. Am. J. Physiol Lung Cell. Mol. Physiol. 2001, 280, L779–L791. [Google Scholar] [CrossRef]
- Calabrese, V.; Testa, G.; Ravagna, A.; Bates, T.E.; Stella, A.M. HSP70 Induction in the Brain Following Ethanol Administration in the Rat: Regulation by Glutathione Redox State. Biochem. Biophys. Res. Commun. 2000, 269, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, G. HSP70 Induction and Oxidative Stress Protection Mediated by a Subtoxic Dose of NMDA in the Retinoic Acid-Differentiated C6 Glioma Cell Line. Brain Res. Bull. 2006, 69, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Miyamoto, Y.; Tanonaka, K.; Han-No, Y.; Yoshida, H.; Takahashi, M.; Takeo, S. Cytoprotective Mechanism of Heat Shock Protein 70 Against Hypoxia/Reoxygenation Injury. J. Mol. Cell. Cardiol. 2000, 32, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, W.; Wu, S.; Perrett, S. Hsp70 in Redox Homeostasis. Cells 2022, 11, 829. [Google Scholar] [CrossRef]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection After Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef]
- Guo, S.; Wharton, W.; Moseley, P.; Shi, H. Heat Shock Protein 70 Regulates Cellular Redox Status by Modulating Glutathione-Related Enzyme Activities. Cell Stress Chaperones 2007, 12, 245–254. [Google Scholar] [CrossRef]
- Chen, S.; Wu, S.; Lin, B. The Potential Therapeutic Value of The Natural Plant Compounds Matrine and Oxymatrine in Cardiovascular Diseases. Front. Cardiovasc. Med. 2024, 11, 1417672. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Puchalski, A.; Wernicki, A.; Dec, M.; Paluch, E. Characterization of Hsp70 Proteins in Bovine Leukocytes Induced by the Temperature 41 Degrees C. Pol. J. Vet. Sci. 2009, 12, 323–328. [Google Scholar]
- Ferreira-Cravo, M.; Moreira, D.C.; Hermes-Lima, M. Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate. Antioxidants 2023, 12, 1197. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Kolesnik, Y.M.; Pavlov, S.V.; Sokolik, E.P.; Bukhtiyarova, N.V. Malate-Aspartate Shunt in Neuronal Adaptation to Ischemic Conditions: Molecular-Biochemical Mechanisms of Activation and Regulation. Neurochem. J. 2012, 6, 22–28. [Google Scholar] [CrossRef]
- Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Makyeyeva, L.; Morozova, O.; Oksenych, V.; Kamyshnyi, O. Methodological Approaches to Experimental Evaluation of Neuroprotective Action of Potential Drugs. Int. J. Mol. Sci. 2024, 25, 10475. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Dinitrosyl Iron Complexes with Thiol-Containing Ligands as a “Working Form” of Endogenous Nitric Oxide. Nitric Oxide 2016, 54, 15–29. [Google Scholar] [CrossRef]
- Henard, C.A.; Vázquez-Torres, A. Nitric Oxide and Salmonella Pathogenesis. Front. Microbiol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Thompho, S.; Fritzsche, S.; Chokbunpiam, T.; Remsungnen, T.; Janke, W.; Hannongbua, S. Adsorption and The Chemical Reaction N2O4 ↔ 2NO2 in the Presence of N2 in a Gas Phase Connected with A Carbon Nanotube. ACS Omega 2021, 6, 17342–17352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Möller, M.N.; Vitturi, D.A. The Chemical Biology of Dinitrogen Trioxide. Redox Biochem. Chem. 2024, 8, 100026. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Shah, F.; Chekman, I.S.; Nagornaya, E.A.; Gorbacheva, S.V.; Gorchakova, N.A.; Bukhtiyarova, N.V. Thiol-Disulfide System: Role in Endogenous Cyto- and Organoprotection, Pathways of Pharmacological Modulation. Kyiv Yuston Publ. House 2020, 232. [Google Scholar]
- Granne, I.; Southcombe, J.H.; Snider, J.V.; Tannetta, D.S.; Child, T.; Redman, C.W.; Sargent, I.L. ST2 and IL-33 In Pregnancy and Pre-Eclampsia. PLoS ONE 2011, 6, E24463. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Reyes, L.M.; Morton, J.S.; Fung, D.; Schneider, J.; Davidge, S.T. Effect of Resveratrol on Metabolic and Cardiovascular Function in Male and Female Adult Offspring Exposed to Prenatal Hypoxia and A High-Fat Diet. J. Physiol. 2016, 594, 1465–1482. [Google Scholar] [CrossRef]
- Qian, S.; Chen, G.; Li, R.; Ma, Y.; Pan, L.; Wang, X.; Wang, X. Disulfide Stress and Its Role in Cardiovascular Diseases. Redox Biol. 2024, 75, 103297. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still A Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Krych, S.; Kramkowski, K.; Jęczmyk, A.; Hrapkowicz, T. Effect of Oxidative Stress on Mitochondrial Damage and Repair in Heart Disease and Ischemic Events. Int. J. Mol. Sci. 2024, 25, 12467. [Google Scholar] [CrossRef]
- Guo, Z.; Tian, Y.; Liu, N.; Chen, Y.; Chen, X.; Yuan, G.; Chang, A.; Chang, X.; Wu, J.; Zhou, H. Mitochondrial Stress as a Central Player in the Pathogenesis of Hypoxia-Related Myocardial Dysfunction: New Insights. Int. J. Med. Sci. 2024, 21, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tian, Y.; Gao, J.; Zhou, B.; Zhou, X.; Chang, X.; Zhou, H. Enhancement of Mitochondrial Homeostasis: A Novel Approach to Attenuate Hypoxic Myocardial Injury. Int. J. Med. Sci. 2024, 21, 2897–2911. [Google Scholar] [CrossRef]
- Song, H.; Polster, B.M.; Thompson, L.P. Chronic Hypoxia Alters Cardiac Mitochondrial Complex Protein Expression and Activity in Fetal Guinea Pigs in a Sex-Selective Manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R912–R924. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St Clair, D.; Batinic-Haberle, I. Manganese Superoxide Dismutase, MnSOD and Its Mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Yuen, R.K.; Chen, B.; Blair, J.D.; Robinson, W.P.; Nelson, D.M. Hypoxia Alters the Epigenetic Profile in Cultured Human Placental Trophoblasts. Epigenetics 2013, 8, 192–202. [Google Scholar] [CrossRef]
- Yan, X.; Fang, Y.; Yuan, Y.; Ding, Y.; Yu, H.; Li, Y.; Shi, Q.; Gao, Y.; Zhou, X.; Zhang, D.; et al. Combined Analysis of The Effects of Hypoxia and Oxidative Stress on DNA Methylation and The Transcriptome In HTR-8/SVneo Trophoblast Cells. J. Cell. Mol. Med. 2024, 28, E18469. [Google Scholar] [CrossRef]
- Leonard, M.O.; Cottell, D.C.; Godson, C.; Brady, H.R.; Taylor, C.T. The Role of HIF-1 Alpha in Transcriptional Regulation of The Proximal Tubular Epithelial Cell Response to Hypoxia. J. Biol. Chem. 2003, 278, 40296–40304. [Google Scholar] [CrossRef]
- Mendoza, E.N.; Ciriolo, M.R.; Ciccarone, F. Hypoxia-Induced Reactive Oxygen Species: Their Role in Cancer Resistance and Emerging Therapies to Overcome It. Antioxidants 2025, 14, 94. [Google Scholar] [CrossRef]
- Giussani, D.A. The Fetal Brain Sparing Response to Hypoxia: Physiological Mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, X.; Liu, Y.; Lu, L.; Zhu, D.; Zhang, Y.; Li, L.; Zhang, P.; Gao, Q.; Lu, X.; et al. Prenatal Hypothyroidism Diminished Exogenous NO-Mediated Diastolic Effects in Fetal Rat Thoracic Aorta Smooth Muscle Via Increased Oxidative Stress. Reprod. Toxicol. 2022, 113, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Korge, P.; Ping, P.; Weiss, J.N. Reactive Oxygen Species Production in Energized Cardiac Mitochondria During Hypoxia/Reoxygenation: Modulation by Nitric Oxide. Circ. Res. 2008, 103, 873–880. [Google Scholar] [CrossRef]
- Spiess, B.D. Perfluorocarbon Emulsions, Platelet Counts, and Inflammation. Shock 2019, 52 (Suppl. S1), 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.C.; Gorman, G.D.; Copeland, J.G.; McDonagh, P.F. Perflubron Emulsion Prevents PMN Activation and Improves Myocardial Functional Recovery After Cold Ischemia and Reperfusion. J. Surg. Res. 2007, 138, 135–140. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Corrigendum to “Therapeutic Oxygen Delivery by Perfluorocarbon-Based Colloids” [Advances in Colloid and Interface Science 294 (2021) 102407]. Adv. Colloid Interface Sci. 2021, 295, 102505. [Google Scholar] [CrossRef]
- Janjua, M.B.; Makepeace, C.M.; Anastacio, M.M.; Schuessler, R.B.; Nichols, C.G.; Lawton, J.S. Cardioprotective Benefits of Adenosine Triphosphate-Sensitive Potassium Channel Opener Diazoxide Are Lost with Administration After the Onset of Stress in Mouse and Human Myocytes. J. Am. Coll. Surg. 2014, 219, 803–813. [Google Scholar] [CrossRef]
- Ahmad, T.; Wang, J.; Velez, A.K.; Suarez-Pierre, A.; Clement, K.C.; Dong, J.; Sebestyen, K.; Canner, J.K.; Murphy, M.P.; Lawton, J.S. Cardioprotective Mechanisms of Mitochondria-Targeted S-Nitrosating Agent and Adenosine Triphosphate-Sensitive Potassium Channel Opener Are Mutually Exclusive. JTCVS Open 2021, 8, 338–354. [Google Scholar] [CrossRef]
- Larrieu, A.J.; Yazdanfar, S.; Redovan, E.; Eftychiadis, A.; Kao, R.; Silver, J.; Ghosh, S.C. Beneficial Effects of Cocarboxylase in the Treatment of Experimental Myocardial Infarction in Dogs. Am. Surg. 1987, 53, 721–725. [Google Scholar] [PubMed]
- Bondarenko, I.P. Effect of Riboxin on Hemodynamics and Microcirculation in Patients with Chronic Ischemic Heart Disease. Vrach Delo 1983, 1, 15–18. (In Russian) [Google Scholar] [PubMed]
- Dunaev, V.V.; Tishkin, V.S.; Evdokimov, E.I.; Belaĭ, I.M. The Mechanism of Action of Riboxin. Farmakol. Toksikol. 1989, 52, 56–58. (In Russian) [Google Scholar] [PubMed]
- Mazur, I.A.; Chekman, I.S.; Belenichev, I.F. Metabolitotropnye Preparaty; Zaporozhye, Ukraine, 2007; p. 309, ISBN 5-225-04144-2. Available online: https://www.researchgate.net/publication/326369488_Metabolitotropnye_preparaty (accessed on 4 March 2024).
- Novikov, V.E.; Levchenkova, O.S.; Ivantsova, E.N.; Vorobieva, V.V. Mitochondrial Dysfunctions and Antihypoxants. Rev. Clin. Pharmacol. Drug Ther. 2019, 17, 31–42. [Google Scholar] [CrossRef]
- Pospelova, M.L.; Barnaulov, O.D. The Antihypoxant and Antioxidant Effects of Medicinal Plants as the Basis for Their Use in Destructive Diseases of The Brain. Hum. Physiol. 2000, 26, 86–91. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Domann, F.E. Regulatory Crosstalk and Interference Between the Xenobiotic and Hypoxia Sensing Pathways at the AhR-ARNT-HIF1α Signaling Node. Chem. Biol. Interact. 2014, 218, 82–88. [Google Scholar] [CrossRef]
- Dow, L.F.; Case, A.M.; Paustian, M.P.; Pinkerton, B.R.; Simeon, P.; Trippier, P.C. The Evolution of Small Molecule Enzyme Activators. RSC Med. Chem. 2023, 14, 2206–2230. [Google Scholar] [CrossRef]
- Weinberg, J.; Liu, K.H.; Lee, C.M.; Crandall, W.J.; Cuevas, A.R.; Druzak, S.A.; Morgan, E.T.; Jarrell, Z.R.; Ortlund, E.A.; Martin, G.S.; et al. Mammalian Hydroxylation of Microbiome-Derived Obesogen, Delta-Valerobetaine, To Homocarnitine, A 5-Carbon Carnitine Analog. J. Biol. Chem. 2025, 301, 108074. [Google Scholar] [CrossRef]
- Stoyanova, S.; Bogdanov, M.G. Rational Design, Synthesis, and In Vitro Activity of Heterocyclic Gamma-Butyrobetaines as Potential Carnitine Acetyltransferase Inhibitors. Molecules 2025, 30, 735. [Google Scholar] [CrossRef]
- Gerashchenko, A.D.; Pozdnyakov, D.I.; Voronkov, A.V. Study of Dose-Dependent Actoprotective Effect of ATACL On Physical Performance and Psychoemotional Status of Animals Under Exhausting Exercise. Res. Results Pharmacol. 2022, 8, 13–22. [Google Scholar] [CrossRef]
- Liepinsh, E.; Vilskersts, R.; Skapare, E.; Svalbe, B.; Kuka, J.; Cirule, H.; Pugovics, O.; Kalvinsh, I.; Dambrova, M. Mildronate Decreases Carnitine Availability and Up-Regulates Glucose Uptake and Related Gene Expression in the Mouse Heart. Life Sci. 2008, 83, 613–619. [Google Scholar] [CrossRef]
- Grigoryan, S.V.; Hazarapetyan, L.G.; Stepanyan, A.A. An Experience of Meldonium Use in Patients with Ventricular Arrhythmias of Ischemic Genesis. Kardiologiia 2019, 59, 26–30. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Sjakste, N.; Gutcaits, A.; Kalvinsh, I. Mildronate: An Antiischemic Drug for Neurological Indications. CNS Drug Rev. 2005, 11, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Bellman, V. Unlocking the Potential of Meldonium: From Performance Enhancement to Therapeutic Insights. Psychoactives 2024, 3, 235–247. [Google Scholar] [CrossRef]
- Vilskersts, R.; Kigitovica, D.; Korzh, S.; Videja, M.; Vilks, K.; Cirule, H.; Skride, A.; Makrecka-Kuka, M.; Liepinsh, E.; Dambrova, M. Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with A Potential Application In COVID-19. Int. J. Mol. Sci. 2022, 23, 45. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Vilskersts, R.; Makarova, E.; Kuka, J.; Liepinsh, E. Pharmacological Effects of Meldonium: Biochemical Mechanisms and Biomarkers of Cardiometabolic Activity. Pharmacol. Res. 2016, 113 Pt B, 771–780. [Google Scholar] [CrossRef]
- Zarubina, I.V.; Lukk, M.V.; Shabanov, P.D. Antihypoxic and Antioxidant Effects of Exogenous Succinic Acid and Aminothiol Succinate-Containing Antihypoxants. Bull. Exp. Biol. Med. 2012, 153, 336–339. [Google Scholar] [CrossRef]
- Zhao, F.; Li, R.; Liu, Y.; Chen, H. Perspectives on Lecithin from Egg Yolk: Extraction, Physicochemical Properties, Modification, and Applications. Front. Nutr. 2023, 9, 1082671. [Google Scholar] [CrossRef]
- Tanaka-Kanegae, R.; Kimura, H.; Hamada, K. Oral Administration of Egg- and Soy-Derived Lysophosphatidylcholine Mitigated Acetylcholine Depletion in the Brain of Scopolamine-Treated Rats. Nutrients 2023, 15, 3618. [Google Scholar] [CrossRef]
- Onaolapo, M.C.; Alabi, O.D.; Akano, O.P.; Olateju, B.S.; Okeleji, L.O.; Adeyemi, W.J.; Ajayi, A.F. Lecithin and Cardiovascular Health: A Comprehensive Review. Egypt. Heart J. 2024, 76, 92. [Google Scholar] [CrossRef]
- Tuteja, N.; Chandra, M.; Tuteja, R.; Misra, M.K. Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology. J. Biomed. Biotechnol. 2004, 2004, 227–237. [Google Scholar] [CrossRef]
- Cannavo, A.; Koch, W.J. GRK2 As Negative Modulator of NO Bioavailability: Implications for Cardiovascular Disease. Cell. Signal. 2018, 41, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q.; Zhang, X.; Zhu, X.; Dong, X.; Shen, L.; Zhang, S.; Niu, L.; Chen, L.; Zhang, M.; et al. L-Arginine Alleviates LPS-Induced Oxidative Stress and Apoptosis Via Activating SIRT1-AKT-Nrf2 and SIRT1-FOXO3a Signaling Pathways in C2C12 Myotube Cells. Antioxidants 2021, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Bielenichev, I.F.; Vizir, V.A.; Mamchur, V.Y.; Kuriata, O.V. Place of Tiotriazoline in the Gallery of Modern Metabolitotropic Medicines. Zaporozhye Med. J. 2019, 21, 1. [Google Scholar] [CrossRef]
- Gureev, A.P.; Nesterova, V.V.; Babenkova, P.I.; Ivanov, M.E.; Plotnikov, E.Y.; Silachev, D.N. L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 2902. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Mazur, I.A.; Chekman, I.S. Report on Preclinical Study of Specific Biological Activity (Anti-Ischemic, Endotelioprotective) of the “Liziniy” Preparation upon Parenteral Administration; Zap Med J: Zaporizhzhya, Ukraine, 2011; 87p. [Google Scholar]
- Belenichev, I.F.; Mazur, I.A.; Chekman, I.S. Report on Preclinical Study of Specific Pharmacological Activity (Cardioprotective, Neuroprotective, Endotelioprotective) of “Angiolin” Tablets. Zap Med J: Zaporizhzhya, Ukraine, 2015; 167p. [Google Scholar]
- Abramov, A.V. Report on Blind Study of Cardioprotective and Endotelioprotective Effects of New “Angiolin” Injection Solution, 25 mg/mL (OJSC "NPO “Farmatron”, Ukraine) Compared with “Tiotriazoline” Injection Solution, 25 mg/mL (JSC "Galichfarm" with Participation of OJSC "NPO "Farmatron", Ukraine) and "Mildronate" Injection Solution, 100 mg/mL (JSC “Grindex”, Latvia) upon Intraperitoneal Administration in Pituitrin-Isadrin Myocardial Infarction Model in Laboratory Rats; Zap Med J: Zaporizhzhya, Ukraine, 2013; 25p. [Google Scholar]
- Mamchur, V.I.; Belenichev, I.F.; Mazur, I.A. Report on Pharmacological Expertise (Acute and Chronic Toxicity) of (S)-2,6-Diaminogexanoic Acid 3-Methyl-1,2,4-Triazolyl-5-Thioacetate (“Liziniy”) and Its 2.5% Solution for Parenteral Use; Zap Med J: Dnipropetrovsk, Ukraine, 2011; 139p. [Google Scholar]
- Belenichev, I.F.; Mazur, I.A.; Abramov, A.V.; Kucherenko, L.I.; Bukhtiyarova, N.V.; Egorov, A.A.; Belenicheva, O.I.; Polyakova, E.N. The Endothelium-Protective Effect of 3-Methyl-1,2,4-Triazolyl-5-Thioacetate (S)-2,6-Diaminohexanic Acid (Lysinium): Effects on the Expression of Vascular Endothelial Growth Factor (VEGF) and The Characteristics of the Endotheliocytes of the Cerebral Vessels of Animals with Cerebral Ischemia. Neurochem. J. 2013, 7, 296–302. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response Against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Lima-Cabello, E.; Martínez-Flórez, S.; Almar, M.; Cuevas, M.J.; González-Gallego, J. Hypoxia-Inducible Factor-1 Modulates the Expression of Vascular Endothelial Growth Factor and Endothelial Nitric Oxide Synthase Induced by Eccentric Exercise. J. Appl. Physiol. 2015, 118, 1075–1083. [Google Scholar] [CrossRef]
- Hood, J.D.; Meininger, C.J.; Ziche, M.; Granger, H.J. VEGF Upregulates EcNOS Message, Protein, and NO Production in Human Endothelial Cells. Am. J. Physiol. 1998, 274, H1054–H1058. [Google Scholar] [CrossRef]
- Tishkin, V.S.; Belenichev, I.F.; Serikov, V.I. Report on Study of General Toxic Effects of “Thiotriazoline Injection Solution 2.5% for Intravenous Administration”; Zap Med J: Zaporizhzhya, Ukraine, 1990; 41p. [Google Scholar]
- Reshetilov, V.I.; Karzov, M.V.; Knysh, E.G. Report on Study of Embryotoxic Properties of New Drug “Thiotriazoline” and Its Pharmaceutical Forms; Zap Med J: Zaporizhzhya, Ukraine, 1994; 66p. [Google Scholar]
- Mazur, I.A.; Voloshin, N.A.; Knysh, E.G. Report on Study of Mutagenic Properties of New Drug “Thiotriazoline” and Its Pharmaceutical Forms; Zap Med J: Zaporizhzhya, Ukraine, 1994; 123p. [Google Scholar]
- Mazur, I.; Belenichev, I.; Kucherenko, L.; Siusiuka, V.; Reznichenko, N.; Kyryliuk, A.; Ryzhenko, V.; Shevchenko, A.; Khromylova, O. Thiotriazoline and Its Today; Jurfond: Dnipro, Ukraine, 2025; 384p, ISBN 978-966-934-648-3. [Google Scholar]
- Belenichev, I.F.; Nagornaia, E.A.; Chekman, I.S.; Bukhtyarova, N.V. Thiol-Disulfide System: Role in Endogenous Cytoprotection and Organoprotection, Paths of Pharmacological Modulation; PE “Yuston Publishing”: Kyiv, Ukraine, 2020; 232p, ISBN 978-617-7854-05-9. [Google Scholar]
- Zvyagintseva, T.V.; Myronchenko, S.I.; Kytsyuk, N.I.; Naumova, O.V. The Effect of Ointments with Antioxidant Activity on The Morphological Structures of Skin of Guinea Pigs Exposed to Local Ultraviolet Irradiation. Precarpathian Bull. Shevchenko Sci. Soc. Pulse 2019, 6, 64–72. [Google Scholar] [CrossRef]
- Manischenkova, Y.; Shkala, L.; Dudich, T.; Litvinova, M.; Ivanova, M. Oxidative Stress in Patients with Diabetes Mellitus Type 2 Associated with Exacerbation of Chronic Pyelonephritis. Int. J. Endocrinol. 2022, 3, 19–22. [Google Scholar] [CrossRef]
- Mukhina, I.V. Report on Independent Preclinical Assessment of Specific Activity of Thiotriazoline Pharmaceutical Forms (2.5% Injection Solution and Tablets). Nizhny Novgorod, Russia, 2009; 57p.
- Kucherenko, L.I.; Hromyleva, O.V.; Mazur, I.A.; Shishkina, S.V. Theoretical Study About L-Arginine Complexes Formation with Thiotriazoline. Zaporozhye Med. J. 2017, 19, 108–112. [Google Scholar] [CrossRef][Green Version]
- Kastanayan, A.A.; Kartashova, E.A.; Zheleznyak, E.I. The Effect of Thiotriazoline on Energy Production in Conditions of Chronic Myocardial Ischemia. South Russ. J. Ther. Pract. 2020, 1, 84–90. [Google Scholar] [CrossRef]
- Dzyak, G.V. Report on Clinical Study: “Double-Blind, Multicenter, Randomized Study in Parallel Groups to Evaluate the Efficacy and Tolerability of Thiotriazoline”; Kyivmedpreparat PJSC Archive: Kyiv, Ukraine, 2010. [Google Scholar]
- Kadin, D.V.; Chumak, B.A.; Filippov, A.E.; Shustov, S.B. Thiotriazoline in the Treatment of Stable Angina II-III Functional Class. Kardiologiia 2015, 55, 26–29. (In Russian) [Google Scholar] [CrossRef]
- Kuka, J.; Vilskersts, R.; Cirule, H.; Makrecka, M.; Pugovics, O.; Kalvinsh, I.; Dambrova, M.; Liepinsh, E. The Cardioprotective Effect of Mildronate Is Diminished After Co-Treatment with L-Carnitine. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 215–222. [Google Scholar] [CrossRef]
- Liepinsh, E.; Vilskersts, R.; Loca, D.; Kirjanova, O.; Pugovichs, O.; Kalvinsh, I.; Dambrova, M. Mildronate, An Inhibitor of Carnitine Biosynthesis, Induces an Increase in Gamma-Butyrobetaine Contents and Cardioprotection in Isolated Rat Heart Infarction. J. Cardiovasc. Pharmacol. 2006, 48, 314–319. [Google Scholar] [CrossRef]
- Berlato, D.G.; Bairros, A.V. Meldonium: Pharmacological, Toxicological, and Analytical Aspects. Toxicol. Res. Appl. 2020, 4, 2397847320915143. [Google Scholar] [CrossRef]
- Gureev, A.P.; Sadovnikova, I.S.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Mildronate Protects Heart MtDNA from Oxidative Stress Toxicity Induced by Exhaustive Physical Exercise. Arch. Biochem. Biophys. 2021, 705, 108892. [Google Scholar] [CrossRef]
- Bielenichev, I.F.; Gorchakova, N.A.; Doroshenko, E.Y.; Samura, I.B.; Ryzhenko, V.P.; Bukhtiiarova, N.V. Use of Metabolites, Metabolithotropic Agents and Nutritional Supplements in Sports and Sports Medicine: A Modern View on the Problem. Mod. Med. Technol. 2023, 4, 76–88. [Google Scholar] [CrossRef]
- Abel, T.; Knechtle, B.; Perret, C.; Eser, P.; Von Arx, P.; Knecht, H. Influence of Chronic Supplementation of Arginine Aspartate in Endurance Athletes on Performance and Substrate Metabolism—A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Sports Med. 2005, 26, 344–349. [Google Scholar] [CrossRef]
- Castell, L.M.; Stear, S.J.; Burke, L.M. (Eds.) Nutritional Supplements in Sport, Exercise and Health: An A-Z Guide; Routledge: London, UK, 2015. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Impact of Arginine Nutrition and Metabolism During Pregnancy on Offspring Outcomes. Nutrients 2019, 11, 1452. [Google Scholar] [CrossRef]
- Bednov, A.; Espinoza, J.; Betancourt, A.; Vedernikov, Y.; Belfort, M.; Yallampalli, C. L-Arginine Prevents Hypoxia-Induced Vasoconstriction in Dual-Perfused Human Placental Cotyledons. Placenta 2015, 36, 1254–1259. [Google Scholar] [CrossRef]
- Suvorava, T.; Nagy, N.; Pick, S.; Lieven, O.; Rüther, U.; Dao, V.T.; Fischer, J.W.; Weber, M.; Kojda, G. Impact of eNOS-Dependent Oxidative Stress on Endothelial Function and Neointima Formation. Antioxid. Redox Signal. 2015, 23, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Parkhomenko, D.; Belenichev, I.; Bukhtiyarova, N.; Kuchkovskyi, O.; Gorchakova, N.; Diachenko, V.; Fedotov, E. Pharmacocorrection of Disturbances in the NO System in Experimental Chronic Generalized Periodontitis. Open Access Maced. J. Med Sci. 2023, 11, 47–52. [Google Scholar] [CrossRef]
- Maslennikov, S.O.; Golovakha, M.L.; Belenichev, I.F.; Makyeyeva, L.V. Regenerative Properties of Polypropylene Mesh Coated with Thiotriazoline and L-Arginine. Biomed. Pharmacol. J. 2022, 15, 1985–1993. [Google Scholar] [CrossRef]
- Malla, R.; Viswanathan, S.; Makena, S.; Kapoor, S.; Verma, D.; Raju, A.A.; Dunna, M.; Muniraj, N. Revitalizing Cancer Treatment: Exploring the Role of Drug Repurposing. Cancers 2024, 16, 1463. [Google Scholar] [CrossRef]
- Cousins, H.; Nayar, G.; Altman, R. Computational Approaches to Drug Repurposing: Methods, Challenges, and Opportunities. Annu. Rev. Biomed. Data Sci. 2024, 7, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5′ AMP-activated Protein Kinase and Its Emerging Potential Beyond Anti-Hyperglycemic Action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Lushchak, O.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin in Antiviral Therapy: Evidence and Perspectives. Viruses 2024, 16, 1938. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.; Kamyshnyi, A. Metformin Therapy Changes Gut Microbiota Alpha-Diversity in COVID-19 Patients with Type 2 Diabetes: The Role of SARS-CoV-2 Variants and Antibiotic Treatment. Pharmaceuticals 2023, 16, 904. [Google Scholar] [CrossRef]
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines 2024, 12, 1630. [Google Scholar] [CrossRef]
- Petakh, P.; Griga, V.; Mohammed, I.B.; Loshak, K.; Poliak, I.; Kamyshnyiy, A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Med. Arch. 2022, 76, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Pavlo, P.; Kamyshna, I.; Kamyshnyi, A. Effects of Metformin on the Gut Microbiota: A Systematic Review. Mol. Metab. 2023, 77, 101805. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a Drug for All Reasons and Diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes. Viruses 2024, 16, 281. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; Kamyshnyi, A. Efficacy of Interferon Alpha for the Treatment of Hospitalized Patients with COVID-19: A Meta-Analysis. Front. Immunol. 2023, 14, 1069894. [Google Scholar] [CrossRef]
- Kamyshnyi, A.; Koval, H.; Kobevko, O.; Buchynskyi, M.; Oksenych, V.; Kainov, D.; Lyubomirskaya, K.; Kamyshna, I.; Potters, G.; Moshynets, O. Therapeutic Effectiveness of Interferon-α2b Against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. Int. J. Mol. Sci. 2023, 24, 6887. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vorobets, I.; Halabitska, I.; Kamyshnyi, O. Modulatory Roles of AHR, FFAR2, FXR, and TGR5 Gene Expression in Metabolic-Associated Fatty Liver Disease and COVID-19 Outcomes. Viruses 2024, 16, 985. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Budarna, O.; Halabitska, I.; Petakh, P.; Kamyshnyi, O. Insight into COVID-19 Severity in MAFLD Patients: A Single-Center Prospective Cohort Study. Front. Genet. 2024, 15, 1460318. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Sheida, A.; Taghizadieh, M.; Memar, M.Y.; Hamblin, M.R.; Bannazadeh Baghi, H.; Sadri Nahand, J.; Asemi, Z.; Mirzaei, H. Paxlovid (Nirmatrelvir/Ritonavir): A New Approach to Covid-19 Therapy? Biomed. Pharmacother. 2023, 162, 114367. [Google Scholar] [CrossRef]
- Kamyshna, I.I.; Pavlovych, L.B.; Maslyanko, V.A.; Kamyshnyi, A.M. Analysis of the Transcriptional Activity of Genes of Neuropeptides and Their Receptors in the Blood of Patients with Thyroid Pathology. J. Med. Life 2021, 14, 243–249. [Google Scholar] [CrossRef]
- Topol, I.; Kamyshny, A. Study of Expression of TLR2, TLR4 and Transcription Factor NF-kB Structures of GALT of Rats in the Conditions of the Chronic Social Stress and Modulation of Structure of Intestinal Microflora. Georgian Med. News 2013, 225, 115–122. [Google Scholar]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus. Nutrients 2024, 16, 3349. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.E.; Kamyshnyi, O. The Interplay of Gut Microbiota, Obesity, and Depression: Insights and Interventions. Cell. Mol. Life Sci. 2024, 81, 443. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Wang, Z.; Gan, J.; Yu, B.; Lu, B.; Jiang, X. Melatonin as a Therapeutic Agent for Alleviating Endothelial Dysfunction in Cardiovascular Diseases: Emphasis on Oxidative Stress. Biomed. Pharmacother. 2023, 167, 115475. [Google Scholar] [CrossRef] [PubMed]
- Dzhuryak, V.; Sydorchuk, L.; Sydorchuk, A.; Kamyshnyi, O.; Kshanovska, A.; Levytska, S.; Knut, R.; Sheremet, M.; Ivashchuk, S.; Petrynych, O.; et al. The cytochrome 11B2 aldosterone synthase gene CYP11B2 (RS1799998) polymorphism associates with chronic kidney disease in hypertensive patients. Biointerface Res. Appl. Chem. 2020, 10, 5406–5411. [Google Scholar] [CrossRef]
- Strumillo, J.; Nowak, K.E.; Krokosz, A.; Rodacka, A.; Puchala, M.; Bartosz, G. The Role of Resveratrol and Melatonin in the Nitric Oxide and Its Oxidation Products Mediated Functional and Structural Modifications of Two Glycolytic Enzymes: GAPDH and LDH. Biochim. Biophys. Acta. Gen. Subj. 2018, 1862, 877–885. [Google Scholar] [CrossRef]
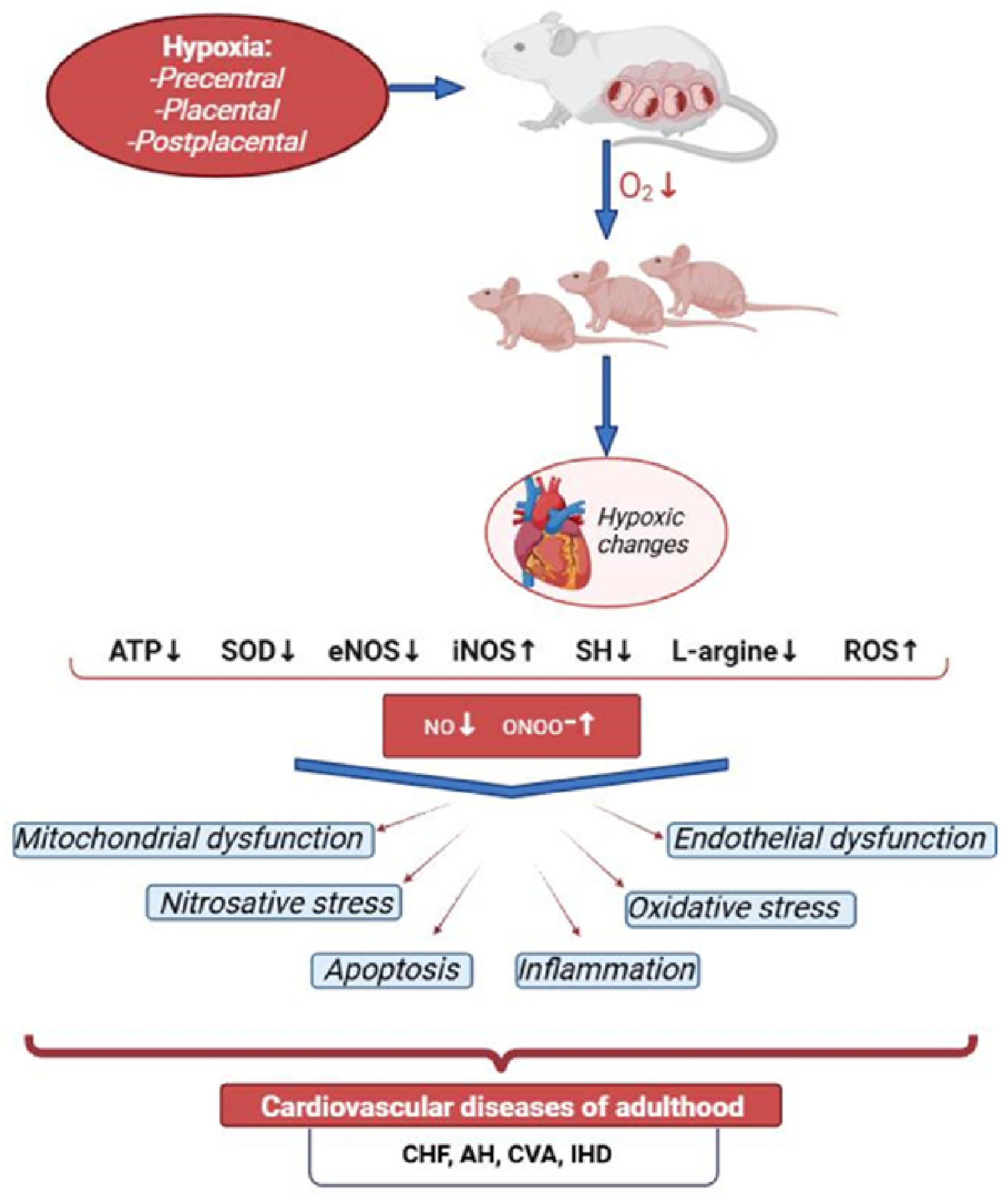
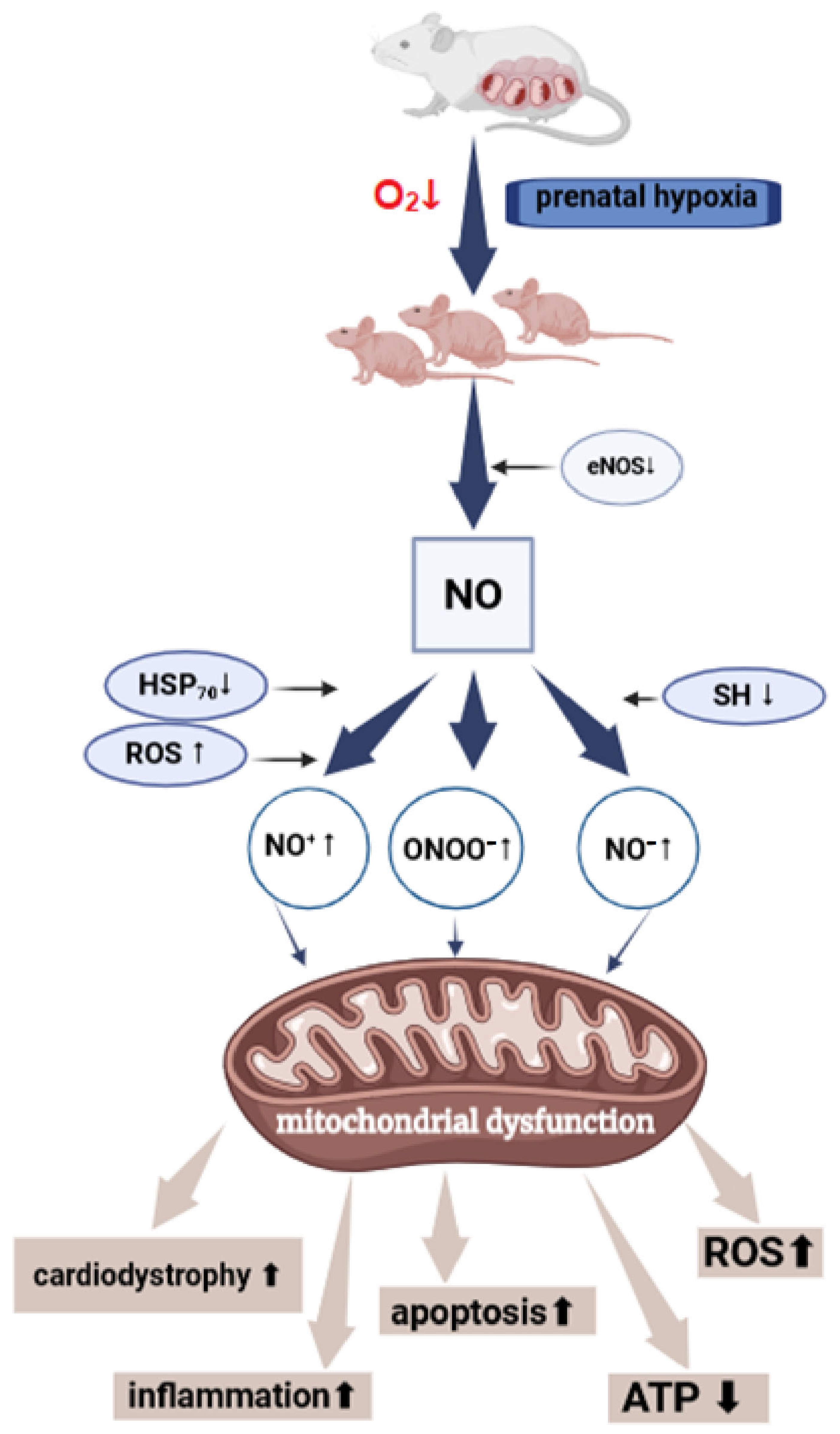
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popazova, O.; Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Gorchakova, N.; Oksenych, V.; Kamyshnyi, O. Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia—Focus on the NO System. Antioxidants 2025, 14, 743. https://doi.org/10.3390/antiox14060743
Popazova O, Belenichev I, Bukhtiyarova N, Ryzhenko V, Gorchakova N, Oksenych V, Kamyshnyi O. Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia—Focus on the NO System. Antioxidants. 2025; 14(6):743. https://doi.org/10.3390/antiox14060743
Chicago/Turabian StylePopazova, Olena, Igor Belenichev, Nina Bukhtiyarova, Victor Ryzhenko, Nadia Gorchakova, Valentyn Oksenych, and Oleksandr Kamyshnyi. 2025. "Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia—Focus on the NO System" Antioxidants 14, no. 6: 743. https://doi.org/10.3390/antiox14060743
APA StylePopazova, O., Belenichev, I., Bukhtiyarova, N., Ryzhenko, V., Gorchakova, N., Oksenych, V., & Kamyshnyi, O. (2025). Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia—Focus on the NO System. Antioxidants, 14(6), 743. https://doi.org/10.3390/antiox14060743









